1. Introduction
1.1. Ocular Nerve Anatomy in Different Species
The extraocular muscles (EOMs) control eye movements, innervated by three pairs of cranial nerves known as the oculomotor nerve (CNIII), trochlear nerve (CNIV), and abducens nerve (CNVI). These nerves arise from their respective motor nuclei in the brainstem and travel through the skull to innervate specific somites, the EOMs. In tetrapods, the oculomotor nerve (CNIII) controls the superior rectus, inferior rectus, medial rectus, and inferior oblique muscles (
Table 1). The trochlear nerve (CNIV) innervates the superior oblique muscle, and the abducens nerve (CNVI) innervates the lateral rectus muscle (LR) and has retractor bulbi. The coordinated action of these EOMs allows precise eye movements such as tracking a moving object or maintaining stable gaze during head movements, provided by the vestibulo-ocular reflect (VOR; [
1,
2,
3,
4,
5]). In tetrapods, oculomotor special somatic motor neurons [
6] project their axons through cranial nerve III to form an inferior division that innervates the ipsilateral inferior rectus (IR), medial rectus (MR), and inferior oblique (IO), and a superior division that innervates the contralateral superior rectus (SR). Trochlear special somatic motor neurons project their axons through cranial nerve IV to innervate the contralateral superior oblique muscle (SO;
Table 1). Abducens somatic motor neurons innervate through cranial nerve VI to reach the lateral rectus muscle (LR).
The extraocular muscles are innervated by the same three cranial nerves and are typically considered identical across vertebrates [
7,
8]. That said, oculomotor, trochlear and abducens targets can vary across species. Lampreys have only three cranial branches to the extraocular muscles whereas gnathostomes have four cranial nerves (
Table 1). The targets of cranial nerve III include the nasal rectus (NR) that is innervated by a dorsal ramus comprising only contralateral fibers in chondrichthyans and lungfish. In contrast, the medial rectus (MR) is innervated by a ventral ramus which originates from ipsilateral fibers in bony fish,
Latimeria and tetrapods [
9,
10,
11,
12,
13,
14,
15,
16,
17]. Moreover, unique to mammals, is the levator palpebrae superioris (LPS) muscle that elevates the eyelid [
18,
19]. Trochlear motor neurons send their axons to exit the brainstem dorsally and then cross the midline to innervate the contralateral IO [
9,
10,
15,
20]. The abducens nucleus (cranial nerve VI) innervates two extraocular muscles in lampreys (the caudal and ventral rectus) but is restricted to a single muscle, the lateral rectus, in gnathostomes (
Table 1). Basicranial muscles are likely present in all sarcopterygians (lobe-finned fishes); these muscles evolved to form the retractor bulbi in most tetrapods [
21,
22,
23,
24,
25,
26,
27,
28]. The retractor bulbi (
Table 1) are reduced or absent in derived microchiropters and primates [
29,
30].
Overall, the three ocular cranial nerves and associated extraocular muscles develop in a stereotyped fashion that is best understood from research in mice [
17,
31,
32].
1.2. Ocular Nerve Growth and Guidance
The growth and guidance of axons is a complex process that involves the coordinated action of many genes, and any disruptions or mutations in these genes can lead to abnormal development of the nervous system and related disorders. Several developmental defects are associated with the
Sema3 gene family, involving genes that interact with neuropilins (
Npn1/2) and
Plexina1/2 to control trochlear decussation [
33,
34]. In addition,
Netrin-1 repels the trochlear axons and
Unc5 [
35,
36,
37]. The loss of genes
Slit1/2 and
Robo1/2 can likewise result in aberrant connections of trochlear axons [
38,
39,
40]. Defects in axon guidance are associated with genes
Robo3, Col25a1, and there are also various
Tubb gene-related defects and gain-of function mutations [
34,
38,
41].
1.3. Vestibular Inputs to the Motor Neurons Which Innervate the Extraocular Muscles
Epithelial mesodermal coeloms are believed to represent serial homolog of somites to generate three extraocular muscles [
32]. The ocular motor neurons are themselves innervated by vestibular nuclei [
1,
5,
42]. Vestibular inputs to ocular motor neurons display different patterns of connections in lampreys, chondrichthyans, and Osteichthyes [
11,
21,
43,
44]. Among teleosts, frogs, birds, and mammals each have a specific pattern of innervation by vestibular inputs [
1,
4,
17,
42,
45]. By contrast, studies in chondrichthyans (cartilaginous fishes) revealed a different pattern with a combination of ipsilateral and contralateral nerve fibers [
11,
12]. Many details of vestibular relationships are incomplete in lampreys (which lack a horizontal canal) but can be compared to the pattern of connections in gnathostomes [
42,
43,
46,
47,
48].
1.4. Perturbation of the Extraocular Muscles
Among these genes are those controlling the projections that can be the source of developmental defects. In mice, certain mutations result in the reduction or gain-of-function of these two muscles, resulting in ptosis due to the inability to lift the eyes and lids [
19]. Perturbations in the growth and guidance of ocular cranial nerves can cause paralytic strabismus, which is one of a number of congenital cranial dysinnervation disorders (CCDDs;[
49]). In addition, axons can make targeting errors implicating aberrant cell body migration and axon extension [
34].
Connection of three ocular motor neurons that innervate distinct EOMs will be described in
Section 2 and
Section 3. Next, the genetic basis of how and why different ocular motor neuron innervate the ocular muscles in vertebrates will be provided (
Section 4 and
Section 5). Vestibular nuclei connections differ between the eyes and ears across vertebrates (
Section 6). Finally, an overview of axonal defects in mutant mice and humans is provided, detailing the congenital cranial dysinnervation disorders (
Section 7).
2. Extraocular Muscles are Innervated by Three Cranial Nerves
Three groups of ocular motor neurons develop that innervate cranial nerve III+IV+VI to reach out the six EOMs.
2.1. Ocular Motor Neurons (CNIII)
Ocular motor neurons projects ipsilaterally, with the exception of the oculomotor superior division, in which they project contralaterally [
50]. In all vertebrates (
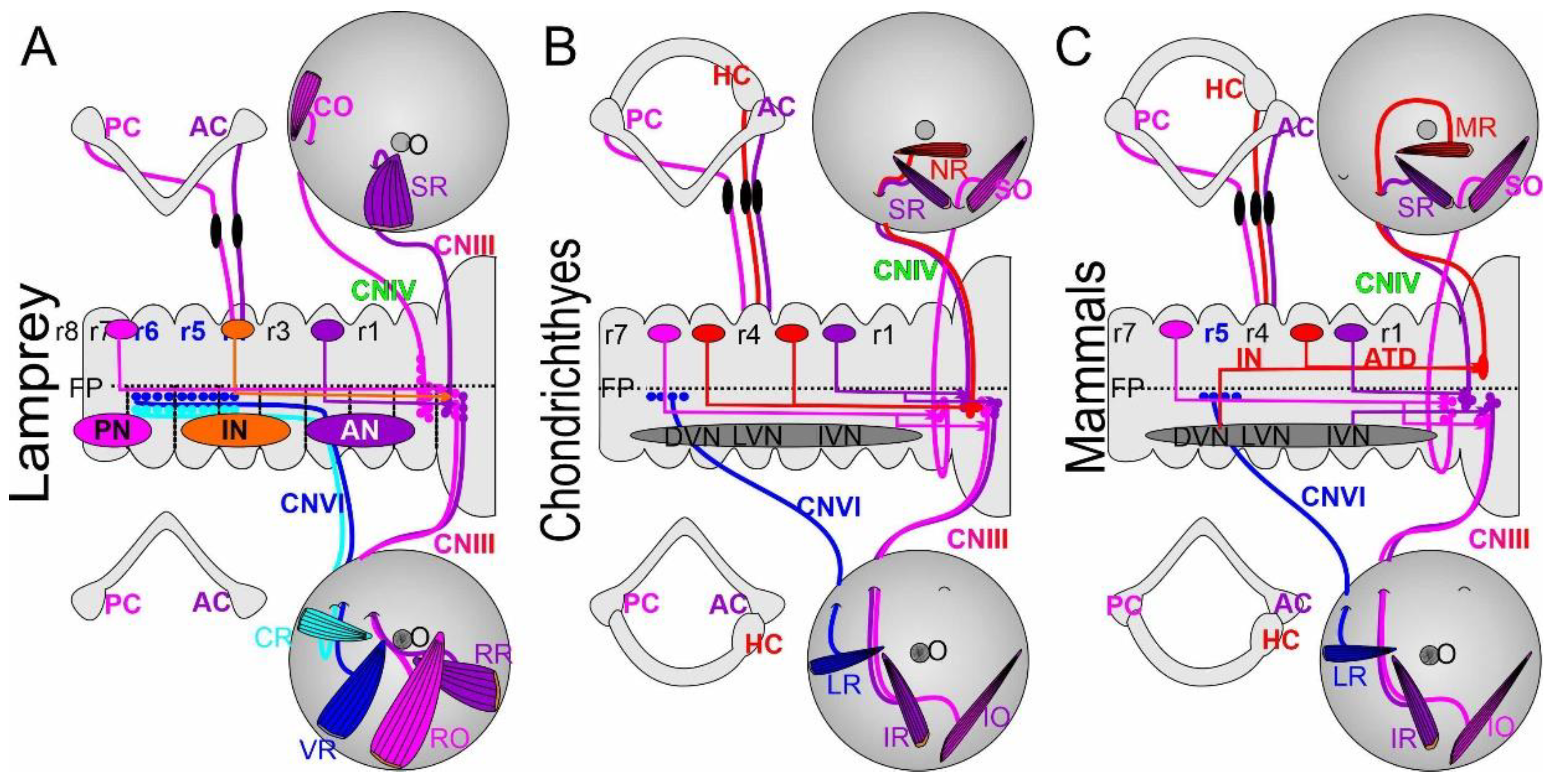
Figure 1), a contralateral motor neuron population arises to join the ipsilateral cranial nerve CNIII [
17,
36,
51,
52,
53]. In addition to the contralateral projections to the superior rectus (SR) is a bilateral innervation of the levator palpebrae superior [LPS; [
19]]. Although all axonal projections exit the brainstem as multiple small bundles, their axons coalesce into a single nerve to form cranial nerve CNIII (
Figure 1). Lampreys have only three branches that innervate the extraocular muscles (
Figure 1): the superior rectus is innervated from contralateral cranial nerve III whereas two ipsilateral branches innervate the rostral rectus and rostral oblique [
9,
16,
53]. In contrast to gnathostomes, chondrichthyans and lungfish (
Figure 1) show a dorsal branch around the optic nerve to innervate the superior rectus and the nasal rectus (NR) whereas only two ventral branches form the inferior rectus and inferior oblique innervation [
11,
12,
13,
16,
54]. Among tetrapods, Latimeria and teleosts exhibit one branch that passes dorsal to the optic nerve to reach the superior rectus [
9,
16,
19,
31], which is innervated from the contralateral cranial nerve CNIII [
19,
36,
52,
55,
56]. In addition, three ventral branches innervate the inferior rectus (IR), medial rectus (MR), and inferior oblique (IO) through ipsilateral projections in cranial nerve III.
2.2. Trochlear Motor Neurons (CNIV)
Trochlear motor neurons have contralateral projections that extend dorsally to cross near the cerebellum and target a single extraocular muscle, the SO/CO (
Figure 1). In lampreys, trochlear motor neurons have a unique dorsal position that develops next to the cerebellum from which they innervate largely bilateral neurons to reach the CO [
9,
16,
53]. These motor neurons also have long dendrites to extend close to the ocular motor neurons. Whether the trochlear nucleus develop close to the cerebellum or migrates there from more ventral origins is debated [
53,
57]. In gnathostomes, trochlear motor neurons form a largely contralateral projection which crosses near the cerebellum and innervates the SO rostrally. The bundle of CNIV can be demonstrated to cross over the contralateral CNIII by dye tracing and other means such as tubulin antibodies [
4,
19,
31,
36,
38,
56,
58]. Studies of mutants and lesions to the trochlear have shown a mix of ipsi- and contralateral fibers. For example, cutting the nerve produces a redirection that interacts with adjacent CIII fibers to innervate the SO in frogs [
59,
60,
61,
62] and chicken [
63,
64].
2.3. Abducens (CNVI)
The abducens projects ipsilaterally to innervate the lateral rectus (LR; also referred to the posterior rectus; PR), which is equivalent to the ventral rectus (VR) in lampreys (
Figure 1;
Table 1). In addition, lampreys have a second extraocular muscle that is referred to as the caudal rectus (CR). It appears that the equivalent to the CR was lost in chondrichthyans, lungfish and actinopterygians [
11,
13]. In contrast,
Latimeria and tetrapods have a unique innervation of a separate abducens extraocular muscle, the basicranial rectus (BR), and the retractor bulbi (RB;
Figure 2). In the sarcopterygian coelacanth
Latimeria, the BR has no connection to the eye [
22,
25,
65,
66]. This innervation provides a very large muscle that likely is common across basal sarcopterygians [
23], including the famous prehistoric sarcopterygian
Eusthenopteron [
67]. Unfortunately, details of CNVI organization in
Latimeria are unclear [
68] and would require modern labeling methods such as antibodies with tubulin [
41,
69]. The retractor bulbi (RB) in tetrapods has a ventral position that is comparable to the CR in lampreys (
Figure 1). Among tetrapods, the RB can be very large (as in chickens,
Figure 2; [
24]), whereas the RB is absent in microchiroptera and primates [
29,
34]. Moreover, a unique formation, known as the tentacular retractor muscle, which is equivalent to the retractor bulbi in amniotes [
21], is present in caecilians [
27,
28]. The distribution of the constituent neurons differs between vertebrates: they extends from r5 to 6 and parts of r4 in lampreys [
8,
43,
70], derive exclusively from r6 in chondrichthyans [
8], and are derived from r5 and 6 in sauropsids and teleosts [
8] but exclusively from r5 in lungfish, amphibians and mammals [
8,
20,
45].
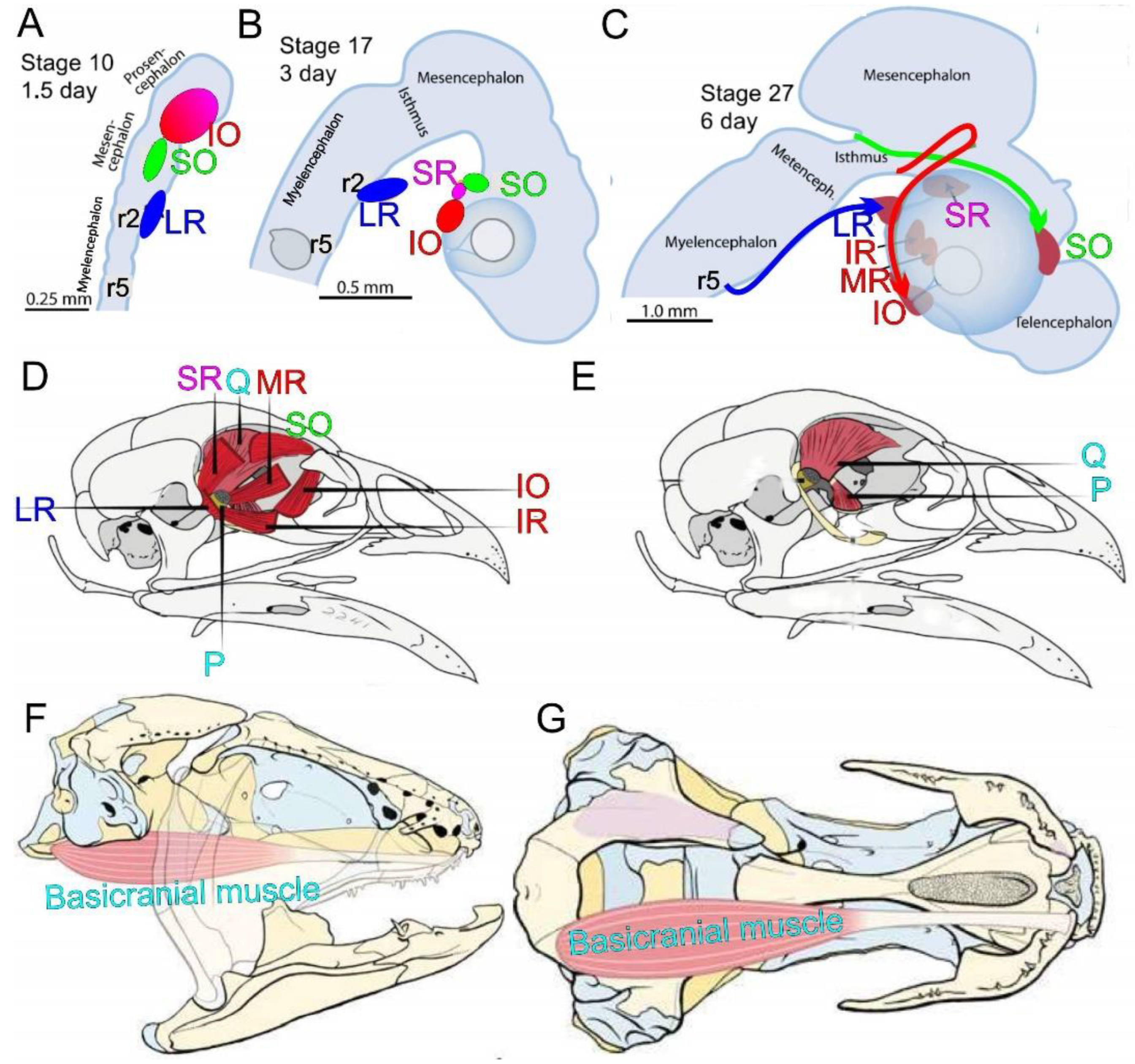
3. Extraocular Muscles are Derived from Several Sources
Extraocular muscles (EOMs) are in many ways unique compared to other skeletal muscles [
32,
71,
72]. Head cavities, or epithelial mesodermal coeloms in the head, are thought to represent serial homologs of somites (
Figure 2). Three pairs of head cavities form each pharyngeal pouches thereby showing a metamerical arrangement that was the basis of three cranial somites [
8,
16,
73]. Extraocular muscles may diverge from different muscle precursors that are under the influence of different extrinsic signals [
71]. The extraocular muscles are not affected from Duchenne muscular dystrophy but are differentially affected by other conditions, including myasthenia gravis [
71]. The extraocular muscles differ in composition from branchial and trunk muscles as they have distinctive fibers and a unique pattern of innervation that allows fine control of movement [
4].
Paraxial mesoderm was proposed as somitomeres that were transformed into epithelial somites [
74]. More recently, it was demonstrated that myoblasts form in paraxial mesoderm that will differentiate into distinct muscle [
32]. Extraocular muscles form relatively close to the midbrain (CNIII), midbrain-hindbrain (CNIV) and hindbrain (CNVI;
Figure 2). Muscle fibers in the chicken will split into 4 extraocular muscles that innervate CNIII, will migrate a long distance to innervate the dorsal SO (CNIV) and develop into CNVI innervated by lateral rectus precursors close to the trigeminal ganglion and next to rhombomere 2 (
Figure 2; [
31,
32,
38]). In addition, CNVI also provides the retractor bulbi and innervation.
Myf5 and
Pitx2 are sequentially active and are essential for myoblasts for all ocular muscles that do not differentiate in a mutation in mice [
31,
38].
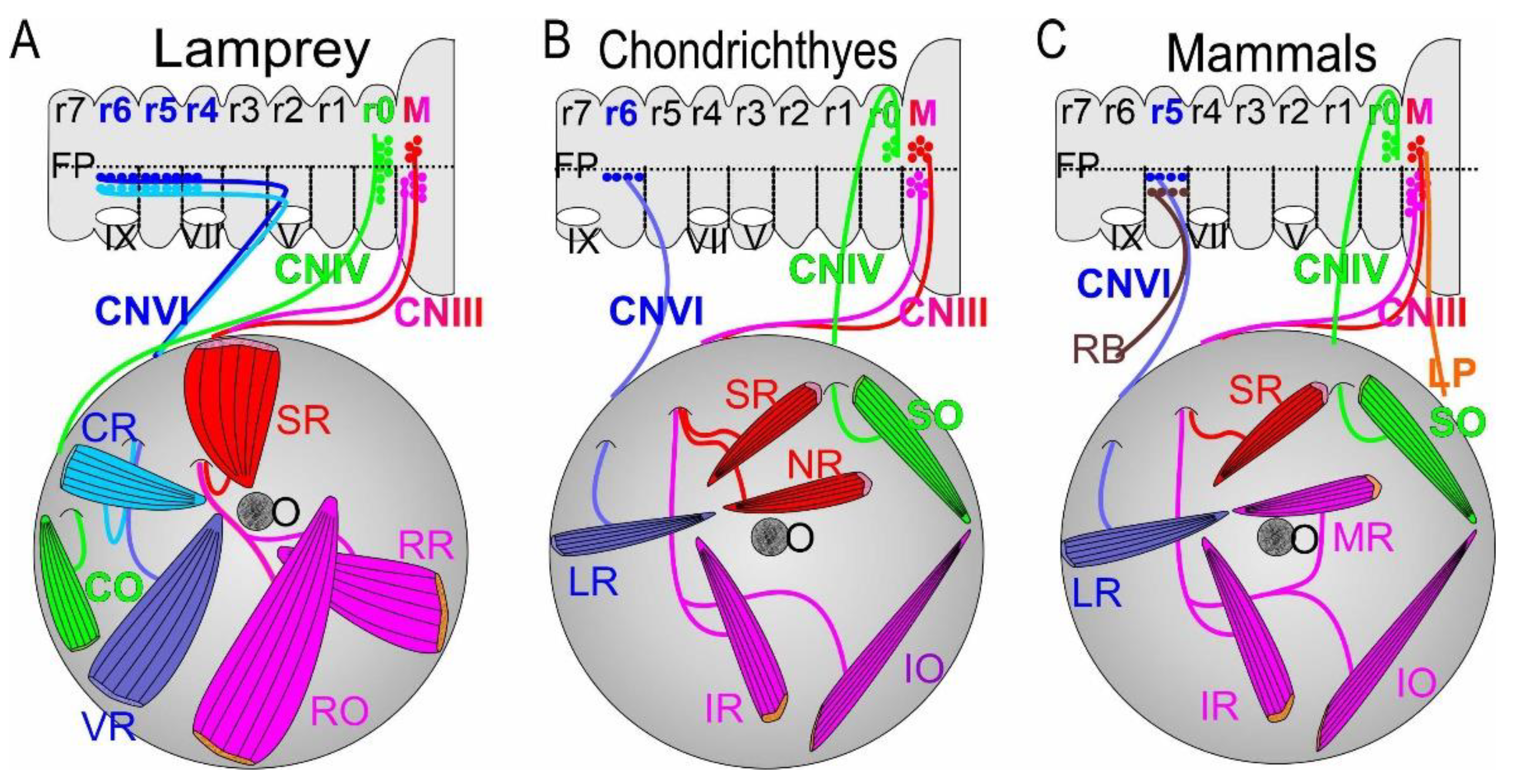
Figure 3.
Cladistic analysis of vertebrate extraocular eye muscles and their cranial innervation, shown over 520 million of years (MYA). Hagfish lack EOMs; lampreys have three CNIII (blue color), whereas four CNIII are common in gnathostomes. Two ipsi- and two contralateral fibers form in chondrichthyans and lungfish, including a unique nasal rectus (NR) muscle (orange color). Three ipsi- and one contralateral innervation from Osteichthyes, inclduing the medial rectus (MR) muscle (lilac color). In addition, lampreys have two abducens muscle fibers whereas only a single abducens is found to the lateral rectus (LR) in all gnathostomes (lilac and orange). A variation of the rhombomeres shows three origins, chondrichthyans is from r6, lungfish, amphibians, and mammals provide r5 CNVI neurons and sauropsids and teleost have two rhombomeres, r5 + 6.
Latimeria have a very large abducens, the basicranial muscle, which requires proper investigation. The retractor bulbi is equivalent to one of the two lamprey eye muscles. Basicranial and tentaculate muscles, as well as quadratus/pyramidalis muscles, seem to be derived from retractor bulbi muscles. A split into the levator palpebrae from the superior rectus is a unique late segregation in mammals. The ages of different lines are indicated as approximations in millions of years. Assembled from [
4,
8,
9,
11,
12,
13,
20,
22,
23,
27,
31,
32,
71,
75,
76].
Figure 3.
Cladistic analysis of vertebrate extraocular eye muscles and their cranial innervation, shown over 520 million of years (MYA). Hagfish lack EOMs; lampreys have three CNIII (blue color), whereas four CNIII are common in gnathostomes. Two ipsi- and two contralateral fibers form in chondrichthyans and lungfish, including a unique nasal rectus (NR) muscle (orange color). Three ipsi- and one contralateral innervation from Osteichthyes, inclduing the medial rectus (MR) muscle (lilac color). In addition, lampreys have two abducens muscle fibers whereas only a single abducens is found to the lateral rectus (LR) in all gnathostomes (lilac and orange). A variation of the rhombomeres shows three origins, chondrichthyans is from r6, lungfish, amphibians, and mammals provide r5 CNVI neurons and sauropsids and teleost have two rhombomeres, r5 + 6.
Latimeria have a very large abducens, the basicranial muscle, which requires proper investigation. The retractor bulbi is equivalent to one of the two lamprey eye muscles. Basicranial and tentaculate muscles, as well as quadratus/pyramidalis muscles, seem to be derived from retractor bulbi muscles. A split into the levator palpebrae from the superior rectus is a unique late segregation in mammals. The ages of different lines are indicated as approximations in millions of years. Assembled from [
4,
8,
9,
11,
12,
13,
20,
22,
23,
27,
31,
32,
71,
75,
76].
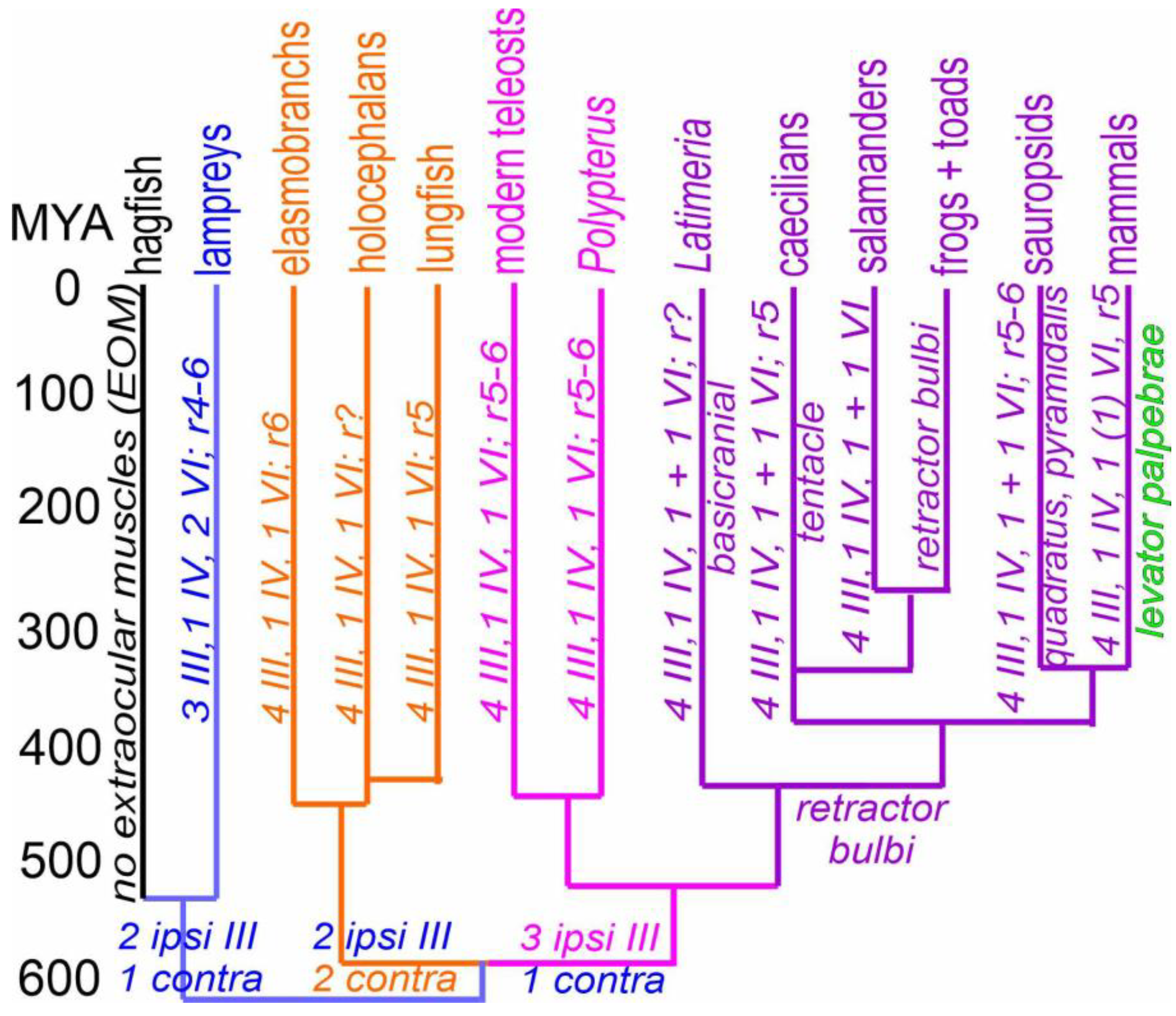
Traditionally, almost all extraocular muscles are suggested to be nearly identical across vertebrates [
32]. However, a few additional or unique extraocular muscles and their innervation are known. Lampreys have three CNIII, a CNIV and two extraocular muscles that are innervated by CNVI (
Figure 1 and
Figure 3; [
9]). In contrast, gnathostomes have four CNIII fibers that split into two combinations: two ipsi- and two contralateral branches of CNIII supplies, inclduing the contralateral nasal rectus (NR; chondrichthyans, lungfish), whereas bony fishes,
Latimeria and tetrapods have one contralateral and three ipsilateral CNIII branches, including projection to ipsilateral medial rectus (MR;
Figure 3). The CNIV in lampreys and gnathostomes is likely equivalent between the CO and SO and migrates late from the midbrian/hinbrain into a different, rostral position in gnathostomes (
Figure 2 and
Figure 3). Whether one CNVI evolved in lampreys to become the retractor bulbi in sarcopterygians remains to be seen (
Figure 2 and
Figure 3). In addition, the retractor bulbi in tetrapods converts into two muscles in birds, the pyramidalis and quadratus, which drive the nictitating movement in almost all amniotes (
Figure 2 and
Figure 3; [
24,
77]). A unique tentacular retractor muscle in amphibians [
27,
28] derives from the retractor bulbi. The basicranial muscle is innervated by the abducens, which is not connected with the eyes (
Figure 2 and
Figure 3; [
22,
23,
25,
65,
66]). Chondrichthyans, teleosts and lungfish do not have an ‘accessory’ muscle but several cases of unique branches of new extraocular muscles are reported [
10,
78]. An additional branch is a nictitating membrane in elasmobranchs, which evolved independently in sauropsids and most mammals [
77,
79,
80]. The nictitating membranes are replaced by eyelids in chiroptera and primates [
29,
30], which evolved into a muscle unique to mammals, the levator palpebrae (LP; [
18,
19]), which is innervated by contralateral cranial nerve III [
34].
In summary, muscle formation is derived from paraxial mesoderm, which generates cranial nerves III, IV and VI to innervate extraocular muscles, including the unique branch of lampreys, Latimeria and tetrapods. A detailed analysis of gains and losses of CNIII and CNVI requires additional work to elucidate the genetic and development of different muscle fibers and their pattern of innervation in vertebrates.
4. Development and Axon Guidance
Dating of rat cranial motor neurons using 3H-thymidine reveal that they form along a caudal-to-rostral gradient, with abducens emerging at E12 and oculomotor and trochlear motor neurons between
E12 and E13 [
81,
82].
The extension of nerve fibers begins at about E10 in mice, reaches the extraocular muscles at about E11 and segregates to each of the six extraocular muscles by about E13 [
19,
31,
38]
. Despite overall similarities in the two special somatic motor neuron groups and the one somatic motor neuron group, each group is unique in its projections through cranial nerves CN
III, CNIV and CNVI. A detailed Edinger-Westphal complex develops in tetrapods [
6,
83]
. A temporal progression of gene expression is evident for cranial nerves CNIII and CNIV in mice, zebrafish and chicken [
55,
83]
. In contrast, abducens nerve development is independent of CNVI [
84,
85,
86]
but depends on Hox genes.
The long-established use of 3H-Thymidine as a marker for proliferation is now complemented by use of the synthetic thymidine analog bromodeoxyuridine (BrdU) [
55]. BrdU expression starts in chicken at developmental stage 11 and ends at about stage 23 [
55]. This pattern of proliferation is confirmed for the abducens in zebrafish [
87]. A gradient is found whereby ocular motor neurons develop slightly later from trochlear motor neuron IV in zebrafish [
17,
75], whereas mammals have a temporal overlap of cranial nerve CNIII/IV/VI cell cycle exits [
81,
88]. Specifically, the innervation of extraocular muscles begins in zebrafish around 30 h postfertilization (hpf) and reaches all six eye muscles by about 54 hpf [
75]. Fluorescent labeling to detail the generation of cranial nerves CNIII and CN IV has demonstrated an earlier projection [
17].
Recent labeling of EdU [
89] could help detail the origin of ocular motor neurons across vertebrates from lampreys through mammals and shows clear dorso-ventral progression in the chicken [
55]. Extraocular muscles innervating neurons that develop spatial and temporal maturity to generate the three ocular motor neurons (CNIII, CNIV, CNVI). Only hagfish have no eye muscles and are lacking all ocular motoneurons [
90], unique among vertebrates [
45]. Cranial nerve III innervates the contralateral superior rectus and ipsilateral inferior oblique, inferior rectus and medial rectus (
Figure 1) in teleosts, chicken, mice [
31,
38,
55,
75,
91] and lampreys [
53,
57]. Cranial nerve III fibers require 1-3 days (zebrafish, mammals) to develop, with a pause prior to expanding to innervate the four extraocular muscles [
38]. Mice show delayed innervation compared to chickens thanks to the superior rectus that migrates across the floor plate to innervate the superior rectus (and levator palpebrae [
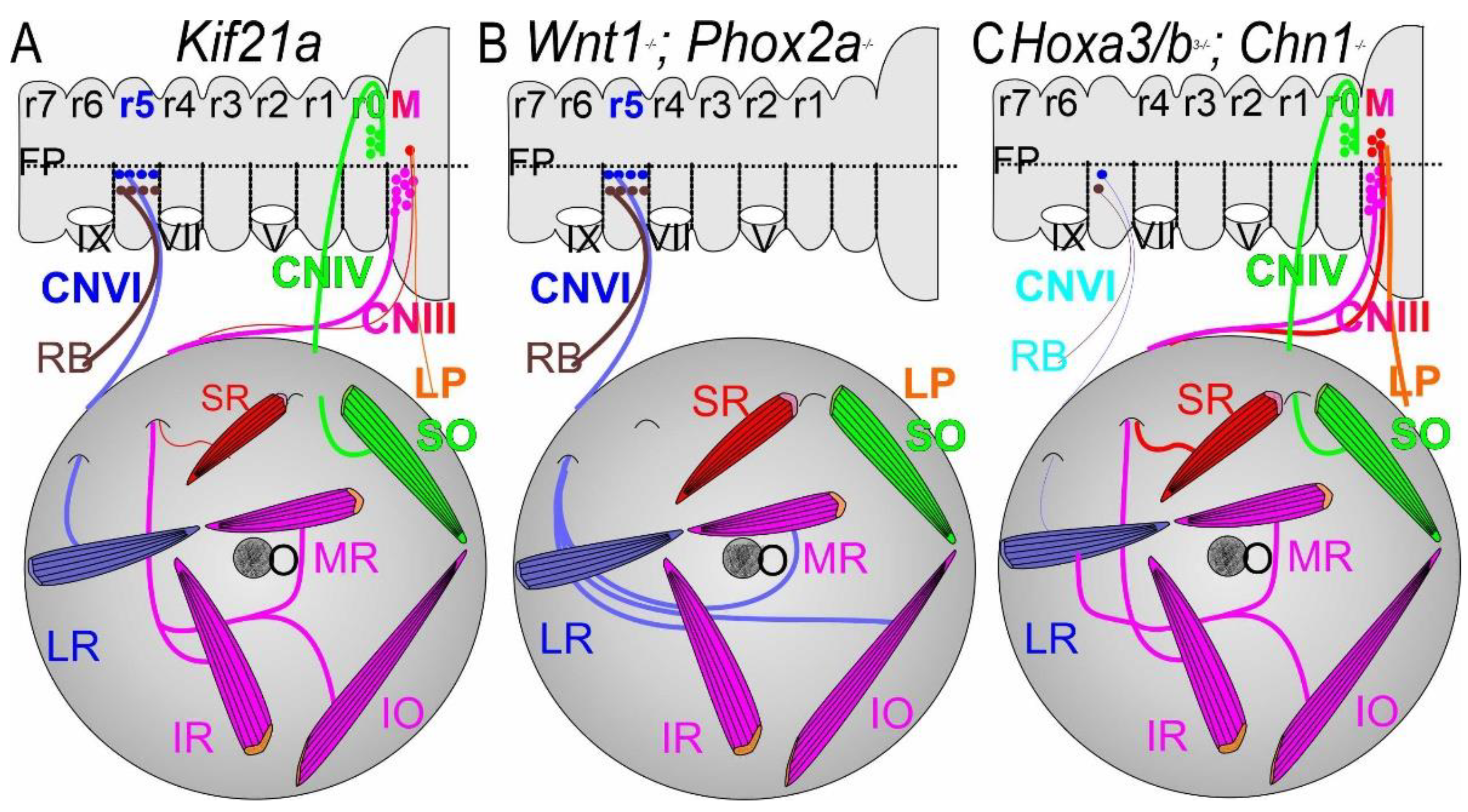
31]). Development of the superior rectus depends on the
Kif21a mouse mutant that eventually becomes reduced by cell death in the contralateral neurons, as documented by using caspase immunofluorescent labeling [
19]. In addition, instead of extending to the superior rectus as in control mice, the axons prematurely exit from the dorsal root but do extend completely to the superior rectus (or levator palpebrae). Consequently, the elevation of levator palpebrae and upward eye movements caused by the superior rectus are reduced and there is partial degeneration of the extraocular muscles [
19]. Moreover, EOMs depend on genes that affect the muscle innervation [
92].
4.1. Ocular Motor Neurons
Deletion of roundabout guidance receptor (
Robo) and slit guidance ligand (
Slit) causes derailed innervation after the incomplete contralateral superior rectus of cranial nerve III [
40]. Mice that are null for
myogenic factor 5 (Myf5) do not have any extraocular muscle formation [
31]. The initial patterns of innervation by ocular nerves develop nearly normally but, in the absence of their EOM targets, the nerves have begun to degenerate by E14.5 [
31]. Loss of
paired-like homeodomain transcription factor 2 (Pitx2) results in disoriented fibers and delayed innervation [
38]. Functions are needed for S
emaphorin3f (Sema3f), which interacts with
Neuropilin 2 (
Nrp2), which requires defasciculating of cranial nerve III [
33,
93].
Sema3a interacts with
Nrp1 to regulate mouse innervation; it requires detailing the ocular motor innervation [
94].
Sema3a/c ligands signal via
Plexin a1 (
Plxna1) and α2-
chimaerin (
Chn1), and can result in defasciculating [
75,
95,
96,
97], which involves a double null for
Sema3a/f and
Nrp1/2 [
93,
94], including
Plxna1 and
Chn1. To fully understand the various ligands that repel or attract different nerve ocular fibers that have an additional role for
Nova2 in various peripheral innervation [
98] requires detailing its effect on extraocular muscles’ innervation.
In summary, three patterns of ocular motor neurons form with three branches to reach from one contra- and two ipsilateral branches in lampreys, two innervate from ipsi- and two contralateral branches in chondrichthyans and lungfish, whereas one contra- and three ipsilateral branches are found in teleosts,
Latimeria and tetrapods (
Figure 3). In mice, the cranial nerve III axons begin to extend by E10, interacts with extraocular muscles by E11, and develops branches around E13 which proceed to innervate four extraocular muscles. Absence of
Wnt1 or
Phox2a results in the loss of innervation of extraocular muscles. The genes
Robo, Slit, Nrp2, Sema3f, Plxn1, Chn1, Myf5 and
Pitx2 influence fiber branching, which is specifically affected by
Kif21a superior rectus and levator palpebrae.
4.2. Trochlear Fibers
Trochlear fibers are involved in several genes controlling projections that plays a role for dysplasia [
99,
100].
Netrin1 (
Ntn1) acts as a bifunctional guidance cue that simultaneously attracts some axons to the floor plate while steering others away [
35].
α- and
β-tubulin monomers of different tubulin are near normal in terms of loss-of-function mutations, but there can be a missense mutation in
Tubb3 [
41] that results in the misrouting of CNIII and CNIV. Downstream,
Ntn1 interacts with
Dcc to create attractive or repulsive signaling.
Unc5 netrin receptor c (Unc5c) displays an incomplete and variable projection to reach the SO bilaterally [
36,
37] and interacts with
Fgf8 [
101,
102].
Nrp2 must interact with
Sema3f because null mutants show loss or absence of CNIV [
34,
93]. Interestingly, exuberant branches form that innervate CNIII after perturbations of
Myf5 and
Pitx2 [
31,
38]. Incomplete crossing results if
α2-chimaerin (Chn1KI/KI) mutants are combined with
Epha4KO/KO, pointing to reversed and bidirectional interactions. A loss-of-function of
Chn1KO/KO with
Epha4KO/KO [
88] documents the interaction with
ephrin A5 (
Efna5). Misexpression of
Smo using
Pax2 results in redirection of trochlear fibers to exit ipsilaterally, adjacent to CNIII [
36]. Unfortunately,
Pax2-cre; Smo overexpressing mice die early around E15 before the extraocular muscles have fully developed, meaning that the pattern of innervation in a redirected ipsilateral SO innervation is not known.
In summary, the trochlear nerve innervates the CO/SO from dorsal (lamprey) or ventral (gnathostomes) origins.
4.3. Abducens Fibers
Abducens fibers depend on
Shh and other genes for differentiation of neurons in r5 of mice [
69,
84,
103,
104,
105,
106]. Downstream,
Sall4 is found in mice [
97,
107].
Robo3 in CNVI show exuberant crossings and combine with ipsilateral projections [
34,
108,
109]. Downstream of
Mafb are several cadherins that lead to misdirections in
Pcdh17 null mutant zebrafish [
110]. Several factors interact with semaphorins, plexins and neuropilins and participate in guidance of the abducens fibers [
93,
94]. Abducens defasciculating occurs after loss of
Npr1 [
33,
34]. In addition,
α2-chimaerin abducens’ guidance in the absence of loss or gain of this gene is described
(Chn1KO/KO; Chn1KI/KI). Moreover,
Eph receptor A4 (
Epha4KO/KO) with
Efna5 interacts to signal forward and reverse; both are critical for abducens signaling, pointing to a bidirectional interaction [
34,
88].
In contrast to CNVI, innervation of CNIII is unaffected by
Hoxa3/b3 and
Mafb [
69,
97,
103]. Likewise,
Hoxa1 loss-of-function deletions results in aberrant innervation of the abducens [
111] interacting with
Sall4 [
105,
107]. Conversely, loss of
Lmx1b, Wnt1 and
Phox2a, which eliminates CNIII and CNIV, results in an expansion of CNVI to innervate the denervation of remaining extraocular muscles [
85,
86,
112], showing a plasticity in the residual innervation.
In summary, CNVI is unique in its size (from r4-6, just r6, just r5 or both r5 and 6) and gives rise to major abducens projections and additional extraocular muscles like retractor bulbi.
Overall, gains and losses are evident in lampreys, chondrichthyans and sarcopterygians, which added two different ocular motoneurons (nasal and medial rectus), whereas the caudal rectus of lampreys evolved into the retractor bulbi in tetrapods. Fiber guidance is presented as a variation of the same theme for the three ocular motoneuron group with certain guidance genes in CNVI compared to CNIII and IV that may lead to dysplasia of innervation [
92]. The split into CNIII and VI requires additional work to elucidate the origin of the additional motoneurons.
5. Molecular Properties of Ocular III, IV and VI Motor Neurons
In vertebrates, sonic hedgehog (
Shh) acts as a long-range morphogen to define three different ventral lineages (V1-3) and all motor neurons. Oculomotor and trochlear motor neurons are special somatic motor neurons (SSM) while abducens are somatic motor neurons (SM) that generate next to the branchial motor neurons, (BM; [
6,
113,
114,
115,
116]). Special somatic motor neurons develop next to the isthmus (between the r0 and the midbrain) to generate ocular motor neurons (cranial nerve III) and trochlear motor neurons (cranial nerve IV) whereas somatic motor neurons are found in the abducens (cranial nerve VI). In addition, somatic motor neurons are continuous with the spinal cord and hypoglossal somatic motor neurons [
114,
117]. Following a discussion of the general role of
Shh, I will detail the genetics for CNIII/IV, followed by CN VI.
5.1. Ocular III + IV are Dependent on a Unique Set of Genes
The sonic hedgehog protein (
Shh) interacts with several genes: Patched (
Ptch), Smoothened (
Smo) and GLI-Kruppel family member
Gli1-3 [
36,
118]. The neurectoderm homeobox genes
Otx2/Gbx2 define the isthmic region that specifies the midbrain-hindbrain barrier, including the CNIII/IV in vertebrates [
119,
120,
121]. Modifying the concentration equilibrium between
Gbx2 and
Otx2 define the location of the midbrain-hindbrain boundary [
116,
121,
122,
123,
124]. Several transcription factors, including
En1/2, Foxa1/2, Lmx1a/b, Nurr1, and
Pitx3, interact with growth factors or morphogens such as
Shh, Fgf8, Tgf, and
Wnt1 [
102,
116,
121,
125,
126].
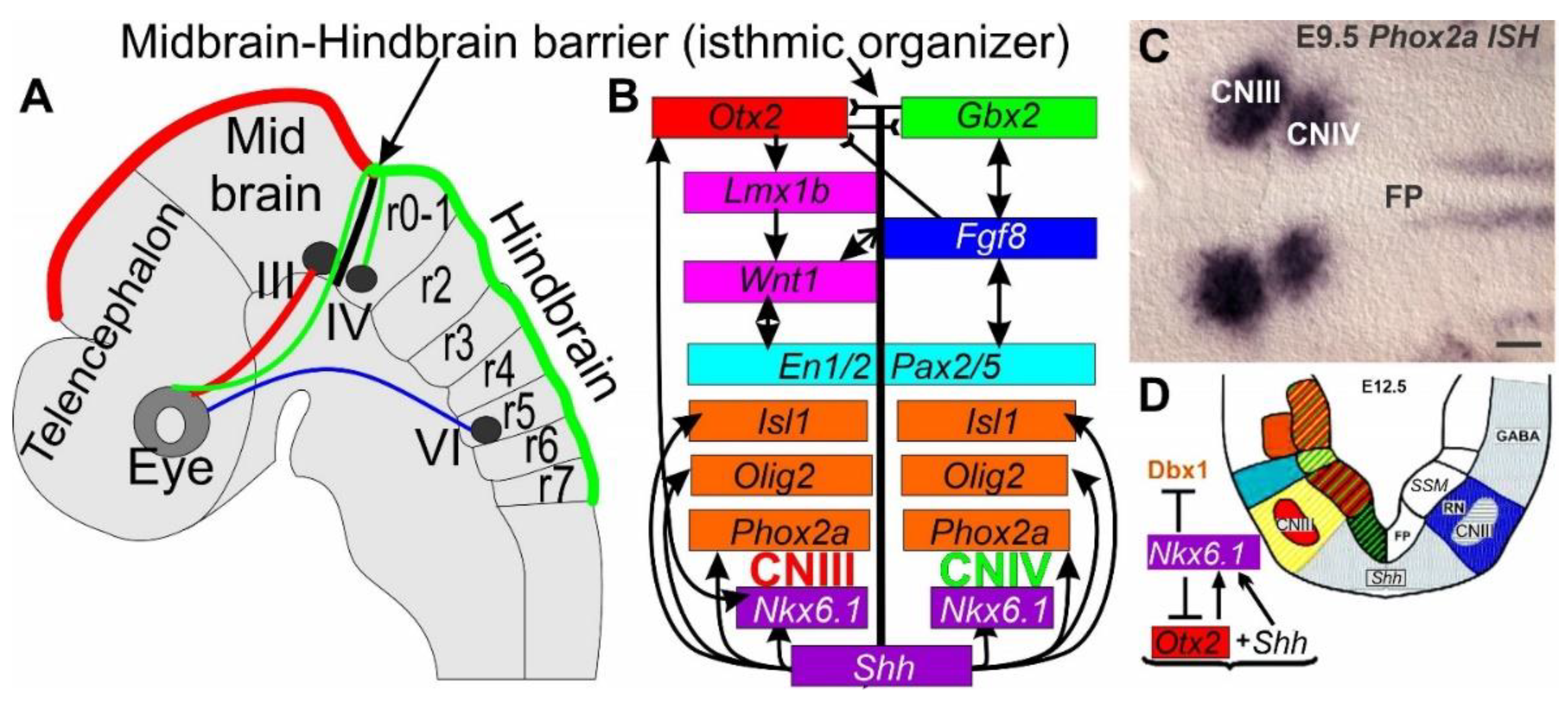
Figure 4.
Defining the isthmic organizer in the mouse. (A) The Midbrain-Hindbrain barrier is established by reciprocal inhibition of
Otx2 (red) and
Gbx2 (green) to regulate the isthmic organizer, including cranial nerves CNIII (midbrain, red) and CNIV (rhombomere 0, green) and cranial nerve VI at rhombomere 5 (blue). (B) Downstream is controlled by
Otx2 such as
Lmx1b and
Wnt1, whereas
Gbx2 is downstream of
Fgf8.
Shh is needed for
Nkx6.1, Phox2a, Olig2 and
Isl1 upregulation and interaction with
Otx2. (C)
Phox2a is expressed in the two adjacent groups of special somatic motor neurons located in cranial nerves (CN) III and IV. (D) Expression of
Shh is needed for upregulation of
Nkx6.1. Modified after [
36,
121,
127,
128,
129].
Figure 4.
Defining the isthmic organizer in the mouse. (A) The Midbrain-Hindbrain barrier is established by reciprocal inhibition of
Otx2 (red) and
Gbx2 (green) to regulate the isthmic organizer, including cranial nerves CNIII (midbrain, red) and CNIV (rhombomere 0, green) and cranial nerve VI at rhombomere 5 (blue). (B) Downstream is controlled by
Otx2 such as
Lmx1b and
Wnt1, whereas
Gbx2 is downstream of
Fgf8.
Shh is needed for
Nkx6.1, Phox2a, Olig2 and
Isl1 upregulation and interaction with
Otx2. (C)
Phox2a is expressed in the two adjacent groups of special somatic motor neurons located in cranial nerves (CN) III and IV. (D) Expression of
Shh is needed for upregulation of
Nkx6.1. Modified after [
36,
121,
127,
128,
129].
In addition to
Wnt1 (
Figure 4), other transcription factors such as
Notch-Delta,
Shh, transforming growth factor 13 (
TGF-13), and bone morphogenetic protein (
BMP), play a role in development [
123]. The transcription factor
Wnt1 is highly conserved across cnidarians, flies, and vertebrates and shows frizzled (
Fzd) and lipoprotein receptor-related protein (
Lrp5/6). β-catenin (
β-cat) activates transcription factors
Wnt, Dvl, Axin, β-cat, β-TrCP, Cki, Gsk, Apc and proteasome degrade
β-cat, which will be blocked by transcription factors through binding with
Graucho [
123]. Previous work showed the absence of cranial nerves III and IV after
Wnt1 loss [
85,
130] and reduced proliferation of CNIII and IV [
102,
123,
125].
Downstream of
Shh in mammals is the transcription factor Nkx6 homeobox 1 (
Nkx6.1), which interacts with oligodendrocyte transcription factor 1/2 (
Olig1/2) in special and somatic motor neurons [
117,
131,
132]. Specifically,
Nkx6.1 is a transcriptional repressor that is required for the development of somatic motor neurons and special somatic motor neurons in the brainstem and the spinal cord [
127].
Nkx6.1 expression requires derepression of the
Shh to develop brain homeobox 1 and 2 (
Dbx1 and
Dbx2) and fate-switching of motor neurons into interneurons [
127].
Otx2 participates in a reciprocal interaction with
Nkx6.1 to develop cranial nerve III (
Figure 4).
Upstream of ocular and trochlear motor neurons III and IV is the boundary of
Otx2/Gbx2 expression that defines the isthmic region [
36,
129]. Once the
Otx2/Gbx2 boundary is established (
Figure 4), the regulatory genes of
Shh/Fgf8/Lmx1b/Phox2a/Wnt1/En2 interact with each other to develop the isthmic region [
119,
124]. In the absence of
Otx2 there is reduced expression of
Nkx6.1, Olig2 and
Isl1 in cranial nerves III and IV. Furthermore,
Nkx6.1 is needed for
Phox2a to develop these cranial nerves while
Olig2,
Isl1 and
Pou4f1 are reduced after
Nkx6.1 null mutation [
112,
127].
Otx2 is upstream of
Lmx1b, which is required for cranial nerve III to develop special somatic motor neurons [
128].
Lmx1b is required for the earliest expression of
Phox2a, suggesting that
Phox2a acts downstream of
Lmx1b [
112,
119,
128]:
Lmx1b null mice show near complete loss of cranial nerves CNIII and CNIV in
Phox2a null mice [
112,
115]. Moreover,
Lmx1b is lost in initially expressed
Wnt1, En1 and
Fgf8 [
128], suggesting a dependence on the isthmic region that allows the formation of cranial nerves III and IV [
36,
119,
124].
Fgf8 expression is dependent on
Lmx1b [
128], which is absent after
Fgf8 null mice [
102,
121,
129]. Interestingly, early upregulation of
Nkx6.1 is evident in
Lmx1b null mice, suggesting partial independence from
Nkx6.1 and
Lmx1b [
112]. The expression of
Phox2a, Olig2 and
Isl1 is likely downstream of
Shh, Lmx1b and
Wnt1 [
112,
127,
132]. The expression of
Lmx1 in lampreys is controlled by a single gene, which could function as both
Lmx1a/b [
119,
133]. To know how many
Wnts are present in lampreys, and which could have a similar expression in the isthmus, requires more work to define the midbrain-hindbrain barrier in lampreys [
36,
124]. The trochlear motor neurons likely depend on
Nkx6.1 and
Phox2a, but studies using
in situ hybridization are required to demonstrate gene expression.
In summary, a complex genetic interplay generates the midbrain-hindbrain boundary, where
Shh, Otx2, Gbx2, Nkx6.1, Lmx1b, Phox2a, Wnt1, Fgf8, Olig2 and Isl1 interact to develop the two special somatic motor neuron of cranial nerves III and IV (
Figure 4).
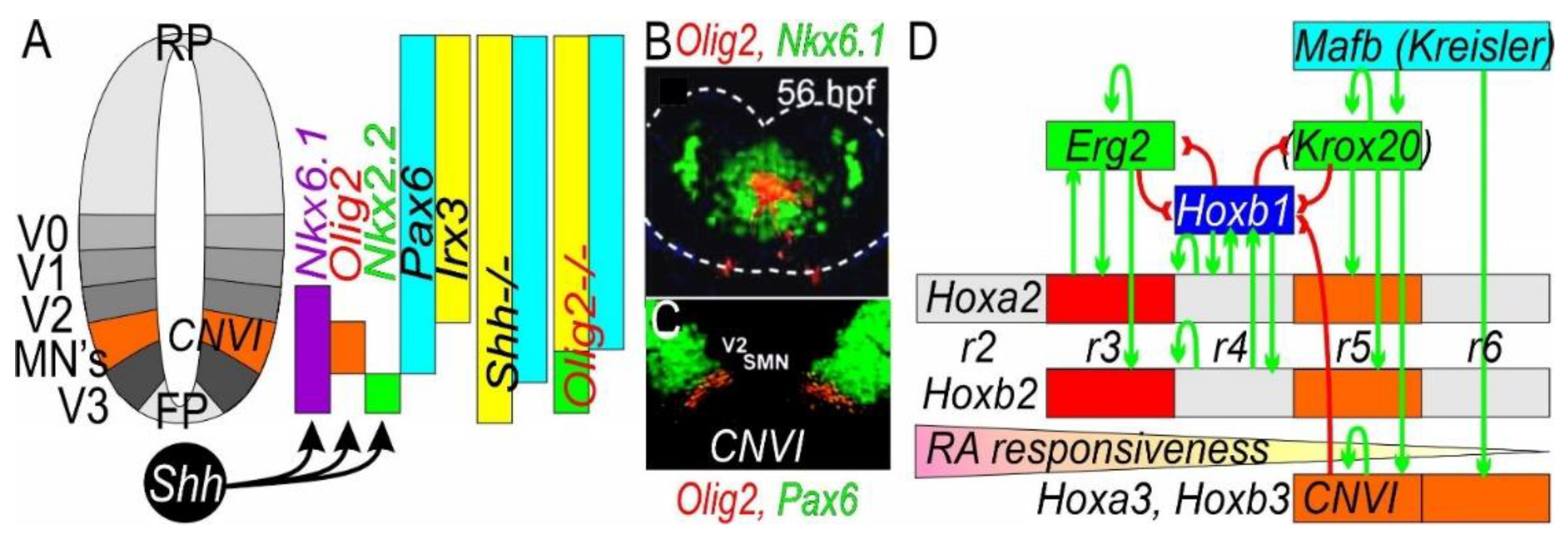
5.2. Abducens Motor Neurons Develop Independent of Oculomotor and Trochlear Motor Neurons
In contrast to the isthmic organizer that defines cranial nerves CNIII and CNIV (
Figure 4), cranial nerve CNVI (
Figure 5) depends on Hox gene expression that ends in rhombomeres [
114,
134]. Obviously,
Shh expression is required to develop motor neurons, including cranial nerve CNVI, to drive
Nkx6.1 [
114,
116]. As with the role for
Nkx6.1 in cranial nerves III and IV, the expression of cranial nerve VI depends on
Nkx6.1, suggesting it acts like a typical somatic motor neuron, comparable to hypoglossal and spinal motor neurons [
84,
117]. Downstream are gene regulatory networks that regulate transcription factors that are specified by cis-regulatory elements to exert their functions [
114]. In addition to requiring the gene
Shh, there are several other genes that are needed for somatic motor neuron formation:
Pax6,
Nkx6.1 and
Olig2 [
84,
103,
127]. Superimposed on this arrangement is the
Hox code control of the hindbrain by regional upregulation of rhombomeres r2-r11, defined by Puelles work [
6,
83,
129,
134].
The
Hox genes have four
Hox clusters that each have a unique expression pattern (
Figure 5.; [
134]). Complex feedback and feedforward pathways that regulates rhombomeres have been identified in fish and mammals. In mice,
early growth response 2 gene (
Egr2, aka Krox20) is expressed in two bands in rhombomeres r3 and r5 and interacts with the
v-maf musculoaponeurotic fibrosarcoma oncogene (Mafb, aka Kreisler; r5 and r6). Further, expression of
Hoxa3/b3 is needed to develop rhombomere r5 (
Figure 5). In addition, retinoic acid (
Figure 5) exerts its effects in a graded manner whereby concentration is typically higher close to the spinal cord, whereas RA are degraded rapidly in later development [
135].
While
Phox2a is needed for the development of ocular and trochlear motor neurons CNIII and CNIV [
112], abducens motor neurons and CNVI can form independently of
Phox2a, suggesting a different molecular dependency of somatic motor neurons such as
Nkx6.1 [
84]. In fact, branchial motor neurons could develop by
Phox2a/b, which could control
Nkx6.1, Olig2 and
Isl1 expression in mice in the brainstem [
84,
103,
115,
117]. In r5,
Hoxa3-1-: Hoxb3-1- double mutant mice show a complete loss of somatic motor neurons including CNVI [
103,
137]. Therefore, it is suggested that genes
Hoxa3 and
Hoxb3 are required for generation of somatic motor neurons [
103]. None of the genes
Pax6, Olig2, Isl1, Tuji or
ChAT are present in double null mutants and/or are reduced in the residual expression of abducent motor neurons CNVI [
103]. All brainstem motor neurons in mice are absent after
Olig2 deletion [
131,
132]. The bHLH gene
Neurog2 is closely related to
Olig2 and is known to interact with
Olig2 to induce somatic motor neurons to differentiate [
131,
138], whereas
Olig2 interacts with
Phox2b to regulate branchial motor neurons [
112,
115,
131].
Hoxa3 gain-of-function in chicken suggests the formation of anterior somatic motor neurons in rhombomeres r1-4 [
139]. In contrast,
Hoxb3Tg mutants exhibit a possible r4 to r2 identity switch, but not an r4 to r5 identity switch [
137]. One has to keep in mind that the chicken hindbrain is generated from two rhombomeres (r5+6) whereas in mammals is generated only in r5 [
8,
103]. Like chicken, bony fish have two rhombomeres – r5 and r6 (
Figure 2) – that are highly positive for
Olig2 and
Isl1 [
87].
In summary, a combination of
Shh, Nkx6.1, Olig2, Isl1, Hoxa3 and
Hoxb3 genetic control is required for the development of the abducens neurons, CNVI (
Figure 5).
6. Vestibulo-Ocular Interactions Across Vertebrates
The vestibulo-ocular reflex (VOR) is a crucial connection that helps vertebrates maintain stable vision during head movements; it involves a complex interplay between the vestibular system (which senses head motion) and the ocular motor system (which controls eye movements). Vertebrates, except for hagfish, have a vestibular connection that innervates - via three ocular motor neuron groups - six eye muscles to help move the eyes. Connections between the vestibular neurons and higher order connections form a three-neuron arc [
3,
4,
44,
45,
140,
141].
The first neuron in this arc receives input from the vestibular ganglion neurons to reach the brainstem, the vestibular nuclei. Vestibular nuclei are composed of four areas, the lateral, inferior, medial, and descending vestibular neurons (LVN, IVN, MVN, and DVN). The second leg of neurons crosses mostly to the contralateral side to reach the three oculomotor cranial nerves (III, IV and VI; [
1,
142]), the third leg. The VOR is a remarkable adaptation that enables vertebrates to maintain stable vision in a dynamic environment and highlights the complex interplay between different sensory and motor systems in the hindbrain [
44,
47].
The majority of tetrapods have been shown to receive predominantly contralateral excitatory connections [
143] with the exception of the medial rectus (MR). The MR involves a contralateral projection of internuclear fibers with ipsilateral fibers of the ascending tract of Deiters (
Figure 6; [
144]. A recent study has described a much simpler connection in a teleost, composed of ipsilateral and contralateral VOR pathways [
17,
42] that are consistent with the VOR connections in chondrichthyans [
11]. Limited information on lamprey vestibular connections suggests receiving of a direct vestibular input into the dorsal cranial nerve IV that reaches out the contralateral cranial nerve III [
42,
43,
47].
In summary, a detailed analysis shows at least three patterns of the vestibulo-ocular reflex: lampreys, chondrichthyans and mammals that split into primary antagonistic extraocular muscles of the left and right side of the eyes (
Figure 6).
6.1. Lampreys Have a Different VOR
Lampreys have a simpler vestibular organization that consists of two canal cristae, the anterior and the posterior cristae (
Figure 6; AC, PC; [
46,
48]), innervated by three populations of vestibular neurons: the anterior, inferior and posterior nuclei (AN, IN, PN; [
42,
43,
53]). Selectively tracing the anterior crista shows fibers projecting mostly caudally whereas the posterior crista projects rostrally [
43]. A direct link may exist between the dorsal CNIV and the posterior crista [
9,
53]. Contralateral vestibular fibers have been traced to project to three CNIII nuclei. Untangling the detailed pattern of connections between the vestibular neurons and the abducens nerves requires further work. Assuming the same configuration is true for gnathostomes one can expect the following pairing of antagonistic interactions: RR/SR, RO/CO, CR/VR (
Figure 6). Essentially, we can assume the same extraocular muscles are present in gnathostomes in terms of function despite the different origin and insertion [
32] between the lamprey and gnathostomes [
47].
Lampreys exhibit robust visuo-vestibular integration, suggesting that visual and vestibular inputs are integrated and respond to saccades and nystagmus. Thus, all basic components of the visuo-vestibular control of gaze have different ocular motor neurons to the eye and lack a horizontal canal [
9,
42,
46,
48]
. Further investigations are needed that can explain the VOR in lampreys fully.
6.2. Elasmobranchs and Lungfish May Have a Unique Connection of the VOR
Chondrichthyans are well described and have the simplest connections between the vestibular output and the ocular motor neurons [
11,
12]. A few things are different about the vestibular organization and the utricle that indicates a separation between chondrichthyans and lungfish [
13] and the remaining gnathostomes [
44,
45]; it evolved an anterior and a posterior branch within elasmobranchs [
11,
145]. Differences from the contralateral CNIII should be named the nasal retractor (NR). A contralateral projection receives from the PC to reach out predominantly to the IO/SO. Likewise, we can assume that a counteraction occurs between SR/IR whereas the counteraction should be between the NR/LR. The detail of this connection remains to be determined in chondrichthyans and lungfish [
11,
12,
13].
6.3. Osteichthyes (Bony Fishes) Share a Separate Connection of the VOR
Tetrapods, coelacanth and teleosts have a unique connection between the medial rectus (MR), compared to the equivalent to the NR [
11], which counteracts with lateral rectus in Osteichthyes [
1,
5,
42]. While most connections are identical among gnathostomes, despite some variation of the abducens origin between r5-6, the connection between the MR evolved differently: In part, the input is provided by abducens internuclear neurons (IN) that project contralaterally via the ipsilateral ascending tract of Deiters (ATD). Simply speaking, this unique arrangement in tetrapods combines a contralateral input, (the internuclear neurons) with an ipsilateral input (the ascending tract of Deiters), to drive the MR movement [
4,
11,
143,
146,
147]. The synergistic muscle pairs are well understood; there is counteraction between lateral rectus and medial rectus extraocular muscles to act as abductors and adductors; a counteraction drives superior rectus and inferior rectus extraocular muscles that act as elevator and depressor; a counteraction of superior oblique and inferior oblique extraocular muscles’ actions executes intorsion and extorsion [
1]. In addition, we know that secondary and tertiary function are known among eye movements in mammals that depend on the position of the eye. For example, the superior rectus has primary action as elevator, secondary as adductor, and third as extorsion [
4]. In contrast to mammals, teleosts have a direct connection of the VOR [
17,
148] without involving the ascending tract of Deiters, which has a comparison to elasmobranchs [
42,
143].
In summary, the VOR shows a common function to drive extraocular muscles in different directions. The six extraocular muscles are different and form into three major groups. Despite all the differences it appears that similar circuitry drives the VOR with the ocular motor neurons to generate coherent movements across vertebrates, as predicted over 70 years ago by Szentágothai [
1,
5,
142].
7. Congenital Cranial Dysinnervation Disorders (CCDD) in Humans
Eye alignment and movement requires the coordination of the six extraocular muscles (EOMs) which are innervated by the three ocular nerves CNIII, IV and VI in humans [
49,
105]. CNIII divides into a superior branch which innervates the superior rectus (SR) and levator palpebrae (LP) muscles; the motor neurons are born ipsilateral to the muscles they innervate, but then the SR motor neurons migrate across the floor plate and the levator palpebrae MNs migrate to the floor plate to innervate the contralateral SR or, bilaterally, the levator palpebrae muscles. The CNIII inferior branch extends from the ipsilateral motor neurons and innervates the ispilateral IO, IR, and MR muscles. Cranial nerve IV innervates the contralateral SO muscle; the motor neurons send their axons dorsally to exit the midbrain and the nerve then loop around the dorsal midbrain/cerebellum junction to innervate the muscles on the contralateral side. CNVI innervates the LR from ipsilateral neurons that form in r5 (
Figure 4 and
Figure 7). Cranial ocular innervation develops in a spatial pattern that aligns the six EOMs and the levator palpebrae.
The human congenital cranial dysinnervation disorders (CCDD) include disorders in which children are born with restricted eye or facial movements secondary to defects in cranial motor neuron development or cranial axon growth or guidance. Most CCDDs are genetic, and many are inherited [
97]. CCDDs that perturb oculomotor development (with or without additional perturbations of trochlear and abducens development) are referred to as congenital fibrosis of the extraocular muscles (CFEOM), while those that perturb abducens development are referred to as Duane syndrome or horizontal gaze palsy (Table 2).
7.1. CFEOM1: KIF21A
CFEOM1 is typically an isolated congenital eye movement disorder. Affected individuals have bilateral ptosis, eyes fixed in a downward position, and absent vertical and variably restricted horizontal eye movements. CFEOM1 results from monoallelic missense variants in
KIF21A that can be inherited as a dominant trait or arise
de novo [
150]
. KIF21A is widely expressed in developing and mature neurons and many other cell types, and it encodes an anterograde kinesin protein with a motor domain that interacts with microtubules, a flexible stalk domain, and a C-terminal WD40 domain. The protein has been shown to transport cargos along microtubules, as well as to regulate microtubule dynamics by serving as a ‘brake’ near the cell periphery [
19,
151,
152,
153,
154]. KIF21A can exist in an autoinhibited conformation in which the stalk domain interacts with the motor domain or can exist in an active conformation in which two molecules dimerize and their motor domains interact with and use ATP to walk down microtubules. CFEOM1 missense variants are clustered in specific regions of the motor and stalk domain that are critical to KIF21A autoinhibition and the disease-causing variants attenuate autoinhibition in vitro [
19,
151]. Therefore, CFEOM1 results from an alteration rather than loss of KIF21A function.
Introduction of one of the most frequent human CFEOM1-
KIF21A variants into a mouse model recapitulated the human eye phenotype (
Figure 7; [
19,
151,
155]). Evaluation of affected embryonic mice revealed stalling of superior division axons within a bulge in the proximal oculomotor nerve; growth cones within the bulge were enlarged, had increased numbers of filopodia, and were randomly directed. These observations are reminiscent of the anatomy of decision region for axon turning or branching that, in this case, has formed prematurely. The SP and LPS muscles markedly attenuated innervation and appeared hypoplastic. It remains to be determined how attenuated KIF21A autoinhibition leads to this very selective error in oculomotor development.
7.2. CFEOM2: PHOX2A
CFEOM2 is an isolated autosomal recessive eye movement disorder that has been identified primarily in consanguineous pedigrees. Affected individuals have bilateral ptosis and their eyes are fixed in an exotropic position at rest with limited residual horizontal and vertical movements; magnetic resonance imaging reveals absent oculomotor nerves and, to the extent it can be detected, absent trochlear nerves [
156]. CFEOM2 results from loss-of-function variants in
PHOX2A [
157]. In mice, PHOX2A is essential for oculomotor and trochlear motor neuron specification and, in its absence, oculomotor and trochlear nuclei and nerves do not form [
85,
112,
115,
157,
158,
159]. Further work detailing the complete absence or partial deletions of
PHOX2A would be helpful.
| CFEOM1 |
bilateral ptosis, infraduction, restricted eye movements KIF21A [19,150]. |
| CFEOM2 |
bilateral ptosis, exotropia, restricted eye movements PHOX2A [157] |
| CFEOM3 |
variable ptosis, infraduction, restricted eye movements TUBB3 [41] |
| DRS |
restricted horizontal eye movements CHN1 [69,88,160,161] |
| Abbreviations |
CFEOM1-3, congenital fibrosis of the extraocular muscles; DRS, Duane retraction syndrome [105]. |
7.3. CFEOM3:TUBB3
Individuals with CFEOM3 can have a variable ocular motility phenotype with mild to severe ptosis and limited upgaze. It is a dominant disorder that results from missense variants in
TUBB3 [
41] that alter amino acid residues in the tubulin beta III isotype, a building block of neuronal microtubules. Remarkably, while some
TUBB3 variants can cause isolated CFEOM, others cause CFEOM together with additional cranial nerve, spinal nerve, and central white matter maldevelopment [
34,
41]. These variants perturb the growth and guidance of oculomotor as well as other peripheral and central axons [
41,
162]. Moreover, a different set of missense variants can cause malformations of cortical development in the absence of CFEOM [
49]. Additional mechanistic studies are necessary to understand the developmental etiologies of these phenotype-genotype correlations.
7.4. Duane Retraction Syndrome (DRS)
Duane retraction syndrome is a CCDD defined by limited abduction or both abduction and adduction, accompanied by globe retraction on attempted adduction. It is inherited in only the minority of cases but can result from dominant altered function variants in
CHN1 [
88,
160], dominant loss-of-function variants in
SALL4 [
161] and
MAFB [
69], or recessive loss-of-function variants in
HOXA1 [
111].
CHN1 variants result in isolated DRS, while
MAFB results in DRS with or without hearing loss, and
SALL4 in DRS with or without radial ray anomalies. By contrast, loss of
HOXA1 results in the most syndromic form of DRS. Mouse models of
MAFB confirm that this form of DRS results from failure of abducens motor neuron specification, while a mouse model of
CHN1 revealed aberrant abducens axon growth and guidance. In both cases, later in embryonic development the abducens nerve is absent and the lateral rectus is secondarily innvervated by aberrant branches of the oculomotor nerve [
69,
88].
Notably, studies
in vitro and in a
CHN1 knock-in mouse harboring a human DRS variant revealed that
CHN1 variants cause DRS through hyperactivation of the encoded alpha2-chimaerin protein. By contrast,
CHN1 and
Epha4 knock-out mice have abducens axon wandering distinct from the
CHN1 knock-in mice and do not have DRS. In addition, several guidance molecules help to fine tune abducens axon guidance, including
Epha2, Efna5, various
Sema’s, Nrp1 and
PlexinA, among others [
105]. Further work is needed to consolidate guidance across all ocular motoneurons.
8. Summary and Conclusions
Ocular motor neurons are the basis of eye movements in vertebrates. Several additional muscles are innervated from the abducens and form the retractor bulbi in tetrapods and the basicranial muscle in Latimeria: the retractor may be derived from a single extraocular muscle in lamprey that was replaced by two independent muscles of gnathostomes: the medial rectus and the nasal rectus. The motor neurons form two groups that have molecularly distinct origins: A) Populations projecting via cranial nerves III and VI to originate at the midbrain-hindbrain boundary and are regulated from Lmx1b, Wnt1, Nkx6.1, Phox2a, Olig2 and Isl1, which are factors controlling special somatic motor neuron development. B) A variable population is associated with r4-6 projects via cranial nerve V and is regulated by Hoxa3/b3, Nkx6.1, Olig2 and Isl1, which are factors controlling the development of general somatic motor neurons (SM). The earliest development of extraocular muscles is independent of cranial nerves, which subsequently interact with the developing muscles to, yield complete differentiation. The circuitry serving the vestibulo-ocular reflex, which remains unclear in detail for lampreys and chondrichthyans, has been clarified in bony fish, frogs, and mammals. Predominantly contralateral vestibular neurons have projections via the internuclear neurons and the ascending tract of Deiters nuclei to innervate the medial rectus in mammals. Several syndromes have been linked to distinct genetic perturbations (mostly incomplete deletions) in specific groups of ocular motor neurons: the lack of one innervation target can allow other branches to sprout and cause misinnervation, leading to aberrant movements, for example the Duane retractor syndrome.
Author Contributions
This paper was written by B.F.
Funding
This work was supported be the NIH/NIA (R01 AG060504) to B.F.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Ethical Approval
All applicable international, national, and/or institution guidelines for the care and use of animals were followed.
Acknowledgments
I thank Paul Martin and Elizabeth Engle to read and provide critiques of the paper. My late friend, Hans Straka, was reading an early draft. E. N. Yamoah, J. Kersigo and K L. Elliott acknowledge for his support to submit the current paper.
Conflicts of Interest
The author declares no conflict of interest.
References
- Straka, H.; Fritzsch, B.; Glover, J.C. Connecting ears to eye muscles: Evolution of a ‘simple'reflex arc. Brain Behav. Evol. 2014, 83, 162–175. [Google Scholar] [CrossRef]
- Elliott, K.L.; Straka, H. Assembly and functional organization of the vestibular system. In Evolution of Neurosensory Cells and Systems; CRC Press: Boca Raton, FL, USA, 2022; pp. 135–174. [Google Scholar]
- Beraneck, M.; Elliott, K.L.; Glover, J.C.; Straka, H. Development of the vestibular system. Front. Neurol. 2023, 14. [Google Scholar] [CrossRef]
- Horn, A.K.; Straka, H. Functional organization of extraocular motoneurons and eye muscles. Annu. Rev. Vis. Sci. 2021, 7, 793–825. [Google Scholar] [CrossRef]
- Straka, H.; Lambert, F.M.; Simmers, J. Role of locomotor efference copy in vertebrate gaze stabilization. Front. Neural Circuits 2022, 16, 1040070. [Google Scholar] [CrossRef]
- Fritzsch, B.; Elliott, K.L.; Glover, J.C. Gaskell revisited: New insights into spinal autonomics necessitate a revised motor neuron nomenclature. Cell Tissue Res. 2017, 370, 195–209. [Google Scholar] [CrossRef]
- Guthrie, S. Patterning and axon guidance of cranial motor neurons. Nat. Rev. Neurosci. 2007, 8, 859–871. [Google Scholar] [CrossRef]
- Gilland, E.; Baker, R.G. Evolutionary Patterns of Cranial Nerve Efferent Nuclei in Vertebrates. Brain Behav. Evol. 2005, 66, 234–254. [Google Scholar] [CrossRef]
- Fritzsch, B.; Sonntag, R.; Dubuc, R.; Ohta, Y.; Grillner, S. Organization of the six motor nuclei innervating the ocular muscles in lamprey. J. Comp. Neurol. 1990, 294, 491–506. [Google Scholar] [CrossRef]
- Nishi, M. Augenmuskulatur. Handbuch der vergleichenden Anatomie der Wirbeltiere 1938, 5, 453–466. [Google Scholar]
- Graf, W.; Brunken, W. Elasmobranch oculomotor organization: Anatomical and theoretical aspects of the phylogenetic development of vestibulo-oculomotor connectivity. J. Comp. Neurol. 1984, 227, 569–581. [Google Scholar] [CrossRef]
- Puzdrowski, R. Innervation of the medial rectus muscle in the ratfish, Hydrolagus colliei. J. Comp. Neurol. 1998, 400, 571–579. [Google Scholar] [CrossRef]
- von Bartheld, C.S. Oculomotor and sensory mesencephalic trigeminal neurons in lungfishes: Phylogenetic implications. Brain Behav. Evol. 1992, 39, 247–263. [Google Scholar] [CrossRef]
- Pinkus, F. Die Hirnnerven des Protopterus annectens. Morphol. Arb. 1895, 4, 275–346. [Google Scholar]
- Haller von Hallerstein, V. Kranialnerven. Handb. Vgl. Anat. Wirbeltiere 1934, 2, 541–684. [Google Scholar]
- Bjerring, H.C. A Contribution to Structural Analysis of the Head of Craniate Animals 1 The orbit and its contents in 20-22-mm embryos of the North American actinopterygian Amia calva L., with particular reference to the evolutionary significance of an aberrant, nonocular, orbital muscle innervated by the oculomotor nerve and notes on the metameric character of the head in craniates. Zool. Scr. 1978, 6, 127–183. [Google Scholar]
- Greaney, M.R.; Privorotskiy, A.E.; D'Elia, K.P.; Schoppik, D. Extraocular motoneuron pools develop along a dorsoventral axis in zebrafish, Danio rerio. J. Comp. Neurol. 2017, 525, 65–78. [Google Scholar] [CrossRef]
- Newell, F.W. The eye and ocular adnexa of the monotreme Ornithorhynchus anatinus. Trans. Am. Ophthalmol. Soc. 1953, 51, 501. [Google Scholar]
- Cheng, L.; Desai, J.; Miranda, C.J.; Duncan, J.S.; Qiu, W.; Nugent, A.A.; Kolpak, A.L.; Wu, C.C.; Drokhlyansky, E.; Delisle, M.M. Human CFEOM1 mutations attenuate KIF21A autoinhibition and cause oculomotor axon stalling. Neuron 2014, 82, 334–349. [Google Scholar] [CrossRef]
- Nieuwenhuys, R. Topological Analysis of the Brainstem of the Australian Lungfish Neoceratodus forsteri. Brain Behav. Evol. 2022, 96, 242–262. [Google Scholar] [CrossRef]
- Evinger, C.; Graf, W.M.; Baker, R. Extra-and intracellular HRP analysis or the organization of extraocular motoneurons and internuclear neurons in the guinea pig and rabbit. J. Comp. Neurol. 1987, 262, 429–445. [Google Scholar] [CrossRef]
- Bemis, W.E.; Northcutt, R.G. Innervation of the basicranial muscle of Latimeria chalumnae. In The Biology of Latimeria Chalumnae and Evolution of Coelacanths; Springer: Berlin/Heidelberg, Germany, 1991; pp. 147–158. [Google Scholar]
- Bjerring, H.C. Facts and thoughts on piscine phylogeny. In Evolutionary Biology of Primitive Fishes; Springer: Berlin/Heidelberg, Germany, 1985; pp. 31–57. [Google Scholar]
- Smith-Paredes, D.; Bhullar, B.-A.S. The Skull and Head Muscles of Archosauria. In Heads, Jaws, and Muscles; Springer: Berlin/Heidelberg, Germany, 2019; pp. 229–251. [Google Scholar]
- Millot, J.; Anthony, J. Anatomie de Latimeria chalumnae. 2. Système nerveux et organes des sens; Ed. du Centre National de la Recherche Scientifique: 1965.
- Meshida, K.; Lin, S.; Domning, D.; Reidenberg, J.S.; Wang, P.C.; Gilland, E. The unique rectus extraocular muscles of cetaceans: Homologies and possible functions. J. Anat. 2022, 240, 1075–1094. [Google Scholar] [CrossRef]
- Wake, M.H. The comparative morphology and evolution of the eyes of caecilians (Amphibia, Gymnophiona). Zoomorphology 1985, 105, 277–295. [Google Scholar] [CrossRef]
- O'Reilly, J.C.; Nussbaum, R.A.; Boone, D. Vertebrate with protrusible eyes. Nature 1996, 382, 33–33. [Google Scholar] [CrossRef]
- Machado, M.; dos Santos Schmidt, E.M.; Margarido, T.C.; Montiani-Ferreira, F. A unique intraorbital osseous structure in the large fruit-eating bat (Artibeus lituratus). Vet. Ophthalmol. 2007, 10, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, H. The innervation of the monkey accessory lateral rectus muscle. Brain Res. 1984, 296, 139–144. [Google Scholar] [CrossRef]
- Michalak, S.M.; Whitman, M.C.; Park, J.G.; Tischfield, M.A.; Nguyen, E.H.; Engle, E.C. Ocular motor nerve development in the presence and absence of extraocular muscle. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2388–2396. [Google Scholar]
- Noden, D.M.; Francis-West, P. The differentiation and morphogenesis of craniofacial muscles. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 1194–1218. [Google Scholar] [CrossRef] [PubMed]
- Chilton, J.K.; Guthrie, S. Axons get ahead: Insights into axon guidance and congenital cranial dysinnervation disorders. Dev. Neurobiol. 2017, 77, 861–875. [Google Scholar] [CrossRef]
- Whitman, M.C. Axonal Growth Abnormalities Underlying Ocular Cranial Nerve Disorders. Annu. Rev. Vis. Sci. 2021, 7, 827–850. [Google Scholar] [CrossRef]
- Colamarino, S.A.; Tessier-Lavigne, M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell 1995, 81, 621–629. [Google Scholar] [CrossRef]
- Jahan, I.; Kersigo, J.; Elliott, K.L.; Fritzsch, B. Smoothened overexpression causes trochlear motoneurons to reroute and innervate ipsilateral eyes. Cell Tissue Res. 2021, 384, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Burgess, R.W.; Jucius, T.J.; Ackerman, S.L. Motor axon guidance of the mammalian trochlear and phrenic nerves: Dependence on the netrin receptor Unc5c and modifier loci. J. Neurosci. 2006, 26, 5756–5766. [Google Scholar] [CrossRef]
- Bjorke, B.; Weller, K.G.; Jones, L.E.; Robinson, G.E.; Vesser, M.; Chen, L.; Gage, P.J.; Gould, T.W.; Mastick, G.S. Oculomotor nerve guidance and terminal branching requires interactions with differentiating extraocular muscles. Dev. Biol. 2021, 476, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Bjorke, B.; Mastick, G.S. Motor neuron migration and positioning mechanisms: New roles for guidance cues. In Proceedings of the Seminars in cell & developmental biology; 2019; pp. 78–83. [Google Scholar]
- Bjorke, B.; Shoja-Taheri, F.; Kim, M.; Robinson, G.E.; Fontelonga, T.; Kim, K.-T.; Song, M.-R.; Mastick, G.S. Contralateral migration of oculomotor neurons is regulated by Slit/Robo signaling. Neural Dev. 2016, 11, 18. [Google Scholar] [CrossRef]
- Tischfield, M.A.; Engle, E.C. Distinct alpha- and beta-tubulin isotypes are required for the positioning, differentiation and survival of neurons: New support for the 'multi-tubulin' hypothesis. Biosci. Rep. 2010, 30, 319–330. [Google Scholar] [CrossRef]
- Straka, H.; Baker, R. Vestibular blueprint in early vertebrates. Front. Neural Circuits 2013, 7, 182. [Google Scholar] [CrossRef]
- Fritzsch, B. Evolution of the vestibulo-ocular system. Otolaryngol. Head Neck Surg. 1998, 119, 182–192. [Google Scholar] [CrossRef]
- Glover, J. Development and Evolution of Vestibulo-Oclar Reflex Circuit. In The Senses; Fritzsch, B., Straka, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 6, pp. 309–325. [Google Scholar]
- Chagnaud, B.P.; Engelmann, J.; Fritzsch, B.; Glover, J.C.; Straka, H. Sensing external and self-motion with hair cells: A comparison of the lateral line and vestibular systems from a developmental and evolutionary perspective. Brain Behav. Evol. 2017, 90, 98–116. [Google Scholar] [CrossRef]
- Fritzsch, B.; Signore, M.; Simeone, A. Otx1 null mutant mice show partial segregation of sensory epithelia comparable to lamprey ears. Dev. Genes Evol. 2001, 211, 388–396. [Google Scholar] [CrossRef]
- Wibble, T.; Pansell, T.; Grillner, S.; Pérez-Fernández, J. Conserved subcortical processing in visuo-vestibular gaze control. Nat. Commun. 2022, 13, 1–18. [Google Scholar] [CrossRef]
- Fritzsch, B.; Kersigo, J.; Rejent, K.; Gherman, W.; Frank, P.W.; Giovannucci, D.R.; Maklad, A. Hair Cell Morphological Patterns and Polarity Organization in the Sea Lamprey Vestibular Cristae. Anat. Rec. 2023, 306, 2170–2184. [Google Scholar] [CrossRef]
- Engle, E.C. Human genetic disorders of axon guidance. Cold Spring Harb. Perspect. Biol. 2010, 2, a001784. [Google Scholar] [CrossRef]
- Fritzsch, B.; Elliott, K.L. Evolution and development of the inner ear efferent system: Transforming a motor neuron population to connect to the most unusual motor protein via ancient nicotinic receptors. Front. Cell. Neurosci. 2017, 11, 114. [Google Scholar] [CrossRef]
- Puelles, L.; Privat, A. Do oculomotor neuroblasts migrate across the midline in the fetal rat brain? Anat. Embryol. 1977, 150, 187–206. [Google Scholar] [CrossRef]
- Naujoks-Manteuffel, C.; Sonntag, R.; Fritzsch, B. Development of the amphibian oculomotor complex: Evidences for migration of oculomotor motoneurons across the midline. Anat. Embryol. 1991, 183, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Pombal, M.A.; Megías, M. Development and functional organization of the cranial nerves in lampreys. Anat. Rec. 2019, 302, 512–539. [Google Scholar] [CrossRef]
- Kuroda, S.; Adachi, N.; Kusakabe, R.; Kuratani, S. Developmental fates of shark head cavities reveal mesodermal contributions to tendon progenitor cells in extraocular muscles. Zool. Lett. 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K.B.; Agarwala, S.; Ragsdale, C.W. PHOX2A regulation of oculomotor complex nucleogenesis. Development 2010, 137, 1205–1213. [Google Scholar] [CrossRef]
- Bohlen, M.O.; Bui, K.; Stahl, J.S.; May, P.J.; Warren, S. Mouse extraocular muscles and the musculotopic organization of their innervation. Anat. Rec. 2019, 302, 1865–1885. [Google Scholar] [CrossRef]
- Fritzsch, B.; Northcutt, R.G. Origin and migration of trochlear, oculomotor and abducent motor neurons in Petromyzon marinus L. Brain Res. Dev. Brain Res. 1993, 74, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Meshida, K.; Lin, S.; Domning, D.P.; Reidenberg, J.S.; Wang, P.C.; Gilland, E. The unique rectus extraocular muscles of cetaceans: Homologies and possible functions. J. Anat. 2022, 240, 1075–1094. [Google Scholar] [CrossRef]
- Fritzsch, B.; Sonntag, R. Oculomotor (N III) motoneurons can innervate the superior oblique muscle of Xenopus after larval trochlear (N IV) nerve surgery. Neurosci. Lett. 1990, 114, 129–134. [Google Scholar] [CrossRef]
- Fritzsch, B.; Sonntag, R. Sequential double labelling with different fluorescent dyes coupled to dextran amines as a tool to estimate the accuracy of tracer application and of regeneration. J. Neurosci. Methods 1991, 39, 9–17. [Google Scholar] [CrossRef]
- Fangboner, R.F.; Luncsford, A.P.; Vanable Jr, J.W. Trochlear nerve regeneration in Xenopus laevis larvae. J. Exp. Zool. 1980, 211, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Fangboner, R.F.; Vanable Jr, J.W. Formation and regression of inappropriate nerve sprouts ing trochlear nerve regeneration in Xenopus laevis. J. Comp. Neurol. 1974, 157, 391–406. [Google Scholar] [CrossRef]
- Sohal, G.; Hirano, S.; Kumaresan, K.; Ali, M. Influence of altered afferent input on the number of trochlear motor neurons during development. J. Neurobiol. 1992, 23, 10–16. [Google Scholar] [CrossRef]
- Sohal, G.; Knox, T.; Allen Jr, J.; Arumugam, T.; Campbell, L.; Yamashita, T. Development of the trochlear nucleus in quail and comparative study of the trochlear nucleus, nerve, and innervation of the superior oblique muscle in quail, chick, and duck. J. Comp. Neurol. 1985, 239, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Dutel, H.; Herrel, A.; Clément, G.; Herbin, M. A reevaluation of the anatomy of the jaw-closing system in the extant coelacanth Latimeria chalumnae. Naturwissenschaften 2013, 100, 1007–1022. [Google Scholar] [CrossRef]
- Dutel, H.; Galland, M.; Tafforeau, P.; Long, J.A.; Fagan, M.J.; Janvier, P.; Herrel, A.; Santin, M.D.; Clément, G.; Herbin, M. Neurocranial development of the coelacanth and the evolution of the sarcopterygian head. Nature 2019, 569, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, H.C. The nervus rarus in Coelacanthiform phylogeny. Zool. Scr. 1972, 1, 57–68. [Google Scholar] [CrossRef]
- Lemire, M. Etude architectonique du rhombencephalon de Latimeria chalumnae. Bulletin du Museum national d’histoire naturelle. Zoologie 1971, 2, 41–96. [Google Scholar]
- Park, J.G.; Tischfield, M.A.; Nugent, A.A.; Cheng, L.; Di Gioia, S.A.; Chan, W.M.; Maconachie, G.; Bosley, T.M.; Summers, C.G.; Hunter, D.G.; et al. Loss of MAFB Function in Humans and Mice Causes Duane Syndrome, Aberrant Extraocular Muscle Innervation, and Inner-Ear Defects. Am J Hum Genet 2016, 98, 1220–1227. [Google Scholar] [CrossRef]
- Murakami, Y.; Pasqualetti, M.; Takio, Y.; Hirano, S.; Rijli, F.M.; Kuratani, S. Segmental development of reticulospinal and branchiomotor neurons in lamprey: Insights into the evolution of the vertebrate hindbrain. Development 2004, 131, 983–995. [Google Scholar] [CrossRef]
- Spencer, R.F.; Porter, J.D. Biological organization of the extraocular muscles. Prog. Brain Res. 2006, 151, 43–80. [Google Scholar] [PubMed]
- Ziermann, J.M.; Diogo, R.; Noden, D.M. Neural crest and the patterning of vertebrate craniofacial muscles. Genesis 2018, 56, e23097. [Google Scholar] [CrossRef]
- Kuratani, S.; Adachi, N. What are head cavities?—A history of studies on vertebrate head segmentation. Zool. Sci. 2016, 33, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.G.; Meier, S. Morphogenesis of the head of a newt: Mesodermal segments, neuromeres, and distribution of neural crest. Dev. Biol. 1984, 106, 181–193. [Google Scholar] [CrossRef]
- Clark, C.; Austen, O.; Poparic, I.; Guthrie, S. α2-Chimaerin regulates a key axon guidance transition during development of the oculomotor projection. J. Neurosci. 2013, 33, 16540–16551. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A resource for timelines, timetrees, and divergence times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef]
- Yeo, C.; Hardiman, M.; Glickstein, M. Classical conditioning of the nictitating membrane response of the rabbit. Exp. Brain Res. 1985, 60, 114–126. [Google Scholar] [CrossRef]
- Collin, S.P. Scene through the eyes of an apex predator: A comparative analysis of the shark visual system. Clin. Exp. Optom. 2018, 101, 624–640. [Google Scholar] [CrossRef]
- Gruber, S. The visual system of sharks: Adaptations and capability. Am. Zool. 1977, 17, 453–469. [Google Scholar] [CrossRef]
- Poscai, A.N.; de Sousa Rangel, B.; da Silva Casas, A.L.; Wosnick, N.; Rodrigues, A.; Rici, R.E.G.; Kfoury Junior, J.R. Microscopic aspects of the nictitating membrane in Carcharhinidae and Sphyrnidae sharks: A preliminary study. Zoomorphology 2017, 136, 359–364. [Google Scholar] [CrossRef]
- Altman, J.; Bayer, S.A. Development of the brain stem in the rat. V. Thymidine-radiographic study of the time of origin of neurons in the midbrain tegmentum. J. Comp. Neurol. 1981, 198, 677–716. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Bayer, S.A. Development of the brain stem in the rat. II. Thymidine-radiographic study of the time of origin of neurons of the upper medulla, excluding the vestibular and auditory nuclei. J. Comp. Neurol. 1980, 194, 37–56. [Google Scholar] [CrossRef]
- Company, V.; Moreno-Bravo, J.A.; Perez-Balaguer, A.; Puelles, E. The amniote oculomotor complex. Anat. Rec. 2019, 302, 446–451. [Google Scholar] [CrossRef]
- Muller, M.; Jabs, N.; Lork, D.E.; Fritzsch, B.; Sander, M. Nkx6. 1 controls migration and axon pathfinding of cranial branchio-motoneurons. Development 2003, 130, 5815–5826. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Nichols, D.; Echelard, Y.; McMahon, A. Development of midbrain and anterior hindbrain ocular motoneurons in normal and Wnt-1 knockout mice. J. Neurobiol. 1995, 27, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.D.; Baker, R.S. Absence of oculomotor and trochlear motoneurons leads to altered extraocular muscle development in the Wnt-1 null mutant mouse. Dev. Brain Res. 1997, 100, 121–126. [Google Scholar] [CrossRef]
- Zannino, D.A.; Appel, B. Olig2+ precursors produce abducens motor neurons and oligodendrocytes in the zebrafish hindbrain. J. Neurosci. 2009, 29, 2322–2333. [Google Scholar] [CrossRef]
- Nugent, A.A.; Park, J.G.; Wei, Y.; Tenney, A.P.; Gilette, N.M.; DeLisle, M.M.; Chan, W.-M.; Cheng, L.; Engle, E.C. Mutant α2-chimaerin signals via bidirectional ephrin pathways in Duane retraction syndrome. J. Clin. Investig. 2017, 127, 1664–1682. [Google Scholar] [CrossRef]
- Kersigo, J.; Gu, L.; Xu, L.; Pan, N.; Vijayakuma, S.; Jones, T.; Shibata, S.B.; Fritzsch, B.; Hansen, M.R. Effects of Neurod1 expression on mouse and human schwannoma cells. Laryngoscope 2021, 131, E259–E270. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Martin, P.R. Vision and retina evolution: How to develop a retina. Neurosci. Rep. 2022, 12, 240–248. [Google Scholar] [CrossRef]
- Goldblatt, D.; Huang, S.; Greaney, M.R.; Hamling, K.R.; Voleti, V.; Perez-Campos, C.; Patel, K.B.; Li, W.; Hillman, E.M.; Bagnall, M.W. Neuronal birthdate reveals topography in a vestibular brainstem circuit for gaze stabilization. Curr. Biol. 2023, 33, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Whitman, M.C.; Gilette, N.M.; Bell, J.L.; Kim, S.A.; Tischfield, M.; Engle, E.C. TWIST1, a gene associated with Saethre-Chotzen syndrome, regulates extraocular muscle organization in mouse. Dev. Biol. 2022, 490, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Molliver, M.E.; Ginty, D.D.; Kolodkin, A.L. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J. Neurosci. 2003, 23, 6671–6680. [Google Scholar] [CrossRef]
- Gu, C.; Rodriguez, E.R.; Reimert, D.V.; Shu, T.; Fritzsch, B.; Richards, L.J.; Kolodkin, A.L.; Ginty, D.D. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 2003, 5, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, J.E.; Baskaran, P.; Clark, C.; Hendry, A.; Lerner, O.; Hintze, M.; Allen, J.; Chilton, J.K.; Guthrie, S. Axon guidance in the developing ocular motor system and Duane retraction syndrome depends on Semaphorin signaling via alpha2-chimaerin. Proc. Natl. Acad. Sci. USA 2012, 109, 14669–14674. [Google Scholar] [CrossRef]
- Chan, W.M.; Miyake, N.; Zhu-Tam, L.; Andrews, C.; Engle, E.C. Two novel CHN1 mutations in 2 families with Duane retraction syndrome. Arch. Ophthalmol. 2011, 129, 649–652. [Google Scholar] [CrossRef]
- Whitman, M.C.; Engle, E.C. Ocular congenital cranial dysinnervation disorders (CCDDs): Insights into axon growth and guidance. Hum. Mol. Genet. 2017, 26, R37–r44. [Google Scholar] [CrossRef]
- Saito, Y.; Miranda-Rottmann, S.; Ruggiu, M.; Park, C.Y.; Fak, J.J.; Zhong, R.; Duncan, J.S.; Fabella, B.A.; Junge, H.J.; Chen, Z. NOVA2-mediated RNA regulation is required for axonal pathfinding during development. Elife 2016, 5, e14371. [Google Scholar] [CrossRef]
- de Sanctis, E.G.; Mesnard, G.; Dejour, D.H. Trochlear dysplasia: When and how to correct. Clin. Sports Med. 2022, 41, 77–88. [Google Scholar] [CrossRef]
- Kazley, J.M.; Banerjee, S. Classifications in brief: The Dejour classification of trochlear dysplasia. Clin. Orthop. Relat. Res. 2019, 477, 2380. [Google Scholar] [CrossRef]
- Irving, C.; Malhas, A.; Guthrie, S.; Mason, I. Establishing the trochlear motor axon trajectory: Role of the isthmic organiser and Fgf8. Development 2002, 129, 5389–5398. [Google Scholar] [CrossRef]
- Lee, S.; Danielian, P.S.; Fritzsch, B.; McMahon, A.P. Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development 1997, 124, 959–969. [Google Scholar] [CrossRef]
- Gaufo, G.O.; Thomas, K.R.; Capecchi, M.R. Hox3 genes coordinate mechanisms of genetic suppression and activation in the generation of branchial and somatic motoneurons. Development 2003, 130, 5191–5201. [Google Scholar] [CrossRef] [PubMed]
- G. Hernández, R.; Calvo, P.M.; Blumer, R.; de la Cruz, R.R.; Pastor, A.M. Functional diversity of motoneurons in the oculomotor system. Proc. Natl. Acad. Sci. USA 2019, 116, 3837–3846. [Google Scholar] [CrossRef]
- Whitman, M.C.; Engle, E.C. Genetics of Strabismus. In Albert and Jakobiec's Principles and Practice of Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 6887–6905. [Google Scholar]
- Zaidi, S.M.H.; Syed, I.N.; Tahir, U.; Noor, T.; Choudhry, M.S. Moebius Syndrome: What We Know So Far. Cureus 2023, 15, e35187. [Google Scholar] [CrossRef]
- Chen, K.Q.; Anderson, A.; Kawakami, H.; Kim, J.; Barrett, J.; Kawakami, Y. Normal embryonic development and neonatal digit regeneration in mice overexpressing a stem cell factor, Sall4. PLoS ONE 2022, 17, e0267273. [Google Scholar] [CrossRef]
- Friocourt, F.; Kozulin, P.; Belle, M.; Suárez, R.; Di-Poï, N.; Richards, L.J.; Giacobini, P.; Chédotal, A. Shared and differential features of Robo3 expression pattern in amniotes. J. Comp. Neurol. 2019, 527, 2009–2029. [Google Scholar] [CrossRef]
- Friocourt, F.; Chédotal, A. The Robo3 receptor, a key player in the development, evolution, and function of commissural systems. Dev. Neurobiol. 2017, 77, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, K.; Kawakami, K. Protocadherin-mediated cell repulsion controls the central topography and efferent projections of the abducens nucleus. Cell Rep. 2018, 24, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Tischfield, M.A.; Bosley, T.M.; Salih, M.A.; Alorainy, I.A.; Sener, E.C.; Nester, M.J.; Oystreck, D.T.; Chan, W.-M.; Andrews, C.; Erickson, R.P. Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat. Genet. 2005, 37, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Andersson, E.; Hedlund, E.; Alekseenko, Z.; Coppola, E.; Panman, L.; Millonig, J.H.; Brunet, J.-F.; Ericson, J.; Perlmann, T. Specific and integrated roles of Lmx1a, Lmx1b and Phox2a in ventral midbrain development. Development 2011, 138, 3399–3408. [Google Scholar] [CrossRef]
- Litingtung, Y.; Dahn, R.D.; Li, Y.; Fallon, J.F.; Chiang, C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature 2002, 418, 979–983. [Google Scholar] [CrossRef]
- Delás, M.J.; Briscoe, J. Repressive interactions in gene regulatory networks: When you have no other choice. Curr Top Dev Biol 2020, 139, 239–266. [Google Scholar]
- Brunet, J.-F.; Pattyn, A. Phox2 genes—From patterning to connectivity. Curr. Opin. Genet. Dev. 2002, 12, 435–440. [Google Scholar] [CrossRef]
- Epstein, D.J.; McMahon, A.P.; Joyner, A.L. Regionalization of Sonic hedgehog transcription along the anteroposterior axis of the mouse central nervous system is regulated by Hnf3-dependent and -independent mechanisms. Development 1999, 126, 281–292. [Google Scholar] [CrossRef]
- Sander, M.; Paydar, S.; Ericson, J.; Briscoe, J.; Berber, E.; German, M.; Jessell, T.M.; Rubenstein, J.L. Ventral neural patterning by Nkx homeobox genes: Nkx6.1 controls somatic motor neuron and ventral interneuron fates. Genes Dev 2000, 14, 2134–2139. [Google Scholar] [CrossRef]
- Briscoe, J.; Thérond, P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013, 14, 416–429. [Google Scholar] [CrossRef]
- Glover, J.C.; Elliott, K.L.; Erives, A.; Chizhikov, V.V.; Fritzsch, B. Wilhelm His’ lasting insights into hindbrain and cranial ganglia development and evolution. Dev. Biol. 2018, 444, S14–S24. [Google Scholar] [CrossRef]
- Wicht, H. The brains of lampreys and hagfishes: Characteristics, characters, and comparisons. Brain Behav. Evol. 1996, 48, 248–261. [Google Scholar] [CrossRef]
- Joyner, A.L.; Liu, A.; Millet, S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol 2000, 12, 736–741. [Google Scholar] [CrossRef]
- Di Nardo, A.A.; Fuchs, J.; Joshi, R.L.; Moya, K.L.; Prochiantz, A. The physiology of homeoprotein transduction. Physiol. Rev. 2018, 98, 1943–1982. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Lowenstein, E.D.; Cui, K.; Hernandez-Miranda, L.R. Regulation of early cerebellar development. FEBS J. 2023, 290, 2786–2804. [Google Scholar] [CrossRef] [PubMed]
- McMahon, A.P.; Joyner, A.L.; Bradley, A.; McMahon, J.A. The midbrain-hindbrain phenotype of Wnt-1-/Wnt-1- mice results from stepwise deletion of engrailed-expressing cells by 9.5 days postcoitum. Cell 1992, 69, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Losos, K.; Joyner, A.L. FGF8 can activate Gbx2 and transform regions of the rostral mouse brain into a hindbrain fate. Development 1999, 126, 4827–4838. [Google Scholar] [CrossRef]
- Prakash, N.; Puelles, E.; Freude, K.; Trümbach, D.; Omodei, D.; Di Salvio, M.; Sussel, L.; Ericson, J.; Sander, M.; Simeone, A.; et al. Nkx6-1 controls the identity and fate of red nucleus and oculomotor neurons in the mouse midbrain. Development 2009, 136, 2545–2555. [Google Scholar] [CrossRef]
- Sherf, O.; Nashelsky Zolotov, L.; Liser, K.; Tilleman, H.; Jovanovic, V.M.; Zega, K.; Jukic, M.M.; Brodski, C. Otx2 requires Lmx1b to control the development of mesodiencephalic dopaminergic neurons. PLoS ONE 2015, 10, e0139697. [Google Scholar] [CrossRef]
- Watson, C.; Shimogori, T.; Puelles, L. Mouse Fgf8-Cre-LacZ lineage analysis defines the territory of the postnatal mammalian isthmus. J. Comp. Neurol. 2017, 525, 2782–2799. [Google Scholar] [CrossRef] [PubMed]
- Mastick, G.S.; Davis, N.M.; Andrew, G.; Easter, S. Pax-6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development 1997, 124, 1985–1997. [Google Scholar] [CrossRef]
- Szu, J.; Wojcinski, A.; Jiang, P.; Kesari, S. Impact of the Olig Family on Neurodevelopmental Disorders. Front. Neurosci. 2021, 15, 659601. [Google Scholar] [CrossRef]
- Zhou, Q.; Anderson, D.J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 2002, 109, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.L.; Pavlínková, G.; Chizhikov, V.V.; Yamoah, E.N.; Fritzsch, B. Development in the Mammalian Auditory System Depends on Transcription Factors. Int. J. Mol. Sci. 2021, 22, 4189. [Google Scholar] [CrossRef]
- Parker, H.J.; Krumlauf, R. A Hox gene regulatory network for hindbrain segmentation. Curr. Top. Dev. Biol. 2020, 139, 169–203. [Google Scholar]
- Glover, J.C.; Renaud, J.S.; Rijli, F.M. Retinoic acid and hindbrain patterning. J. Neurobiol. 2006, 66, 705–725. [Google Scholar] [CrossRef]
- Comai, G.E.; Tesařová, M.; Dupé, V.; Rhinn, M.; Vallecillo-García, P.; da Silva, F.; Feret, B.; Exelby, K.; Dollé, P.; Carlsson, L. Local retinoic acid signaling directs emergence of the extraocular muscle functional unit. PLoS Biol. 2020, 18, e3000902. [Google Scholar] [CrossRef]
- Wong, E.Y.; Wang, X.A.; Mak, S.S.; Sae-Pang, J.J.; Ling, K.W.; Fritzsch, B.; Sham, M.H. Hoxb3 negatively regulates Hoxb1 expression in mouse hindbrain patterning. Dev. Biol. 2011, 352, 382–392. [Google Scholar] [CrossRef]
- Dennis, D.J.; Han, S.; Schuurmans, C. bHLH transcription factors in neural development, disease, and reprogramming. Brain Res. 2019, 1705, 48–65. [Google Scholar] [CrossRef]
- Guidato, S.; Prin, F.; Guthrie, S. Somatic motoneurone specification in the hindbrain: The influence of somite-derived signals, retinoic acid and Hoxa3. Development 2003, 130, 2981–2996. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.A.; Burgess, H.A. A critical review of zebrafish neurological disease models–2. Application: Functional and neuroanatomical phenotyping strategies and chemical screens. Oxf. Open Neurosci. 2023, 2, kvac019. [Google Scholar] [CrossRef] [PubMed]
- Dlugaiczyk, J.; Gensberger, K.D.; Straka, H. Galvanic vestibular stimulation: From basic concepts to clinical applications. J. Neurophysiol. 2019, 121, 2237–2255. [Google Scholar] [CrossRef] [PubMed]
- Szentágothai, J. The elementary vestibulo-ocular reflex arc. J. Neurophysiol. 1950, 13, 395–407. [Google Scholar] [CrossRef]
- Elliott, K.L.; Straka, H. Assembly and functional organization of the vestibular system. In Evolution of Neurosensory Cell and Systems; Fritzsch, B., Elliott, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2022; Volume 6, pp. 135–174. [Google Scholar]
- Highstein, S.M.; Holstein, G.R. The anatomy of the vestibular nuclei. Prog. Brain Res. 2006, 151, 157–203. [Google Scholar]
- Fritzsch, B.; Beisel, K.; Jones, K.; Farinas, I.; Maklad, A.; Lee, J.; Reichardt, L. Development and evolution of inner ear sensory epithelia and their innervation. J. Neurobiol. 2002, 53, 143–156. [Google Scholar] [CrossRef]
- McCrea, R.A.; Strassman, A.; Highstein, S.M. Anatomical and physiological characteristics of vestibular neurons mediating the vertical vestibulo-ocular reflexes of the squirrel monkey. J Comp Neurol 1987, 264, 571–594. [Google Scholar] [CrossRef]
- Diaz, C.; Glover, J.C. The vestibular column in the mouse: A rhombomeric perspective. Front. Neuroanat. 2022, 15, 806815. [Google Scholar] [CrossRef]
- Schoppik, D.; Bianco, I.H.; Prober, D.A.; Douglass, A.D.; Robson, D.N.; Li, J.M.B.; Greenwood, J.S.F.; Soucy, E.; Engert, F.; Schier, A.F. Gaze-Stabilizing Central Vestibular Neurons Project Asymmetrically to Extraocular Motoneuron Pools. J. Neurosci. 2017, 37, 11353–11365. [Google Scholar] [CrossRef]
- Hanai, K.; Tabuchi, H.; Nagasato, D.; Tanabe, M.; Masumoto, H.; Miya, S.; Nishio, N.; Nakamura, H.; Hashimoto, M. Automated detection of enlarged extraocular muscle in Graves’ ophthalmopathy with computed tomography and deep neural network. Sci. Rep. 2022, 12, 16036. [Google Scholar] [CrossRef]
- Yamada, K.; Andrews, C.; Chan, W.-M.; McKeown, C.A.; Magli, A.; De Berardinis, T.; Loewenstein, A.; Lazar, M.; O'Keefe, M.; Letson, R. Heterozygous mutations of the kinesin KIF21A in congenital fibrosis of the extraocular muscles type 1 (CFEOM1). Nat. Genet. 2003, 35, 318–321. [Google Scholar] [CrossRef]
- van der Vaart, B.; van Riel, W.E.; Doodhi, H.; Kevenaar, J.T.; Katrukha, E.A.; Gumy, L.; Bouchet, B.P.; Grigoriev, I.; Spangler, S.A.; Yu, K.L. CFEOM1-associated kinesin KIF21A is a cortical microtubule growth inhibitor. Dev. Cell 2013, 27, 145–160. [Google Scholar] [CrossRef]
- Bianchi, S.; van Riel, W.E.; Kraatz, S.H.; Olieric, N.; Frey, D.; Katrukha, E.A.; Jaussi, R.; Missimer, J.; Grigoriev, I.; Olieric, V. Structural basis for misregulation of kinesin KIF21A autoinhibition by CFEOM1 disease mutations. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Al-Haddad, C.; Boustany, R.-M.; Rachid, E.; Ismail, K.; Barry, B.; Chan, W.-M.; Engle, E. KIF21A pathogenic variants cause congenital fibrosis of extraocular muscles type 3. Ophthalmic Genet. 2021, 42, 195–199. [Google Scholar] [CrossRef]
- Xia, C.; Shi, L.; Nan, J.; Hao, Y.; Jia, Y. Identification of a novel KIF21A gene mutation in a Chinese family with congenital fibrosis of the extraocular muscles. [Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2022, 58, 213–214. [Google Scholar]
- Desai, J.; Velo, M.P.R.; Yamada, K.; Overman, L.M.; Engle, E.C. Spatiotemporal expression pattern of KIF21A during normal embryonic development and in congenital fibrosis of the extraocular muscles type 1 (CFEOM1). Gene Expr. Patterns 2012, 12, 180–188. [Google Scholar] [CrossRef]
- Demer, J.L.; Clark, R.A.; Tischfield, M.A.; Engle, E.C. Evidence of an asymmetrical endophenotype in congenital fibrosis of extraocular muscles type 3 resulting from TUBB3 mutations. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4600–4611. [Google Scholar] [CrossRef]
- Nakano, M.; Yamada, K.; Fain, J.; Sener, E.C.; Selleck, C.J.; Awad, A.H.; Zwaan, J.; Mullaney, P.B.; Bosley, T.M.; Engle, E.C. Homozygous mutations in ARIX (PHOX2A) result in congenital fibrosis of the extraocular muscles type 2. Nat. Genet. 2001, 29, 315–320. [Google Scholar] [CrossRef]
- Pattyn, A.; Morin, X.; Cremer, H.; Goridis, C.; Brunet, J.-F. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development 1997, 124, 4065–4075. [Google Scholar] [CrossRef]
- Dong, J.M.; Shen, Q.; Li, J.; Du, W.; Pang, H.L.; Lin, S.F.; Bu, J. [Identification of a novel PHOX2A gene mutation in a Chinese family with congenital fibrosis of extraocular muscles type 2]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2012, 29, 5–8. [Google Scholar]
- Miyake, N.; Chilton, J.; Psatha, M.; Cheng, L.; Andrews, C.; Chan, W.-M.; Law, K.; Crosier, M.; Lindsay, S.; Cheung, M. Human CHN1 mutations hyperactivate α2-chimaerin and cause Duane's retraction syndrome. Science 2008, 321, 839–843. [Google Scholar] [CrossRef]
- Al-Baradie, R.; Yamada, K.; Hilaire, C.S.; Chan, W.-M.; Andrews, C.; McIntosh, N.; Nakano, M.; Martonyi, E.J.; Raymond, W.R.; Okumura, S. Duane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am. J. Hum. Genet. 2002, 71, 1195–1199. [Google Scholar] [CrossRef]
- Grant, P.E.; Im, K.; Ahtam, B.; Laurentys, C.T.; Chan, W.-M.; Brainard, M.; Chew, S.; Drottar, M.; Robson, C.D.; Drmic, I. Altered white matter organization in the TUBB3 E410K syndrome. Cereb. Cortex 2019, 29, 3561–3576. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).