1. Introduction
Diabetes mellitus (DM), widely known as “diabetes”,is a metabolic illness resulting from the deterioration in insulin hormone secretion or activitybecause ofglucose levels higher than expected in the blood [
1]. DM ranks the eighth among the mortality causes in the world [
2], and it affects humans’ health negatively besides leading to severe financial problems for individuals, families, and health systems of countries [
3]. The type of diabetes in which insulin resistance and insulin insufficiency co-exist is called type2 diabetes. Although no certain age range can be attributed to this disease, it is usually diagnosed after 30. On the other hand, DM tends to increase at 45 years of ageand later.While the incidence of DM is high in individuals who have problems with overweight or obesity, it can also be seen in people who do not have problems in terms of weight [
4].
Exercise is a set of movements implemented within a specified plan, program, and continuity to maintainpeople’s physical development and protection [
5]. It creates more energy consumption than usual by contracting the musculoskeletal system. Exercising has positive effects on chronic pains that occur in our daily lives and on diseases such as cardiovascular disorders, depression, obesity, and diabetes [
6]. In addition, exercise is among the most recommended methods by health institutions for the glucose metabolism, skeletal muscle function, and psychological health of individuals and the functioning of other organs [
7]. Anaerobic exercise has recently become an important physiological concept in the field of sports sciences [
8]. It can be defined as a set of exercises and physical activities that should be carried out intensely in a short time. The anaerobic energy system in the body demonstrates its effectiveness during a maximal level of exercise where the amount of oxygen the athlete receives is insufficient [
9,
10,
11].
The experimentalanimalmodel is anessential method that allows research on biological or behavioural aspects and examines pathologies that originate by themselves or that are induced through specific stimulations on non-human species that are biologically human-like [
12]. Researching diabetic animal models provides critical information about the physiopathology of the disease, and progress can be made to prevent the disease by developing new treatment methods [
13].
Glut 4, which has the characteristic of a glucose transporter regulated through insulin, is found in tissues of the skeletal and heart muscle (adipose tissue and striped muscle). Besides, the gene that codes Glut4 was cloned and mapped in 1989 [
14]. Glucose, whose function in the body is to provide energy for cell function, is a simple sugar. After the food consumed is digested, glucose enters the bloodstream, and insulin becomes secreted in the pancreas. The reason for this processis to cause glucose intake in the fat and muscle cells.These cells take energy from glucose and convert it into fat for long-term storage. Insulin becomes connected receptors on the cell membrane andcauses Glut4 molecules to recruit on the membrane.The Glut4 protein takes on the responsibility to transfer glucose inside the cell [
15].
This study aimedtoprovide alternative solutions to control diabetes that has an increased prevalence by demonstrating effectiveness of a differentiated type of exercise otherthan aerobic exercise on diabetes and to contribute to the literature to guide future research in this field.
2. Materials and Methods
2.1. Animals
Animals were kept in cages with a room temperature of 22±2°C and 60% humidity in units with a 12-hour light/dark cycle, and food and water were provided ad libitum. For this study, permission was obtained from Fırat University Experimental Animals Ethics Committee with the date and number 2020/10.
2.2. Initiation of diabetes
The diabetes model was induced in rats by a single intraperitoneal injection of STZ (60 mg/kg) at a single administration frequency. The daily food and water consumption ratios of the animals were tracked regularly throughout the experimental process.
2.3. Creating the groups
A total of 45 eight-week-old Wistar Albino rats (n=15 per group) were used in this study. The animals are divided into three sections research groups. Group 1 was the control group, Group 2 was the sedentary diabetes group, and Group 3 was thediabetes + anaerobic exercise group. After assigning the rats into these groups, the rats in Group 1 were fed a standard diet, and no exercise protocol was applied.Diabetes was induced in rats in Group 2 with STZ administration, and these rats were also fed the standard diet with no exercise. Lastly, STZ-induced diabetes was produced in the rats in Group 3, they were fed the standard diet, and a 6-week exercise program with five days of vigorous exercise per week was implemented.During the six weeks of the study, rats were fed ad libitum with pellet food and water and kept in rooms with temperature and humidity control.
2.4. The Exercise protocol
The rats underwent running testsfive days a week forsixweeks,and an exhaustion exercise was given on the last day as theanaerobic exercise. A treadmill adjustable at 0-15o of inclination was used for the exercises. In the initial phase, rats ran at a speed of 10 m/min and reached a speed of 30 m/min at the end of the 2-week adaptation period with controlled gradual increases.The running test was performed between 08:00-10:00 in the morning to rule out basal glucocorticoid activity. Rats were subjected to a 30-minute running test every day.At the end of six weeks/After six weeks, the animals were decapitated, and the Glut4 gene expression levels in the muscle tissues were examined.
Figure 1.
Control and Experimental Group Animals during application of exercise protocol.
Figure 1.
Control and Experimental Group Animals during application of exercise protocol.
2.5. Measuring blood glucose levels
One week after STZ administration, blood glucose levels were measured using blood glucose test strips.The rats that developed diabetes were identified, and their blood glucose levels were measured two times in a week for 6 weeks.
2.6. Collection of tissues
The rats were sacrificed under appropriate conditions, and muscle tissues weretransferred into RNA Later solution for preservation of RNA. After incubating the solution for one day at 4ºC, the excess RNA Later solution was discarded.Within two weeks, total RNA was isolated from the tissue examples, which stored at at -20 ºC.
2.7. RNA isolation from tissue examples
RNA isolation of the samples was performed using Invitrogen PureLink Total RNA Kit and the protocol recommended by the manufacturer was manipulated. Briefly; Tissue samples of 100 mg that were crushed on ice wereplaced into 2-mL Eppendorf tubes. Each tube with samples was added 1000 mL of trizol and 5 µL of DTT, and they were homogenized by being vortexed for 5 minutes withone measure of zirconium beads added. The samples incubated on ice were then vortexed at intervals of 5 min. After incubation, the samples were centrifuged at 14000 rpm for 10 minutes in 4Co. The supernatants were added to 200 µl of chloroform in each Eppendorf tube and vortexed until the tubes changed colour. Following another 10-minute incubation on ice, liquids consisting of three phases were obtained (DNA in the lower phase, protein in the mid-section, and RNA in the upper phase). With each sample, the RNA part was transferred to another Eppendorf tube, and it was diluted withisopropyl alcoholat a 1:1 volume ratio.The solution was vortexed for 3-5 minutes and incubated on ice for 10 minutes.The sample was centrifuged at 14,000 rpm for 15 minutes at 4Co. Subsequently, the whole supernatant was removed from the pellet to which 1 ml of 75% ethyl alcohol was added,and they were vortexed. The final 5-minute centrifugation was made at 14,000 rpm. The precipitating pellet obtained was removed and incubated on ice for 6 minutes with the lids open. Diethylpyrocarbonate (DEPC) was added according to the amount of RNA solution. The RNA sample solution was kept on ice for 15-20 minutes.
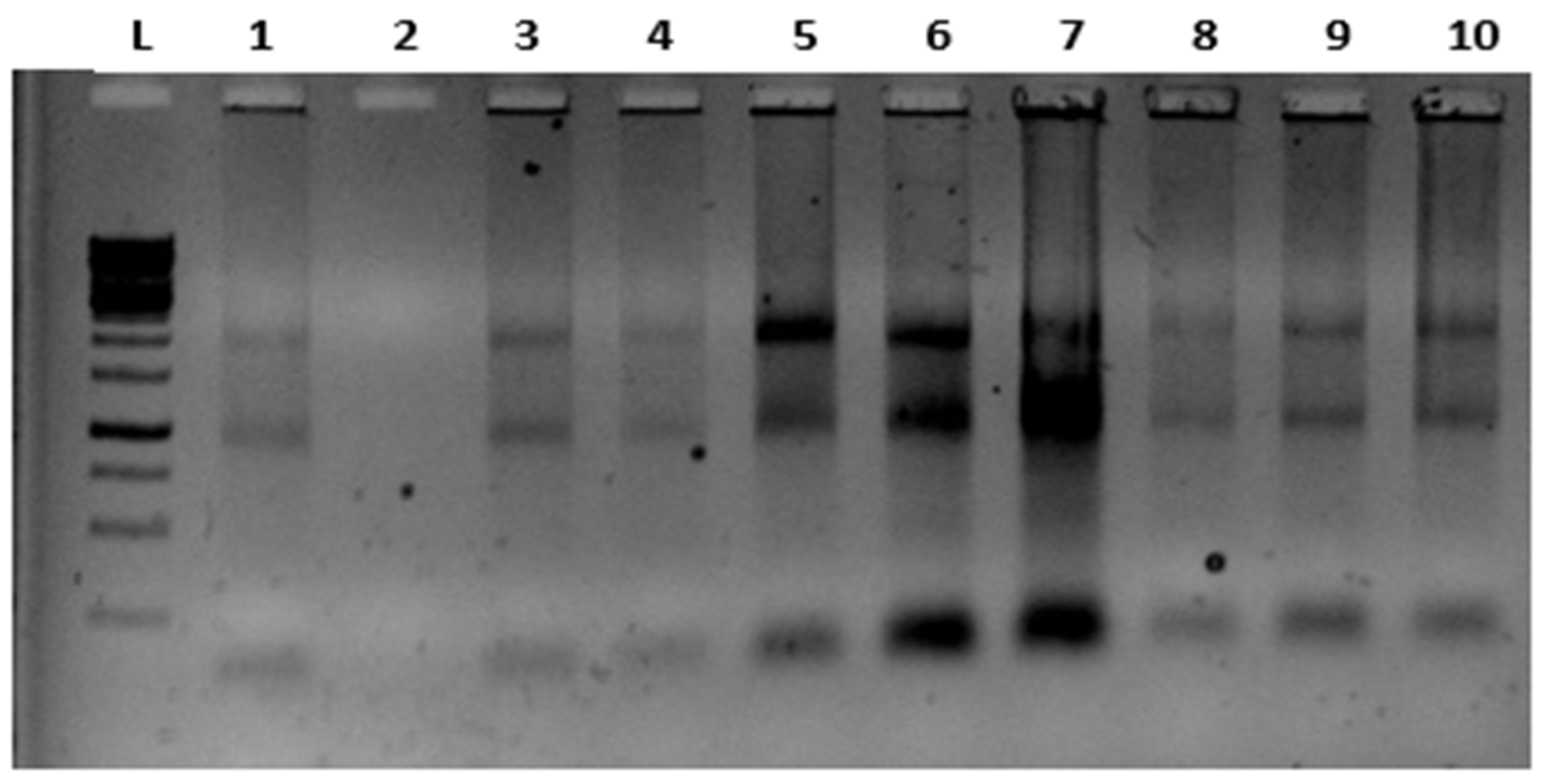
2.8. Quality and control of RNAs
Gels with 1% agarose and 5 µl of RedSafe stain were used to run the isolated RNA samples for 20-30 minutes. The RNA 18S 28S bands were projected on the InvitrogenIBright 750 UV gel imaging system to examine the degradation of RNAs. The RNA samples that were found suitable were used for cDNA synthesis. Further, the concentrations of RNA samples were assessed in a Qubit Fluorometer instrument using Qubit HS RNA Assay Kit. Based on the results, an average value for concentrations was set, and from this initial concentration, cDNA synthesis was carried out. Complementary DNA was synthesized from suitable quality RNA samples using Applied Biosystems High-Capacity cDNA Reverse Transcription Kits with processes of 10minutes at 25°C, 120 minutes at 37°C, 5 minutes at 85°C, and 4°C.
2.9. Quantitative Real-Time (qRT)-PCR
GAPDH (glyceraldehyde 3-phosphate dehydrogenase) gene was used as housekeeping gene during Real-Time PCR for mRNA gene normalization.mRNA gene expression level was determined by qRT-PCR reaction after cDNA synthesis. The Applied Biosystem Step One Plus Real-Time instrument performed the PCR in the synthesized cDNA samples. Conditions for qRT-PCR were 3 minutes at 95 °C, 15 seconds at 95 °C, 10 seconds at 54 °C, and 40 cycles of 15 seconds at 72 °C and 20 seconds at 95 °C.The cycle was set at 60°C for 1 minute and 95°C for 15 seconds. Relative Gene Expressional algorithm was used to determine the expression level of each gene during qRT-PCR. The results obtained from PCR were evaluated by making calculations using the Pfaffl analysis method according to 2004. Fold changes (Fc) were used to convey changes in gene expression.
2.10. Statistical analysis
SPSS (version 22 for Windows) and Bioconductor were employed to analyse selections among data sets. For data analysis, one-way ANOVA was utilized with the level of important set at p<0.05.
3. Results
3.1. Image of Isolated RNAs
RNA samples that were run on 1% agarose gel using a 1kb ladder (for 20-30 min.) wereimaged by staining them with RedSafe solution. The images obtained for the 18S 28S RNA bands are presented in (
Figure 2)
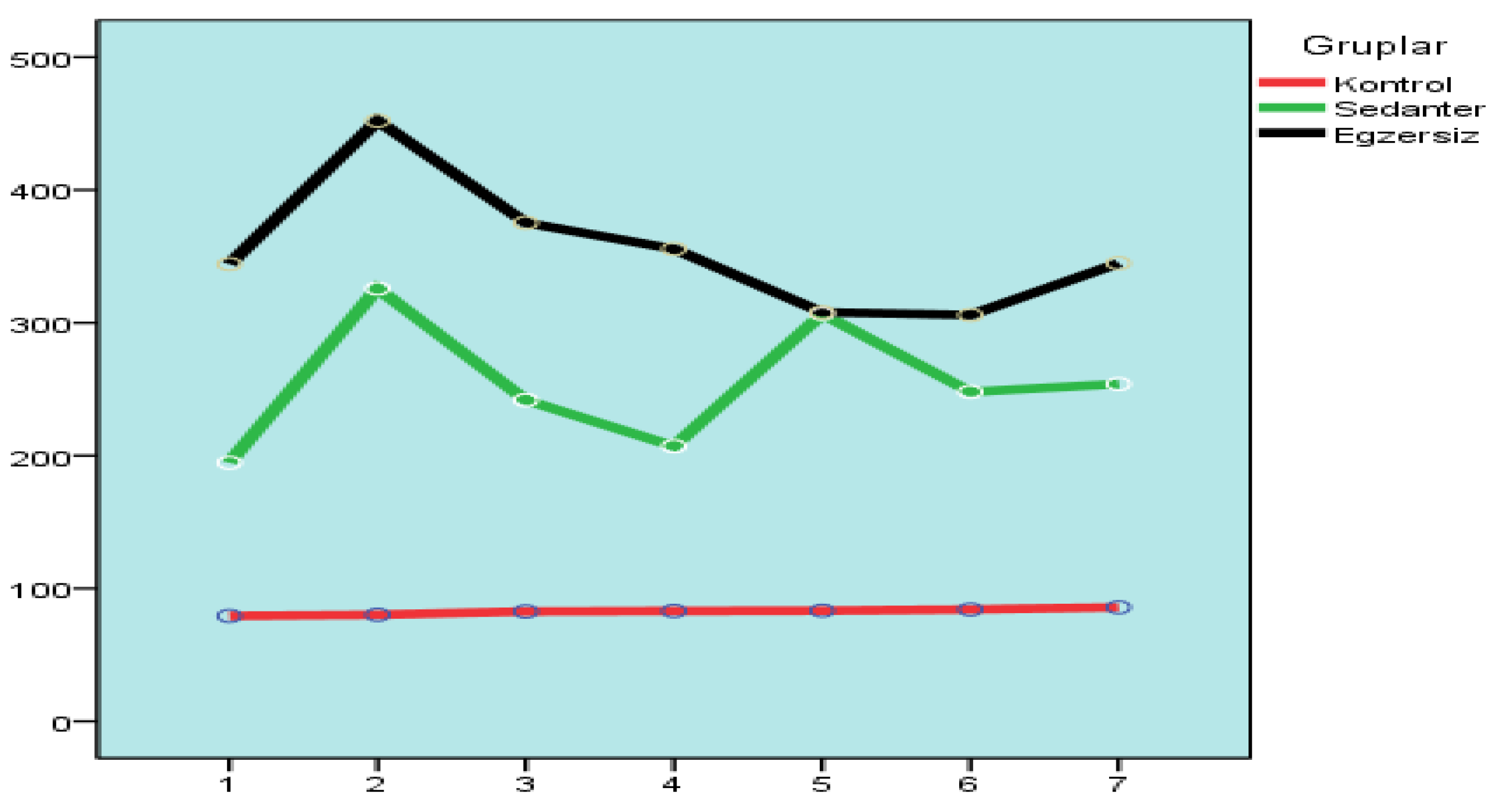
3.2. Measuring of Blood Glucose Levels
Blood glucose levels of rats with diabetes induced for six weeks were measured. Blood glucose was measured in diabetic rats for six weeks.
Repeated measures ANOVA test was used to analyze whether there were important differences among the groups in terms of blood glucose level measurements. ANOVA test results showed that there were important differences among the groups (p<0.05).
The Games-Howell test was applied to investigate whether the groups differed significantly. When the analysis results of the control group and the sedentary and exercise group were analyzed, important differences were observed, while no important differences were observed among the sedentary and exercise group.
According to the Games-Howell test results, the relationship among the control group with the sedentary and exercise group appears to be significant. It was concluded that there was no important difference among the sedentary group and the exercise group (
Figure 3).
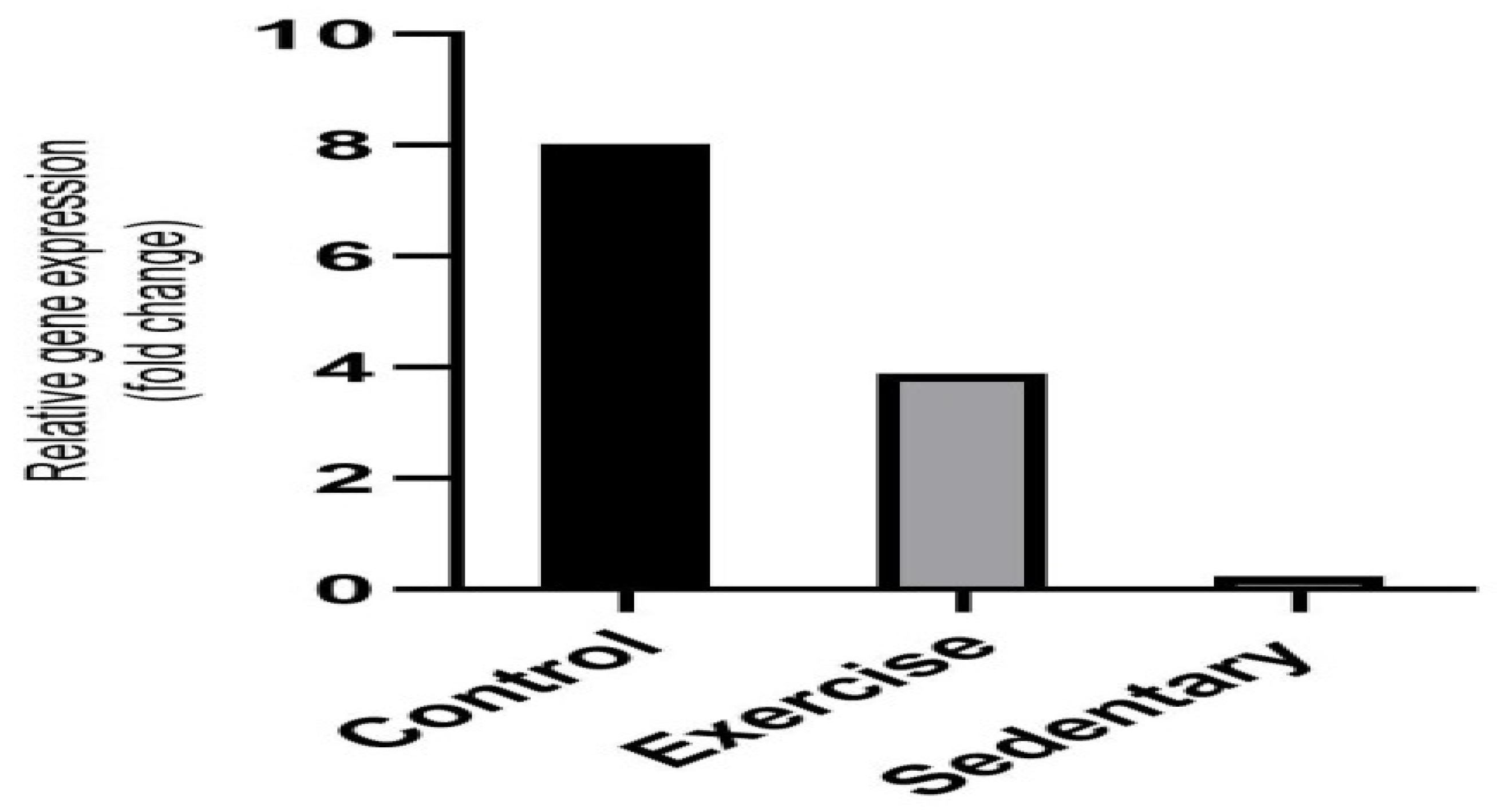
3.3. Determination of Gene Expression
The expression level of the Glut4 gene was analysed in the skeletal muscle tissue. An increased expression of the glucose transporter 4 (Glut4) was observed in the animals in the exercise group. It was notable that the lowest level of expression was seen in the sedentary group, whereas the level in the control group was normal. To conclude, based on the results of theexercisegroup,it can be said thatanaerobic exercise affects the gene expressionunder focus positively (
Figure 4).
The high level of fold changes among the control group, the sedentary group, and the exercise group indicate the effectiveness on the Glut4 gene expression.
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
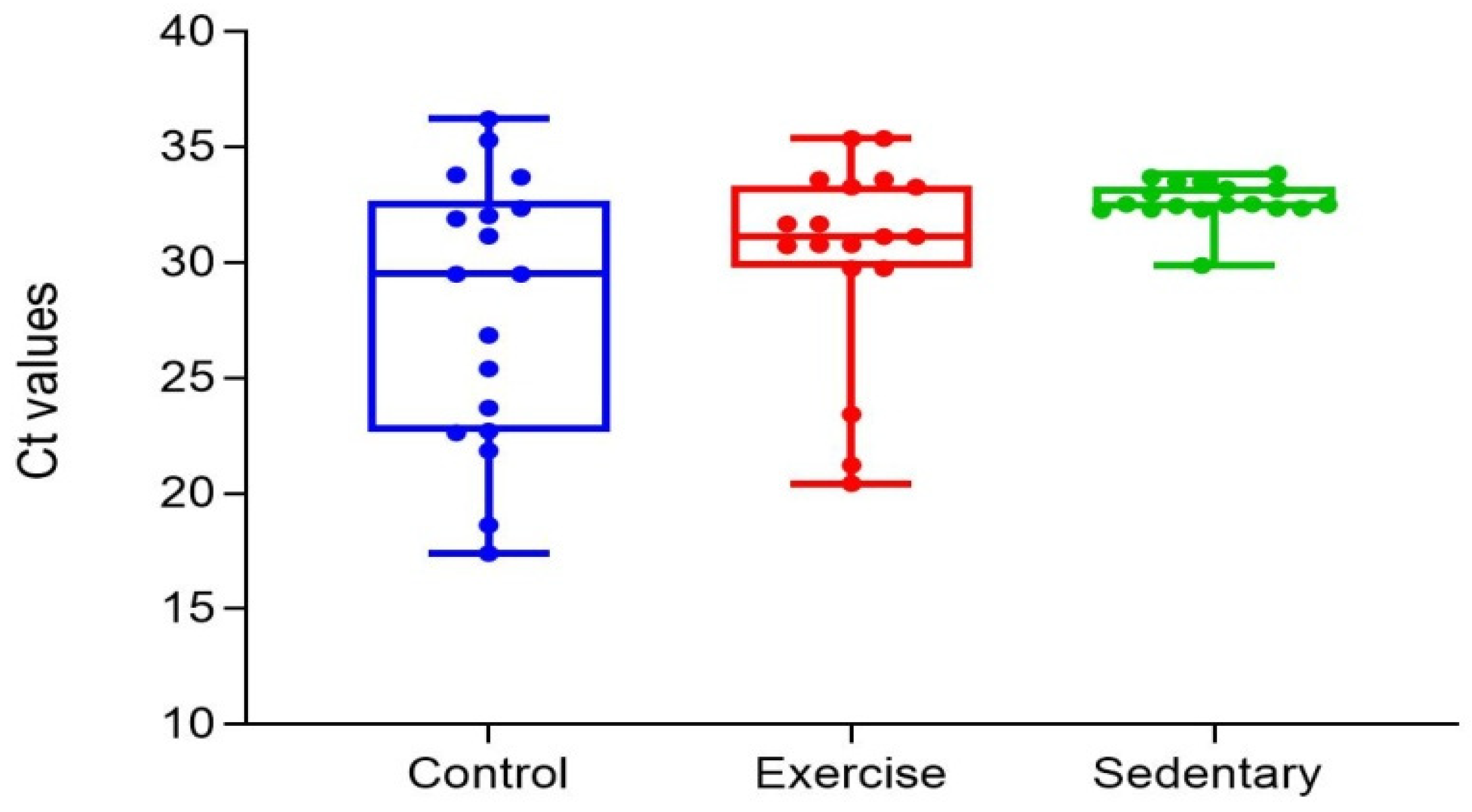
Figure 5.
CT Distribution Chart of Changes in the Exercise, Control and Sedentary Groups.
Figure 5.
CT Distribution Chart of Changes in the Exercise, Control and Sedentary Groups.
4. Discussion
Previous studies have pointed out that anaerobic exercise protocol has effects on blood glucose levels [
16,
17]. But, study results that examined the relationship between anaerobic exercise practice and blood glucose levels are contradictory [
16]. In this study, we tried to determine the effect of anaerobic exercise on blood glucose. The analysis of the measurements exhibited that there was an increase in the arithmetic averages of all three groups after the second measurement. It was considered that the reason for this increase was the fact that STZ might have demonstrated its effect in rats between 3-7 days. Rats are accepted as diabetic when their blood glucose level is around 200 ml/dL and above in the blood obtained from the tail vein [
18]. In this study, blood glucose levels were assessed four days after the STZ injection. After the second measurement, no change was observed in the mean blood glucose levels in the control group, while measurements in the sedentary and exercise group showed a decreasing trend. Measurements were made during the 6-week study, whereas blood glucose levels were tested twice in the last week. Some metabolic changes are demonstrated in rats when they feel stressed. One of these changes is the possibility of blood glucose levels increasing up to two times [
19]. It was thought that this possibility was the cause of the increase between the sixth and seventh measurements. In general, it was seen that important differences existed among measurements.
Blood glucose analysis results in the groups revealed significant differences in the study. The Games-Howell test was applied to assess which groups had significant differences, and an important difference was found among the control and the sedentary group (p<0.05). In addition, an important difference was also found among the control and the exercise group (p<0.05), while the sedentary and the exercise group did not differ significantly (p>0.05). In light of this information, anaerobic exercise can be said to have a healing effect on blood glucose levels when the exercise group was compared to the control group. However, when the sedentary and the exercise group were compared, the anaerobic exercise applied was found to be insufficient in decreasing blood glucose.
Diabetes was experimentally induced in rats and the effects of aerobic and resistance exercise on inflammatory markers as well as some metabolic parameters were compared. At the end of their 6-week study, a reduction in the blood glucose levels was determined in the 5
th and 8
th weeks compared to the beginning in rats that underwent aerobic exercise, while no decrease was observed in the resistance exercise group. Furthermore, while the plasma insulin level of the aerobic exercise group was within the normal interval at the end of the 8
th week, the measurements were above the normal level in the resistance exercise group [
20]. This finding of the research shows a similarity with this current study. Although a decrease occurred in the blood glucose levels of the rats at the end of the 6-week anaerobic exercise, there was no important difference among the anaerobic exercise group and the sedentary group.
Investigated the effects on metabolic control of a 12-week (3 days per week) bicycle ergometer aerobic exercise protocol with type 2 diabetic patients, seven resistance exercise protocols to strengthen lower and upper extremities, and the combined exercise training comprising these two exercise protocols. According to the study results, no important difference was found among the blood glucose values between the groups. However, they observed that the metabolic impact was more effective in the group where aerobic exercise was performed compared to other groups [
17]. They examined the effects of various types of exercise on metabolic parameters, inflammatory markers, and histologic structure of muscles in type 2 diabetic male rats. The types of exercises applied to rats were aerobic exercise, combined exercise and, resistance exercise. After the six-week exercise protocol, analyses revealed significant differences in body weight and blood glucose levels in the aerobic and combined exercise groups. However, there was no significant difference in blood glucose levels in the resistance exercise group compared to the aerobic and combined exercise groups. Yet, a important difference was found among the resistance exercise group and the control group in terms of blood glucose levels [
21]. Similarly, in this study, besides finding a important difference among the control and the exercise group, a important difference was not found among the sedentary and the anaerobic exercise group. The results of the current study also did not demonstrate significant differences in blood glucose levels among sedentary and exercise groups (p>0.005). While there are similar results between this study and the research results in the literature, contradictory findings are also present in other studies. Research examining the effects of anaerobic exercise on diabetes shows that exercise significantly reduces blood glucose levels even though the durations and methods may vary [
22,
23]. In their study, after dividing 48 people with type 2 diabetes into four different groups, i.e., control, aerobic, resistance, and aerobic + resistance, concluded that the exercise protocol applied reduced blood glucose levels significantly in all groups compared to the control group [
24]. Determined that acute and chronic exercises have positive effects on balancing blood glucose levels [
25].
Exercise is one of the most effective stimulators of Glut4 gene expression in skeletal muscle [
26]. Fold changes are present in the gene expression graph between the three study groups in this research. The high level of expressions in these fold changes means that the deterioration in the Glut4 gene expression has recovered. The reason for the high expression level in the control group is that diabetes was not induced in this group.While the expression level of the sedentary group was the lowest, anaerobic exercise appears to regulate Glut4 gene expression in the exercise group. High values in the CT distribution chart mean that the gene expression level is low. This shows that the deteriorated Glut4 gene expression that is created through induced diabetes was not restored. When the figure containing CT values was examined, the values in the control group were found low because diabetes was not induced in this group. The highest values were observed in the sedentary group, whereas the values were normal in the exercise group.
Briefly, anaerobic exercise seems to improve the gene expression that is investigated in this research. Analysed the Glut4 structure in the muscle tissues in the diabetes-induced rats, which underwent daily 45 minutes of chronic exercise for four weeks. Diabetes suppresses the Glut4 level in the muscle tissue.The study points out that exercise removed this pressure, causing the Glut4 expression to exceed normal levels. In conclusion, exercise has been shown to play an important role in the prevention of diabetes [
27]. Applied a galanin antagonist (M35) and a 4-week walking exercise to investigate whether these will increase the Glut4 concentration in the plasma membrane of the skeletal muscle in the back extremities of the elevated galanin streptozotocin-induced diabetic rats. After the walking exercise, the plasma galanin levels were found to be higher compared to the sedentary control group. Increases in the Glut4 mRNA expression and Glut4 protein levels in the skeletal muscles of hind limbs were observed in all exercise groups [
28]. In a study investigating the effects of strength training on health, it was determined that resistance exercise decreased the fat in internal organs and HbA1c, increased Glut4 density, prevented type 2 diabetes with the improvement in insulin sensitivity, and assisted the treatment stage [
29]. Considering the results of the studies in the literature, the effects of various exercise protocols on Glut4 gene expression have been proven and in these aspects, they are similar to the results of the present study.
5. Conclusions
According to the results of the study, at the end of 6 weeks of regular anaerobic exercise, positive effects were observed among the exercise group and the control group, while no important results were observed in terms of blood glucose levels among the exercise group and the sedentary group. Although it was observed that blood glucose levels of the exercise group decreased, it was determined that anaerobic exercise did not have a statistically significant benefit in lowering blood glucose levels. When the relationship between the anaerobic exercise applied and the Glut4 expression was considered, the Glut4 gene was expressed at a normal level in the control group.In addition, the lowest level of expression was found in the sedentary group. However, as mentioned in the hypothesis, regularly performing anaerobic exercisepositively affected the expression level of this gene. In conclusion, the information that anaerobic exercise has a healing effect on the impaired Glut4 gene expression in diabetic patients isretained in the study.
Author Contributions
S.H. and Y.K..; methodology, R.P.; software, S.D.; validation, S.H., L.K.D. and F.M.U.; formal analysis, Y.K.; investigation, R.P.; resources, S.D.; data curation, S.H.; writing—original draft preparation, L.K.D. and D.A.A.; writing—review and editing, Y.K.; visualization, F.M.U.; supervision, S.D.; project administration, Y.K.; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was supported by Fırat University Scientific Research Projects program, Turkey (Project no. BSY.20.04). We express our gratitude to Fırat University.
This article was derived from the Master’s Thesis of the author, SezginHepsert.
Conflicts of Interest
Not applicable.
References
- Motahari Tabari, N.; Ahmad Shirvani, M.; Shirzad-E-Ahoodashty, M.; Yousefi-Abdolmaleki, E.; Teimourzadeh, M. The effect of 8 weeks aerobic exercise on insulin resistance in type 2 diabetes: a randomized clinical trial. Glob. J. Health. Sci. 2014, 7(1), 115–121. [Google Scholar] [CrossRef]
- World Health Organization 2017: The top ten causes of death. Available from: http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death, 2017 (accessed 23 November 2019).
- Shaw, J.E.; de Courten, M.; Boyko, E.J.; Zimmet, P.Z. Impact of new diagnostic criteria for diabetes on different populations. Diabetes Care. 1999, 22(5), 762–766. [Google Scholar] [CrossRef]
- Pan, C.; Shang, S.; Kirch, W.; Thoenes, M. Burden of diabetes in the adult Chinese population: A systematic literature review and future projections. Int. J. Gen. Med. 2010, 3, 173–179. [Google Scholar] [CrossRef]
- World Health Organization 2010. World health statistics 2010. Available from:https://www.who.int/publications/i/item/9789241563987 (accessed 10 September 2019).
- Robison, J.; Miller, W.C. Exercise, physical activity, weight and health. Health at Every Size 2004, 18(4), 49–50. [Google Scholar]
- Ardıç, F. Egzersiz reçetesi. Turk. J. Phys. Med. Rehab. 2014, 60(2), 1–8. [Google Scholar] [CrossRef]
- Vasconcellos, F.; Seabra, A.; Katzmarzyk, P.T.; Kraemer-Aguiar, L.G.; Bouskela, E.; Farinatti, P. Physical activity in overweight and obese adolescents: systematic review of the effects on physical fitness components and cardiovascular risk factors. Sports Med. 2014, 44(8), 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Özkan, A.; Mitat, K.; Ersöz, G. 2011. Wingate anaerobik güç testinde optimal yükün belirlenmesi. Spormetre Beden Eğitimi ve Spor Bilimleri Dergisi. 2011, 9(1), 1–5. [Google Scholar] [CrossRef]
- Morton, J.P.; Maclaren, D.P.; Cable, N.T.; Campbell, I.T.; Evans, L.; Bongers, T.; Griffiths, R.D.; Kayani, A.C.; McArdle, A.; Drust, B. Elevated core and muscle temperature to levels comparable to exercise do not increase heat shock protein content of skeletal muscle of physically active men. Acta Physiologica. 2007, 190(4), 319–327. [Google Scholar] [CrossRef] [PubMed]
- Åstrand, P.O.; Rodahl, K.; Dahl, H.A.; Strømme, S.B. Textbook of Work Physiology: Physiological Bases of Exercise, 3 rd ed.; Human kinetics: Leeds, United Kingstom, 2003; pp. 71–127. [Google Scholar]
- Salén, J.C.W. Animal models: Principles and Problems. Handbook of Laboratory Animal Science. Boca Raton, Fla. (USA): CRC Press, 1994.- ISBN 08-493-4378X (v. 1: acid-free paper). p. 1-6.
- Çiçek, Z.; Koçaklı, Z.G.; Akıllıoğlu, K.; Doğan, A. Diyabetik hayvan modelleri ve önemi. Arch. Med. Rev. J. 2018, 27(3), 311–327. [Google Scholar] [CrossRef]
- Gibala, M.J.; McGee, S.L. Metabolic adaptations to short-term high-intensity interval training: a little pain for a lot of gain? Exerc. Sport. Sci. Rev. 2008, 36(2), 58–63. [Google Scholar] [CrossRef]
- Stuart, C.A.; Yin, D.; Howell, M.E.; Dykes, R.J.; Laffan, J.J.; Ferrando, A.A. Hexose transporter mRNAs for GLUT4, GLUT5, and GLUT12 predominate in human muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 291(5), E1067–E1073. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Missbach, B.; Dias, S.; König, J.; Hoffmann, G. 2014. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetologia 2014, 57(9), 1789–1797. [Google Scholar] [CrossRef]
- de Oliveira, V.N.; Bessa, A.; Jorge, M.L.; Oliveira, R.J.; de Mello, M.T.; De Agostini, G.G.; Jorge, P.T.; Espindola, F.S. The effect of different training programs on antioxidant status, oxidative stress, and metabolic control in type 2 diabetes. Appl. Physiol. Nutr. Metab. 2012, 37(2), 334–344. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, ıts practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12(1), 60. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, O. Deneysel diyabet modelleri. D. J. Med. Sci. 2015, 1(1). [Google Scholar] [CrossRef]
- Özköslü, M.A.; Sönmezer, E.; Arıkan, H.; Bayraktar, N. Deneysel olarak oluşturulan tip 2 diyabet modelinde farklı egzersiz modellerinin inflamatuar belirleyiciler ve metabolik parametreler üzerine etkisi. Journal of Exercise Therapy and Rehabilitation 2019, 6(1), 10–18. [Google Scholar]
- Özköslü, M.A. Sıçan Tip 2 Diyabet Modelinde Farklı Egzersiz Tiplerinin Metabolik Parametreler, Inflamatuar Belirleyiciler ve Kasın Histolojik Yapısı Üzerine Etkisi. Doktora Tezi, Hacettepe Üniversitesi, 2019.
- Dunstan, D.W.; Daly, R.M.; Owen, N.; Jolley, D.; Vulikh, E.; Shaw, J.; Zimmet, P. Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care. 2005, 28(1), 3–9. [Google Scholar] [CrossRef]
- Byrne, H.; Caulfield, B.; De Vito, G. Effects of self-directed exercise programmes on ındividuals with type 2 diabetes mellitus: a systematic review evaluating their effect on hba1c and other metabolic outcomes, physical characteristics, cardiorespiratory fitness and functional outcomes. Sports Med. 2017, 47(4), 717–733. [Google Scholar] [CrossRef] [PubMed]
- Jorge, M.L.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S.; Jorge, P.T.; Geloneze, B. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011, 60(9), 1244–1252. [Google Scholar] [CrossRef]
- Tibana, R.A.; da Cunha Nascimento, D.; Frade de Souza, N.M.; de Souza, V.C.; de Sousa Neto, I.V.; Voltarelli, F.A.; Pereira, G.B.; Navalta, J.W.; Prestes, J. Irisin levels are not associated to resistance training-ınduced alterations in body mass composition in older untrained women with and without obesity. J. Nutr. Health. Aging. 2017, 21(3), 241–246. [Google Scholar] [CrossRef]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93(3), 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Bakum, A. Deneysel Diyabet Oluşturulmuş Sıçanlarda Kronik Egzersizin Kas Dokusundaki Glut-4 ve Çinko Düzeylerine Etkisi. Yüksek Lisans Tezi, Selçuk Üniversitesi, 2019.
- He, B.; Shi, M.; Zhang, L.; Li, G.; Zhang, L.; Shao, H.; Li, J.; Fang, P.; Ma, Y.; Shi, Q.; Sui, Y. Beneficial effect of galanin on insulin sensitivity in muscle of type 2 diabetic rats. Physiol Behav. 2011, 103(3-4), 284–289. [Google Scholar] [CrossRef] [PubMed]
- Westcott, W.L. Resistance training is medicine: effects of strength training on health. Sports Med. Rep. 2012, 11(4), 209–216. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).