Submitted:
05 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
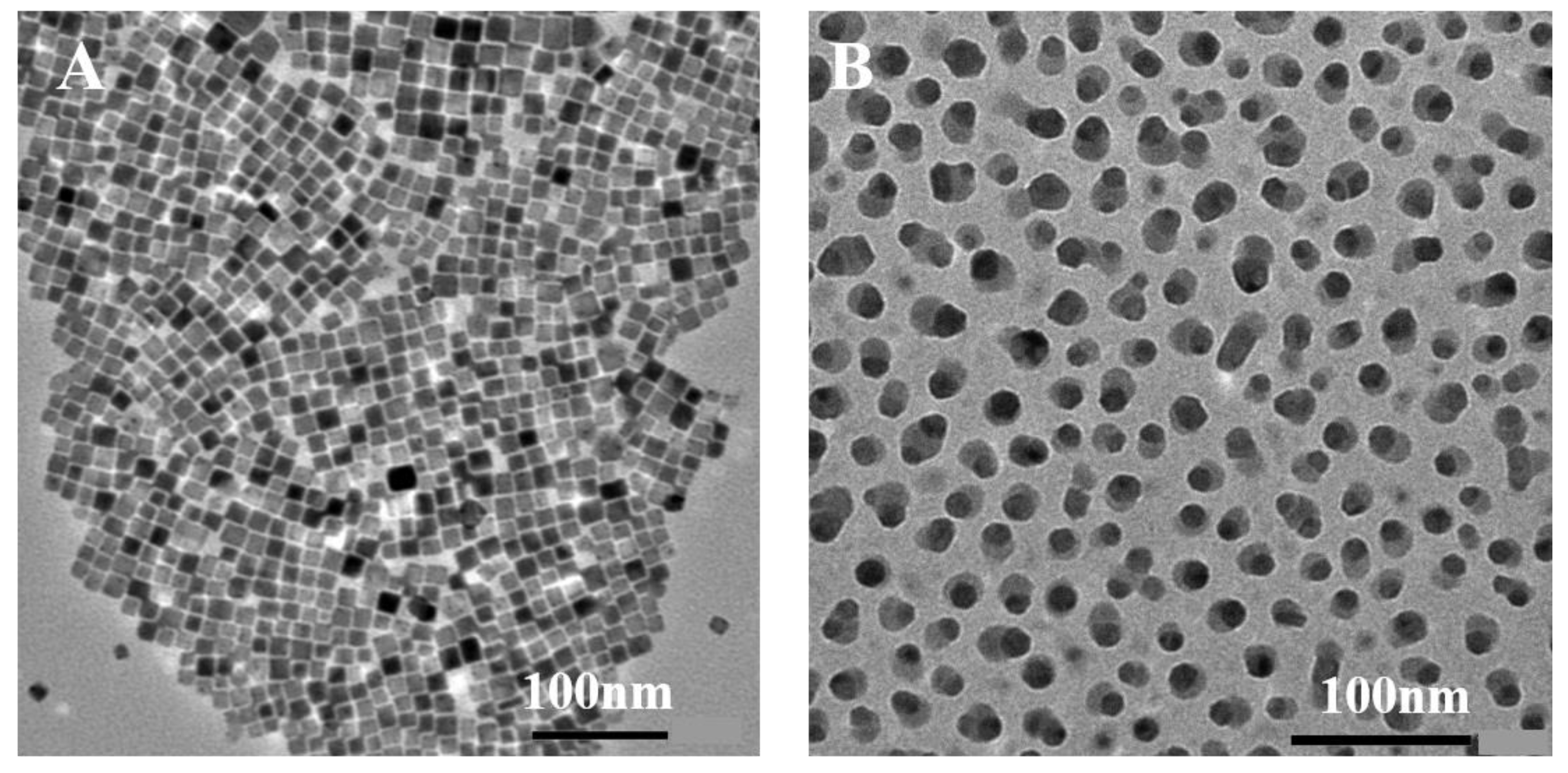
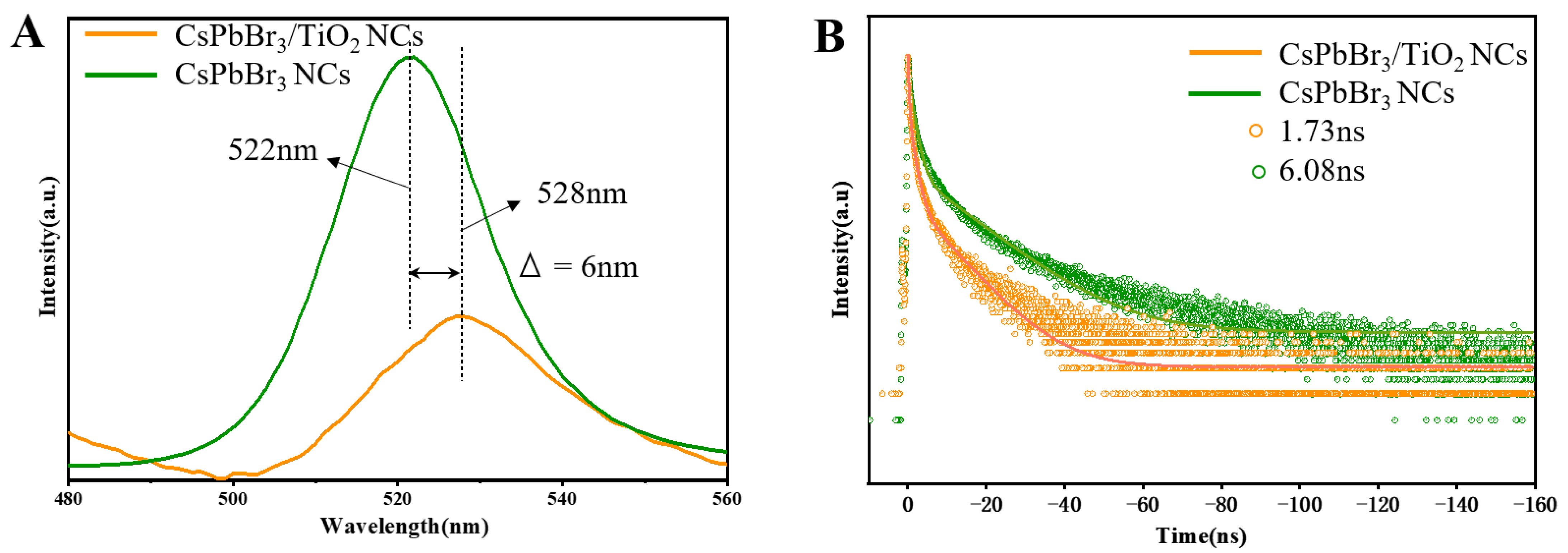
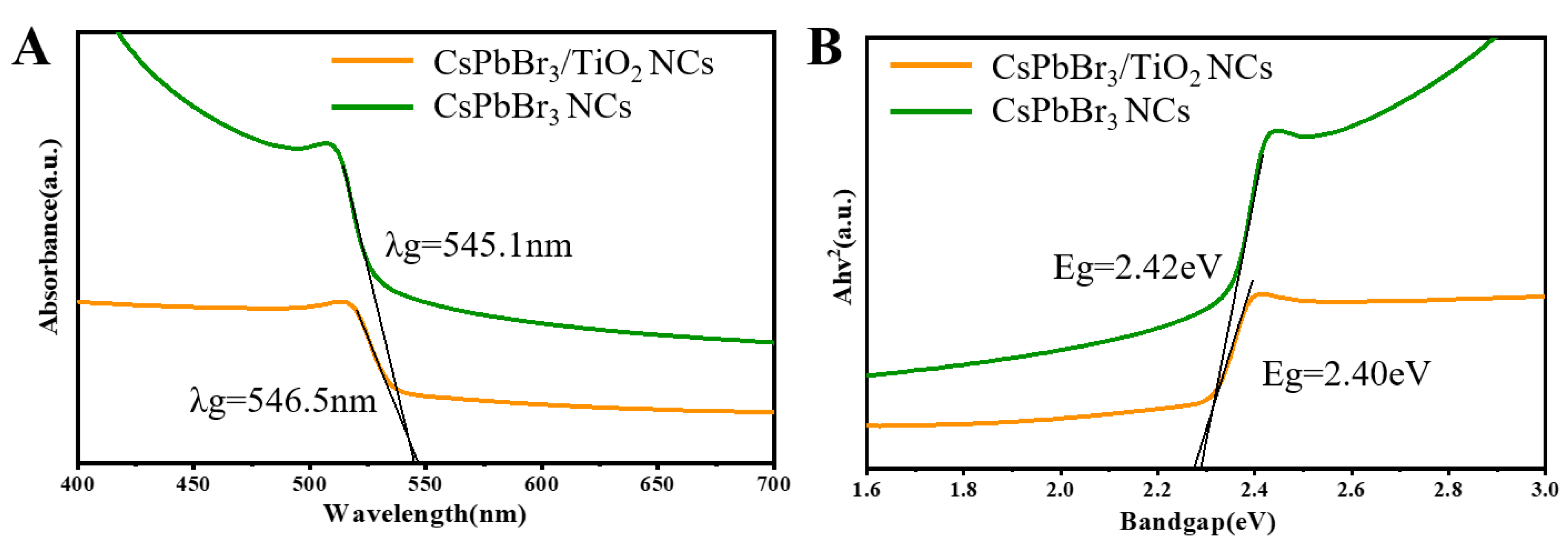
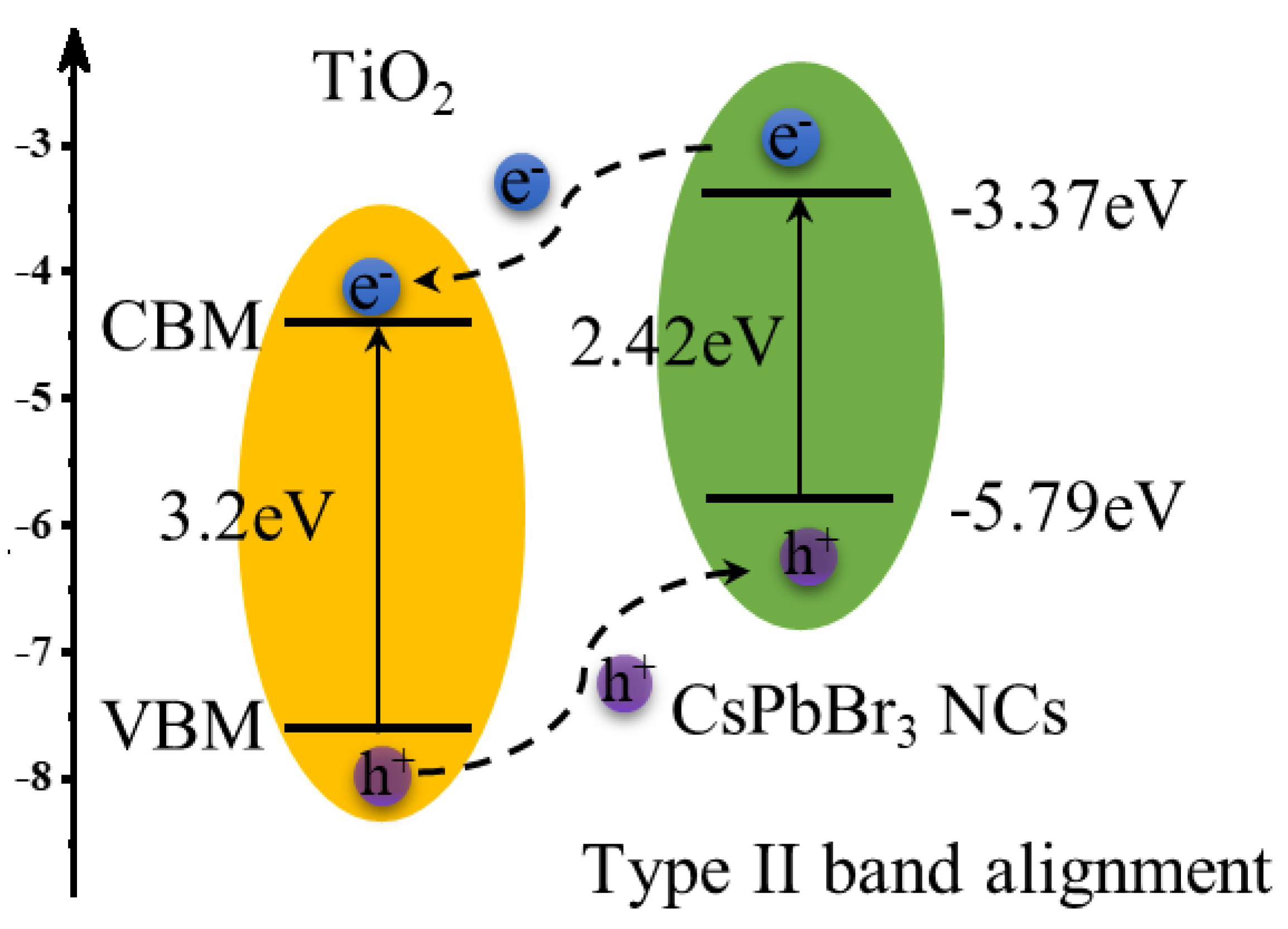
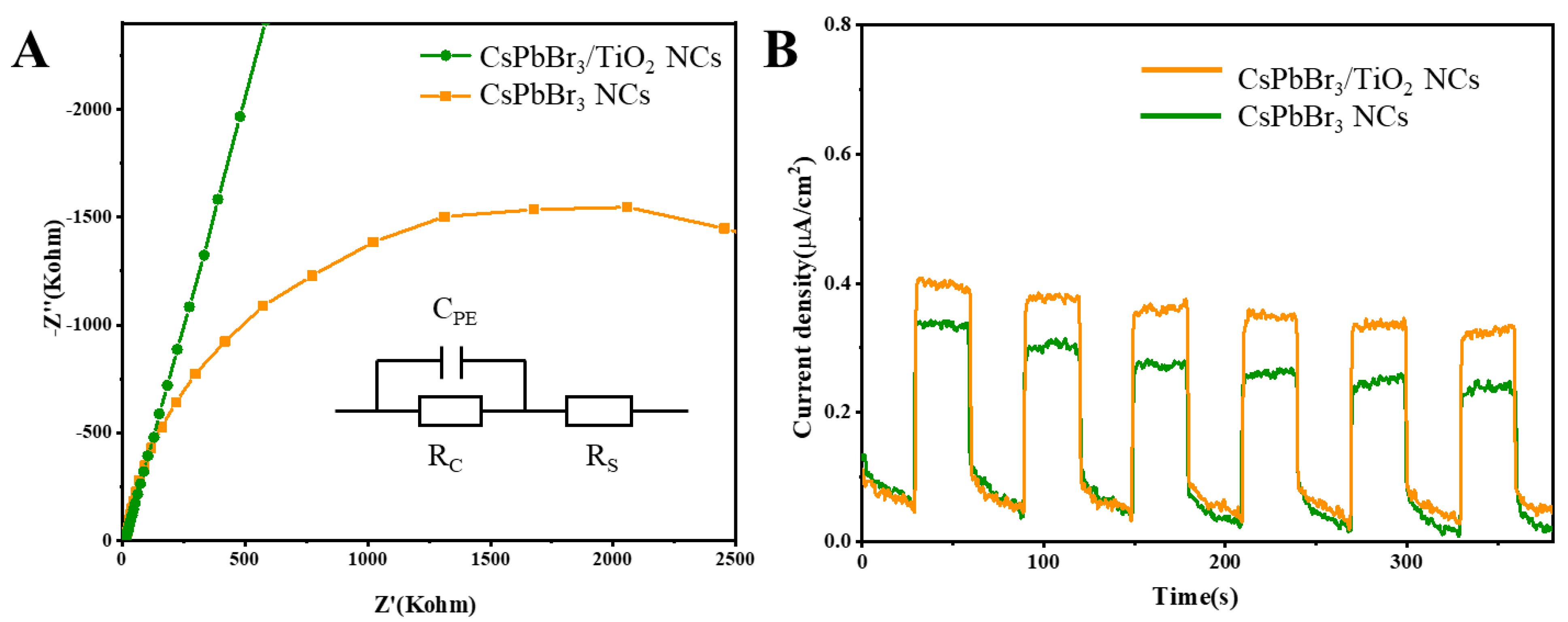
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2. Characterization
3.2.1. Materials Characterization
3.2.2. Optical Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–1. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N. J.; Noh, J. H.; Yang, W. S.; Kim, Y. C.; Ryu, S.; Seo, J.; Seok, S. Compositional engineering of perovskite materials for high-performance solar cells. Nature. 2015, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Marshall, A. R.; Sanehira, E. M.; Chernomordik, B. D.; Moore, D. T.; Christians, J. A.; Chakrabarti, T.; Luther, J. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. S.; Park, B. W.; Jung, E. H.; Jeon, N. J.; Kim, Y. C.; Lee, D. U.; Shin, S. S.; Seo, J.; Kim, E. K.; Noh, J. H. J. S. , Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science. 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2016, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Chen, J.; Dong, J.; Shan, Y. V50-fold EQE improvement up to 6.27% of solution-processed all-inorganic perovskite CsPbBr3 QLEDs via surface ligand density control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef]
- Zhi-Kuang, T.; Reza Saberi, M.; May Ling, L.; Pablo, D.; Ruben, H.; Felix, D.; Michael, P.; Aditya, S.; Pazos, L. M.; Dan, C. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2018, 9, 687–692. [Google Scholar]
- Zhang, X.; Xu, B.; Zhang, J.; Gao, Y.; Zheng, Y.; Wang, K.; Sun, X. All-inorganic perovskite nanocrystals for high-efficiency light emitting diodes: dual-phase CsPbBr3-CsPb2Br5 Composites. Adv. Funct. Mater. 2016, 26, 4595–4600. [Google Scholar] [CrossRef]
- Song, T.; Cheng, H.; Fu, C.; He, B.; Zou, B. Influence of the active layer nanomorphology on device performance for ternary PbSXSe1-X quantum dots based solution-processed infrared photodetector. Nanotechnology 2016, 27, 165202. [Google Scholar] [CrossRef]
- Chiba, T.; Hoshi, K.; Pu, Y. J.; Takeda, Y.; Kido, J. High efficiency perovskite quantum-dot light-emitting devices by effective washing process and interfacial energy level alignment. ACS Appl. Mater. Inter. 2017, 9, 18054. [Google Scholar] [CrossRef]
- Dong, Y.; Gu, Y.; Zou, Y.; Song, J.; Xu, L.; Li, H. Improving all-inorganic perovskite photodetectors by preferred orientation and plasmonic effect. Small 2016, 12, 5622–5632. [Google Scholar] [CrossRef] [PubMed]
- Chien, T. ;Mathews;Nripan;Yantara;Natalia;Mingjie;Mhaisalkar;Subodh, G.;Boix;Pablo, P., Perovskite materials for light-emitting diodes and lasers. Adv. Mater. 2016, 28, 6804–6834. [Google Scholar]
- Fu, Y.; Zhu, H.; Schrader, A. W.; Liang, D.; Jin, S. Nanowire lasers of formamidinium lead halide perovskites and their stabilized alloys with improved stability. Nano.Lett. 2016, 16, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M. V.; Trinh, M. T.; Jin, S.; Zhu, X. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Lim, S. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat.Mater. 2014, 13, 476–480. [Google Scholar] [CrossRef]
- Zhong, Q.; Cao, M.; Hu, H.; Di, Y.; Chen, M.; Li, P.; Wu, L.; Qiao, Z. One-pot synthesis of highly stable CsPbBr3@SiO2 core-shell nanoparticles. Acs Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano.Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Xu, Y. F.; Yang, M. Z.; Chen, H. Y.; Liao, J. F.; Kuang, D. Enhanced solar-driven gaseous CO2 conversion by CsPbBr3 nanocrystal/Pd nanosheet Schottky-junction photocatalyst. ACS Appl. Energy Mater. 2018, 1, 5083–5089. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M. I.; Grotevent, M. J.; Kovalenko, M. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liao, Q. , Embedding perovskite nanocrystals into a polymer matrix for tunable luminescence probes in cell imaging Adv. Funct. Mater. 2017, 27, 1604382. [Google Scholar] [CrossRef]
- Wei,Y.; Deng, X.;Xie, Z.; Cai, X.; Ma, S. Enhancing the stability of perovskite quantum dots by encapsulation in crosslinked polystyrene beads via a swelling-shrinking strategy toward superior water resistance. Adv. Funct. Mater. 2017, 27, 1703535. [CrossRef]
- Wang, Y.; Zhu, Y.; Huang, J.; Cai, J.; Zhu, J.; Yang, X.; Shen, J.; Jiang, H.; Li, C. CsPbBr3 perovskite quantum dots-bsed monolithic electrospun fiber membrane as an ultrastable and utrasensitive fluorescent sensor in aqueous medium. J.Phys. Chem. Lett. 2016, 7, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Raja, S. N.; Bekenstein, Y.; Koc, M. A.; Fischer, S.; Zhang, D.; Lin, L.; Ritchie, R. O.; Yang, P.; Alivisatos, A. Interfaces, Encapsulation of perovskite nanocrystals into macroscale polymer matrices: enhanced stability and polarization. ACS Appl. Mater. Inter.Inter. 2016, 8, 35523–35533. [Google Scholar] [CrossRef]
- Hou, S.; Guo, Y.; Tang, Y.; Quan, Q. Interfaces, synthesis and stabilization of colloidal perovskite nanocrystals by multidentate polymer micelles. ACS Appl. Mater. Inter. 2017, 9, 18417. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Guo, S.; Cao, S.; Wang, L.; Gao, F.; Yang, Z.; Zheng, J.; Yang, W. General strategy for In situ growth of all-Inorganic CsPbX3 (X = Br, I, and Cl) perovskite nanocrystals in polymer fibers toward significantly enhanced water/thermal stabilities. Adv. Opt. Mater. 2018, 6(15), 1800346. [Google Scholar] [CrossRef]
- Yang, X.; Xu, T.; Zhu, Y.; Cai, J.; Gu, K; Zhu, J. Devices, E., Preparation of CsPbBr3@PS composite microspheres with high stability by electrospraying. J. Mater. Chem. C 2018, 6, 7971–7975. [Google Scholar] [CrossRef]
- Meyns, M.; Perálvarez, M.; Heuer-Jungemann, A.; Hertog, W.; Ibáñez, M.; Nafria, R.; Genç, A.; Arbiol, J.; Kovalenko, M. V.; Carreras, J. Interfaces, polymer-enhanced stability of inorganic perovskite nanocrystals and their application in color conversion LEDs. ACS Appl. Mat.Inte. 2016, 8, 19579–19586. [Google Scholar] [CrossRef]
- Li, Z.; Long, K.; Huang, S.; Liang, L. Highly luminescent and ultrastable CsPbBr3 perovskite quantum dots incorporated into a silica/alumina monolith. Angew. Chem. 2017, 129, 8246–8250. [Google Scholar] [CrossRef]
- Wang, H. C.; Lin, S. Y.; Tang, A. C.; Singh, B. P.; Tong, H. C.; Chen, C. Y.; Lee, Y. C.; Tsai, T. L.; Liu, S. Mesoporous silica particles integrated with all-Inorganic CsPbBr3 perovskite quantum-dot nanocomposites (MP-PQDs) with high stability and wide color gamut used for backlight display. Angew. Chem. Int. Ed. 2016, 55, 7924–7929. [Google Scholar] [CrossRef]
- Dirin, D. N.; Protesescu, L.; Trummer, D.; Kochetygov, I. V.; Yakunin, S.; Krumeich, F.; Stadie, N. P.; Kovalenko, M. V. Harnessing defect-tolerance at the nanoscale: highly luminescent lead halide perovskite nanocrystals in mesoporous silica matrixes. Nano Lett. 2016, 16, 5866–5874. [Google Scholar] [CrossRef]
- Chun;Sun;Yu;Zhang;Cheng;Ruan;Chunyang;Yin;Xiaoyong;Materials, W. Efficient and stable white lEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater. 2016, 28, 10088–10094. [CrossRef] [PubMed]
- Loiudice, A.; Saris, S.; Oveisi, E.; Alexander, D.; Buonsanti, R. CsPbBr3 QD/AlOx inorganic nanocomposites with exceptional stability in water, light, and heat. Angew. Chem. 2017, 129, 10696–10701. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. ;Hofman, E;Li, J.; Davis A. Photoelectrochemically active and environmentally stable CsPbBr3/TiO2 core/shell nanocrystals. Adv. Funct. Mater. 2017, 28, 1704288. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, M.; Yang, Z.; Qiu, H.; Bhatti, A. Trioctylphosphine-assisted pre-protection low-temperature solvothermal synthesis of highly stable CsPbBr3/TiO2 Nanocomposites. J. Phys. Chem. Lett. 2021, 12(15), 3786–3794. [Google Scholar] [CrossRef]
- Long, R.; Fang, W. H.; Prezhd, O. V. Strong interaction at the perovskite/TiO2 interface facilitates ultrafast photoinduced charge separation: a nonadiabatic molecular dynamics sudy. J. Phys. Chem. C 2017, 121, 3797–3806. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Chen, Y.; Gao, Y. Improved electroluminescence performance of quantum dot light-emitting diodes: A promising hole injection layer of Fe-doped NiO nanocrystals. Opt. Mater. 2020, 107, 110158. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



