Submitted:
05 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
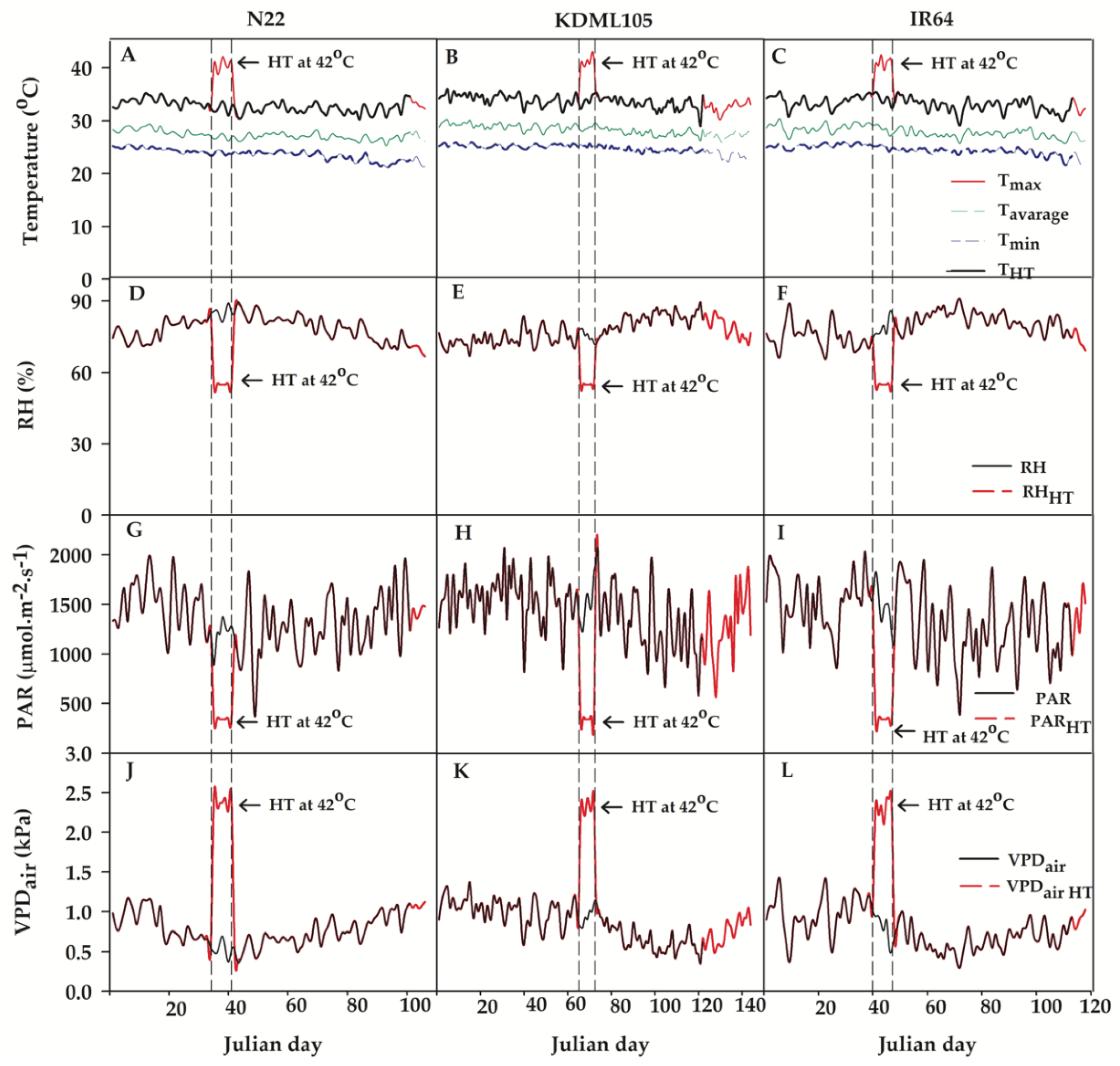
2.1. Rice experimental growing period under various climatic conditions
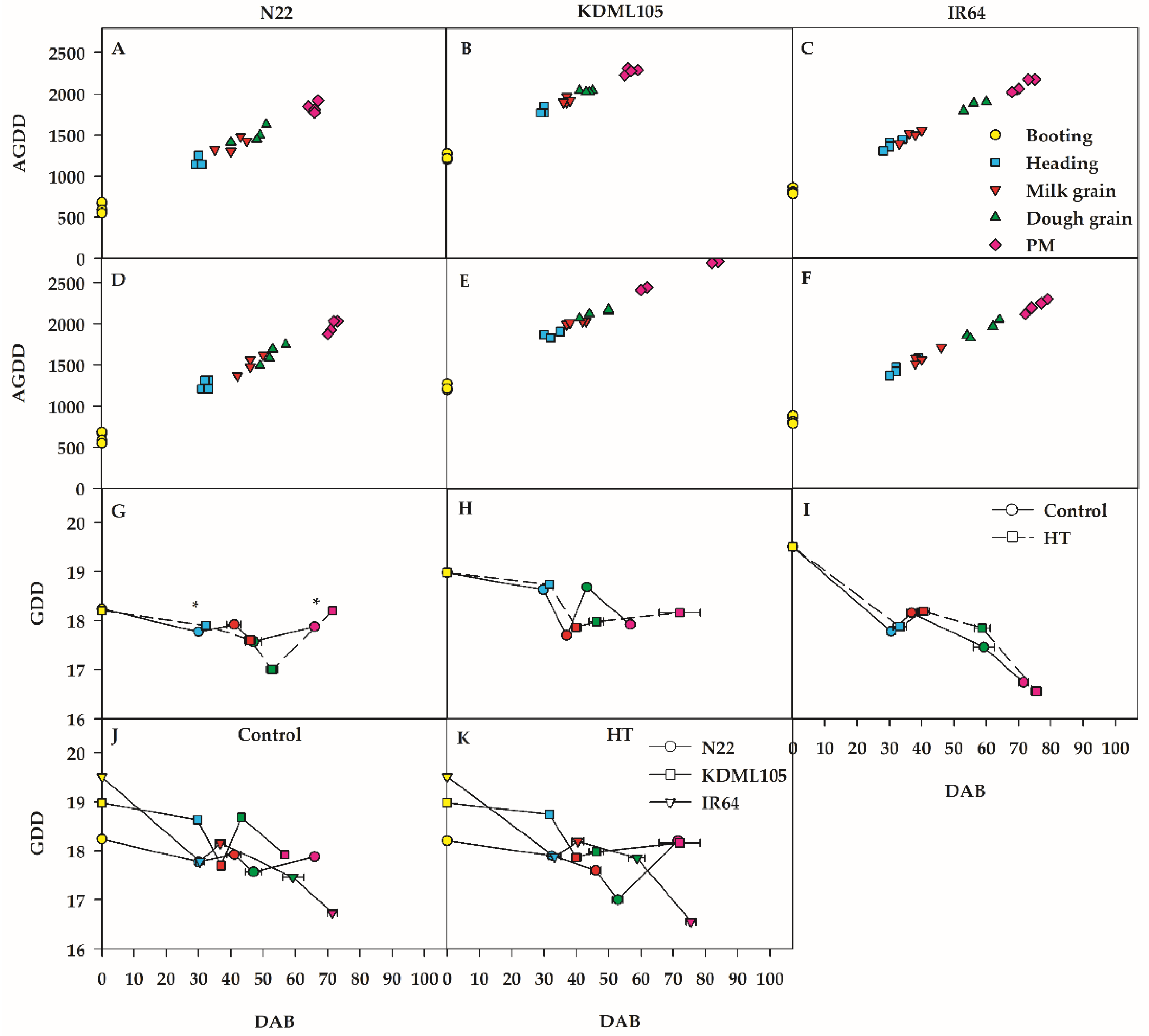
2.2. AGDD and GDD of booting heat stress influence rice phenology shift
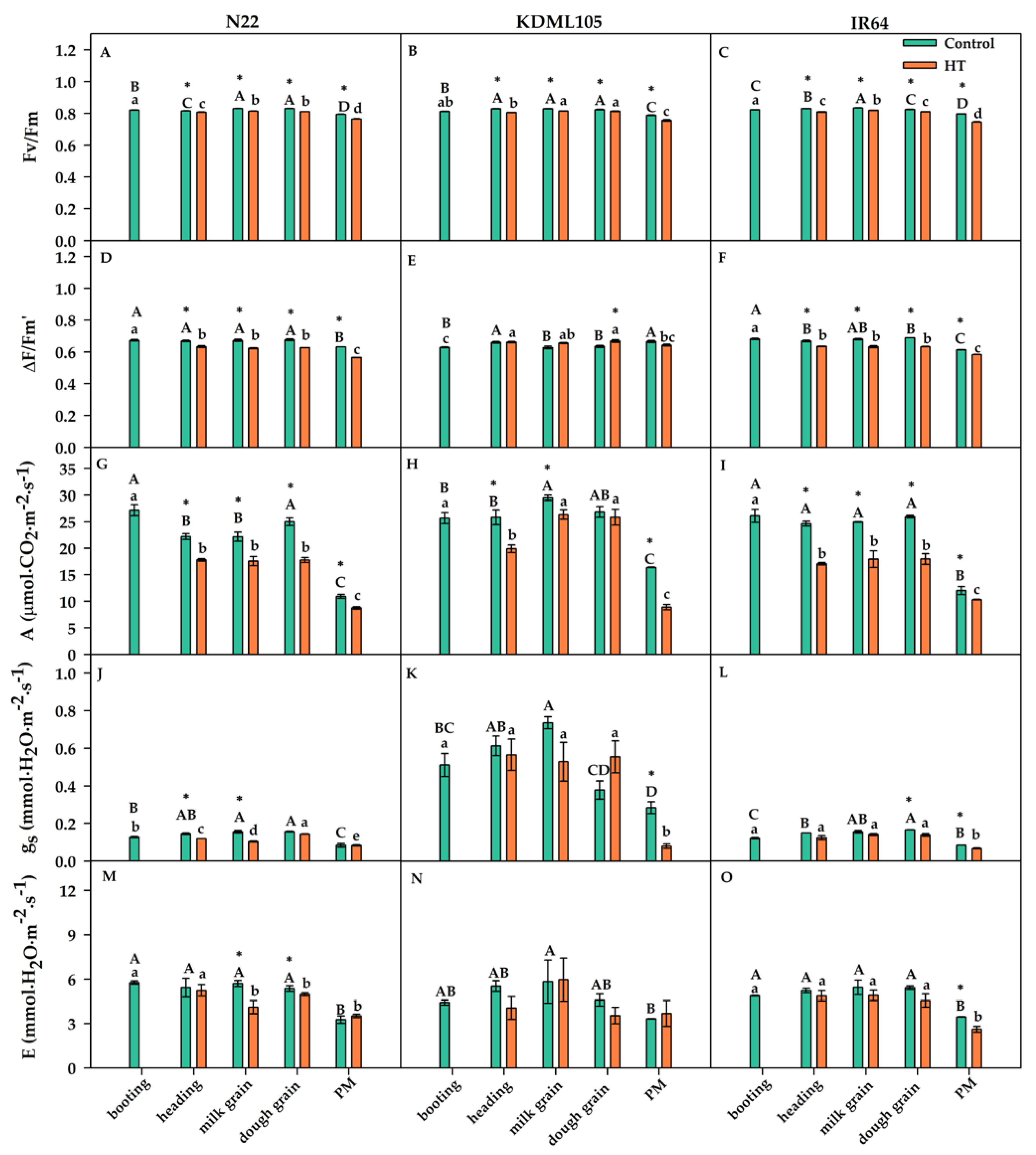
2.3. Rice photosynthesis as affected by booting heat stress
2.3.1. PSII efficiency
2.3.2. A, gs, and E
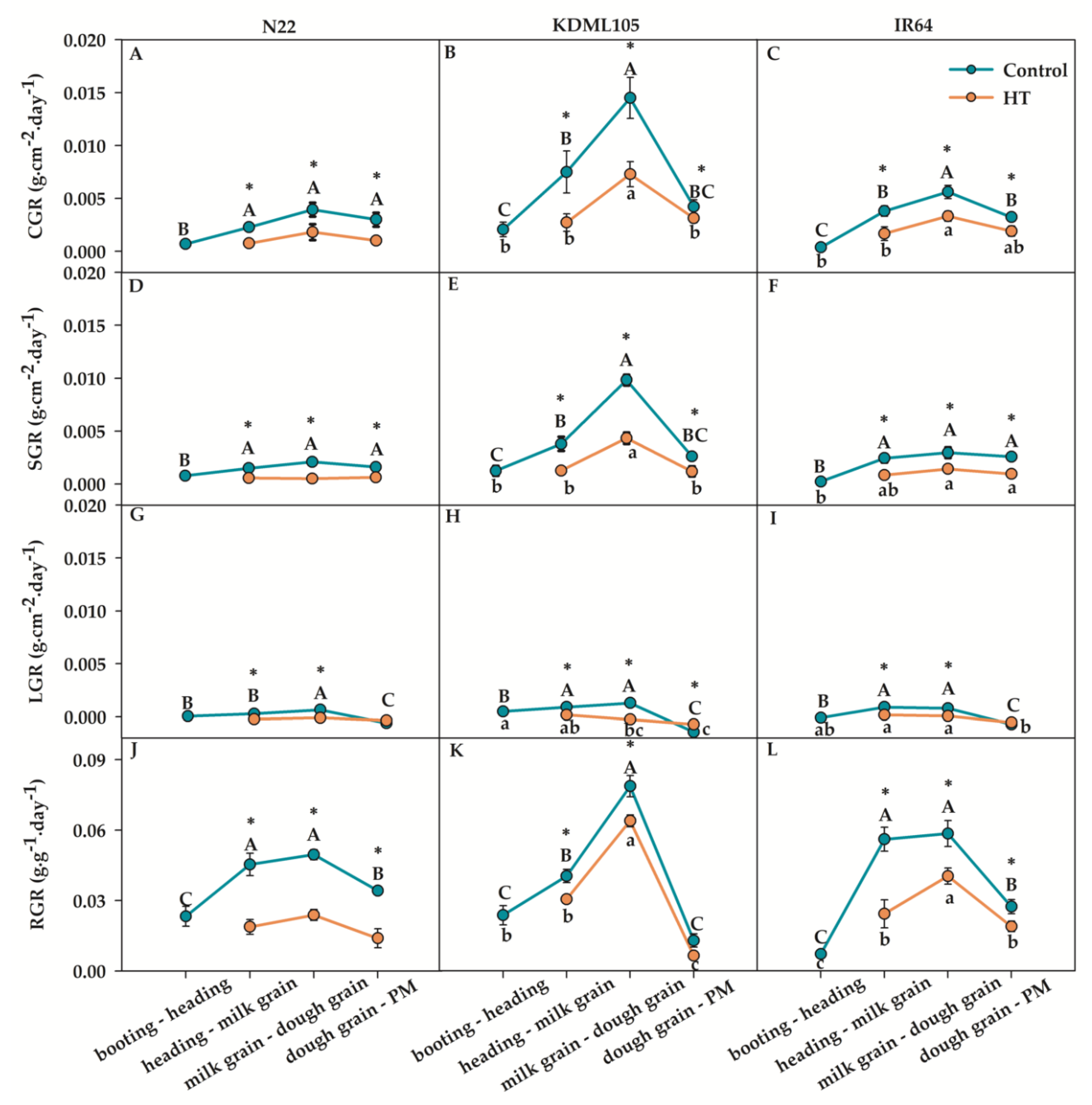
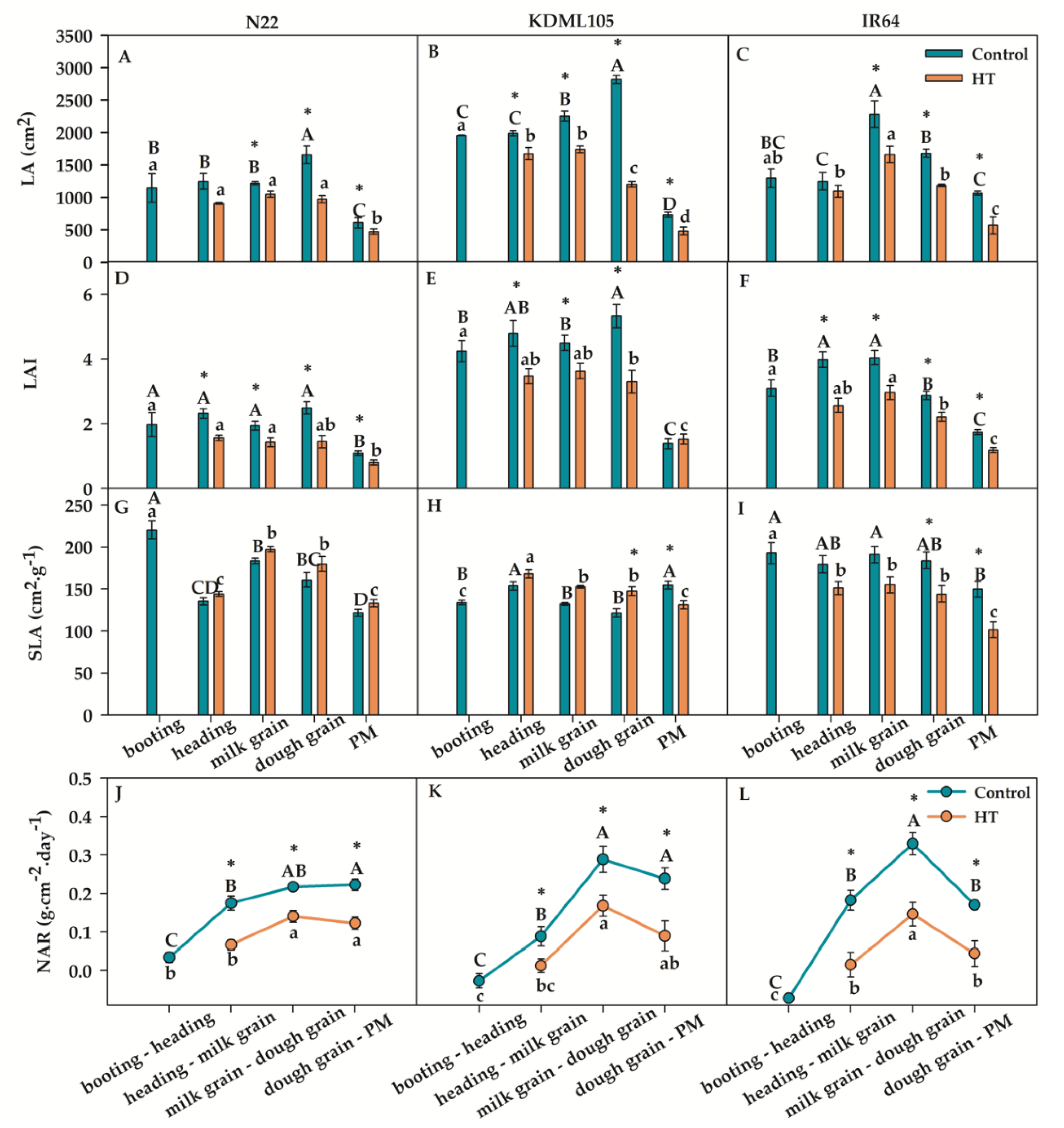
2.4. Rice growth performance under booting heat stress
2.5. Rice yield components and yield response to booting heat stress
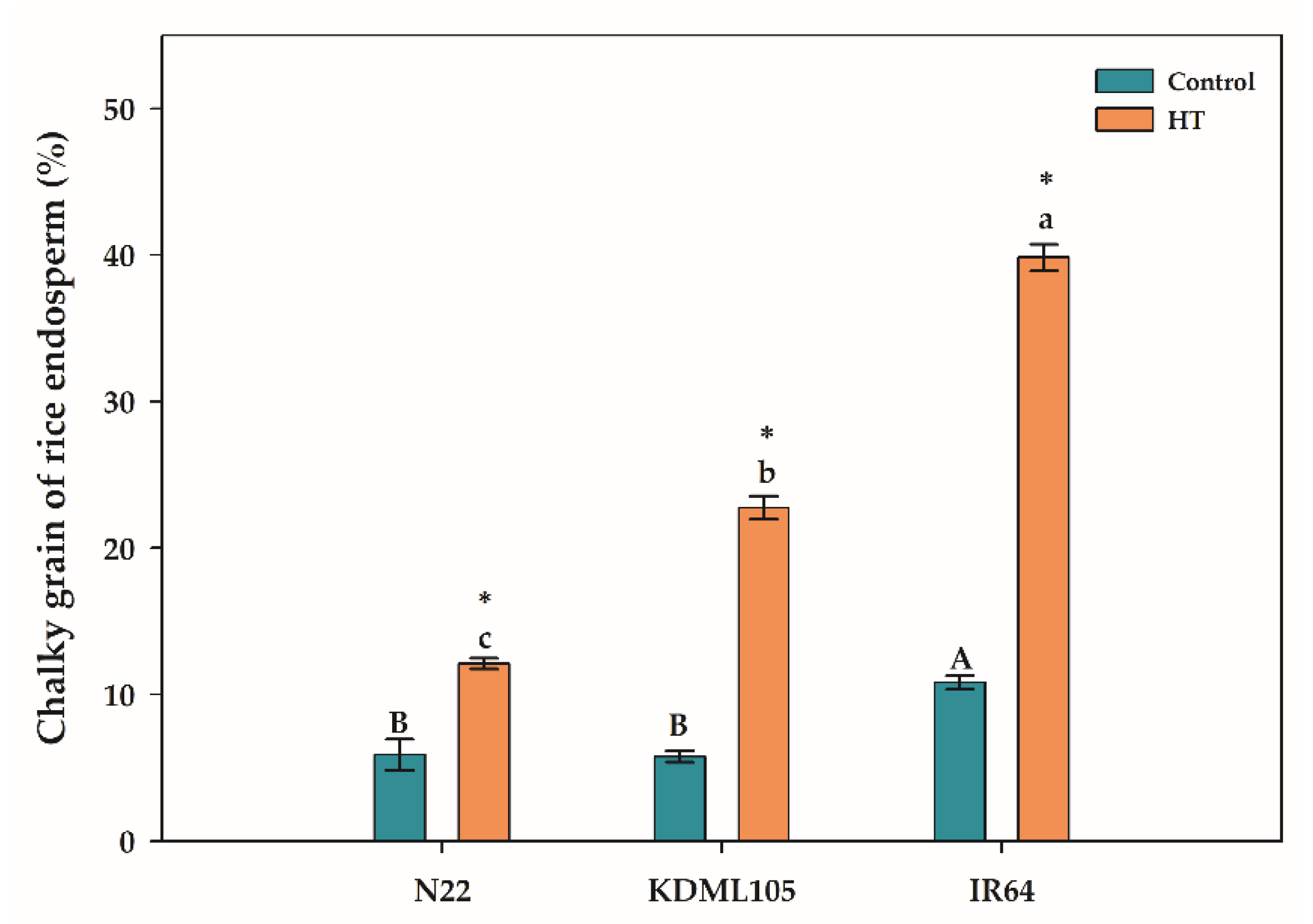
2.6. Chalky rice grain due to booting heat stress
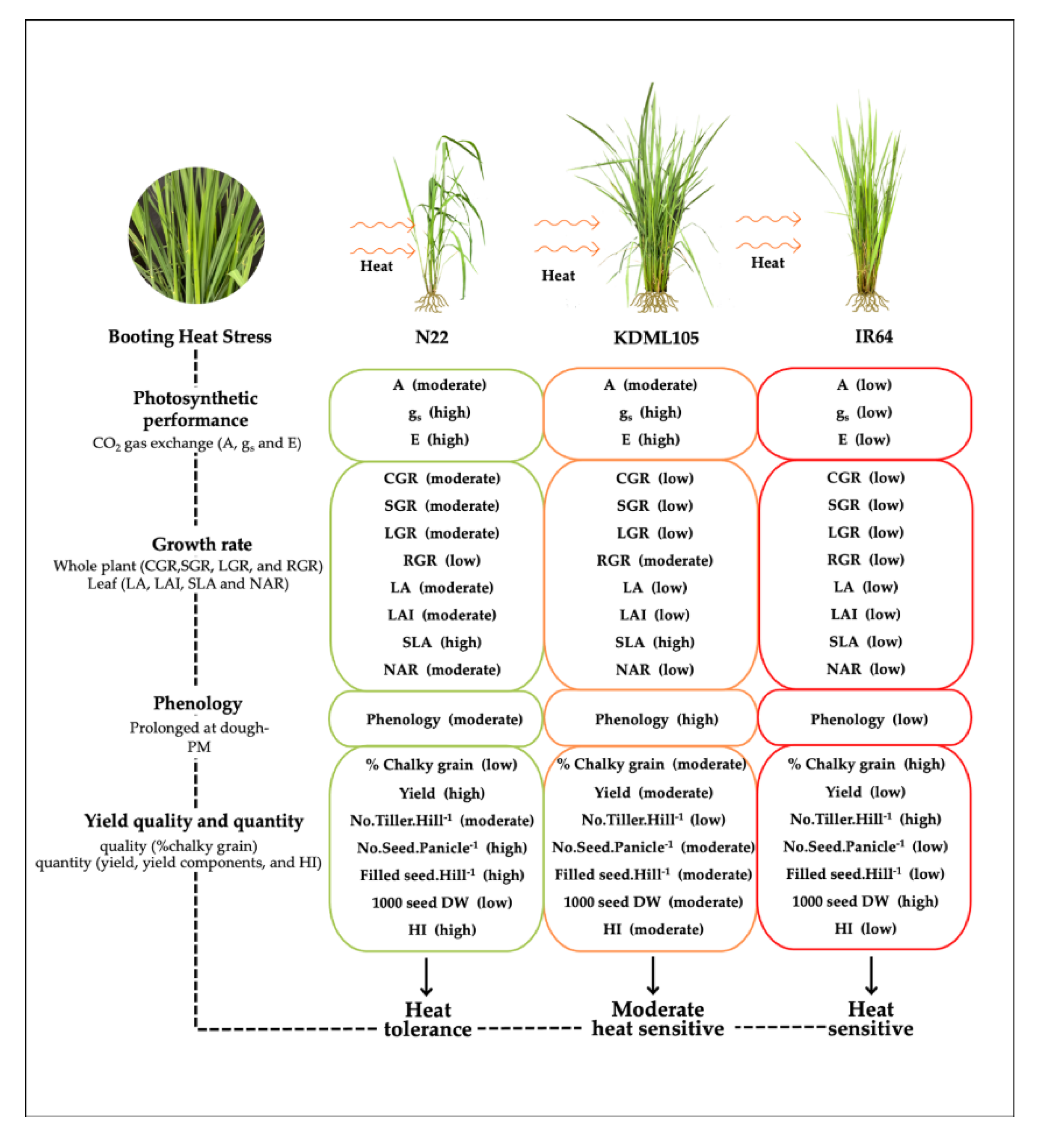
3. Discussion
3.1. Booting heat stress initiating forward phenological development in rice
3.2. Booting heat stress altering leaf characteristic and leaf function in rice
3.3. Booting heat stress impacting on biomass in rice
3.4. Booting heat stress affecting rice yield and grain quality
4. Materials and Methods
4.1. Plant materials
4.2. Phenological development
4.3. AGDD calculation
4.4. Photosynthetic determination
4.4.1. PSII efficiency
4.4.2. CO2 gas exchange
4.5. Growth analysis
4.6. Yield components and yield
4.7. Chalky grain
4.8. Experimental design and statistical analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jagadish, S.K. Heat stress during flowering in cereals-effects and adaptation strategies. New Phytol 2020, 226, 1567–1572. [Google Scholar] [CrossRef]
- Lyman, N.B.; Jagadish, K.S.; Nalley, L.L.; Dixon, B.L.; Siebenmorgen, T. Neglecting rice milling yield and quality underestimates economic losses from high-temperature stress. PLoS ONE 2013, 8, e72157. [Google Scholar] [CrossRef] [PubMed]
- FAS. Thailand Rice: Recent Dry Conditions after a Promising Start; Optimism Still Remains for this Crop Season; Commodity Intelligence Report, Foreign Agricultural Service, United States Department of Agriculture: Washington, DC, USA, 2021; p. 14. [Google Scholar]
- OAE. Agricultural Production Data: Off-Season Rice Varieties—Cultivated Area Harvested Area Productivity and Yield per Rai in 2020; Office of Agricultural Economics: Bangkok, Thailand, 2022. [Google Scholar]
- Xu, Y.; Chu, C.; Yao, S. The Impact of High-Temperature Stress on Rice: Challenges and Solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Dutta, S.; Mohanty, S.; Tripathy, B. C. Role of Temperature Stress on Chloroplast Biogenesis and Protein Import in Pea. Plant Physiol. 2009, 150, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Chen, J.H.; He, N.Y.; Guo, F.Q. Metabolic Reprogramming in Chloroplasts under Heat Stress in Plants. Int. J. Mol. Sci. 2018, 19, 849. [Google Scholar] [CrossRef] [PubMed]
- Beena, R.; Vighneswaran, V.; Sindhumole, P.; Narayankutty, M.; Voleti, S. Impact of High Temperature Stress during Reproductive and Grain Filling Stage in Rice. ORYZA- Int. J. Rice 2018, 55, 126. [Google Scholar] [CrossRef]
- Thuy, T.; Saitoh, K. Responses of Fourteen Vietnamese Rice (Oryza Sativa L.) Cultivars to High Temperatures during Grain Filling Period under Field Conditions. Agronomy 2017, 7, 57. [Google Scholar] [CrossRef]
- Mahmood, A.; Wang, W.; Ali, I.; Zhen, F.; Osman, R.; Liu, B.; Liu, L.; Zhu, Y.; Cao, W.; Tang, L. Individual and Combined Effects of Booting and Flowering High-Temperature Stress on Rice Biomass Accumulation. Plants 2021, 10, 1021. [Google Scholar] [CrossRef]
- Bahuguna, R. N.; Solis, C. A.; Shi, W.; Jagadish, K. S. V. Post-Flowering Night Respiration and Altered Sink Activity Account for High Night Temperature-Induced Grain Yield and Quality Loss in Rice (Oryza Sativa L.). Physiol. Plant. 2017, 159, 59–73. [Google Scholar] [CrossRef]
- Zhen, F.; Zhou, J.; Mahmood, A.; Wang, W.; Chang, X.; Liu, B.; Liu, L.; Cao, W.; Zhu, Y.; Tang, L. Quantifying the Effects of Short-Term Heat Stress at Booting Stage on Nonstructural Carbohydrates Remobilization in Rice. Crop J. 2020, 8, 194–212. [Google Scholar] [CrossRef]
- Morita, S.; Wada, H.; Matsue, Y. Countermeasures for Heat Damage in Rice Grain Quality under Climate Change. Plant Prod. Sci. 2016, 19, 1–11. [Google Scholar] [CrossRef]
- Shi, P.; Tang, L.; Wang, L.; Sun, T.; Liu, L.; Cao, W.; Zhu, Y. Post-Heading Heat Stress in Rice of South China during 1981–2010. PLOS ONE 2015, 10, e0130642. [Google Scholar] [CrossRef] [PubMed]
- Rani, B.A.; Maragatham, N. Effect of elevated temperature on rice phenology and yield. Indian J. Sci. Technol. 2013, 6, 5095–5097. [Google Scholar]
- Sanwong, P.; Sanitchon, J.; Dongsansuk, A.; Jothityangkoon, D. High Temperature Alters Phenology, Seed Development and Yield in Three Rice Varieties. Plants 2023, 12, 666. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Silk, W. K.; Schurr, U. Environmental Effects on Spatial and Temporal Patterns of Leaf and Root Growth. Annu. Rev. Plant Biol. 2009, 60, 279–304. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Tao, F.; Palosuo, T.; Rötter, R. P. Impacts of Heat Stress on Leaf Area Index and Growth Duration of Winter Wheat in the North China Plain. Field Crops Res. 2018, 222, 230–237. [Google Scholar] [CrossRef]
- Nagai, T.; Makino, A. Differences Between Rice and Wheat in Temperature Responses of Photosynthesis and Plant Growth. Plant Cell Physiol. 2009, 50, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Hurkman, W. J.; Vensel, W. H.; Tanaka, C. K.; Whitehand, L.; Altenbach, S. B. Effect of High Temperature on Albumin and Globulin Accumulation in the Endosperm Proteome of the Developing Wheat Grain. J. Cereal Sci. 2009, 49, 12–23. [Google Scholar] [CrossRef]
- Piveta, L. B.; Roma-Burgos, N.; Noldin, J. A.; Viana, V. E.; Oliveira, C. de; Lamego, F. P.; Avila, L. A. de. Molecular and Physiological Responses of Rice and Weedy Rice to Heat and Drought Stress. Agriculture 2020, 11, 9. [Google Scholar] [CrossRef]
- Hilty, J.; Muller, B.; Pantin, F.; Leuzinger, S. Plant Growth: The What, the How, and the Why. New Phytol. 2021, 232, 25–41. [Google Scholar] [CrossRef]
- Paethaisong, W.; Lontom, W.; Dongsansuk, A. Impact of Short-Term Exposure to Elevated Temperatures on Physiology of Thai Rice (Cv. Riceberry). IOP Conf. Ser. Earth Environ. Sci. 2019, 346, 012083. [Google Scholar] [CrossRef]
- Prasertthai, P.; Paethaisong, W.; Theerakulpisut, P.; Dongsansuk, A. High Temperature Alters Leaf Lipid Membrane Composition Associated with Photochemistry of PSII and Membrane Thermostability in Rice Seedlings. Plants 2022, 11, 1454. [Google Scholar] [CrossRef]
- 25. Dongsansuk ,A.; Theerakulpisut, P.; Pongdontri, P. Short-term heat exposure effect on PSII efficiency and growth of rice (Oryza sativa L.). Pertanika J Trop Agric Sci. 2017, 40, 621–628.
- Pansarakham, P.; Pongdontri, P.; Theerakulpisut, P.; Dongsansuk, A. Effect of Short-Term Heat Exposure on Physiological Traits of Indica Rice at Grain-Filling Stage. Acta Physiol. Plant. 2018, 40, 173. [Google Scholar] [CrossRef]
- Prasad, P. V. V.; Boote, K. J.; Allen, L. H.; Sheehy, J. E.; Thomas, J. M. G. Species, Ecotype and Cultivar Differences in Spikelet Fertility and Harvest Index of Rice in Response to High Temperature Stress. Field Crops Res. 2006, 95, (2–3). [Google Scholar] [CrossRef]
- Sakata, T.; Takahashi, H.; Nishiyama, I.; Higashitani, A. Effects of High Temperature on the Development of Pollen Mother Cells and Microspores in Barley Hordeum Vulgare L. J. Plant Res. 2000, 113, 395–402. [Google Scholar] [CrossRef]
- Abiko, M.; Akibayashi, K.; Sakata, T.; Kimura, M.; Kihara, M.; Itoh, K.; Asamizu, E.; Sato, S.; Takahashi, H.; Higashitani, A. High-Temperature Induction of Male Sterility during Barley (Hordeum Vulgare L.) Anther Development Is Mediated by Transcriptional Inhibition. Sex. Plant Reprod. 2005, 18, 91–100. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K.; Horie, T. High Temperature-Induced Spikelet Sterility of Japonica Rice at Flowering in Relation to Air Temperature, Humidity and Wind Velocity Conditions. Jpn. J. Crop Sci. 1997, 66, 449–455. [Google Scholar] [CrossRef]
- Jeng, T. L.; Tseng, T. H.; Wang, C. S.; Chen, C. L.; Sung, J. M. Starch Biosynthesizing Enzymes in Developing Grains of Rice Cultivar Tainung 67 and Its Sodium Azide-Induced Rice Mutant. Field Crops Res. 2003, 84, 261–269. [Google Scholar] [CrossRef]
- Aghamolki, M. T. K.; Yusop, M. K.; Oad, F. C.; Zakikhani, H.; Jaafar, H. Z.; Kharidah, S.; Musa, M. H. Heat Stress Effects on Yield Parameters of Selected Rice Cultivars at Reproductive Growth Stages. 2014. [Google Scholar]
- Tao, L.X.; Tan, H.J.; Wang, X.; Cao, L.Y.; Cheng, S.H. Effects of high temperature stress on super hybrid rice Guodao 6 during flowering and filling phases. China J. Rice Sci. 2007, 21, 518–524. [Google Scholar]
- Matsui, T.; Omasa, K.; Horie, T. The Difference in Sterility Due to High Temperatures during the Flowering Period among Japonica-Rice Varieties. Plant Prod. Sci. 2001, 4, 90–93. [Google Scholar] [CrossRef]
- Fitzgerald, M. A.; McCouch, S. R.; Hall, R. D. Not Just a Grain of Rice: The Quest for Quality. Trends Plant Sci. 2009, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, T.; Horigane, A. K.; Ida, M.; Iwasawa, N.; San-oh, Y. A.; Nakazono, M.; Nishizawa, N. K.; Masumura, T.; Kondo, M.; Yoshida, M. Formation of Grain Chalkiness and Changes in Water Distribution in Developing Rice Caryopses Grown under High-Temperature Stress. J. Cereal Sci. 2009, 50, 166–174. [Google Scholar] [CrossRef]
- Lang, N. T.; Ha, P. T. T.; Tru, P. C.; Toan, T. B.; Buu, B. C.; Cho, Y.-C. Breeding for Heat Tolerance Rice Based on Marker-Assisted Backcrosing in Vietnam. Plant Breed. Biotechnol. 2015, 3, 274–281. [Google Scholar] [CrossRef]
- Coast, O.; Murdoch, A. J.; Ellis, R. H.; Hay, F. R.; Jagadish, K. S. V. Resilience of Rice (O Ryza Spp.) Pollen Germination and Tube Growth to Temperature Stress: Resilience of Rice Pollen to Temperature Stress. Plant Cell Environ. 2016, 39, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Alduchov, O. A.; Eskridge, R. E. Improved Magnus Form Approximation of Saturation Vapor Pressure. J. Appl. Meteorol. 1996, 35, 601–609. [Google Scholar] [CrossRef]
- Redona, D. Standard Evaluation System (SES) for Rice, 5th ed.; International Rice Research Institute, Los Baños: Laguna, Phillppines, 2013; p. 55. [Google Scholar]
- Mcmaster, G. Growing Degree-Days: One Equation, Two Interpretations. Agric. For. Meteorol. 1997, 87, 291–300. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview. In Chlorophyll a Fluorescence; Papageorgiou, G. C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer Netherlands: Dordrecht, 2004. [Google Scholar] [CrossRef]
- Pandey, R.; Paul, V.; Madurima, D.; Meena, M.; Meena, R.C. Plant Growth Analysis. Manual of ICAR Sponsored Training Programme on Physiological Techniques to Analyze the Impact of Climate Change on Crop Plants. , Division of Plant Physiology.; IARI.; New Delhi pp.103–107. 16–25 January. [CrossRef]
- Chen, X.; Chen, M.; Lin, G.; Yang, Y.; Yu, X.; Wu, Y.; Xiong, F. Structural Development and Physicochemical Properties of Starch in Caryopsis of Super Rice with Different Types of Panicle. BMC Plant Biol. 2019, 19, 482. [Google Scholar] [CrossRef]

| Geno-types | Growth stage | A | gs | E | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | HT | % change | Control | HT | % change | Control | HT | % change | ||
| N22 | Booting | 27.18±1.02a | ND | ND | 0.127±0.00b | ND | ND | 5.765±0.11a | ND | ND |
| heading | 22.22±0.56b* | 17.79±0.22b | -19.90 | 0.145±0.00ab* | 0.119±0.00c | -18.33 | 5.423±0.63a | 5.243±0.38a | -3.32 | |
| Milk grain | 22.18±0.88b* | 17.54±0.87b | -20.92 | 0.155±0.01a* | 0.104±0.00d | -33.03 | 5.703±0.20a* | 4.119±0.44b | -27.77 | |
| Dough grain | 25.02±0.72a* | 17.76±0.46b | -29.02 | 0.156±0.00a | 0.143±0.00a | -8.18 | 5.356±0.19a* | 4.197±0.10b | -21.64 | |
| PM | 10.90±0.38c* | 8.75±0.23c | -19.67 | 0.084±0.01c | 0.083±0.00e | -1.22 | 3.265±0.25b | 3.520±0.12b | +7.81 | |
| KDML105 | Booting | 25.70±1.03b | ND | ND | 0.512±0.06bc | ND | ND | 4.748±0.18ab | ND | ND |
| heading | 25.83±1.38b* | 19.890±0.73b | -23.01 | 0.613±0.05ab | 0.566±0.08a | -7.59 | 5.932±0.39ab | 4.356±0.83 | -26.56 | |
| Milk grain | 29.49±0.52a* | 26.349±0.89a | -10.66 | 0.736±0.03a | 0.529±0.10a | -28.12 | 6.260±1.57a | 6.398±1.57 | +2.21 | |
| Dough grain | 26.84±0.10ab | 24.853±1.49a | -7.41 | 0.379±0.05cd | 0.555±0.08a | +46.29 | 4.927±0.44ab | 3.808±0.59 | -22.71 | |
| PM | 16.37±0.07c* | 8.891±0.49c | -45.72 | 0.285±0.03d* | 0.080±0.01b | -71.89 | 3.574±0.02b | 3.957±0.94 | +10.72 | |
| IR64 | Booting | 26.12±1.19a | ND | ND | 0.122±0.00c | ND | ND | 5.235±0.03a | ND | ND |
| heading | 24.65±0.46a* | 17.06±0.22b | -30.80 | 0.149±0.00b | 0.124±0.01a | -16.69 | 5.971±0.16a | 5.277±0.37a | -11.62 | |
| Milk grain | 24.97±0.07a* | 17.92±1.58b | -28.23 | 0.155±0.01ab | 0.141±0.01a | -8.94 | 5.840±0.52a | 5.261±0.37a | -9.92 | |
| Dough grain | 25.97±0.25a* | 17.95±0.10b | -30.88 | 0.166±0.00a* | 0.139±0.01a | -16.28 | 5.803±0.13a | 4.888±0.47a | -15.77 | |
| PM | 12.01±0.75b* | 10.32±0.05c | -14.07 | 0.084±0.00d* | 0.067±0.00b | -20.68 | 3.709±0.02b* | 2.800±0.21b | -24.52 | |
| Geno–types | No.Tiller.Hill–1 | No.Seed.Panicle–1 | Filled seed.Hill–1 | 1000 seed DW | Yield (kg.rai–1) | HI | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| control | heat | % change | control | heat | % change | control | heat | % change | control | heat | % change | control | heat | % change | control | heat | % change | |||
| N22 | 21.50±0.29c | 23.67±0.88c* | +10.23a | 103.00±0.58b* | 82.00±1.15a | -20.38a | 715.50±28.57c* | 558.50±8.95ab | -21.75a | 19.35±1.07b* | 13.5±0.69b | –29.89b | 431.44±25.53b* | 230.21±6.65c | –46.17a | 0.31±0.01b* | 0.29±0.01a | –10.06a | ||
| KDML105 | 38.00±0.58a* | 33.00±0.00a | –13.12b | 103.50±2.06b* | 77.00±1.73b | –25.58b | 1300.6±59.03a* | 720.50±79.39a | –43.92b | 32.38±2.39a* | 23.2±0.81a | –27.46b | 849.44±51.42a* | 439.15±5.98a | –47.97a | 0.39±0.01a* | 0.26±0.01b | –32.83c | ||
| IR64 | 26.00±1.15b | 29.5±0.87b* | +14.05a | 115.00±0.00a* | 74.00±1.15b | –35.63c | 1123.5±89.26b* | 429.33±14.85b | –61.70b | 23.06±0.71b | 23.0±0.40a | +0.13a | 703.68±44.63a* | 252.49±1.76b | –65.20b | 0.37±0.02a* | 0.28±0.00a | –24.30b | ||
| Means±SE | 28.50±2.49 | 28.72±1.41 | +3.72 | 107.17±2.06 | 77.67±1.35 | –27.20 | 1064.5±89.26 | 569.44±48.21 | –42.46 | 24.93±2.09 | 19.9±1.63 | –19.08 | 670.52±65.57 | 307.28±33.23 | –53.12 | 0.36±0.01 | 0.28±0.01 | -22.40 | ||
| No. | Timing (h) |
Temperature (°C ) |
Relative humidity (RH) (%) |
Timing (h) |
Light intensity* (µmol.m–2. s–1) |
|---|---|---|---|---|---|
| 1 | 00.00–03.00 | 27 | 66 | 06.00–07.00 | 70 |
| 2 | 03.00–07.00 | 26 | 70 | 07.00–08.00 | 115 |
| 3 | 07.00–09.00 | 29 | 61 | 08.00–09.00 | 200 |
| 4 | 09.00–10.00 | 35 | 51 | 09.00–10.00 | 265 |
| 5 | 10.00–12.00 | 38 | 46 | 10.00–11.00 | 340 |
| 6 | 12.00–15.00 | 42 | 41 | 11.00–13.00 | 390 |
| 7 | 15.00–17.00 | 40 | 43 | 13.00–14.00 | 340 |
| 8 | 17.00–18.00 | 37 | 50 | 14.00–15.00 | 265 |
| 9 | 18.00–21.00 | 33 | 57 | 15.00–16.00 | 200 |
| 10 | 21.00–00.00 | 32 | 62 | 16.00–17.00 | 115 |
| 11 | 17.00–18.00 | 70 | |||
| 12 | 18.00–06.00 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





