Submitted:
05 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
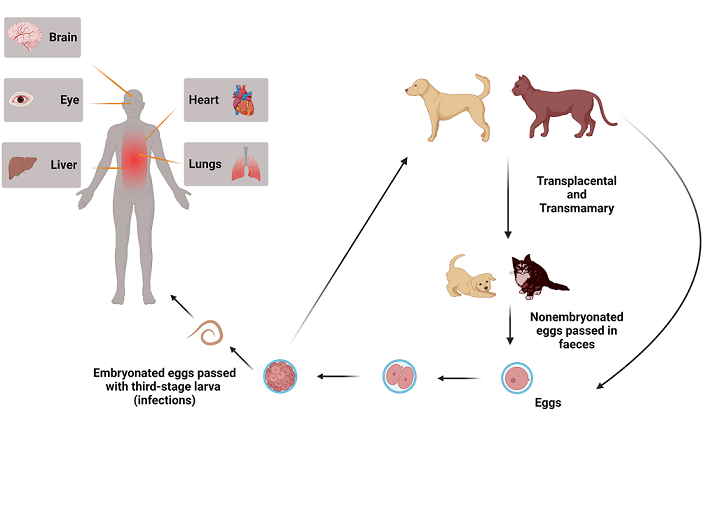
1. Introduction
2. Terminology
3. Epidemiology
4. Pathophysiology
5. Clinical presentations
5.1. Visceral larva migrans
5.2. Ocular larval toxocarosis
5.3. Neurotoxocarosis or cerebral toxocarosis
5.4. Covert and common toxocarosis
6. Diagnosis of toxocariasis in humans
6. Treatment
7. Prevention
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gasser, R.B.; et al. Harnessing the Toxocara Genome to Underpin Toxocariasis Research and New Interventions. Adv Parasitol 2016, 91, 87–110. [Google Scholar] [PubMed]
- Mizgajska-Wiktor, H.; Jarosz, W. [A comparison of soil contamination with Toxocara canis and Toxocara cati eggs in rural and urban areas of Wielkopolska district in 2000-2005]. Wiad Parazytol 2007, 53, 219–25. [Google Scholar] [PubMed]
- Gawor, J.; Borecka, A. The contamination of the environment with Toxocara eggs in Mazowieckie voivodship as a risk of toxocarosis in children. Wiad Parazytol 2004, 50, 237–41. [Google Scholar] [PubMed]
- Fan, C.K.; et al. Cerebral Toxocariasis: Silent Progression to Neurodegenerative Disorders? Clin Microbiol Rev 2015, 28, 663–86. [Google Scholar] [CrossRef] [PubMed]
- Beaver, P.C.; et al. Chronic eosinophilia due to visceral larva migrans; report of three cases. Pediatrics 1952, 9, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.K.; Liao, C.W.; Cheng, Y.C. Factors affecting disease manifestation of toxocarosis in humans: genetics and environment. Vet Parasitol 2013, 193, 342–52. [Google Scholar] [CrossRef]
- Overgaauw, P.A.; van Knapen, F. Veterinary and public health aspects of Toxocara spp. Vet Parasitol 2013, 193, 398–403. [Google Scholar] [CrossRef]
- Morimatsu, Y.; et al. A familial case of visceral larva migrans after ingestion of raw chicken livers: appearance of specific antibody in bronchoalveolar lavage fluid of the patients. Am J Trop Med Hyg 2006, 75, 303–6. [Google Scholar] [CrossRef]
- Choi, G.Y.; et al. Acute drug-induced hepatitis caused by albendazole. J Korean Med Sci 2008, 23, 903–5. [Google Scholar] [CrossRef]
- Rubinsky-Elefant, G.; et al. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol 2010, 104, 3–23. [Google Scholar] [CrossRef]
- Schantz, P.M.; Glickman, L.T. Toxocaral visceral larva migrans. N Engl J Med 1978, 298, 436–9. [Google Scholar] [CrossRef] [PubMed]
- Despommier, D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev 2003, 16, 265–72. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, O.; et al. [Meningoencephalitis caused by Toxocara canis]. Ann Med Interne (Paris) 1998, 149, 391–2. [Google Scholar]
- Gueglio, B.; et al. Epidemiologic approach to human toxocariasis in western France. Parasitol Res 1994, 80, 531–6. [Google Scholar] [CrossRef]
- Moiyadi, A.; et al. Visceral larva migrans presenting as multiple intracranial and intraspinal abscesses. Neuropathology 2007, 27, 371–4. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Auer, H. Neurotoxocarosis. Rev Inst Med Trop Sao Paulo 2007, 49, 279–87. [Google Scholar] [CrossRef] [PubMed]
- Kazek, B.; et al. The cerebral form of toxocarosis in a seven-year-old patient. Folia Neuropathol 2006, 44, 72–6. [Google Scholar] [PubMed]
- Poulsen, C.S.; et al. Differential serodiagnostics of Toxocara canis and Toxocara cati--is it possible? Parasite Immunol 2015, 37, 204–7. [Google Scholar] [CrossRef] [PubMed]
- Bachli, H.; Minet, J.C.; Gratzl, O. Cerebral toxocariasis: a possible cause of epileptic seizure in children. Childs Nerv Syst 2004, 20, 468–72. [Google Scholar] [CrossRef]
- Quattrocchi, G.; et al. Toxocariasis and epilepsy: systematic review and meta-analysis. PLoS Negl Trop Dis 2012, 6, e1775. [Google Scholar] [CrossRef]
- Smith, H.; et al. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol 2009, 25, 182–8. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.R.; et al. Clinical features of covert toxocariasis. Scand J Infect Dis 1987, 19, 693–6. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.; et al. Meningitis by Toxocara canis after ingestion of raw ostrich liver. J Korean Med Sci 2012, 27, 1105–8. [Google Scholar] [CrossRef]
- Radman, N.E.; et al. Human toxocarosis. Its seroprevalence in the city of La Plata. Mem Inst Oswaldo Cruz 2000, 95, 281–5. [Google Scholar] [CrossRef]
- Xinou, E.; et al. CT and MR imaging findings in cerebral toxocaral disease. AJNR Am J Neuroradiol 2003, 24, 714–8. [Google Scholar] [PubMed]
- Auer, H.; Walochnik, J. Toxocariasis and the clinical spectrum. Adv Parasitol 2020, 109, 111–130. [Google Scholar] [CrossRef]
- Deshayes, S.; Bonhomme, J.; de La Blanchardiere, A. Neurotoxocariasis: a systematic literature review. Infection 2016, 44, 565–74. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, C.N. The epidemiology and public health importance of toxocariasis: a zoonosis of global importance. Int J Parasitol 2013, 43, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Pelloux, H.; Faure, O. [Toxocariasis in adults]. Rev Med Interne 2004, 25, 201–6. [Google Scholar] [CrossRef]
- Cianferoni, A.; et al. Visceral larva migrans associated with earthworm ingestion: clinical evolution in an adolescent patient. Pediatrics 2006, 117, e336–9. [Google Scholar] [CrossRef]
- Chen, J.; et al. Advances in molecular identification, taxonomy, genetic variation and diagnosis of Toxocara spp. Infect Genet Evol 2012, 12, 1344–8. [Google Scholar] [CrossRef]
- Woodhall, D.M.; Fiore, A.E. Toxocariasis: A Review for Pediatricians. J Pediatric Infect Dis Soc 2014, 3, 154–9. [Google Scholar] [CrossRef]
- Li, M.W.; et al. The complete mitochondrial genomes for three Toxocara species of human and animal health significance. BMC Genomics 2008, 9, 224. [Google Scholar] [CrossRef]
- Zhu, X.Q.; et al. Genetic blueprint of the zoonotic pathogen Toxocara canis. Nat Commun 2015, 6, 6145. [Google Scholar] [CrossRef] [PubMed]
- Kassai, T.; et al. Standardized nomenclature of animal parasitic diseases (SNOAPAD). Vet Parasitol 1988, 29, 299–326. [Google Scholar] [CrossRef] [PubMed]
- Strube, C.; et al. Seroprevalence of human toxocarosis in Europe: A review and meta-analysis. Adv Parasitol 2020, 109, 375–418. [Google Scholar]
- Petithory, J.C. Can Ascaris suum cause visceral larva migrans? Lancet 1996, 348, 689. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, W.; et al. Optimized DNA-based identification of Toxocara spp. eggs in soil and sand samples. Parasit Vectors 2021, 14, 426. [Google Scholar]
- Ma, G.; et al. Global and regional seroprevalence estimates for human toxocariasis: A call for action. Adv Parasitol 2020, 109, 275–290. [Google Scholar]
- Fakhri, Y.; et al. Toxocara eggs in public places worldwide - A systematic review and meta-analysis. Environ Pollut 2018, 242(Pt B), 1467–1475. [Google Scholar] [CrossRef]
- Azam, D.; et al. Temperature and the development and survival of infective Toxocara canis larvae. Parasitol Res 2012, 110, 649–56. [Google Scholar] [CrossRef] [PubMed]
- Roddie, G.; et al. Contamination of dog hair with eggs of Toxocara canis. Vet Parasitol 2008, 152, 85–93. [Google Scholar] [CrossRef] [PubMed]
- El-Tras, W.F.; Holt, H.R.; Tayel, A.A. Risk of Toxocara canis eggs in stray and domestic dog hair in Egypt. Vet Parasitol 2011, 178, 319–23. [Google Scholar] [CrossRef] [PubMed]
- Salem, G.; Schantz, P. Toxocaral visceral larva migrans after ingestion of raw lamb liver. Clin Infect Dis 1992, 15, 743–4. [Google Scholar] [CrossRef]
- Yoshikawa, M.; et al. A familial case of visceral toxocariasis due to consumption of raw bovine liver. Parasitol Int 2008, 57, 525–9. [Google Scholar] [CrossRef]
- Keegan, J.D.; Holland, C.V. Contamination of the hair of owned dogs with the eggs of Toxocara spp. Vet Parasitol 2010, 173, 161–4. [Google Scholar] [CrossRef] [PubMed]
- Taira, K.; et al. Zoonotic risk of Toxocara canis infection through consumption of pig or poultry viscera. Vet Parasitol 2004, 121, 115–24. [Google Scholar] [CrossRef] [PubMed]
- Dutra, G.F.; et al. Risk of infection by the consumption of liver of chickens inoculated with low doses of Toxocara canis eggs. Vet Parasitol 2014, 203, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.R.; et al. The expanded spectrum of toxocaral disease. Lancet 1988, 1, 692–5. [Google Scholar] [CrossRef]
- Pawlowski, Z. Toxocariasis in humans: clinical expression and treatment dilemma. J Helminthol 2001, 75, 299–305. [Google Scholar] [CrossRef]
- Del Prete, G.F.; et al. Purified protein derivative of Mycobacterium tuberculosis and excretory-secretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper) profile of cytokine production. J Clin Invest 1991, 88, 346–50. [Google Scholar] [CrossRef]
- Sil, A.; et al. Loeffler's Syndrome and Multifocal Cutaneous Larva Migrans: Case report of an uncommon occurrence and review of the literature. Sultan Qaboos Univ Med J 2023, 23, 104–108. [Google Scholar] [CrossRef]
- Takamoto, M.; et al. Occurrence of interleukin-5 production by CD4- CD8- (double-negative) T cells in lungs of both normal and congenitally athymic nude mice infected with Toxocara canis. Immunology 1995, 85, 285–91. [Google Scholar]
- Ackerman, S.J.; Bochner, B.S. Mechanisms of eosinophilia in the pathogenesis of hypereosinophilic disorders. Immunol Allergy Clin North Am 2007, 27, 357–75. [Google Scholar] [CrossRef] [PubMed]
- Dent, L.A.; et al. Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infect Immun 1999, 67, 989–93. [Google Scholar] [CrossRef]
- Kay, A.B. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med 2005, 11, 148–52. [Google Scholar] [CrossRef]
- Kay, A.B.; Phipps, S.; Robinson, D.S. A role for eosinophils in airway remodelling in asthma. Trends Immunol 2004, 25, 477–82. [Google Scholar] [CrossRef]
- Gomes, I.; et al. Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: implications in fibrogenesis. J Allergy Clin Immunol 2005, 116, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012, 130, 607–612. [Google Scholar] [CrossRef]
- van Balkum, M.; et al. Hypereosinophilia: a diagnostic challenge. Neth J Med 2018, 76, 431–436. [Google Scholar] [PubMed]
- Gillespie, S.H. Human toxocariasis. J Appl Bacteriol 1987, 63, 473–9. [Google Scholar] [CrossRef]
- Pivetti-Pezzi, P. Ocular toxocariasis. Int J Med Sci 2009, 6, 129–30. [Google Scholar] [CrossRef]
- Kuenzli, E.; et al. Toxocariasis-associated cardiac diseases--A systematic review of the literature. Acta Trop 2016, 154, 107–20. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, B.; et al. Cerebral toxocariasis after consumption of raw duck liver. Am J Trop Med Hyg 2007, 76, 600–2. [Google Scholar] [CrossRef] [PubMed]
- Dousset, V.; Sibon, I.; Menegon, P. [Case no 6. Cerebral vasculitis due to Toxocara canis (or catis) origin]. J Radiol 2003, 84, 89–91. [Google Scholar] [PubMed]
- Lompo, L.D.; et al. [Toxocara canis cerebral vasculitis revealed by iterative strokes]. Rev Neurol (Paris) 2012, 168, 533–7. [Google Scholar] [CrossRef]
- Fellrath, J.M.; Magnaval, J.F. Toxocariasis after slug ingestion characterized by severe neurologic, ocular, and pulmonary involvement. Open Forum Infect Dis 2014, 1, ofu063. [Google Scholar] [CrossRef]
- Hamidou, M.A.; et al. Systemic vasculitis with lymphocytic temporal arteritis and Toxocara canis infection. Arch Intern Med 2002, 162, 1521–4. [Google Scholar] [CrossRef]
- de Boysson, H.; et al. Vasculitis secondary to anti-C1q antibodies induced by Toxocariasis. Infection 2015, 43, 755–8. [Google Scholar] [CrossRef]
- Maiga, Y.; et al. [Presentation of cerebral toxocariasis with mental confusion in an adult: case report and review of the literature]. Bull Soc Pathol Exot 2007, 100, 101–4. [Google Scholar]
- Ardiles, A.; et al. [Toxocariasis in an adult manifested as hypereosinophilic syndrome with predominant neurological involvement Clinical case]. Rev Med Chil 2001, 129, 780–5. [Google Scholar] [PubMed]
- Oujamaa, L.; et al. [Cerebral vasculitis secondary to Toxocara canis and Fasciola hepatica co-infestation]. Rev Neurol (Paris) 2003, 159, 447–50. [Google Scholar] [PubMed]
- Stiles, C.W. The determination of generic types, and a list of roundworm genera with their original and type species, in Bulletin 79, C.W. Stiles, Hassall, A., Editor. 1905, Bureau of Animal Industry, United States Department of Agriculture. p. 1–150.
- Wilder, H.C. Nematode endophthalmitis. Trans Am Acad Ophthalmol Otolaryngol 1950, 55, 99–109. [Google Scholar] [PubMed]
- Chandler, A.C. The helminthic parasites of cats in Calcutta and the relation of cats to human helminthic infections. Indian Journal of Medical Research 1925, 23, 213–227. [Google Scholar]
- Fülleborn, F. Askarisinfektion durch Verzehren eingekapselter Larven und übergelungene intrauterine Askarisinfektion. Arch. Schiffs-u. Tropen-Hyg. 1921, 25, 367–375. [Google Scholar]
- Schwartz, Some parasites of dogs and cats transmissible to human beings and domesticated animals. Vet. Alumni Quart. 1932. Ohio State Univ..
- Nichols, R.L. The etiology of visceral larva migrans I. Diagnostic morphology of infective second-stage Toxocara larvae. J Parasitol 1956, 42, 349–62. [Google Scholar]
- Magnaval, J.F.; et al. Highlights of human toxocariasis. Korean J Parasitol 2001, 39, 1–11. [Google Scholar] [CrossRef]
- Moreira, G.M.; et al. Human toxocariasis: current advances in diagnostics, treatment, and interventions. Trends Parasitol 2014, 30, 456–64. [Google Scholar] [CrossRef]
- Akao, N.; Ohta, N. Toxocariasis in Japan. Parasitol Int 2007, 56, 87–93. [Google Scholar] [CrossRef]
- Raistrick, E.R.; Hart, J.C. Ocular toxocariasis in adults. Br J Ophthalmol 1976, 60, 365–70. [Google Scholar] [CrossRef] [PubMed]
- Stoicescu, R.M.; Mihai, C.M.; Giannakopoulou, A.D. Marked hypereosinophilia in a toddler: a case report. J Med Life 2011, 4, 105–8. [Google Scholar]
- Musso, C.; et al. Prevalence of Toxocara-induced liver granulomas, detected by immunohistochemistry, in a series of autopsies at a Children's Reference Hospital in Vitoria, ES, Brazil. Virchows Arch 2007, 450, 411–7. [Google Scholar] [CrossRef] [PubMed]
- Hartleb, M.; Januszewski, K. Severe hepatic involvement in visceral larva migrans. Eur J Gastroenterol Hepatol 2001, 13, 1245–9. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; et al. Myocarditis associated with visceral larva migrans due to Toxocara canis. Intern Med 2002, 41, 706–8. [Google Scholar] [CrossRef]
- Enko, K.; et al. Fulminant eosinophilic myocarditis associated with visceral larva migrans caused by Toxocara canis infection. Circ J 2009, 73, 1344–8. [Google Scholar] [CrossRef]
- Aghaei, S.; et al. Toxocara spp. infection and risk of childhood asthma: A systematic review and meta-analysis. Acta Trop 2018, 182, 298–304. [Google Scholar] [CrossRef]
- Humbert, P.; et al. Skin manifestations associated with toxocariasis: a case-control study. Dermatology 2000, 201, 230–4. [Google Scholar] [CrossRef]
- Marx, C.; et al. Toxocariasis of the CNS simulating acute disseminated encephalomyelitis. Neurology 2007, 69, 806–7. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J. Visceral and ocular larva migrans. Semin Neurol 1993, 13, 175–9. [Google Scholar] [CrossRef]
- Keller, M.; Pavia, A.T.; Byington, C.L. Possible intrafamilial transmission of Toxocara causing eosinophilic meningitis in an infant. Pediatr Infect Dis J 2008, 27, 849–50. [Google Scholar] [CrossRef] [PubMed]
- Mukund, A.; et al. Eosinophilic abscesses: a new facet of hepatic visceral larva migrans. Abdom Imaging 2013, 38, 774–7. [Google Scholar] [CrossRef] [PubMed]
- Raffray, L.; Le Bail, B.; Malvy, D. Hepatic visceral larva migrans presenting as a pseudotumor. Clin Gastroenterol Hepatol 2013, 11, e42. [Google Scholar] [CrossRef]
- Woodhall, D.; et al. Ocular toxocariasis: epidemiologic, anatomic, and therapeutic variations based on a survey of ophthalmic subspecialists. Ophthalmology 2012, 119, 1211–7. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, T.J.; Lee, K.W. Pulmonary Toxocariasis: Initial and Follow-Up CT Findings in 63 Patients. AJR Am J Roentgenol 2015, 204, 1203–11. [Google Scholar] [CrossRef]
- Sharghi, N.; et al. Environmental exposure to Toxocara as a possible risk factor for asthma: a clinic-based case-control study. Clin Infect Dis 2001, 32, E111–6. [Google Scholar] [CrossRef]
- Buijs, J.; Egbers, M.W.; Nijkamp, F.P. Toxocara canis-induced airway eosinophilia and tracheal hyporeactivity in guinea pigs and mice. Eur J Pharmacol 1995, 293, 207–15. [Google Scholar] [CrossRef]
- Pinelli, E.; Aranzamendi, C. Toxocara infection and its association with allergic manifestations. Endocr Metab Immune Disord Drug Targets 2012, 12, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Desowitz, R.S.; Rudoy, R.; Barnwell, J.W. Antibodies to canine helminth parasites in asthmatic and nonasthmatic children. Int Arch Allergy Appl Immunol 1981, 65, 361–6. [Google Scholar] [CrossRef]
- Li, L.; et al. Asthma and toxocariasis. Ann Allergy Asthma Immunol 2014, 113, 187–92. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Barankin, B.; Hon, K.L.E. Cutaneous Larva Migrans. Recent Pat Inflamm Allergy Drug Discov 2017, 11, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Caumes, E. Treatment of cutaneous larva migrans. Clin Infect Dis 2000, 30, 811–4. [Google Scholar] [CrossRef]
- Brenner, M.A.; Patel, M.B. Cutaneous larva migrans: the creeping eruption. Cutis 2003, 72, 111–5. [Google Scholar]
- Del Giudice, P.; et al. Autochthonous Cutaneous Larva Migrans in France and Europe. Acta Derm Venereol 2019, 99, 805–808. [Google Scholar] [CrossRef]
- Gavignet, B.; et al. Cutaneous manifestations of human toxocariasis. J Am Acad Dermatol 2008, 59, 1031–42. [Google Scholar] [CrossRef]
- Bahnea, R.G.; et al. [Cutaneous manifestations on toxocariasis cases hospitalized in the Paediatric Diseases Clinic of Iasi, between 2005-2008]. Rev Med Chir Soc Med Nat Iasi 2009, 113, 428–31. [Google Scholar]
- Rayes, A.A.; et al. Human toxocariasis and pyogenic liver abscess: a possible association. Am J Gastroenterol 2001, 96, 563–6. [Google Scholar] [CrossRef]
- Smith, H.V. Antibody reactivity in human toxocariasis., in Toxocara and Toxocariasis: Clinical, Epidemiological, and Molecular Perspectives, J.W. Lewis, Maizels, R. M., Editor. 1993, Institute of Biology and the British Society for Parasitology: London, UK. p. 91-109.
- Dinning, W.J.; et al. Toxocariasis: a practical approach to management of ocular disease. Eye (Lond) 1988, 2 ( Pt 5), 580–2. [Google Scholar] [CrossRef]
- Stewart, J.M.; Cubillan, L.D.; Cunningham, E.T. Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina 2005, 25, 1005–13. [Google Scholar] [CrossRef] [PubMed]
- Krasny, J.; Sach, J. Forms of Ocular Larval Toxocariasis in Childhood. A Review. Cesk Slov Oftalmol 2022, 2, 1001–1009. [Google Scholar] [PubMed]
- Good, B.; et al. Ocular toxocariasis in schoolchildren. Clin Infect Dis 2004, 39, 173–8. [Google Scholar] [CrossRef]
- Shields, J.A. Ocular toxocariasis. A review. Surv Ophthalmol 1984, 28, 361–81. [Google Scholar] [CrossRef]
- Small, K.W.; et al. Surgical management of retinal traction caused by toxocariasis. Am J Ophthalmol 1989, 108, 10–4. [Google Scholar] [CrossRef]
- Moore, M.T. Human Toxocara canis encephalitis with lead encephalopathy. J Neuropathol Exp Neurol 1962, 21, 201–18. [Google Scholar] [CrossRef]
- Beautyman, W.; et al. Review of a case previously reported as showing an ascarid larva in the brain. J Pathol Bacteriol 1966, 91, 271–3. [Google Scholar] [CrossRef]
- Schochet, S.S. Human Toxocara canis encephalopathy in a case of visceral larva migrans. Neurology 1967, 17, 227–9. [Google Scholar] [CrossRef]
- Hill, I.R.; Denham, D.A.; Scholtz, C.L. Toxocara canis larvae in the brain of a British child. Trans R Soc Trop Med Hyg 1985, 79, 351–4. [Google Scholar] [CrossRef]
- Mikhael, N.Z.; et al. Toxocara canis infestation with encephalitis. Can J Neurol Sci 1974, 1, 114–20. [Google Scholar] [CrossRef]
- Sanchez, S.S.; Garcia, H.H.; Nicoletti, A. Clinical and Magnetic Resonance Imaging Findings of Neurotoxocariasis. Front Neurol 2018, 9, 53. [Google Scholar] [CrossRef]
- Vidal, J.E.; Sztajnbok, J.; Seguro, A.C. Eosinophilic meningoencephalitis due to Toxocara canis: case report and review of the literature. Am J Trop Med Hyg 2003, 69, 341–3. [Google Scholar] [CrossRef]
- Caldera, F.; et al. Toxocara encephalitis presenting with autonomous nervous system involvement. Infection 2013, 41, 691–4. [Google Scholar] [CrossRef]
- Ruttinger, P.; Hadidi, H. MRI in cerebral toxocaral disease. J Neurol Neurosurg Psychiatry 1991, 54, 361–2. [Google Scholar] [CrossRef] [PubMed]
- Singer, O.C.; et al. Severe meningoencephalomyelitis due to CNS-Toxocarosis. J Neurol 2011, 258, 696–8. [Google Scholar] [CrossRef] [PubMed]
- Quinnell, R.J. Genetics of susceptibility to human helminth infection. Int J Parasitol 2003, 33, 1219–31. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.V.; et al. Radiologic surveillance for retinoblastoma metastases unexpectedly showed disseminated toxocariasis in liver, lung, and spinal cord. Can J Ophthalmol 2010, 45, 185–6. [Google Scholar] [CrossRef]
- Lee, J.Y.; et al. Toxocariasis might be an important cause of atopic myelitis in Korea. J Korean Med Sci 2009, 24, 1024–30. [Google Scholar] [CrossRef] [PubMed]
- Moshe, S.L.; et al. Epilepsy: new advances. Lancet 2015, 385, 884–98. [Google Scholar] [CrossRef]
- Sack, U.; et al. Multiplex analysis of cytokines in exhaled breath condensate. Cytometry A 2006, 69, 169–72. [Google Scholar] [CrossRef]
- Wendler, H. [The visceral larva migrans syndrome due to Toxocara canis]. Munch Med Wochenschr 1972, 114, 1634–40. [Google Scholar]
- Kazacos, K.R. Visceral and ocular larva migrans. Semin Vet Med Surg Small Anim 1991, 6, 227–35. [Google Scholar]
- Sumner, D.; Tinsley, E.G. Encephalopathy due to visceral larva migrans. J Neurol Neurosurg Psychiatry 1967, 30, 580–4. [Google Scholar] [CrossRef]
- Chen, J.; et al. Toxocariasis: a silent threat with a progressive public health impact. Infect Dis Poverty 2018, 7, 59. [Google Scholar] [CrossRef]
- Jones, J.L.; et al. Toxoplasma gondii and Toxocara spp. co-infection. Am J Trop Med Hyg 2008, 78, 35–9. [Google Scholar] [CrossRef]
- Fillaux, J.; Magnaval, J.F. Laboratory diagnosis of human toxocariasis. Vet Parasitol 2013, 193, 327–36. [Google Scholar] [CrossRef] [PubMed]
- Magnaval, J.F.; et al. Application of the western blotting procedure for the immunodiagnosis of human toxocariasis. Parasitol Res 1991, 77, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Roig, J.; et al. Acute eosinophilic pneumonia due to toxocariasis with bronchoalveolar lavage findings. Chest 1992, 102, 294–6. [Google Scholar] [CrossRef] [PubMed]
- Watthanakulpanich, D.; et al. Application of Toxocara canis excretory-secretory antigens and IgG subclass antibodies (IgG1-4) in serodiagnostic assays of human toxocariasis. Acta Trop 2008, 106, 90–5. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; et al. PCR tools for the verification of the specific identity of ascaridoid nematodes from dogs and cats. Mol Cell Probes 2007, 21, 349–54. [Google Scholar] [CrossRef] [PubMed]
- Magnaval, J.-F.; Glickman, L.T.; Dorchies, P. La toxocarose, une zoonose helminthique majeure. Rev Med Vet. 1994, 145, 611–627. [Google Scholar]
- in LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012: Bethesda (MD).
- Magnaval, J.F. Apparent weak efficacy of ivermectin for treatment of human toxocariasis. Antimicrob Agents Chemother 1998, 42, 2770. [Google Scholar] [CrossRef] [PubMed]
- Magnaval, J.F. Comparative efficacy of diethylcarbamazine and mebendazole for the treatment of human toxocariasis. Parasitology 1995, 110 ( Pt 5), 529–33. [Google Scholar] [CrossRef]
- Magnaval, J.F.; Charlet, J.P. [Comparative efficacy of thiabendazole and mebendazole in the treatment of toxocariasis]. Therapie 1987, 42, 541–4. [Google Scholar] [PubMed]
- Sturchler, D.; et al. Thiabendazole vs. albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol 1989, 83, 473–8. [Google Scholar] [CrossRef] [PubMed]
- Horton, J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology 2000, 121, S113–32. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Smith, P.G. Albendazole in hydatid disease--hepatocellular toxicity. Trans R Soc Trop Med Hyg 1987, 81, 343–4. [Google Scholar] [CrossRef] [PubMed]
- Ben Fredj, N.; et al. Albendazole-induced associated acute hepatitis and bicytopenia. Scand J Infect Dis 2014, 46, 149–51. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, B.O.; Haznedaroglu, I.C.; Coplu, L. Albendazole-induced amegakaryocytic thrombocytopenic purpura. Ann Pharmacother 1998, 32, 842. [Google Scholar] [CrossRef] [PubMed]
- Polat, C.; et al. Dual treatment of albendazole in hepatic hydatidosis: New therapeutic modality in 52 cases. J Gastroenterol Hepatol 2005, 20, 421–5. [Google Scholar] [CrossRef]
- Bilgic, Y.; et al. Albendazole Induced Recurrent Acute Toxic Hepatitis: A Case Report. Acta Gastroenterol Belg 2017, 80, 309–311. [Google Scholar]
- Marin Zuluaga, J.I.; et al. Albendazole-induced granulomatous hepatitis: a case report. J Med Case Rep 2013, 7, 201. [Google Scholar] [CrossRef]
- Dayan, A.D. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop 2003, 86, 141–59. [Google Scholar] [CrossRef] [PubMed]
- Magnaval, J.F.; Charlet, J.P. Etude double aveugle de l’efficacité du mébendazole dans le traitement de la toxocarose humaine. Therapie 1992, 47, 145–148. [Google Scholar] [PubMed]
- Braithwaite, P.A.; Thomas, R.J.; Thompson, R.C. Hydatid disease: the alveolar variety in Australia. A case report with comment on the toxicity of mebendazole. Aust N Z J Surg 1985, 55, 519–23. [Google Scholar] [PubMed]
- Miskovitz, P.F.; Javitt, N.B. Leukopenia associated with mebendazole therapy of hydatid disease. Am J Trop Med Hyg 1980, 29, 1356–8. [Google Scholar] [CrossRef] [PubMed]
- Puente, S.; et al. Imported Mansonella perstans infection in Spain. Infect Dis Poverty 2020, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Boussinesq, M.; Prod, J.; Chippaux, J.P. Onchocerca volvulus: striking decrease in transmission in the Vina valley (Cameroon) after eight annual large scale ivermectin treatments. Trans R Soc Trop Med Hyg 1997, 91, 82–6. [Google Scholar] [CrossRef]
- Marti, H.; et al. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am J Trop Med Hyg 1996, 55, 477–81. [Google Scholar] [CrossRef]
- Ahn, S.J.; Ryoo, N.K.; Woo, S.J. Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia Pac Allergy 2014, 4, 134–41. [Google Scholar] [CrossRef]
- Inagaki, K.; et al. Case Report: Ocular Toxocariasis: A Report of Three Cases from the Mississippi Delta. Am J Trop Med Hyg 2019, 100, 1223–1226. [Google Scholar] [CrossRef]
- Rubin, M.L.; et al. An intraretinal nematode (a case report). Trans Am Acad Ophthalmol Otolaryngol 1968, 72, 855–66. [Google Scholar]
- Seong, S.; et al. A case of ocular toxocariasis successfully treated with albendazole and triamcinolon. Korean J Parasitol 2014, 52, 537–40. [Google Scholar] [CrossRef]
- Hrˇckova, G. Novel approaches to immunoprophylaxis in toxocariasis, in Toxocara: the enigmatic parasite, C.V. Holland, Smith, H.V., Editor. 2006, CAB International: Oxfordshire. p. 174–194.
- Martinez-Pulgarin, D.F.; et al. Ocular toxocariasis: new diagnostic and therapeutic perspectives. Recent Pat Antiinfect Drug Discov 2015, 10, 35–41. [Google Scholar] [CrossRef]
- Frazier, M.; Anderson, M.L.; Sophocleous, S. Treatment of ocular toxocariasis with albendezole: a case report. Optometry 2009, 80, 175–80. [Google Scholar] [CrossRef]
- Barisani-Asenbauer, T.; et al. Treatment of ocular toxocariasis with albendazole. J Ocul Pharmacol Ther 2001, 17, 287–94. [Google Scholar] [CrossRef] [PubMed]
- Giuliari, G.P.; Ramirez, G.; Cortez, R.T. Surgical treatment of ocular toxocariasis: anatomic and functional results in 45 patients. Eur J Ophthalmol 2011, 21, 490–4. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; et al. Clinical features and course of ocular toxocariasis in adults. PLoS Negl Trop Dis 2014, 8, e2938. [Google Scholar] [CrossRef]
- el Matri, L.; et al. [Toxocara canis in apparently bilateral ocular site]. J Fr Ophtalmol 1990, 13, 303–8. [Google Scholar]
- Choi, K.D.; et al. Toxocara optic neuropathy: clinical features and ocular findings. Int J Ophthalmol 2018, 11, 520–523. [Google Scholar] [PubMed]
- Gass, J.D.; Braunstein, R.A. Further observations concerning the diffuse unilateral subacute neuroretinitis syndrome. Arch Ophthalmol 1983, 101, 1689–97. [Google Scholar] [CrossRef]
- Zygulska-Machowa, H.; Ziobrowski, S. [A case of ocular toxocariasis treated by xenon photocoagulation]. Klin Oczna 1987, 89, 213–4. [Google Scholar]
- Garcia, H.H. Neurocysticercosis. Neurol Clin 2018, 36, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, O.; et al. Eosinophilic meningomyelitis in toxocariasis: case report and review of the literature. Clin Neurol Neurosurg 2005, 107, 432–8. [Google Scholar] [CrossRef] [PubMed]
- Graeff-Teixeira, C.; da Silva, A.C.; Yoshimura, K. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev 2009, 22, 322–48, Table of Contents. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.; Jung, H. Pharmacokinetic optimisation of the treatment of neurocysticercosis. Clin Pharmacokinet 1998, 34, 503–15. [Google Scholar] [CrossRef] [PubMed]
- Hombu, A.; et al. Treatment of larva migrans syndrome with long-term administration of albendazole. J Microbiol Immunol Infect 2019, 52, 100–105. [Google Scholar] [CrossRef]
- Jabbour, R.; Atweh, L.A.; Atweh, S. Migration of Toxocara canis into the spinal cord in poorly treated patients. Neurology 2015, 84. [Google Scholar] [CrossRef]
- Goffette, S.; et al. Eosinophilic pleocytosis and myelitis related to Toxocara canis infection. Eur J Neurol 2000, 7, 703–6. [Google Scholar] [CrossRef]
- Wang, J.L.; et al. Major parasitic diseases of poverty in mainland China: perspectives for better control. Infect Dis Poverty 2016, 5, 67. [Google Scholar] [CrossRef]
- Matsangos, M.; et al. Health Status of Afghan Refugees in Europe: Policy and Practice Implications for an Optimised Healthcare. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef]
- Deplazes, P.; et al. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet Parasitol 2011, 182, 41–53. [Google Scholar] [CrossRef]
- Paul, M.; King, L.; Carlin, E.P. Zoonoses of people and their pets: a US perspective on significant pet-associated parasitic diseases. Trends Parasitol 2010, 26, 153–4. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.C. Ascarid infections of cats and dogs. Vet Clin North Am Small Anim Pract 1987, 17, 1307–39. [Google Scholar] [CrossRef]
- Chomel, B.B. Control and prevention of emerging parasitic zoonoses. Int J Parasitol 2008, 38, 1211–7. [Google Scholar] [CrossRef] [PubMed]
- Action to reduce human health hazards arising from animals. WHO Chron 1978, 32, 307–10.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




