1. Introduction
Chemoradiotherapy (CRT) is currently the standard treatment for locally advanced head and neck cancer [
1]. However, CRT causes mucositis, dermatitis, muscle and nerve dysfunction, and tissue fibrosis, resulting in post-treatment dysphagia [
2]. Early rehabilitation intervention is recommended to prevent dysphagia, and electrical stimulation is another option [
3]. The efficacy of neuromuscular electrical stimulation (NMES) in treating dysphagia after CRT has been demonstrated in several studies. In recent years, transcutaneous electrical sensory stimulation (TESS), which is similar to NMES, has been developed for treating dysphagia. Interference-wave electrical stimulation (IFES) is used for performing TESS, whereas pulsed-wave electrical stimulation is used for performing NMES. IFES stimulates deep tissues with two electrical stimuli that have slightly different frequencies, creating undulations owing to local wave interference. In general, TESS causes lesser irritation to the skin than NMES. Clinical trials evaluating the use of TESS for dysphagia have reported its efficacy in patients with various types of dysphagia, including sequelae of cerebrovascular disorders [
3,
4,
5]. Based on this evidence, an interferential current device (IFCD) was developed in Japan and received medical device certification under the trade name “Gentle-stim” in July 2015. It is now commercially available and is used at many medical facilities in Japan for swallowing rehabilitation. Although the use of TESS with IFCD for dysphagia has been clinically studied in several fields, its safety and efficacy in patients with head and neck cancer have not yet been investigated.
IFCD has been recognized as a noninvasive medical device, and its likelihood of causing serious adverse events (AEs) is extremely low. However, the insert instructions for IFCD recommend that it should be used with caution under a physician’s guidance in inpatient settings, not outpatient settings, as its safety has not been established in patients with malignant tumors or acute illnesses. Similarly, its use in acutely injured or inflamed areas should be avoided as its safety has not been established. Thus, due to these safety considerations, IFCDs are only approved for use under the supervision of a healthcare professional and are not recommended for use by the patient alone, such as at home.
In this study, the safety of TESS in patients with head and neck cancers undergoing CRT was evaluated. This study aimed to determine whether TESS with IFCD exacerbates the disease status, pain, and feasibility of CRT treatment. Additionally, we reported the effectiveness of electrical stimulation therapy as rehabilitation for dysphagia after CRT, along with a discussion of the literature.
2. Materials and Methods
2.1. Ethics
The study protocol was approved by the Certified Clinical Research Committee of Hiroshima University (certification number: CRB210005), registered with the Japan Registry of Clinical Trials (jRCTs062220008), and submitted to the Ministry of Health, Labour and Welfare. Written informed consent was obtained from each participant, and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
2.2. Study Objectives and Eligibility Criteria
This single-center, exploratory, single-arm prospective study was conducted to evaluate the safety of TESS in patients enrolled and treated between April 13, 2022, and March 30, 2023. Ten patients with locally advanced head and neck cancer who underwent CRT were selected from the Hiroshima University Hospital. The eligibility criteria were as follows: 1) patients who underwent CRT for head and neck cancer at the Hiroshima University Hospital, 2) patients who received 70 Gy of radiation to the laryngopharyngeal area, 3) patients over 20 years of age at the time of obtaining consent, and 4) patients who could provide written consent for participation in this study. The exclusion criteria were as follows: 1) patients with a history of radiation therapy in the head and neck area, 2) patients with tracheostomy, 3) patients in whom mainly a part other than the laryngopharyngeal area has been irradiated, 4) patients with pacemakers and implantable cardioverter-defibrillators, 5) patients with difficulty wearing IFCD on the neck, 6) patients with many inconveniences in daily life (Performance Status 2 or higher), 7) patients who are pregnant, may become pregnant, or are breastfeeding, and 8) patients who are judged to be inappropriate by the principal investigator or the research coordinator.
2.3. Medical Device Used
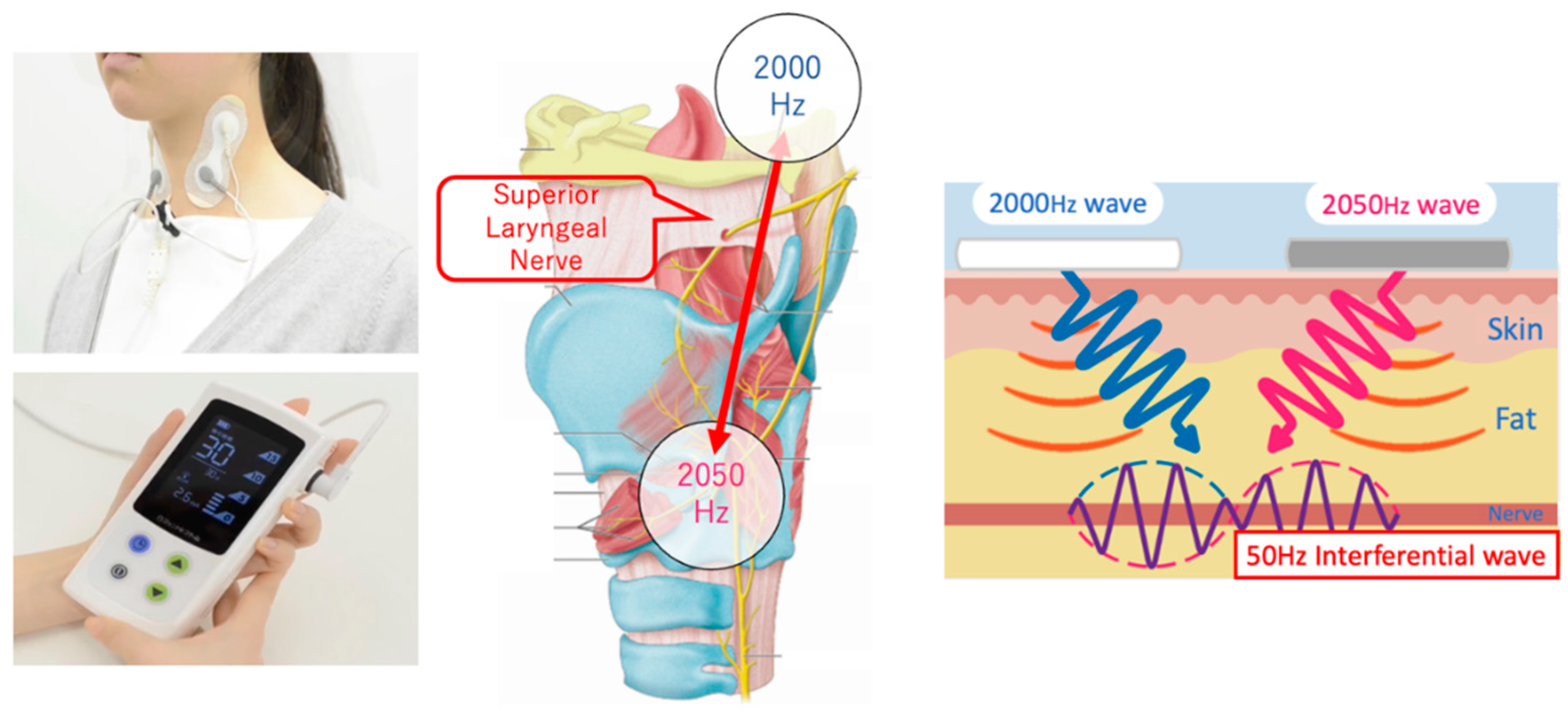
We used an IFCD named “Gentle-Stim” (Food Care Co., Ltd., Sagamihara, Japan,
https://www.food-care.co.jp/lng_en/message.html, medical device certification number: 227AHBZX00026000) for TESS in this study. The indications and effects of this device include percutaneous nerve and muscle stimulation for analgesia and amelioration of muscle atrophy (
Figure 1).
2.4. Interventions
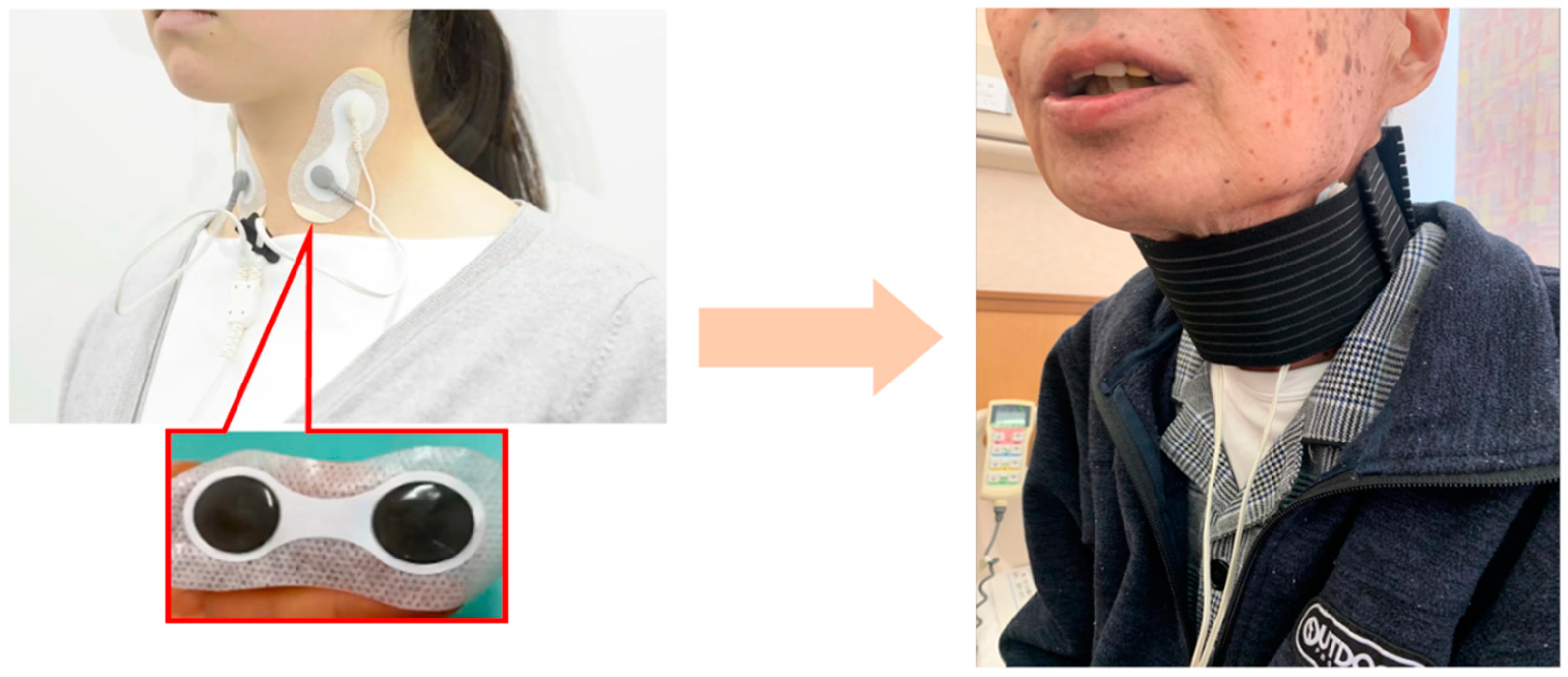
The IFCD was placed during swallowing rehabilitation. After removing sebum, sweat, and dirt from the cervical skin, the stimulating electrodes were placed with a neck band, as adhesive tape can cause skin deprivation when removed (
Figure 2). The stimulation duration was 30 min, and the stimulation output level was adjusted after assessing the patient. In cases where swallowing rehabilitation could not be performed owing to medical conditions, IFCD placement without rehabilitation was allowed.
2.5. Definition of “feasible”
If the patient was able to wear IFCD with a 10 Gy irradiation period of at least 3 out of 5 days and if it was used for at least 20 min at a power exceeding level 1, it was considered “feasible.” If the patient refused to wear the device or if the physician determined it as unsuitable, it was considered “not feasible.” The presence or absence of swallowing rehabilitation did not affect the use of IFCD.
2.6. Outcome Measures
As irradiation progressed, AEs, such as dermatitis, mucositis, and pain, were expected to become more severe owing to cumulative toxicity. AEs were classified according to the Common Terminology Criteria for Adverse Events (version 5.0; translated into Japanese by the Japan Clinical Oncology Group) [
6]. Even if IFCD placement could be performed without complications in the early period, IFCD placement was assumed to be difficult in the final stages. Therefore, we performed an evaluation at every 10 Gy irradiation dose and examined the feasibility rate and duration. In addition, the number of days of swallowing rehabilitation intervention, number of days of IFCD implementation, output power, intervention duration, and AEs were evaluated.
2.7. Primary Endpoint and Statistical Analysis
The primary endpoint of this study was the rate of feasibility of IFCD. Kaplan–Meier survival time analysis was performed using a time axis of 10 Gy units for the cumulative irradiation dose to account for termination. Treatment was terminated if irradiation was discontinued for any reason. To evaluate the feasibility rate in units of 10 Gy, the cumulative irradiation dose used as the time axis was rounded off to the nearest 10 Gy. The feasibility rate of IFCD was acceptable if it exceeds 70% at a 10 Gy unit cumulative irradiation dose. If more than seven of 10 enrolled patients are feasible, the lower limit of the 90% one-sided confidence interval of the rate of feasibility of IFCD exceeds 45%. Sample size of 10 patients was required to obtain sufficient precision for this exploratory trial for safety. All statistical analyses were performed using JMP Pro ver.16.2.0 (SAS Institute Inc., USA).
3. Results
The 10 enrolled patients were all males, with an average age of 64.8 years. Among them, seven patients had hypopharyngeal cancer, one had nasopharyngeal cancer, one had laryngeal cancer, and one had unknown primary cancer. Two patients had stage II cancer, four had stage IVA cancer, and four had stage IVB cancer. Six patients received induction chemotherapy (docetaxel, cisplatin, and 5-fluorouracil) before CRT, four patients received two cycles of tri-weekly cisplatin, five received three cycles of tri-weekly cisplatin, and one received seven cycles of weekly cetuximab during irradiation. As for radiation completion, nine patients received an irradiation dose of 70 Gy, whereas one patient was terminated after an irradiation dose of 66Gy due to the patient’s request (
Table 1).
Swallowing rehabilitation intervention and IFCD implementation were performed on almost all days throughout CRT. Days of IFCD had the average output power of the IFCD ranged from 7–8, which was unrelated to the accumulated radiation dose (p=0.7926). In addition, almost all patients underwent TESS for 30 min. Swallowing rehabilitation and IFCD were performed at doses of up to 64 Gy in one case, up to 66 Gy in three cases, up to 68 Gy in two cases, and up to 70 Gy in four cases. Patients who had difficulty implementing IFCD at the end of treatment also had difficulty with the swallowing rehabilitation intervention (
Table 2).
Although non-blood toxicity AEs, such as dermatitis, mucositis, dry mouth, and abnormal taste, occurred during CRT, the severity was less than Grade 3 (
Table 3). None of the patients complained of pain caused by IFCD electrical stimulation.
All patient was able to wear IFCD more than 3 out of 5 days, more than 20 min, and at a power exceeding level 1 until in units of 60Gy. Therefore, IFCD implementation was feasible for all patients until in units of 60Gy. In units of 70Gy, seven patients were able to accept IFCD implementation more than 3 days. However, two patients refused to wear IFCD because of the contact pain and one patient was terminated irradiation after 66Gy because of general fatigue and pain. Survival time analysis using the Kaplan–Meier method for IFCD implementation rates showed a feasibility of 100% for up to 60 Gy and a feasibility of 78% for up to 70 Gy (
Table 4). For primary endpoint, it was concluded that IFCD implementation was feasible until 70Gy irradiation dose units.
4. Discussion
Surgery is the mainstay of treatment for early-stage head and neck cancer. In contrast, advanced head and neck cancers are treated with a combination of radiation therapy, chemotherapy, and surgery. Although radical surgery for advanced head and neck cancer is highly curative, it requires the simultaneous removal of the surrounding organs, muscles, vascular vessels, and nerves and may result in permanent loss of speech, swallowing function, and changes in appearance. Pignon et al. reported on a large meta-analysis in 2000 and 2009 that demonstrated the efficacy of CRT, which is now recognized as the standard treatment for advanced head and neck cancer “for organ-sparing purposes” [
7]. Although CRT causes mucositis, dermatitis, and dysgeusia during treatment, tissue scarring, muscle atrophy, sensory loss, dysphagia, and aspiration are often observed [
2]. In some cases of severe dysphagia, patients have difficulty with oral intake due to repeated aspiration pneumonia even though “organs could be preserved.” In other words, “organ preservation” in CRT may not always lead to “function preservation” [
8]. Therefore, in CRT, for the purpose of “organ preservation,” established methods to obtain “functional preservation” as well as cancer treatment have been explored. The main causes of dysphagia after CRT are “weakness of the swallowing muscles” and “decreased sensation in the pharynx.” Evidence accumulated in recent years has shown that the use of various neuromuscular electrical stimulators in combination with general swallowing rehabilitation has an additive effect [
3].
NMES is recognized as a rehabilitation technique for the weakness of the swallowing muscles. In a randomized controlled trial of patients with nasopharyngeal cancer after radiation therapy, Lin et al. reported that swallowing rehabilitation with NMES significantly improved the swallowing function [
9]. Long et al. also reported a significant improvement in the swallowing angiography parameters in the NEMS plus balloon dilation group in a randomized controlled trial including the same participants [
10]. These studies were performed during the post-treatment period with some concerns that its use during CRT may cause pain as NMES using “pulsed-wave electrical stimulation” is intended to produce passive muscle contraction. TESS is recognized as a rehabilitation method for the loss of sensation in the pharynx. TESS is an IFC-based treatment that stimulates deep tissues by applying two medium-frequency electrical stimuli of slightly different frequencies to a local area, creating undulations owing to wave interference. As it is a new treatment, there is no evidence of its efficacy in the head and neck region.
Although TESS is considered to cause lesser irritation to the skin than NMES and can be performed during CRT, it is necessary to confirm its safety. The timing of the therapeutic intervention was selected during inpatient treatment, which allowed for the most intensive therapeutic intervention. Safety was determined during CRT, which is the most severe situation. IFCD was 100% feasible up to 60 Gy and 78% feasible up to 70 Gy, and the IFCD performance time was 30 min, exceeding the prescribed duration of 20 min in almost all cases. During the 70 Gy irradiation dose unit period while the pain of dermatitis was most severe at the end of the treatment period, some patients refused to wear IFCD. It was thought to be caused by contact pain rather than by electrical stimulation itself. The range of cumulative irradiation doses that exceeded the 70% feasibility rate was defined as the feasible range. Thus, IFCD was considered feasible until the end of CRT treatment. Although the output power seemed to change in relation to the radiation dose accumulation, no significant correlation was observed. Alternative methods, such as fixing the electrodes with a band, have been used to avoid the risk of the adhesive tape causing cervical skin peeling. Additionally, the IFCD stimulation did not cause pain during the study period. All AEs during treatment had a severity of Grade 3 or less, and no serious AEs related to the IFCD intervention occurred.
Several studies have explored the treatment of dysphagia in patients with head and neck cancer undergoing CRT. Kotz et al. reported in a randomized controlled study that swallowing function after CRT was significantly better in the rehabilitation group than that in the no-rehabilitation group, as assessed by the Functional Oral Intake Scale [
11]. Mann et al. conducted a randomized controlled trial for swallowing rehabilitation during CRT and reported that the rehabilitation group had significantly better results [
12]. Tang et al. also reported in a randomized controlled trial of patients with nasopharyngeal cancer that the post-treatment rehabilitation group had a significantly better ability to swallow than the no-treatment group, as assessed by a water drinking test [
13]. Kulbersh et al. studied the adequate timing of swallowing rehabilitation and reported that quality of life scores related to dysphagia were significantly higher in the group that received prophylactic swallowing rehabilitation than those in the group that received rehabilitation after treatment [
14].
In advanced head and neck cancer, rehabilitation intervention is not always possible before treatment as some patients experience swelling, pain on swallowing, and dyspnea. Prophylactic rehabilitation, similar to post-treatment rehabilitation, largely depends on the patient’s proactivity, and rehabilitation in outpatient clinics has limitations in terms of duration and means. Therefore, patients must continue their rehabilitation after discharge from the hospital. In Japan, TESS is recommended to be used under the guidance of a medical healthcare provider, and self-administration by patients has not been confirmed to be safe [
15]. The findings of the present study demonstrate that TESS could be performed safely during CRT, thereby supporting the expansion of the range of indications for TESS. A limitation of this study is that the results do not guarantee the safety of TESS for all patients in all situations. It should be noted that the range of irradiation and dose of radiation to the neck differ depending on the primary lesion and metastatic neck lymph nodes. As CRT may cause severe dermatitis and mucositis, the use of TEES continues to require medical supervision and careful follow-up during CRT. Thus, TEES can be used safely with prudent measures.
Intensive swallowing rehabilitation interventions should be preferably performed during hospitalization for CRT and after radical treatment. Although the effect of TESS on dysphagia is not yet clear, its continued use by patients themselves at home has great potential to contribute to the improvement of sensory disturbance. As TESS is an easy, passive, and harmless rehabilitation technique, we suggest that the restrictions surrounding its use be lifted to enable its use by outpatients after discharge. In order to examine its safety and effectiveness on swallowing function after CRT, the patients treated with TESS will continue to be followed up. Based on its safety in patients with head and neck cancer after CRT, we aim to assess the improvement in swallowing function before and after treatment in patients with dysphagia after CRT.
5. Conclusions
This study confirmed the feasibility and safety of TESS with IFCD in the head and neck region, even during CRT. Although the effectiveness of this method needs to be further investigated in more cases, TESS may be another treatment option for hyposensitivity after CRT.
Author Contributions
Conceptualization, methodology, validation, investigation, data curation, formal analysis, project administration, writing-original draft, Takao H.; Investigation, Kazuki S., Kohei Y., Nobuyuki C., Takayuki T.; Validation; Yuichiro H., Supervision; Takashi I., Tsutomu U., Writing - review and editing, Sachio T, Formal analysis; Kenichi Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Certified Clinical Research Committee of Hiroshima University (certification number: CRB210005), registered with the Japan Registry of Clinical Trials (jRCTs062220008), and submitted by the Ministry of Health, Labour and Welfare.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
We thank Taizo Hirata for their support in conceptualizing this study. We also thank Yuka Nagano, Mikako Kato and Kohei Yoshikawa for performing TESS rehabilitation.
Conflicts of Interest
The Food Care Corporation provided the consumables used for attaching the IFCDs. Conflicts related to this study were properly managed using standards for the management of Conflicts of Interest in Clinical Research under the Clinical Research Act. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- NCCN Clinical Practice Guidelines in Oncology, Head and Neck Cancers, 2023. Available online: https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations/GetFileFromFileManagerGuid?FileManagerGuidId=c0d39f8d-46a2-4662-b373-467ca4cacd96 (accessed on 18 May 2023).
- Yang, W.; Nie, W.; Zhou, X.; Guo, W.; Mou, J.; Yong, J.; Wu, T.; Liu, X. Review of prophylactic swallowing interventions for head and neck cancer. Int. J. Nurs. Stud. 2021, 123, 104074. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Takemura, M.; Tsujita, J.; Oku, Y. Interferential electric stimulation applied to the neck increases swallowing frequency. Dysphagia 2012, 27, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Oku, Y.; Sugishita, S.; Imai, T.; Matsui, T.; Daimon, T.; Nozaki, S.; Yoshikawa, H. Effects of short term interferential current stimulation on swallowing reflex in dysphagic patients. Int. J. Speech Lang. Pathol. Audiol. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Gallas, S.; Marie, J.P.; Leroi, A.M.; Verin, E. Sensory transcutaneous electrical stimulation improves post-stroke dysphagic patients. Dysphagia 2010, 25, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Homma, Y.; Flouzat-Lachaniette, C.H.; Poignard, A.; Chevallier, N.; Rouard, H. Cancer risk is not increased in patients treated for orthopaedic diseases with autologous bone marrow cell concentrate. J. Bone Joint Surg. Am. 2013, 95, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Pignon, J.P.; le Maître, A.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer: an update on 93 randomised trials and 17346 patients. Radiother Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Wall, L.R.; Ward, E.C.; Cartmill, B.; Hill, A.J. Physiological changes to the swallowing mechanism following (chemo)radiotherapy for head and neck cancer: a systematic review. Dysphagia 2013, 28, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.B.; Wu, X.P. A randomized controlled trail of combination therapy of neuromuscular electrical stimulation and balloon dilatation in the treatment of radiation-induced dysphagia in nasopharyngeal carcinoma patients. Disabil. Rehabil. 2013, 35, 450–454. [Google Scholar] [CrossRef]
- Lin, P.H.; Hsiao, T.Y.; Chang, Y.C.; Ting, L.L.; Chen, W.S.; Chen, S.C.; Wang, T.G. Effects of functional electrical stimulation on dysphagia caused by radiation therapy in patients with nasopharyngeal carcinoma. Support Care Cancer 2011, 19, 91–99. [Google Scholar] [CrossRef]
- Kotz, T.; Federman, A.D.; Kao, J.; Milman, L.; Packer, S.; Lopez-Prieto, C.; Forsythe, K.; Genden, E.M. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 376–382. [Google Scholar] [PubMed]
- Carnaby-Mann, G.; Crary, M.A.; Schmalfuss, I.; Amdur, R. “Pharyngocise”: randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 210–219. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, Q.; Wang, Y.; Lu, K.; Wang, Y.; Peng, Y. A randomized prospective study of rehabilitation therapy in the treatment of radiation-induced dysphagia and trismus. Strahlenther Oncol. 2011, 187, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kulbersh, B.D.; Rosenthal, E.L.; McGrew, B.M.; Duncan, R.D.; McColloch, N.L.; Carroll, W.R.; Magnuson, J.S. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope 2006, 116, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Iizumi, Y.; Ihara, Y.; Koike, J.; Takahashi, K. Effects of interferential current electrical stimulation (IFCS) on mastication and swallowing function in healthy young adults: A preliminary study. Clin. Exp. Dent. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).