Submitted:
07 June 2023
Posted:
07 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Results and Discussion
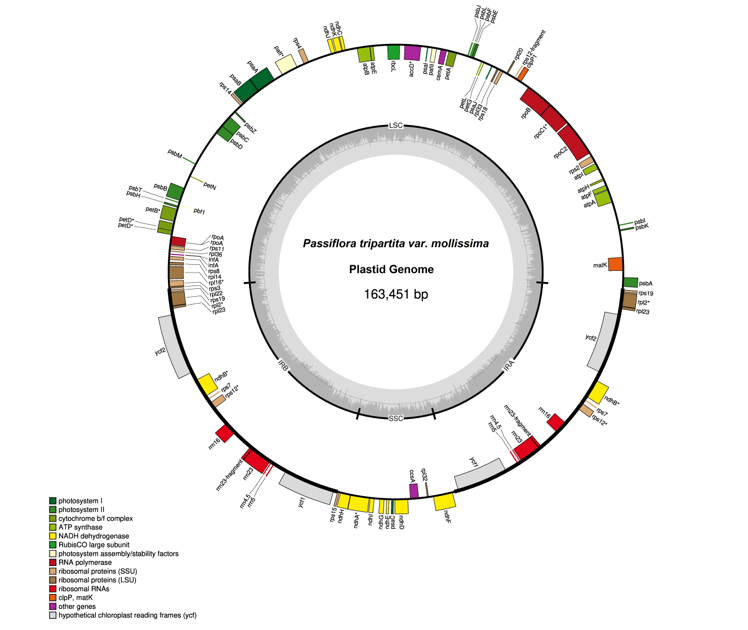
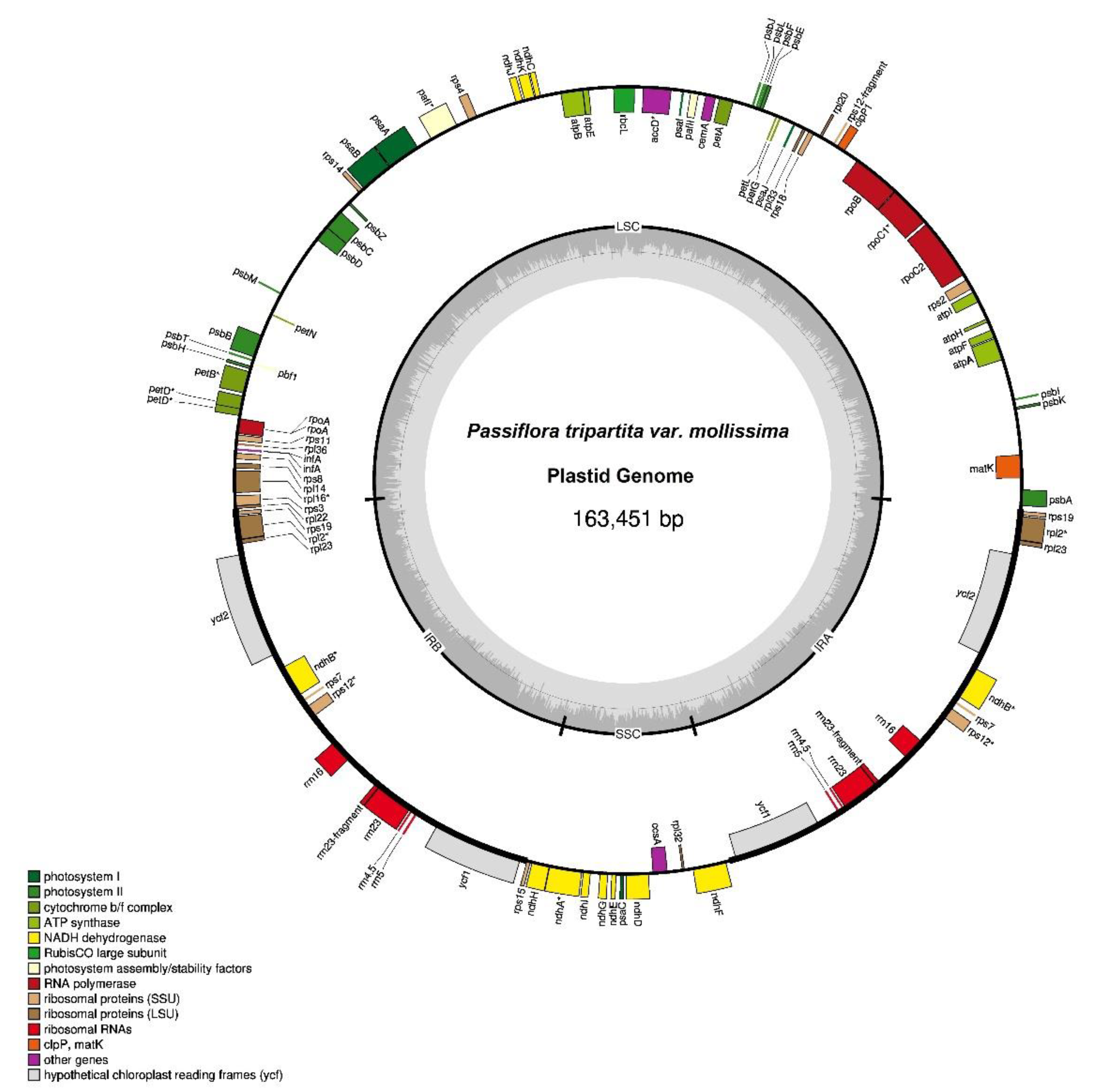
Plastome of Passiflora tripartiva var. mollisima
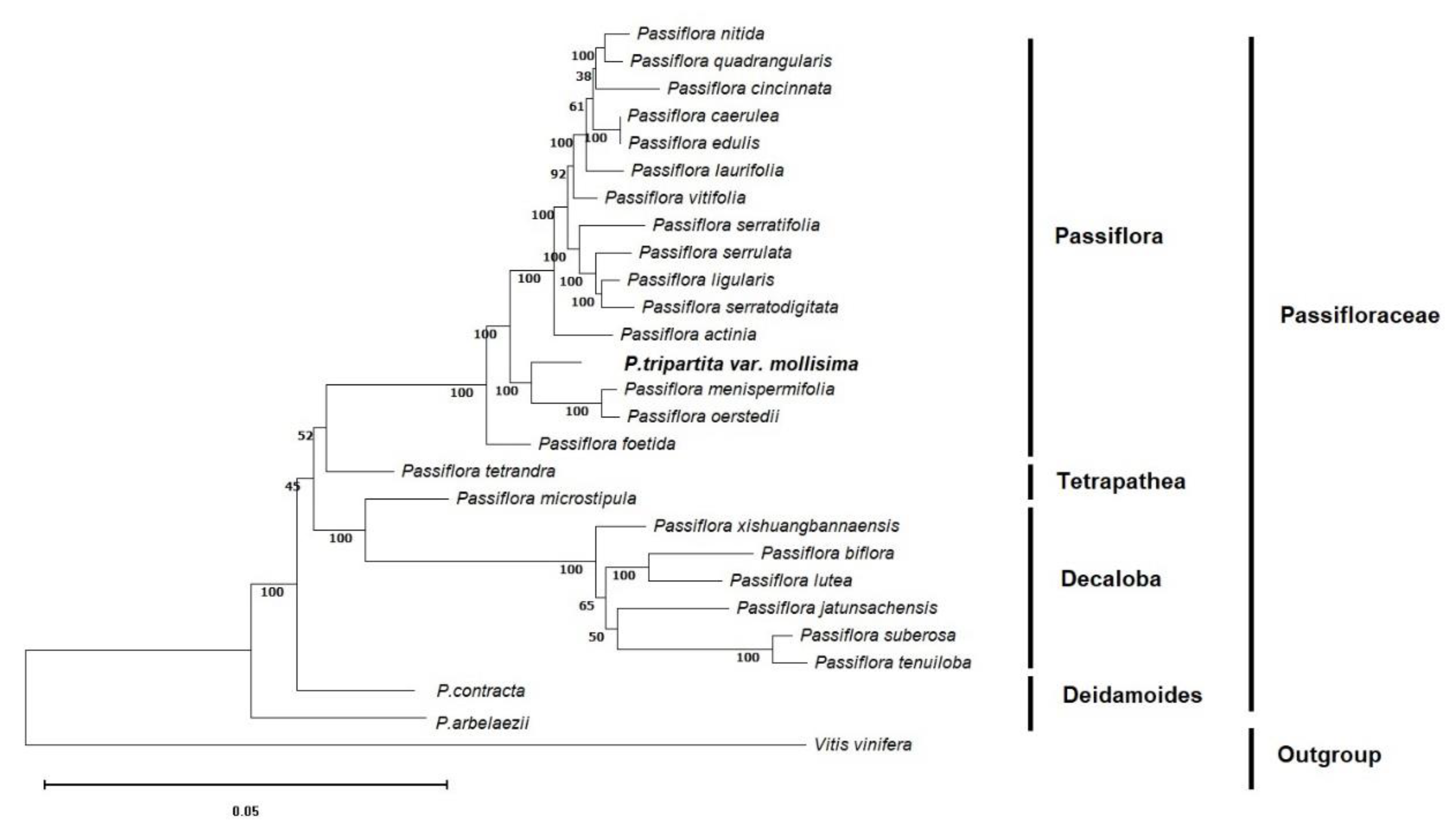
Phylogenetic Reconstruction
Conclusions
Materials and Methods
Plant Materials
DNA Extraction
Genome Sequencing, Assembly, and Annotation
Phylogenetic Analysis
Supplementary information
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ITIS (2022) Passiflora tripartita var. mollisima (Kunth) Holms-Niels. & P.M. Jørg. Available online: https://www.itis.gov/ (accessed on 01 Jun 2023).
- Primot S, Coppens D’Eeckenbrugge G, Rioux V, Ocampo JA, Garcin F (2005) Variación morfológica de tres especies de curubas (Passiflora tripartita var. mollissima, P. tarminiana y P. mixta) y sus híbridos en el Valle del Cauca (Colombia). Rev Bras Frutic 27: 467–471, doi: 10.1590/S0100-29452005000300030. [CrossRef]
- Mayorga M, Fischer G, Melgarejo LM, Parra-Coronado A (2020) Growth, development and quality of Passiflora tripartita var. mollissima fruits under two environmental tropical conditions. J Appl Bot Food Qual 93: 66–75, doi: 10.5073/JABFQ.2020.093.009. [CrossRef]
- Coppens D’Eeckenbrugge G (2001) Conservación y utilización de recursos geneticos de pasifloras. COLCIENCIAS Informe final 1999-2001. Available online: https://agritrop.cirad.fr/576977 (accessed on 01 Jun 2023).
- egura SD, D’Eeckenbrugge GC, Ocampo CH, Ollitrault P (2005) Isozyme variation in Passiflora subgenus tacsonia: Geographic and interspecific differentiation among the three most common species. Genet Resour Crop Evol 52: 455–463, doi: 10.1007/s10722-005-2255-z. [CrossRef]
- Ocampo J, Coppens d’Eeckenbrugge GC (2017) Morphological characterization in the genus passiflora l.: an approach to understanding its complex variability. Plant Syst Evol 303: 531–558, doi: 10.1007/s00606-017-1390-2. [CrossRef]
- Tapia ME, Fries AM (2007) Guía de campo de los cultivos andinos, 1st ed.; FAO: Roma, Italy, pp. 22–114.
- Ríos-García G (2017) Nivel de aceptabilidad del vino de tumbo serrano (Passiflora mollissima) elaborado con los parámetros tecnológicos óptimos en la ciudad de Huánuco 2015. Master thesis, Universidad Nacional Hermilio Valdizán de Huánuco: Huánuco.
- Leterme P, Buldgen A, Estrada F, Londoño AM (2006) Mineral content of tropical fruits and unconventional foods of the Andes and the rain forest of Colombia. Food Chem 95: 644-652.
- Chaparro-Rojas DC, Maldonado ME, Franco-Londoño MC, Urango-Marchena LA (2014) Características nutricionales y antioxidantes de la fruta curuba larga (Passifora mollissima Bailey). Perspect Nutr Hum 16: 203-212, doi: 10.17533/udea.penh.v16n2a07. [CrossRef]
- Giambanelli E, Gómez-Caravaca AM, Ruiz-Torralba A, Guerra-Hernández EJ, Figueroa-Hurtado JG, García-Villanova B, Verardo V (2020) New advances in the determination of free and bound phenolic compounds of banana passion fruit pulp (Passiflora tripartita, var. mollissima (kunth) l.h. bailey) and their in vitro antioxidant and hypoglycemic capacities. Antioxidants 9: 628, doi: 10.3390/antiox9070628. [CrossRef]
- Luo Y, Jian Y, Liu Y, Jiang S, Muhammad D, Wang W (2022) Flavanols from nature: A phytochemistry and biological activity review. Molecules 27: 719, doi: 10.3390/molecules27030719. [CrossRef]
- Daniell H, Lin CS, Yu M, Chang WJ (2016) Chloroplast genomes: diversity, evolution, and applications in genetic engineering. Genome Biol 17: 134, doi: 10.1186/s13059-016-1004-2. [CrossRef]
- Dobrogojski J, Adamiec M, Lucinski R (2020) The chloroplast genome: A review. Acta Physiol Plant 42: 98, doi: 10.1007/s11738-020-03089-x. [CrossRef]
- 15. Ozeki H, Umesono K, Inokuchi H, Kohchi T, Ohyama K (1989) The chloroplast genome of plants: a unique origin. Genome. 31: 169–174, doi: 10.1139/g89-029. [CrossRef]
- Wang W, Lanfear R (2019) Long-reads reveal that the chloroplast genome exists in two distinct versions in most plants. Genome Biol Evol 11: 3372–3381, doi: 10.1093/gbe/evz256. [CrossRef]
- Marks RA, Hotaling S, Frandsen PB, VanBuren R (2021) Representation and participation across 20 years of plant genome sequencing. Nat Plants 7: 1571–1578, doi: 10.1038/s41477-021-01031-8. [CrossRef]
- Sun Y, Shang L, Zhu QH, Fan L, Guo L (2022) Twenty years of plant genome sequencing: achievements and challenges. Trends Plant Sci 27: 391–401, doi: 10.1016/j.tplants.2021.10.006. [CrossRef]
- Cauz-Santos LA, Munhoz CF, Rodde N, Cauet S, Santos AA, Penha HA, Dornelas MC, Varani AM, Oliveira GCX, Gergès H, Vieira MLC (2017) The Chloroplast Genome of Passiflora edulis (Passifloraceae) Assembled from Long Sequence Reads: Structural Organization and Phylogenomic Studies in Malpighiales. Front Plant Sci 8: 334, doi: 10.3389/fpls.2017.00334. [CrossRef]
- Hao C, Wu F (2021) The complete chloroplast genome sequence of Passiflora xishuangbannaensis (Passifloraceae), a vine endemic to Yunnan, China. Mitochondrial DNA B Resour 6: 1763–1764, doi: 10.1080/23802359.2021.1928562. [CrossRef]
- Niu YF, Ni SB, Liu SH, Liu J (2021) The complete chloroplast genome of Passiflora caerulea, a tropical fruit with a distinctive aroma. Mitochondrial DNA B Resour 6: 488–490, doi: 10.1080/23802359.2021.1872442. [CrossRef]
- Mou HF, Huang WH, Liu JY, Wen F, Tian QL, Wu YY, Fu LF, Zhang YJ, Wei YG (2021) Complete chloroplast genome sequence of Passiflora serrulata Jacq. (Passifloraceae). Mitochondrial DNA B Resour 6: 191–193, doi: 10.1080/23802359.2020.1860711. [CrossRef]
- Hopley T, Webber BL, Raghu S, Morin L, Byrne M (2021) Revealing the Introduction History and Phylogenetic Relationships of Passiflora foetida sensu lato in Australia. Front Plant Sci 12: 651805, doi: 10.3389/fpls.2021.651805. [CrossRef]
- Shrestha B, Weng ML, Theriot EC, Gilbert LE, Ruhlman TA, Krosnick SE, Jansen RK (2019) Highly accelerated rates of genomic rearrangements and nucleotide substitutions in plastid genomes of Passiflora subgenus Decaloba. Mol Phylogenet Evol 138: 53–64, doi: 10.1016/j.ympev.2019.05.030. [CrossRef]
- Gioppato HA, da Silva MB, Carrara S, Palermo BRZ, de Souza Moraes T, Dornelas MC (2019) Genomic and transcriptomic approaches to understand Passiflora physiology and to contribute to passionfruit breeding. Theor Exp Plant Physiol 31: 173–181, doi: 10.1007/s40626-018-0134-1. [CrossRef]
- Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Shiki Y, Takeuchi M, Chang Z, Aota SI, Inokuchi H, Ozeki H (1986) Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322: 572–574, doi: 10.1038/322572a0. [CrossRef]
- Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Yamaguchi-Shinozaki K, Ohto C, Torazawa K, Meng BY, Sugita M, Deno H, Kamogashira T, Yamada K, Kusuda J, Takaiwa F, Kato A, Tohdoh N, Shimada H, Sugiura M (1986) The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J 5: 2043–2049, doi: 10.1002/j.1460-2075.1986.tb04464.x. [CrossRef]
- Nguyen HQ, Nguyen TNL, Doan TN, Nguyen TTN, Phạm MH, Le TL, Sy DT, Chu HH, Chu HM (2021) Complete chloroplast genome of novel Adrinandra megaphylla Hu species: molecular structure, comparative and phylogenetic analysis. Sci Rep 11: 11731, doi: 10.1038/s41598-021-91071-z. [CrossRef]
- 29. Kikuchi S, Bédard J, Hirano M, Hirabayashi Y, Oishi M, Imai M, Takase M, Ide T, Nakai M (2013) Uncovering the protein translocon at the chloroplast inner envelope membrane. Science 339: 571–574, doi: 10.1126/science.1229262. [CrossRef]
- Kikuchi S, Asakura Y, Imai M, Nakahira Y, Kotani Y, Hashiguchi Y, Nakai Y, Takafuji K, Bédard J, Hirabayashi-Ishioka Y, Mori H, Shiina T, Nakai MA (2018) Ycf2-FtsHi Heteromeric AAA-ATPase Complex Is Required for Chloroplast Protein Import. Plant Cell 30: 2677–2703, doi: 10.1105/tpc.18.00357. [CrossRef]
- Gabaldón T. Evolution of proteins and proteomes: a phylogenetics approach. Evol. Bioinform. Online. 2005, 1, 51–61, doi: 10.1177/117693430500100004. [CrossRef]
- Zhang N, Zeng L, Shan H, Ma H (2012) Highly conserved low-copy nuclear genes as effective markers for phylogenetic analyses in angiosperms. New Phytol 195: 923–937, doi: 10.1111/j.1469-8137.2012.04212.x. [CrossRef]
- Emms DM, Kelly S (2019) OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol 20: 238, doi:10.1186/s13059-019-1832-y. [CrossRef]
- Cauz-Santos LA, da Costa ZP, Callot C, Cauet S, Zucchi MI, Bergès H, van den Berg C, Vieira MLC (2020) A Repertory of Rearrangements and the Loss of an Inverted Repeat Region in Passiflora Chloroplast Genomes.Genome Biol Evol12: 1841–1857, doi: 10.1093/gbe/evaa155. [CrossRef]
- Pacheco TG, Lopes AS, Welter JF, Yotoko KSC, Otoni WC, Vieira LDN, Guerra MP, Nodari RO, Balsanelli E, Pedrosa FO, de Souza EM, Rogalski M (2020) Plastome sequences of the subgenus Passiflora reveal highly divergent genes and specific evolutionary features. Plant Mol Biol 104: 21–37, doi: 10.1007/s11103-020-01020-z. [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19,11–15.
- Modi A, Vai S, Caramelli D, Lari M (2021) The illumina sequencing protocol and the novaseq 6000 system. In: Bacterial Pangenomics: Methods and Protocols, 2nd edn; Mengoni, A.; Bacci, G.; Fondi, M., Eds.; Springer: New York, USA, Volume 2242, pp. 15–42, ISBN: 978-1-0716-1099-2.
- Wingett SW, Andrews S (2018) FastQ Screen: A tool for multi-genome mapping and quality control. F1000Res 7: 1338, doi: 10.12688/f1000research.15931.2. [CrossRef]
- Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17: 10-12, doi: 10.14806/ej.17.1.200. [CrossRef]
- Gurevich A, Saveliev V, Vyahhi N, Tesler G (2013) QUAST: quality assessment tool for genome assemblies. Bioinformatics 29: 1072–1075, doi: 10.1093/bioinformatics/btt086. [CrossRef]
- Dierckxsens N, Mardulyn P, Smits G (2017) NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Res45: e18, doi: 10.1093/nar/gkw955. [CrossRef]
- Tillich M, Lehwark P, Pellizzer T, Ulbricht-Jones ES, Fischer A, Bock R, Greiner S (2017) GeSeq - versatile and accurate annotation of organelle genomes. Nucleic Acids Res 45: W6–W11, doi: 10.1093/nar/gkx391. [CrossRef]
- Shi L, Chen H, Jiang M, Wang L, Wu X, Huang L, Liu C (2019) CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res 47: W65–W73, doi: 10.1093/nar/gkz345. [CrossRef]
- Greiner S, Lehwark P, Bock R (2019) OrganellarGenomeDRAW (OGDRAW) version 1.3.1: expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res 47: W59–W64, doi: 10.1093/nar/gkz238. [CrossRef]
- Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780, doi: 10.1093/molbev/mst010. [CrossRef]
- Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313, doi: 10.1093/bioinformatics/btu033. [CrossRef]
- Tamura K, Stecher G, Kumar S (2021) MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 38: 3022-3027, doi: 10.1093/molbev/msab120. [CrossRef]
| Features | Poro-poro 1 |
|---|---|
| Genome size (bp) | 163,451 |
| LSC length (bp) | 85, 525 |
| SSC length (bp) | 13,518 |
| IR length (bp) | 32,204 |
| Total GC content (%) | 36.87 |
| A content (%) | 30.79 |
| T(U) content (%) | 32.34 |
| G content (%) | 18.20 |
| C content (%) | 18.67 |
| Total number of genes | 128 |
| Protein-coding genes | 84 |
| rRNA coding genes | 7 |
| tRNA coding genes | 37 |
| Genes duplicated in IR regions | 17 |
| Total introns | 14 |
| Single introns (gene) | 12 |
| Double introns (gene) | 2 |
| Group of genes | Gene names |
|---|---|
| Photosystem I | psaA, psaB, psaC, psaI, psaJ, ycf3 **, ycf4 |
| Photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ |
| Cytochrome b/f complex | petA, petB, petD *, petG, petL, petN |
| ATP synthase | atpA, atpB, atpE, atpF, atpH, atpI |
| NADH dehydrogenase | ndhA*, ndhB * (X2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK |
| RubisCO large subunit | rbcL |
| DNA-dependent RNA polymerase | rpoA, rpoB, rpoC1 *, rpoC2 |
| Ribosomal proteins (SSU) | rps2, rps3, rps4, rps8, rps11, rps12 ** (X2), rps14, rps15, rps16, rps18, rps19 (X2) |
| Ribosomal proteins (LSU) | rpl2 * (X2), rpl14, rpl16 *, rpl20, rpl22, rpl23 (X2), rpl32, rpl33, rpl36 |
| Acetyl-CoA carboxylase | accD |
| C-type cytochrome synthesis | ccsA |
| Envelope membrane protein | cemA |
| Protease | clpP |
| Translational initiation factor IF-1 | infA |
| Maturase | matK |
| Component of TIC complex | yct1, ycf2 |
| Unknown function protein-coding | ycf15 (X2) |
| Ribosomal RNAs | rrn4.5, rrn5 (X2), rrn16 (X2), rrn23 (X2) |
| Transfer RNAs | trnA-UGC * (X2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnG-GCC, trnG-UCC *, trnH-GUG, trnI-CAU (X2), trnI-GAU * (X2), trnK-UUU *, trnL-CAA (X2), trnL-UAA *, trnL-UAG, trnM-CAU (X2), trnN-GUU (X2), trnP-UGG, trnQ-UUG, trnR-ACG (X2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC (X2), trnV-UAC *, trnW-CCA, trnY-GUA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





