Submitted:
02 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
Key Points
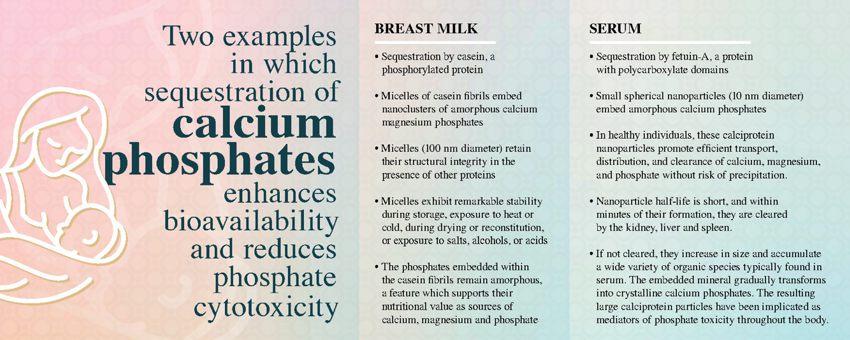
- Sequestration of nascent clusters of ternary phosphates composed of magnesium, calcium, and phosphate by intrinsically disordered proteins play key roles in physiological calcium phosphate management. Sequestration results in the formation of soluble colloids: large fibrous amorphous calcium magnesium phosphate nanoclusters in milk and small spherical amorphous calcium phosphate nanoclusters in serum.
- Breast milk is composed of large fibrous micelles in which casein embeds amorphous calcium magnesium phosphate nanoclusters. These casein micelles exhibit structural integrity, even in the presence of other proteins. The casein micelles remain remarkably stable during storage as liquids or solids, exposure to heat and cold, drying and reconstitution, and exposure to salts, alcohols, or acids. The phosphates embedded within the casein molecules remain amorphous, a feature which supports their nutritional value as sources of calcium, magnesium and phosphate.
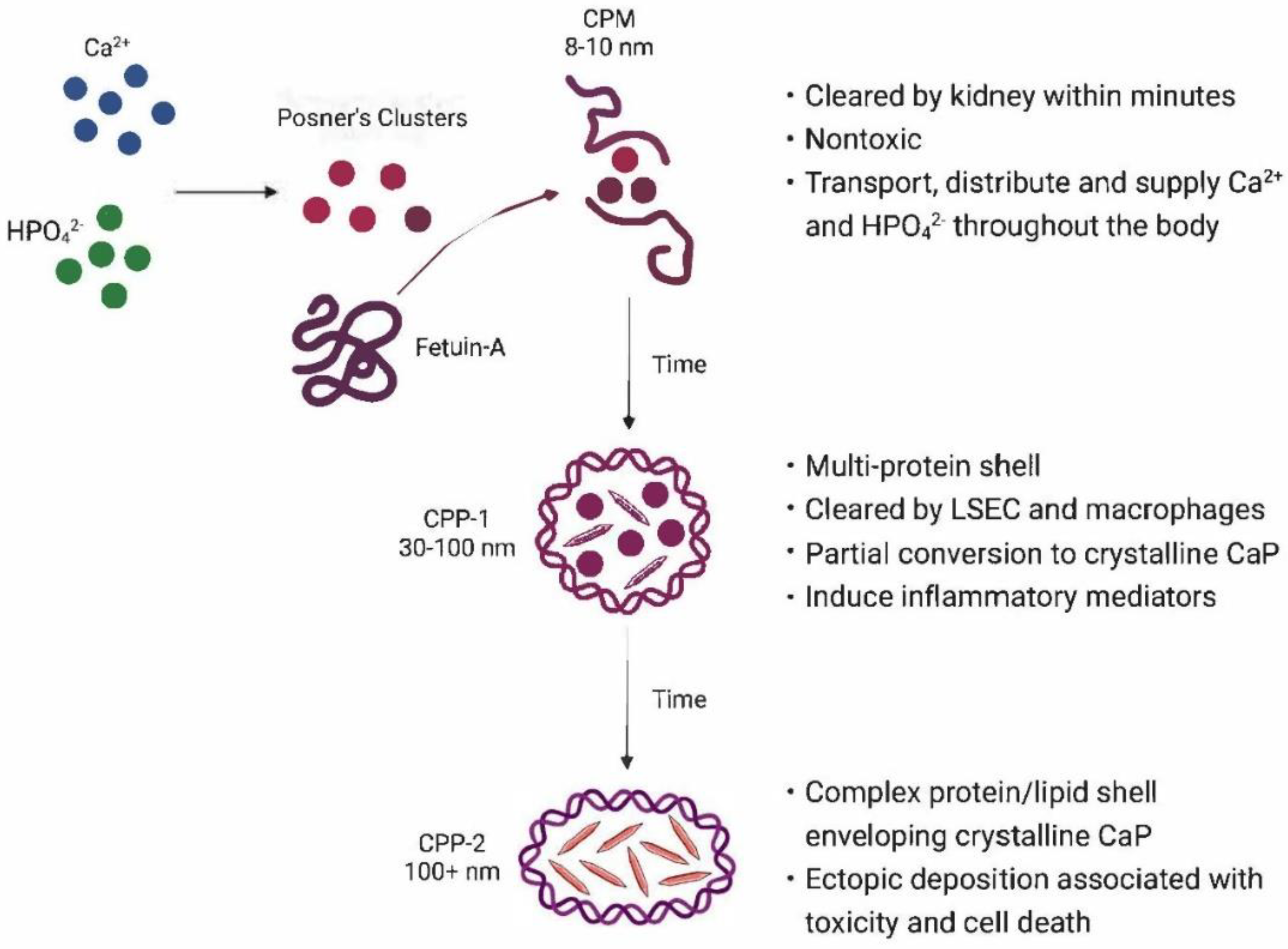
- In healthy individuals, calciprotein nanoparticles, small nanoscale aggregates of amorphous calcium phosphates embedded in binding proteins, promote efficient transport, distribution, and clearance of calcium, magnesium, and phosphate without risk of precipitation. The half-life of these nanoparticles in serum is short, and if not utilized for calcium/phosphate replenishment within minutes of their formation, they are cleared by the kidney, liver and spleen. Some of the nanoparticles may be sufficiently small to pass through renal tissues into urine. As circulating biomaterials, they may couple dietary mineral exposure with endocrine control of mineral metabolism in bone, kidney and intestine. Conversely, when calcium and phosphate homeostasis is disrupted, these particles emerge as mediators of phosphate toxicity throughout the body. If the particles are not scavenged, they increase in size and accumulate a wide variety of organic species typically found in the biofluid environment. The embedded mineral gradually transforms into nanoparticles composed of sequestered mixtures of amorphous and crystalline calcium phosphates. As this ripening occurs, the particles become less soluble in the biofluid and the phosphates become less soluble in acidic media. Ectopic particle deposition on soft tissues is associated with cellular toxicity. Cytotoxicity may be related, at least in part, to the crystallopathy of crystalline calcium phosphates that induce inflammation, injury, and cell death.
1. Introduction
2. The Problem: Calcium Phosphate Solubility
2.1. Reactions of Calcium Ion and Phosphate Ions in Aqueous Solution
| Calcium Phosphate | Empirical Formula | Molar Ratio Ca/P | -Log Ks | pH |
|---|---|---|---|---|
| Amorphous calcium phosphate 1 | - | 1.35 | 10.6 | ~ 6.4 |
| Amorphous calcium phosphate 2 | - | 1.35 | 11.5 | ~ 5.7 |
| Octacalcium phosphate | Ca8H2(PO4)6 ∙ 5 H2O | 1.35 | 11.7 | ~ 5.4 |
2.2. Maturation
2.3. Calcium and phosphate status in biofluids
3. The physiological solution to mineral management
4. IDPs, the physiological sequestration agents in milk
| Species | Mineral content, mmol/L | Ca:P Ratio | Source | ||
|---|---|---|---|---|---|
| Ca | P | Mg | |||
| Donkey | 13.6 | 9.8 | 1.1 | 1.4 | Malacarne [62] |
| Donkey | 12.3 | 8.2 | 1.1 | 1.5 | Fantuz [63] |
| Cow | 13.0 | 8.8 | 1.1 | 1.5 | Fox [64] |
| Human (Early lactation) | 7.4 | 3.9 | 1.4 | 1.9 | Sanchez [65] |
| Human (Late lactation) | 6.3 | 3.9 | 1.4 | 1.6 | |
| Human (Early lactation) | 6.9 | (3.9) | 1.0 | (1.8) | Li [66] |
| Human (Late lactation) | 6.6 | (3.9) | 0.9 | (1.7) | |
| Human (Established feeding) | 6.7 | (3.9) | 1.5 | (1.7) | |
| Human | 7 | 4.7 | 1.3 | 1.5 | Sanchez [65] |
| Species | Mineral content in casein micelles (as a percentage of total mineral content in milk) | Reference | ||
|---|---|---|---|---|
| Ca | P | Mg | ||
| Donkey | 69.3% | 63.8% | 31.2% | Malacarne [62] |
| Donkey | 62.9% | 53.1% | 32.6% | Fantuz [63] |
| Cow | 66.5% | 57% | 33% | Fox [64] |
5. CaP Sequestration in serum
5.1. Sequestration allows calcium and phosphate transport and distribution throughout the body.
5.2. Sequestration allows rapid clearance of CaP as calciproteins.
5.3. Additional changes during circulation of CPPs
5.4. Sequestration enables controlled transfer of calcium and phosphate to other templates.
5.5. Sequestration provides bioavailable calcium and phosphate supporting numerous physiological functions.
5.6. Changes in Sequestered Entities in Health and Disease
5.7. Sequestration protects cells from toxicities associated with CaP crystal deposition.
5.8. Fetuin-A Sequestered ACP and Phosphate Toxicity
6. Current Status & Future Perspectives
- What is the relationship between changes in the populations of CPMs and CPPs and decreases in renal function? The kidney constitutes the principal organ for absorbing minerals from serum and processing them to recover calcium, magnesium and phosphate in the proximal tubules and/or excreting excesses in the urine [125]. Several groups of investigators have confirmed that CPMs and small CPP-1 nanoparticles are sufficiently small to pass through the renal filtration barrier into the Bowman’s space via the fenestrated endothelium of the glomerular capillaries and the filtration slits of the podocytes. When the kidney is functioning normally, the particles are acid-hydrolyzed into their ionic components, enabling ion recovery or excretion of excesses in urine. It is interesting to note that the concentrations of calcium and phosphate ions within the kidney tubules remain at or above saturation. Deposition of calcium phosphates in the kidney is prevented by adequate concentrations of Mg and citrate ions, as well as sequestration by IDPs such as Tamm-Horsfall protein [125]. However, because current test methods are insufficient to monitor CPMs, few data are available to correlate changes in the populations of CPMs and CPPs with declines in renal function and tissue damage until kidney disease has progressed to organ failure.
- What is the relationship between the sequestered nanoparticles and aberrant deposition, including ectopic deposition in soft tissues? Ectopic calcification is associated with aging as well as chronic diseases such as diabetes, metabolic syndrome, and kidney disease. Each of these chronic conditions is a major cause of blindness, kidney failure, heart attacks, stroke and lower limb amputation, and death. Thus, as populations age, these maladies pose an increasingly heavy economic burden. Therefore, continuing investigations into the relationship between sequestered phosphate nanoparticles and their aberrant deposition in soft tissues are needed to fill knowledge gaps and direct innovation for prevention and treatment.
- What roles do sequestered nanoparticles play in bone remodeling? Despite the observations that skeletal modelling necessarily takes place both in utero and in childhood, and skeletal remodeling continuously repairs defects and supports bone health throughout an individual’s lifetime, detailed mechanisms of bone mineralization remain to be elucidated. Cellular aspects of bone biology are actively being studied and highlight the complex interplay between cells, paracrine and endocrine factors and their balance and communication, as well the actions of cytokines and inflammatory mediators [126,127,128,129]. In general, however, hydroxyapatite formation is described as calcium and phosphate deposition in the holes and pores of collagen fibrils. Classically, deposition of crystalline calcium phosphate or crystalline carboxy calcium phosphate is postulated, wherein the latter gradually loses carbonate content as the crystals mature. In contrast, more recent reports describe deposition of amorphous calcium phosphate, perhaps within collagen matrix vesicles, followed by maturation into crystalline calcium phosphate. Despite the recognition of roles that known IDPs (e.g., osteocalcin, osteonectin, and sclerostin) play in these processes, there is little acknowledgement that these IDPs may act as key sequestration agents, facilitating and regulating maintenance of bone physiology in concordance with the other players.
- Bone dysfunction, particularly the changes in bone resilience associated with osteoporosis, is associated with increased risk and prevalence of fractures. Fractures of hip, spine, vertebrae, wrist or femur are a major cause of morbidity and mortality. The risk of a fracture increases with age and is greatest in women. Looking ahead, the lifetime risk of fractures will increase for all ethnic groups as people live longer. Therefore, increased understanding of the roles that sequestered nanoclusters play in bone remodeling and fracture healing constitutes another area where the knowledge gained through research will significantly impact both prevention and treatment.
- Several years ago, Powell and his colleagues reported extensive constitutive formation of porous amorphous magnesium-substituted calcium phosphate particles with diameters averaging 75-100 nm in both the human and murine gastrointestinal tract [20]. These nanominerals were associated with macromolecules, including bacterial peptidoglycan and dietary proteins. Synthetic analogs of these natural aggregates have been prepared both by Powell and his colleagues and Gelli and her group [100,124]. The apparent roles that calciprotein particles such as these play in immunosurveillance in the gut highlights the need for additional study of the actions of these particles in healthy humans and in those experiencing disruptions in intestinal health.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
| 1 | As a result of differences in material sourcing and differing capabilities for characterization, observers have given these nanoparticles different labels, including fetuin-mineral complexes, nanons, nanobes, bions, calcifying nanoparticles, biomimetic mineralo-protein or mineralo-organic nanoparticles, and nanobacteria. In the discussion that follows, the particles will be identified as calciprotein particles (CPPs). |
References
- Lowenstam HA, Weiner S. Mineralization by organisms and the evolution of biomineralization. In: Biomineralization and Biological Metal Accumulation: Biological and Geological Perspectives, Westbroek P, De Jong EW, Eds., D Reidel Publishing Co., Dordrecht, Holland, 1983.
- Kawasaki K, Weiss KM. Mineralized tissue and vertebrate evolution: the secretory calcium-binding phosphoprotein gene cluster. Proc Natl Acad Sci U S A. 2003 Apr 1; 100(7): 4060-5. [CrossRef]
- Kawasaki K, Suzuki T, Weiss KM. Genetic basis for the evolution of vertebrate mineralized tissue. Proc Natl Acad Sci U S A. 2004 Aug 3; 101(31): 11356-61. [CrossRef]
- Rao A, Drechsler M, Schiller S, Scheffner M, Gebauer D, Cölfen H. Stabilization of mineral precursors by intrinsically disordered proteins. Adv. Funct. Mater. 2018; 28: 1802063. [CrossRef]
- Martel J, Wu CY, Peng HH, Young JD. Mineralo-organic nanoparticles in health and disease: an overview of recent findings. Nanomedicine (Lond). 2018 Jul 1;13(14):1787-1793. [CrossRef] [PubMed]
- Gelli R, Ridi F, Baglioni P. The importance of being amorphous: calcium and magnesium phosphates in the human body. Adv Colloid Interface Sci. 2019 Jul;269:219-235. [CrossRef] [PubMed]
- Smith ER, Hewitson TD, Jahnen-Dechent W. Calciprotein particles: mineral behaving badly? Curr Opin Nephrol Hypertens. 2020 Jul; 29(4): 378-386. [CrossRef] [PubMed]
- Miura Y, Iwazu Y, Shiizaki K, Akimoto T, Kotani K, Kurabayashi M, Kurosu H, Kuro-O M. Identification and quantification of plasma calciprotein particles with distinct physical properties in patients with chronic kidney disease. Sci Rep. 2018 Jan 19; 8(1): 1256. [CrossRef]
- Jahnen-Dechent W, Smith ER. Nature's remedy to phosphate woes: calciprotein particles regulate systemic mineral metabolism. Kidney Int. 2020 Apr; 97(4): 648-651. [CrossRef] [PubMed]
- Wu CY, Martel J, Young JD. Comprehensive organic profiling of biological particles derived from blood. Sci Rep. 2018 Jul 27; 8(1): 11310. [CrossRef]
- Price PA, Nguyen TM, Williamson MK. Biochemical characterization of the serum fetuin-mineral complex. J Biol Chem. 2003 Jun 13; 278(24): 22153-60. [CrossRef] [PubMed]
- Jahnen-Dechent W, Heiss A, Schäfer C, Ketteler M. Fetuin-A regulation of calcified matrix metabolism. Circ Res. 2011 Jun 10; 108(12): 1494-509. [CrossRef]
- Brylka L, Jahnen-Dechent W. The role of fetuin-A in physiological and pathological mineralization. Calcif Tissue Int. 2013 Oct; 93(4): 355-64. [CrossRef]
- Heiss A, Eckert T, Aretz A, Richtering W, van Dorp W, Schäfer C, Jahnen-Dechent W. Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J Biol Chem. 2008 May 23; 283(21): 14815-25. [CrossRef] [PubMed]
- Akiyama K, Kimura T, Shiizaki K. Biological and Clinical Effects of Calciprotein Particles on Chronic Kidney Disease-Mineral and Bone Disorder. Int J Endocrinol. 2018 Mar 27; 2018: 5282389. [CrossRef]
- Kutikhin AG, Feenstra L, Kostyunin AE, Yuzhalin AE, Hillebrands JL, Krenning G. Calciprotein Particles: Balancing Mineral Homeostasis and Vascular Pathology. Arterioscler Thromb Vasc Biol. 2021 May 5; 41(5): 1607-1624. [CrossRef]
- Babler A, Schmitz C, Buescher A, Herrmann M, Gremse F, Gorgels T, Floege J, Jahnen-Dechent W. Microvasculopathy and soft tissue calcification in mice are governed by fetuin-A, magnesium and pyrophosphate. PLoS One. 2020 Feb 19; 15(2): e0228938. [CrossRef]
- Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, Muller-Esterl W, Schinke T, Jahnen-Dechent W. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003 Aug; 112(3): 357-66.
- Bäck M, Aranyi T, Cancela ML, Carracedo M, Conceição N, Leftheriotis G, Macrae V, Martin L, Nitschke Y, Pasch A, Quaglino D, Rutsch F, Shanahan C, Sorribas V, Szeri F, Valdivielso P, Vanakker O, Kempf H. Endogenous Calcification Inhibitors in the Prevention of Vascular Calcification: A Consensus Statement From the COST Action EuroSoftCalcNet. Front Cardiovasc Med. 2019 Jan 18; 5: 196. [CrossRef]
- Powell JJ, Thomas-McKay E, Thoree V, Robertson J, Hewitt RE, Skepper JN, Brown A, Hernandez-Garrido JC, Midgley PA, Gomez-Morilla I, Grime GW, Kirkby KJ, Mabbott NA, Donaldson DS, Williams IR, Rios D, Girardin SE, Haas CT, Bruggraber SF, Laman JD, Tanriver Y, Lombardi G, Lechler R, Thompson RP, Pele LC. An endogenous nanomineral chaperones luminal antigen and peptidoglycan to intestinal immune cells. Nat Nanotechnol. 2015 Apr; 10(4): 361-9. [CrossRef]
- Chow, LC. Solubility of calcium phosphates. Monogr Oral Sci. 2001; l13: 94-111.
- Tung MS, Eidelman N, Sieck B, Brown WE. Octacalcium phosphate solubility product from 4 to 37 °C. J Res Nat Bur Stand 1988; 93(5): 613-624.
- Millán Á, Lanzer P, Sorribas V. The Thermodynamics of Medial Vascular Calcification. Front Cell Dev Biol. 2021 Apr 14; 9: 633465. [CrossRef]
- Combes C, Rey C. Amorphous calcium phosphates: synthesis, properties and uses in biomaterials. Acta Biomater. 2010 Sep; 6(9): 3362-78. [CrossRef]
- Edén, M. Structure and formation of amorphous calcium phosphate and its role as surface layer of nanocrystalline apatite: Implications for bone mineralization. Materialia 2021 April; 17(52–54): 101107. [CrossRef]
- Christoffersen MR, Christoffersen J, Kibalczyc W. Apparent solubilities of two amorphous calcium phosphates and of octacalcium phosphate in the temperature range 30-42 °C. J Cryst Growth 1990; 106: 349-354.
- Lenton S, Wang Q, Nylander T, Teixeira S, Holt C. Structural biology of calcium phosphate nanoclusters sequestered by phosphoproteins. Crystals. 2020; 10(9): 755. [CrossRef]
- Boskey AL, Posner AS. Conversion of amorphous calcium phosphate to microcrystalline hydroxyapatite. A pH-dependent, solution-mediated, solid-solid conversion. J Phys Chem 1973; 77: 2313-2317.
- De Yoreo JJ, Gilbert PU, Sommerdijk NA, Penn RL, Whitelam S, Joester D, Zhang H, Rimer JD, Navrotsky A, Banfield JF, Wallace AF, Michel FM, Meldrum FC, Cölfen H, Dove PM. Crystal Growth. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science. 2015 Jul 31; 349(6247): aaa6760. [CrossRef] [PubMed]
- Querido W, Shanas N, Bookbinder S, Oliveira-Nunes MC, Krynska B, Pleshko N. Fourier transform infrared spectroscopy of developing bone mineral: from amorphous precursor to mature crystal. Analyst. 2020 Feb 3; 145(3): 764-776. [CrossRef]
- Nývlt, J. The Ostwald Rule of Stages. Cryst. Res. Technol. 1995; 30: 443-449. [CrossRef]
- Levin A, Mason TO, Adler-Abramovich L, Buell AK, Meisl G, Galvagnion C, Bram Y, Stratford SA, Dobson CM, Knowles TP, Gazit E. Ostwald's rule of stages governs structural transitions and morphology of dipeptide supramolecular polymers. Nat Commun. 2014 Nov 13; 5: 5219. [CrossRef] [PubMed]
- Ostwald,W. Studien über die bildung und umwandlung fester körper. Z. Phys. Chem. 1897; 22: 289.
- Threlfall, T. Structural and thermodynamic explanations of Ostwald’s Rule. Org. Process Res. Dev. 2003; 7(6): 1017–1027. [CrossRef]
- Sharma V, Srinivasan A, Nikolajeff F, Kumar S. Biomineralization process in hard tissues: The interaction complexity within protein and inorganic counterparts. Acta Biomater. 2021 Jan 15; 120: 20-37. [CrossRef] [PubMed]
- Yamada H, Kuro-O M, Ishikawa SE, Funazaki S, Kusaka I, Kakei M, Hara K. Daily variability in serum levels of calciprotein particles and their association with mineral metabolism parameters: A cross-sectional pilot study. Nephrology (Carlton). 2018 Mar; 23(3): 226-230. [CrossRef] [PubMed]
- Moreno EC, Gregory TM, Brown WE. Solubility of CaHPO4 · 2 H2O and formation of ion pairs in the system Ca(OH)2 - H3PO4 - H2O at 37.5 °C. J Res Natl Bur Stand A Phys Chem. 1966 Nov-Dec; 70A(6): 545-552. [CrossRef]
- Sun L, Chow LC, Frukhtbeyn SA, Bonevich JE. Preparation and properties of nanoparticles of calcium phosphates with various Ca/P ratios. J Res Natl Inst Stand Technol. 2010 Jul-Aug; 115(4): 243-255. [CrossRef]
- Garcia AC, Vavrusova M, Skibsted LH. Supersaturation of calcium citrate as a mechanism behind enhanced availability of calcium phosphates by presence of citrate. Food Res Int. 2018 May; 107: 195-205. [CrossRef] [PubMed]
- Holt LE, Pierce JA, Kajdi CN. The solubility of the phosphates of strontium, barium, and magnesium and their relation to the problem of calcification. J Colloid Sci 1954; 9(5): 409-426. [CrossRef]
- Lenton S, Nylander T, Teixeira SC, Holt C. A review of the biology of calcium phosphate sequestration with special reference to milk. Dairy Sci Technol. 2015; 95: 3-14. [CrossRef]
- Holt, C, Raynes, JK, Carver, JA. Sequence characteristics responsible for protein-protein interactions in the intrinsically disordered regions of caseins, amelogenins, and small heat-shock proteins. Biopolymers 2019; 110: e23319. [CrossRef]
- Boskey AL, Villarreal-Ramirez E. Intrinsically disordered proteins and biomineralization. Matrix Biol. 2016 May-Jul; 52-54: 43-59. [CrossRef]
- Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol. 2015 Jan; 16(1): 18-29. [CrossRef]
- Piovesan D, Tabaro F, Mičetić I, Necci M, Quaglia F, Oldfield CJ, Aspromonte MC, Davey NE, Davidović R, Dosztányi Z, Elofsson A, Gasparini A, Hatos A, Kajava AV, Kalmar L, Leonardi E, Lazar T, Macedo-Ribeiro S, Macossay-Castillo M, Meszaros A, Minervini G, Murvai N, Pujols J, Roche DB, Salladini E, Schad E, Schramm A, Szabo B, Tantos A, Tonello F, Tsirigos KD, Veljković N, Ventura S, Vranken W, Warholm P, Uversky VN, Dunker AK, Longhi S, Tompa P, Tosatto SC. DisProt 7.0: a major update of the database of disordered proteins. Nucleic Acids Res. 2017 Jan 4; 45(D1): D219-D227. [CrossRef]
- Sugase K, Dyson HJ, Wright PE. Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature. 2007 Jun 21; 447(7147): 1021-5. [CrossRef] [PubMed]
- Arai M, Sugase K, Dyson HJ, Wright PE. Conformational propensities of intrinsically disordered proteins influence the mechanism of binding and folding. Proc Natl Acad Sci U S A. 2015 Aug 4; 112(31): 9614-9.
- Shoemaker BA, Portman JJ, Wolynes PG. Speeding molecular recognition by using the folding funnel: the fly-casting mechanism. Proc Natl Acad Sci U S A. 2000 Aug 1; 97(16): 8868-73. [CrossRef]
- Huang LH, Han J, Ouyang JM, Gui BS. Shape-dependent adhesion and endocytosis of hydroxyapatite nanoparticles on A7R5 aortic smooth muscle cells. J Cell Physiol. 2020 Jan; 235(1): 465-479. [CrossRef] [PubMed]
- Huang LH, Sun XY, Ouyang JM. Shape-dependent toxicity and mineralization of hydroxyapatite nanoparticles in A7R5 aortic smooth muscle cells. Sci Rep. 2019 Dec 12; 9(1): 18979. [CrossRef]
- Cai MM, Smith ER, Holt SG. The role of fetuin-A in mineral trafficking and deposition. Bonekey Rep. 2015 May 6; 4: 672. [CrossRef]
- de Kruif CG, Huppertz T, Urban VS, Petukhov AV. Casein micelles and their internal structure. Adv Colloid Interface Sci. 2012 Mar-Apr; 171-172: 36-52. [CrossRef] [PubMed]
- Holt, C. The Milk Salts and Their Interaction with Casein. In Advanced Dairy Chemistry; Fox PF, Ed.; Chapman and Hall: London, UK, 1997; pp. 233–254. [Google Scholar]
- Ingham B, Erlangga GD, Smialowska A, Kirby NM, Wang C, Matia-Merino L, Haverkamp RG, Carr AJ. Solving the mystery of the internal structure of casein micelles. Soft Matter. 2015 Apr 14; 11(14): 2723-5. [CrossRef] [PubMed]
- Kamigaki T, Ito Y, Nishino Y, Miyazawa A. Microstructural observation of casein micelles in milk by cryo-electron microscopy of vitreous sections (CEMOVIS). Microscopy (Oxf). 2018 Jun 1; 67(3): 164-170. [CrossRef] [PubMed]
- Hettiarachchi CA, Swulius MT, Harte FM. Assessing constituent volumes and morphology of bovine casein micelles using cryo-electron tomography. J Dairy Sci. 2020 May; 103(5): 3971-3979. [CrossRef] [PubMed]
- McMahon DJ, Oommen BS. Supramolecular structure of the casein micelle. J Dairy Sci. 2008 May; 91(5): 1709-21. [CrossRef] [PubMed]
- Jenness, R.; Sloan, R.E. The Composition of Milk of Various Species: A Review. Dairy Sci. Abstr. 1970, 32, 599–612. [Google Scholar]
- Eisert, R.; Oftedal, O.T. Capital expenditure and income (foraging) during pinniped lactation: The example of the weddell seal (Leptonychotes weddellii). In Smithsonian at the Poles: Contributions to International Polar Year Science; Lang, M.A., Miller, S.E., Krupnik, I., Eds.; Smithsonian Institute: Washington, DC, USA, 2009; pp. 335–346. [Google Scholar]
- Jenness R, Holt C. Casein and lactose concentrations in milk of 31 species are negatively correlated. Experientia. 1987 Sep 15; 43(9): 1015-8. [CrossRef] [PubMed]
- Aoki T, Yamada N, Tomita I, Kako Y, Imamura T. Caseins are cross-linked through their ester phosphate groups by colloidal calcium phosphate. Biochim Biophys Acta. 1987 Jan 30; 911(2): 238-43. [CrossRef] [PubMed]
- Malacarne M, Criscione A, Franceschi P, Bordonaro S, Formaggioni P, Marletta D, Summer A. New Insights into Chemical and Mineral Composition of Donkey Milk throughout Nine Months of Lactation. Animals (Basel). 2019 Dec 17; 9(12): 1161. [CrossRef]
- Fantuz F, Ferraro S, Todini L, Cimarelli L, Fatica A, Marcantoni F, Salimei E. Distribution of calcium, phosphorus, sulfur, magnesium, potassium, and sodium in major fractions of donkey milk. J Dairy Sci. 2020 Oct; 103(10): 8741-8749. [CrossRef] [PubMed]
- Fox, P.F. , Uniacke-Lowe, T., McSweeney, P.L.H., O'Mahony, J.A. Dairy Chemistry and Biochemistry. 2nd Ed., 2015. Springer International Publishing Switzerland. Chem, Switzerland.
- Sánchez C, Fente C, Barreiro R, López-Racamonde O, Cepeda A, Regal P. Association between Breast Milk Mineral Content and Maternal Adherence to Healthy Dietary Patterns in Spain: A Transversal Study. Foods. 2020 May 20; 9(5): 659. [CrossRef]
- Li C, Solomons NW, Scott ME, Koski KG. Minerals and Trace Elements in Human Breast Milk Are Associated with Guatemalan Infant Anthropometric Outcomes within the First 6 Months. J Nutr. 2016 Oct; 146(10): 2067-2074. [CrossRef] [PubMed]
- Dalgleish DG, Parker TG. Binding of calcium ions to bovine alpha-S1-casein and precipitability of the protein-calcium ion complexes. J. Dairy Res. 1980; 47(1): 113–122. [CrossRef]
- Parker TG, Dalgleish DG. Binding of calcium ions to bovine beta-casein. J Dairy Res. 1981 Feb; 48(1): 71-6. [CrossRef] [PubMed]
- Sleigh RW, Mackinlay AG, Pope JM. NMR studies of the phosphoserine regions of bovine alpha s1- and beta-casein. Assignment of 31P resonances to specific phosphoserines and cation binding studied by measurement of enhancement of 1H relaxation rate. Biochim Biophys Acta. 1983 Jan 12; 742(1): 175-83. [CrossRef] [PubMed]
- Sleigh RW, Sculley TB, Mackinlay AG. The binding of beta-casein to hydroxyapatite: the effect of phosphate content and location. J Dairy Res. 1979 Apr; 46(2): 337-42. [CrossRef] [PubMed]
- Bijl E, Huppertz T, van Valenberg H, Holt C. A quantitative model of the bovine casein micelle: ion equilibria and calcium phosphate sequestration by individual caseins in bovine milk. Eur Biophys J. 2019 Jan; 48(1): 45-59. [CrossRef] [PubMed]
- Holt C, Sawyer L. Caseins as rheomorphic proteins - Interpretation of primary and secondary structures of the alpha-S1-caseins, beta-caseins and kappa-caseins. J Chem Soc. Faraday Trans. 1993; 89: 2683–2692. [CrossRef]
- Heiss A, DuChesne A, Denecke B, Grötzinger J, Yamamoto K, Renné T, Jahnen-Dechent W. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem. 2003 Apr 11; 278(15): 13333-41. [CrossRef] [PubMed]
- Häusler M, Schäfer C, Osterwinter C, Jahnen-Dechent W. The physiologic development of fetuin-a serum concentrations in children. Pediatr Res. 2009 Dec; 66(6): 660-4. [CrossRef] [PubMed]
- Šimják P, Cinkajzlová A, Anderlová K, Kloučková J, Kratochvílová H, Lacinová Z, Kaválková P, Krejčí H, Mráz M, Pařízek A, Kršek M, Haluzík M. Changes in plasma concentrations and mRNA expression of hepatokines fetuin A, fetuin B and FGF21 in physiological pregnancy and gestational diabetes mellitus. Physiol Res. 2018 Nov 28; 67(Suppl 3): S531-S542. [CrossRef] [PubMed]
- Ren G, Kim T, Papizan JB, Okerberg CK, Kothari VM, Zaid H, Bilan PJ, Araya-Ramirez F, Littlefield LA, Bowers RL, Mahurin AJ, Nickles MM, Ludvigsen R, He X, Grandjean PW, Mathews ST. Phosphorylation status of fetuin-A is critical for inhibition of insulin action and is correlated with obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2019 Aug 1; 317(2): E250-E260. [CrossRef] [PubMed]
- Jahnen-Dechent W, Büscher A, Köppert S, Heiss A, Kuro-O M, Smith ER. Mud in the blood: the role of protein-mineral complexes and extracellular vesicles in biomineralisation and calcification. J Struct Biol. 2020 Oct 1; 212(1): 107577. [CrossRef] [PubMed]
- Gebauer D, Kellermeier M, Gale JD, Bergström L, Cölfen H. Pre-nucleation clusters as solute precursors in crystallisation. Chem Soc Rev. 2014 Apr 7; 43(7): 2348-71. [CrossRef] [PubMed]
- Köppert S, Büscher A, Babler A, Ghallab A, Buhl EM, Latz E, Hengstler JG, Smith ER, Jahnen-Dechent W. Cellular Clearance and Biological Activity of Calciprotein Particles Depend on Their Maturation State and Crystallinity. Front Immunol. 2018 Sep 4; 9: 1991. [CrossRef]
- Koeppert S, Ghallab A, Peglow S, Winkler CF, Graeber S, Büscher A, Hengstler JG, Jahnen-Dechent W. Live Imaging of Calciprotein Particle Clearance and Receptor Mediated Uptake: Role of Calciprotein Monomers. Front Cell Dev Biol. 2021 Apr 29;9:633925. [CrossRef]
- Satchell SC, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009 May; 296(5): F947-56.
- Herrmann M, Schäfer C, Heiss A, Gräber S, Kinkeldey A, Büscher A, Schmitt MM, Bornemann J, Nimmerjahn F, Herrmann M, Helming L, Gordon S, Jahnen-Dechent W. Clearance of fetuin-A-containing calciprotein particles is mediated by scavenger receptor-A. Circ Res. 2012 Aug 17; 111(5): 575-84. [CrossRef] [PubMed]
- Wu CY, Martel J, Young JD. Ectopic calcification and formation of mineralo-organic particles in arteries of diabetic subjects. Sci Rep. 2020 May 22; 10(1): 8545. [CrossRef]
- Kumon H, Matsuura E, Nagaoka N, Yamamoto T, Uehara S, Araki M, Matsunami Y, Kobayashi K, Matsumoto A. Ectopic calcification: importance of common nanoparticle scaffolds containing oxidized acidic lipids. Nanomedicine. 2014 Feb; 10(2): 441-50. [CrossRef] [PubMed]
- Perovic I, Chang EP, Lui M, Rao A, Cölfen H, Evans JS. A nacre protein, n16.3, self-assembles to form protein oligomers that dimensionally limit and organize mineral deposits. Biochemistry. 2014 Apr 29;53(16):2739-48. [CrossRef] [PubMed]
- Szasz CS, Alexa A, Toth K, Rakacs M, Langowski J, Tompa P. Protein disorder prevails under crowded conditions. Biochemistry. 2011 Jul 5; 50(26): 5834-44. [CrossRef] [PubMed]
- Stawski TM, Roncal-Herrero T, Fernandez-Martinez A, Matamoros-Veloza A, Kröger R, Benning LG. "On demand" triggered crystallization of CaCO3 from solute precursor species stabilized by the water-in-oil microemulsion. Phys Chem Chem Phys. 2018 May 23; 20(20): 13825-13835. [CrossRef] [PubMed]
- Jain G, Pendola M, Rao A, Cölfen H, Evans JS. A Model Sea Urchin Spicule Matrix Protein Self-Associates To Form Mineral-Modifying Protein Hydrogels. Biochemistry. 2016 Aug 9; 55(31): 4410-21. [CrossRef] [PubMed]
- Perovic I, Davidyants A, Evans JS. Aragonite-Associated Mollusk Shell Protein Aggregates To Form Mesoscale "Smart" Hydrogels. ACS Omega. 2016 Nov 30; 1(5): 886-893. [CrossRef]
- Pendola M, Jain G, Evans JS. Skeletal development in the sea urchin relies upon protein families that contain intrinsic disorder, aggregation-prone, and conserved globular interactive domains. PLoS One. 2019 Oct 1; 14(10): e0222068. [CrossRef]
- Hołubowicz R, Wojtas M, Taube M, Kozak M, Ożyhar A, Dobryszycki P. Effect of calcium ions on structure and stability of the C1q-like domain of otolin-1 from human and zebrafish. FEBS J. 2017 Dec; 284(24): 4278-4297. [CrossRef] [PubMed]
- George A, Veis A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem Rev. 2008 Nov; 108(11): 4670-93. [CrossRef]
- Farvadi F, Ghahremani MH, Hashemi F, Reza Hormozi-Nezhad M, Raoufi M, Zanganeh S, Atyabi F, Dinarvand R, Mahmoudi M. Cell shape affects nanoparticle uptake and toxicity: An overlooked factor at the nanobio interfaces. J Colloid Interface Sci. 2018 Dec 1; 531: 245-252. [CrossRef] [PubMed]
- Moreland KT, Hong M, Lu W, Rowley CW, Ornitz DM, De Yoreo JJ, Thalmann R. In vitro calcite crystal morphology is modulated by otoconial proteins otolin-1 and otoconin-90. PLoS One. 2014 Apr 18; 9(4): e95333. [CrossRef]
- Poundarik AA, Boskey A, Gundberg C, Vashishth D. Biomolecular regulation, composition and nanoarchitecture of bone mineral. Sci Rep. 2018 Jan 19; 8(1): 1191. [CrossRef]
- Kunishige R, Mizoguchi M, Tsubouchi A, Hanaoka K, Miura Y, Kurosu H, Urano Y, Kuro-O M, Murata M. Calciprotein particle-induced cytotoxicity via lysosomal dysfunction and altered cholesterol distribution in renal epithelial HK-2 cells. Sci Rep. 2020 Nov 18; 10(1): 20125. [CrossRef]
- Ewence AE, Bootman M, Roderick HL, Skepper JN, McCarthy G, Epple M, Neumann M, Shanahan CM, Proudfoot D. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res. 2008 Aug 29; 103(5): e28-34. [CrossRef] [PubMed]
- Herrmann M, Babler A, Moshkova I, Gremse F, Kiessling F, Kusebauch U, Nelea V, Kramann R, Moritz RL, McKee MD, Jahnen-Dechent W. Lumenal calcification and microvasculopathy in fetuin-A-deficient mice lead to multiple organ morbidity. PLoS One. 2020 Feb 19; 15(2): e0228503. [CrossRef]
- Shook LL, Buhimschi CS, Dulay AT, McCarthy ME, Hardy JT, Duzyj Buniak CM, Zhao G, Buhimschi IA. Calciprotein particles as potential etiologic agents of idiopathic preterm birth. Sci Transl Med. 2016 Nov 9;8(364):364ra154. [CrossRef] [PubMed]
- Pele LC, Haas CT, Hewitt RE, Robertson J, Skepper J, Brown A, Hernandez-Garrido JC, Midgley PA, Faria N, Chappell H, Powell JJ. Synthetic mimetics of the endogenous gastrointestinal nanomineral: Silent constructs that trap macromolecules for intracellular delivery. Nanomedicine. 2017 Feb; 13(2): 619-630. [CrossRef]
- Gelli R, Tempesti P, Ridi F, Baglioni P. Formation and properties of amorphous magnesium-calcium phosphate particles in a simulated intestinal fluid. J Colloid Interface Sci. 2019 Jun 15;546:130-138. [CrossRef] [PubMed]
- Gelli R, Martini F, Geppi M, Borsacchi S, Ridi F, Baglioni P. Exploring the interplay of mucin with biologically-relevant amorphous magnesium-calcium phosphate nanoparticles. J Colloid Interface Sci. 2021 Mar 16; 594: 802-811. [CrossRef] [PubMed]
- Akiyama KI, Miura Y, Hayashi H, Sakata A, Matsumura Y, Kojima M, Tsuchiya K, Nitta K, Shiizaki K, Kurosu H, Kuro-O M. Calciprotein particles regulate fibroblast growth factor-23 expression in osteoblasts. Kidney Int. 2020 Apr; 97(4): 702-712. [CrossRef] [PubMed]
- Kuro-O, M. Klotho and endocrine fibroblast growth factors: markers of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transplant. 2019 Jan 1; 34(1): 15-21. [CrossRef] [PubMed]
- Kuro-O, M. Aging and FGF23-klotho system. Vitam Horm. 2021; 115: 317-332. [CrossRef] [PubMed]
- Price PA, Thomas GR, Pardini AW, Figueira WF, Caputo JM, Williamson MK. Discovery of a high molecular weight complex of calcium, phosphate, fetuin, and matrix gamma-carboxyglutamic acid protein in the serum of etidronate-treated rats. J Biol Chem. 2002 Feb 8; 277(6): 3926-34. [CrossRef] [PubMed]
- Wong TY, Wu CY, Martel J, Lin CW, Hsu FY, Ojcius DM, Lin PY, Young JD. Detection and characterization of mineralo-organic nanoparticles in human kidneys. Sci Rep. 2015 Oct 26; 5: 15272. [CrossRef]
- Smith ER, Hewitson TD, Hanssen E, Holt SG. Biochemical transformation of calciprotein particles in uraemia. Bone. 2018 May; 110: 355-367. [CrossRef] [PubMed]
- Aghagolzadeh P, Bachtler M, Bijarnia R, Jackson C, Smith ER, Odermatt A, Radpour R, Pasch A. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-α. Atherosclerosis. 2016 Aug; 251: 404-414. [CrossRef] [PubMed]
- Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019 Aug; 19(8): 477-489. [CrossRef]
- Honarpisheh M, Foresto-Neto O, Desai J, Steiger S, Gómez LA, Popper B, Boor P, Anders HJ, Mulay SR. Phagocytosis of environmental or metabolic crystalline particles induces cytotoxicity by triggering necroptosis across a broad range of particle size and shape. Sci Rep. 2017 Nov 14;7(1):15523. [CrossRef]
- Mulay SR, Honarpisheh MM, Foresto-Neto O, Shi C, Desai J, Zhao ZB, Marschner JA, Popper B, Buhl EM, Boor P, Linkermann A, Liapis H, Bilyy R, Herrmann M, Romagnani P, Belevich I, Jokitalo E, Becker JU, Anders HJ. Mitochondria Permeability Transition versus Necroptosis in Oxalate-Induced AKI. J Am Soc Nephrol. 2019 Oct; 30(10): 1857-1869. [CrossRef]
- Dautova Y, Kozlova D, Skepper JN, Epple M, Bootman MD, Proudfoot D. Fetuin-A and albumin alter cytotoxic effects of calcium phosphate nanoparticles on human vascular smooth muscle cells. PLoS One. 2014 May 21; 9(5): e97565. [CrossRef]
- Dai XY, Zhao MM, Cai Y, Guan QC, Zhao Y, Guan Y, Kong W, Zhu WG, Xu MJ, Wang X. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int. 2013 Jun; 83(6): 1042-51. [CrossRef] [PubMed]
- Wåhlén E, Olsson F, Söderberg O, Lennartsson J, Heldin J. Differential impact of lipid raft depletion on platelet-derived growth factor (PDGF)-induced ERK1/2 MAP-kinase, SRC 22and AKT signaling. Cell Signal. 2022 Aug; 96: 110356. Erratum in: Cell Signal. 2022 Jul 22;98:110411. [CrossRef] [PubMed]
- Brown, RB. Diabetes, Diabetic Complications, and Phosphate Toxicity: A Scoping Review. Curr Diabetes Rev. 2020; 16(7): 674-689. [CrossRef] [PubMed]
- Merx MW, Schäfer C, Westenfeld R, Brandenburg V, Hidajat S, Weber C, Ketteler M, Jahnen-Dechent W. Myocardial stiffness, cardiac remodeling, and diastolic dysfunction in calcification-prone fetuin-A-deficient mice. J Am Soc Nephrol. 2005 Nov; 16(11): 3357-64. [PubMed]
- Villa-Bellosta R, O'Neill WC. Pyrophosphate deficiency in vascular calcification. Kidney Int. 2018 Jun; 93(6): 1293-1297. [CrossRef] [PubMed]
- Villa-Bellosta R, Sorribas V. Prevention of vascular calcification by polyphosphates and nucleotides- role of ATP. Circ J. 2013; 77(8): 2145-51. [CrossRef] [PubMed]
- Icer MA, Yıldıran H. Effects of fetuin-A with diverse functions and multiple mechanisms on human health. Clin Biochem. 2021 Feb;88:1-10. [CrossRef] [PubMed]
- Boskey AL, Posner AS. Magnesium stabilization of amorphous calcium phosphate: A kinetic study. Mat Res Bull 1974; 9: 907-916.
- Holt C, Van Kemenade MJJM, Harries JE, Nelson LS, Bailey RT, Hukins DWL, Hasnain SS, De Bruyn PL. Preparation of amorphous calcium magnesium phosphates at pH 7 and characterization by X-ray absorption and Fourier transform infrared spectroscopy. J Cryst Growth 1988; 92: 239-252.
- Kibalczyc W, Christoffersen J, Christoffersen MR, Zielenkiewicz A, Zielenkiewicz W. The effect of magnesium ions on the precipitation of calcium phosphates. J Cryst Growth 1990; 106: 355-366.
- Gelli R, Scudero M, Gigli L, Severi M, Bonini M, Ridi F, Baglioni P. Effect of pH and Mg2+ on Amorphous Magnesium-Calcium Phosphate (AMCP) stability. J Colloid Interface Sci. 2018 Dec 1; 531: 681-692. [CrossRef] [PubMed]
- Rodelo-Haad C, Pendón-Ruiz de Mier MV, Díaz-Tocados JM, Martin-Malo A, Santamaria R, Muñoz-Castañeda JR, Rodríguez M. The Role of Disturbed Mg Homeostasis in Chronic Kidney Disease Comorbidities. Front Cell Dev Biol. 2020 Nov 12; 8: 543099. [CrossRef]
- Diaz-Tocados JM, Peralta-Ramirez A, Rodríguez-Ortiz ME, Raya AI, Lopez I, Pineda C, Herencia C, Montes de Oca A, Vergara N, Steppan S, Pendon-Ruiz de Mier MV, Buendía P, Carmona A, Carracedo J, Alcalá-Díaz JF, Frazao J, Martínez-Moreno JM, Canalejo A, Felsenfeld A, Rodriguez M, Aguilera-Tejero E, Almadén Y, Muñoz-Castañeda JR. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017 Nov;92(5):1084-1099. [CrossRef] [PubMed]
- Ter Braake AD, Smit AE, Bos C, van Herwaarden AE, Alkema W, van Essen HW, Bravenboer N, Vervloet MG, Hoenderop JGJ, de Baaij JHF. Magnesium prevents vascular calcification in Klotho deficiency. Kidney Int. 2020 Mar; 97(3): 487-501. [CrossRef] [PubMed]
- ter Braake AD, Eelderink C, Zeper LW, Pasch A, Bakker SJL, de Borst MH, Hoenderop JGJ, de Baaij JHF. Calciprotein particle inhibition explains magnesium-mediated protection against vascular calcification. Nephrol Dial Transplant. 2020 May 1;35(5):765-773. [CrossRef]
| Salt | Formula | Ca/P | -log Ksp) at 25°C1 | -log (Ksp) at 37°C2 | pH |
|---|---|---|---|---|---|
| Monocalcium phosphate monohydrate | Ca(H2PO4)2 ∙ H2O | 0.5 | Highly soluble | Soluble | < 2 |
| Dicalcium phosphate dihydrate | Ca2(HPO4)2 ∙ 2 H2O | 1.0 | 6.59 | 6.66 | 3.5 – 6.8 |
| Octacalcium phosphate | Ca8H2(PO4)6 ∙ 5 H2O | 1.33 | 48.4 | 48.7 | ~ 6 |
| α-Tricalcium phosphate | α-Ca3(PO4)2 | 1.5 | 25.5 | ||
| β-Tricalcium phosphate | β-Ca3(PO4)2 | 1.5 | 28.9 | ||
| Hydroxyapatite | Ca10(PO4)6(OH)2 | 1.67 | 58.4 | 117.3 | 9.5 – 12 |
| Fluoroapatite | Ca5(PO4)3F | 1.67 | 60.5 | ||
| Tetracalcium phosphate | Ca4(PO4)2O | 2.0 | 38.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





