1. Introduction
Cardiovascular Magnetic Resonance (CMR) plays an increasingly crucial role in diagnosing cardiac diseases, serving as the gold standard imaging technique for assessing cardiac function and accurately measuring ventricular volumes and mass. What sets CMR apart is its unique ability to perform soft-tissue characterization [
1]. CMR employs various conventional techniques such as T1-fast spin echo (FSE) with/without Fat Saturation, T2-Short-tau-inversion recovery (STIR) pulse sequence, and late gadolinium enhancement (LGE) technique to evaluate myocardial tissue. These techniques are effective in detecting fat infiltration/metaplasia, edema, and fibrosis, respectively. They have proven to be robust and reproducible. The integration of findings from these conventional techniques with morphological and functional features enables comprehensive diagnosis of myocardial diseases.
Certain features obtained by this technique, such as the presence, pattern of presentation, and extent of fibrosis, have been demonstrated to play a significant prognostic role in various cardiac conditions [
2]. However, a notable limitation of these conventional techniques, particularly LGE and T2-STIR, is their qualitative or semiquantitative nature, allowing only a comparative analysis (hypointense, isointense, hyperintense) between normal and diseased myocardium.
The T1 mapping technique may overcome these limitations by measuring the intrinsic myocardial T1 relaxation time on a pixel-wise basis [
3]. This means that every pixel on a T1 map represents an absolute T1 value, allowing for precise quantification. Abnormalities in myocardial T1, whether they are global or regional, are determined by comparing them to reference values expressed in milliseconds [
4]. Consequently, T1 mapping has the potential to detect diffuse structural changes in the myocardium that may not be assessable by other non-invasive techniques, including LGE. This capability makes T1 mapping a valuable tool for identifying and characterizing subtle myocardial alterations that could have gone unnoticed using conventional imaging methods.
Non-contrast myocardial T1, often referred to as "native" T1, is a term used to distinguish it from post-contrast T1. The most important biological determinants of an increase in “native” T1 are edema (increase of tissue water in i.e. acute infarction, inflammation or acute toxic damage), and the increase of interstitial space for fibrosis (infarction, chronic myocarditis, cardiomyopathy etc) or for amyloidotic proteins deposition [
5]. On contrast, the two most important causes of low native T1 values are lipid overload (i.e. Anderson-Fabry disease, lipomatous metaplasia) and iron overload [
6].
T1 mapping technique may be performed using two different families of pulse sequence: those based on inversion recovery (IR), including the standard Look-Locker sequence, the MOdified Look-Locker Inversion Recovery (MOLLI) sequence, and the Shortened MOLLI (ShMOLLI) sequence; and those, less frequently used, based on a saturation recovery as the saturation recovery single-shot acquisition (SASHA) and the Saturation Pulse Prepared Heart-rate-independent Inversion Recovery (SAPPHIRE) [
5]. The MOLLI technique is the most frequently used. It uses a steady state free precession (SSFP) readout that drives the IR to recover more quickly and reaches a steady state that is less than the equilibrium magnetization (M0). The effect of the readout is an apparent recovery time referred to as T1* which is less than the actual longitudinal recovery time, T1. The MOLLI method samples the IR curve at multiple inversion times using single shot imaging spaced at heart beat intervals. Multiple inversions are used with different trigger delays in order to acquire measurements at different inversion times to sample the IR curve more evenly. Recovery periods are needed between the inversions to ensure that samples from the different inversions are from the same recovery curve. The first method for MOLLI described is the 3(3)3(3)5, where number in parenthesis represents the number of heart beats for recovery of magnetization and the others the numbers of images acquired in different heart beats following a single IR pulse. Nowadays the most used approach is the 5(3)3. A number of protocol modifications (as the ShMOLLI technique), have been proposed to shorten the acquisition duration or to improve the accuracy or precision [
4].
Post-processing methods play a crucial role in T1 mapping. To begin, the signal intensity of each voxel or group of voxels is measured in every image acquired at different inversion times [
7]. These signal intensity values are then plotted against the inversion time, resulting in a curve. In IR-based techniques, the initial part of the curve exhibits a descending pattern until reaching the null point. To transform the curve into a negative exponential curve, this descending portion is inverted by assigning negative values. Next, a mathematical fitting process is applied to the curve. By fitting a mathematical equation to the curve, the T1 value is derived. This equation describes the behavior of the curve and allows for the calculation of T1 relaxation time. Finally, a gray-scale T1 map is generated by assigning a signal value corresponding to the respective T1 value at each voxel or group of voxels [
3]. In visualization, these T1 maps are typically presented as parametric color maps, which can be displayed on workstations. The color map allows for a visual representation of T1 values, aiding in the interpretation and identification of variations in tissue characteristics.
T1 Mapping technique has some limitations: 1) the variability due to factors such as heart rate, motion artifacts, arrhythmias and imaging parameters and, mostly magnetic field inhomogeneity; 2) the lack of standardized reference values for myocardial T1 in the myocardium for which each CMR laboratory must create its own reference values; 3) in many cardiac conditions different phenomena may coexist as fibrosis, edema, inflammation, and even opposite phenomena (as fat and fibrosis) that may affect the myocardial T1 relaxation leading to false-positive or false-negative findings; 4) the additional time required for T1 mapping sequences can lead to longer scan durations, by which SCMR position document suggest to acquire only ventricular 3 short axis views for T1 mapping; 5) the differences in acquisition schemes have a direct effect on the range of normal and abnormal T1 with a given technique, which means that absolute T1 values can only be directly compared when they were obtained with the same acquisition scheme at the same field strength using the same post-processing methods [
5].
The aim of the present study was to compare the diagnostic role of conventional CMR technique to native T1 mapping in a real-life cohort of non selected consecutive patients undergoing CMR.
3. Results
The final population included 323 patients, 206 males (64%), mean age 54±18 years. The baseline characteristics are showed in table 3. Briefly, the main indications for CMR were: suspect of arrhythmogenic cardiomyopathy (ARC) in 20% of cases; non-ischemic dilated cardiomyopathy (DCM) in 19%; hypertrophic cardiomyopathy (HCM) in 16%; chest pain without obstructive coronary artery in 14% of patients; other indications (amyloidosis, scleroderma, previous myocardial infarction, pericarditis, LV non compaction etc) in the remaining of cases. We also included 27(8%) of age- and sex- matched healthy controls. As evident in table 4, conventional T2-STIR images, that were acquired in 154 patients, showed myocardial hyperintensity compatible for edema in 41 patients (27%) of patients. Myocardial fat infiltration/metaplasia in T1-FSE with/without Fat Sat was found in 20 patients, all of them having a positive non-ischemic LGE. LGE was positive in 206 (64%) of patients with an ischemic pattern of distribution in 20 (6%), non-ischemic in 183(57%), while a specific LGE pattern for cardiac amyloidosis was found the remaining 3 patients (1%).
At T1 mapping, the average native T1 was 1040±55 msec. 65(20%) showed a global increase of native T1 (mean T1 1232±85 msec), while it was decreased in 11 (3%) patients (mean T1 885±85 msec). A regional increase of native T1 was found in 160 (46%), 52 of them with global increase of T1 values.
3.1. Conventional CMR vs T1 mapping
Among the 41 patients with signs of myocardial edema at T2-STIR, 32(78%) had also increase of regional native T1, then in 9 patients, edema was not associated with a consistent increase of T1. Overall, in 185(57%) patients a matching between T2-STIR and native T1 was found.
T1 was normal in 74 (36%) patients with positive LGE and abnormal in 33 with negative LGE. Overall a perfect matching between these 2 techniques was found in 206 (64%) patients (both positive in 132, both negative in 74 patients). No significant differences were found for the pattern of LGE: native T1 was increased in 118 out of 186 patients (63%) with non-ischemic LGE vs 14 out of 20 (65%) with ischemic pattern (p = 0.81). Native T1 was normal in 2 out of 10 patients without gadolinium injection.
Among the 33 patients with abnormal T1 and negative LGE, only 4 (12%) had edema at T2 STIR images.
Table 1.
Clinical indications and diagnostic features (part 1).
Table 1.
Clinical indications and diagnostic features (part 1).
| Clinical Indication |
Morphologic features (Cine-SSFP) |
Edema/Fat |
LGE |
Native T1 |
| DCM |
LV dilation and dysfunction |
Nonspecific pattern |
- -
Absent (75%) - -
Non-ischemic (20-25%) |
Slight diffuse increase |
| HCM |
Asymmetrical hypertrophy (septal, apical, septal-apical, diffuse, lateral) Secondary features (intramyocardial coronary artery bridge, apical aneurism, crypts, mitral anterior leaflet elongation, papillary muscles abnormalities) |
Midwall edema in hypertrophied segments associated with LGE (40%) |
Midwall distribution in hypertrophied segments (55-95%) |
Focal increase of T1 only in scar region (55-95%) |
| ARC |
- -
Regional wall motion abnormalities and/or dilation and/or dysfunction - -
of RV in RV presentation or in biventricular presentation - -
of LV in LV-dominant presentation - -
RV or LVintramyocardial india-ink in fat infiltration |
Fat infiltration /metaplasia |
|
|
| Myocarditis |
- -
None - -
Regional wall motion abnormalities (in coronary territory) - -
Global LV dysfunction |
Non-ischemic pattern (85-100%) |
Non-ischemic pattern (85-100%) |
Increased |
| MINOCA |
- -
Regional wall motion abnormalities - -
Potentially LV dysfunction |
Ischemic-like pattern (100% in acute phase) |
Ischemic presentation with potential no-reflow (hypointensity within hyperintense regions) (100%) |
- -
Increased - -
Decrease in presence of haemorrhagic infarction |
| Tako-tsubo |
Regional wall motion abnormalities in apical segments (apical ballooning) |
Transmural in apical regions (100% in acute phase) |
Absent |
Increased in apical regions |
Table 2.
Clinical indications and diagnostic features (part 2).
Table 2.
Clinical indications and diagnostic features (part 2).
| Clinical Indication |
Morphologic features (Cine-SSFP) |
Edema |
LGE |
Native T1 |
| Scleroderma |
Non specific |
Diffuse/Focal edema in active inflammation (possible) |
- -
Normal - -
Diffuse mild-enhanced - -
Focal hyper-enhanced LGE (% unknown) |
Diffuse/Focal increase |
| Amyloidosis |
- -
Concentric hypertrophy - -
Left atrial wall thickening - -
Pericardial effusion |
Nonspecific pattern |
Specific pattern:
- -
Diffuse subendocardial pattern - -
Nulling defect of myocardium - -
Early post-contrast darkening of blood
|
Diffuse increase |
| Myocardial infarction |
- -
Regional wall motion abnormalities (in coronary territory) - -
Potentially LV dysfunction |
- -
Ischemic-like pattern in acute (100%) - -
Absent in chronic |
Ischemic presentation
|
- -
Increased in scar and acute infarction - -
Decrease in presence of haemorrhagic infarction |
| Fabry disease |
- -
Concentric hypertrophy (90%) - -
Asymmetric hypertrophy (10%) |
Nonspecific pattern |
|
- -
Diffusely decreased - -
In late stage with extensive LGE, T1 may increase (pseudonormalization). |
| Dystrophy |
- -
Normal - -
LV dysfunction - -
Regional wall motion abnormalities - -
India ink |
Nonspecific pattern |
Non-ischemic pattern (10-20%) |
- -
Increased in scar region - -
Decreased in fat infiltration |
| Pericarditis |
- -
Pericardial effusion - -
Thickening of pericardial layers |
Hyperintensity of pericardial layers in pericarditis (80%) |
- -
Enhancement of layer in pericarditis - -
No enhancement in non-inflammatory effusion (80%) |
Unknown |
Table 3.
functional characteristics.
Table 3.
functional characteristics.
| Indication |
n. |
Males |
Age |
LV EF |
LV EDVi |
RV EF |
RV EDVi |
| DCM |
61 (18.9) |
47 (77) |
59±14 |
43.15±14.58 |
112.56±35.31 |
56.16±11.54 |
78.21±21.39 |
| HCM |
51 (15.8) |
35 (69) |
60±13 |
67.43±11.31 |
73.80±21.64 |
65.29±7.94 |
69.59±21.74 |
| ARC |
63 (19.5) |
43 (68) |
40±17 |
65.08±7.64 |
87.79±16.22 |
60.74±7.38 |
91.79±19.85 |
| Myocarditis |
44 (13.6) |
29 (66) |
50±20 |
61.34±9.04 |
77.86±18.39 |
61.61±5.93 |
76.09±17.55 |
| Scleroderma |
21 (6.5) |
2 (10) |
56±15 |
61.29±16.71 |
76.81±16.97 |
62.38±7.51 |
75.19±16.48 |
| Amyloidosis |
17 (5.3) |
9 (53) |
76±8 |
63.06±14.25 |
70.94±20.64 |
66.76±9.48 |
59.94±12.43 |
| Myocardial infarction (acute/chronic) |
15 (4.6) |
11 (73) |
62±15 |
49.73±16.42 |
89.60±27.13 |
63.80±8.17 |
61.47±14.73 |
| Dystrophy/Miasteny/Mitochondrial |
8 (2.8) |
7 (85) |
41±17 |
62.00±9.51 |
70.67±20.56 |
62.78±9.68 |
65.44±15.62 |
| Pericarditis-Pericardial effusion |
6 (1.9) |
2 (33) |
46±13 |
66.50±10.21 |
80.50±15.14 |
66.67±6.95 |
71.83±13.06 |
| LV Non-compaction |
4 (1.2) |
2 (50) |
39±21 |
65.50±10.63 |
83.25±11.21 |
58.75±7.41 |
87.75±16.32 |
| Systemic sarcoidosis |
3 (0.9) |
1 (33) |
56±9 |
46.67±21.01 |
74.00±3.61 |
38.00±22.61 |
97.00±32.51 |
| Fabry |
1(0.3) |
1(100) |
45 |
65 |
70 |
63 |
72 |
| Pulmonary hypertension |
1 (0.3) |
1 (100) |
58 |
57 |
83 |
49 |
81 |
| Valvular disease |
1 (0.3) |
1 (100) |
77 |
58 |
88 |
70 |
78 |
| Healthy controls |
27 (8.4) |
16 (59) |
51±18 |
67.15±6.73 |
78.44±18.25 |
63.59±7.00 |
78.07±19.48 |
3.2. Diagnostic role of T1 mapping
Overall, the CMR findings confirmed the initial suspicion in 149 patients (50%), yielded an alternative diagnosis in 41(14%), were nonspecific (not allowing a definitive diagnosis) in 78(26%) and completely negative in 28(10%) (table 5).
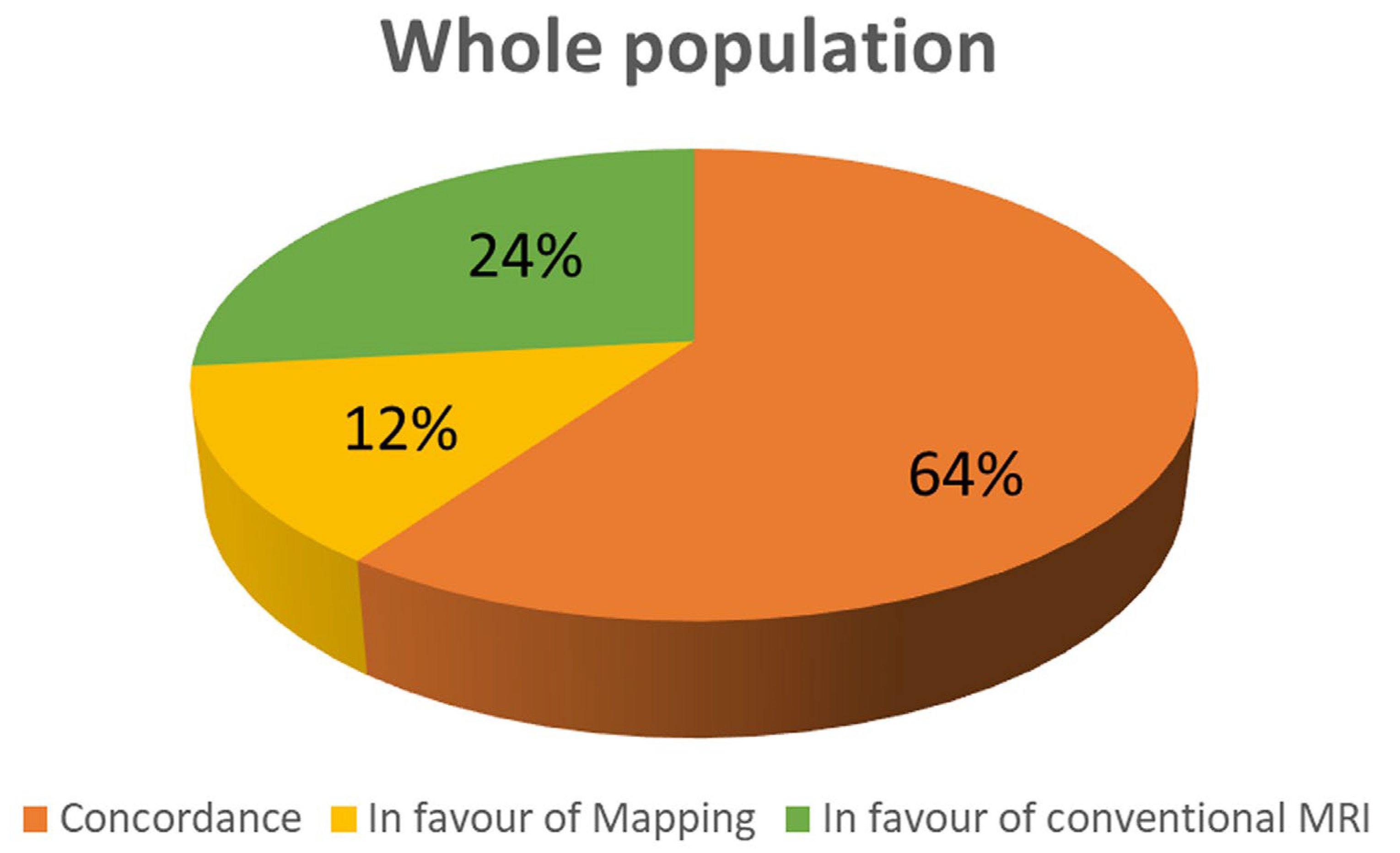
Figure 1.
Concordance between conventional technique and T1 mapping in the whole population.
Figure 1.
Concordance between conventional technique and T1 mapping in the whole population.
Conventional CMR techniques (LGE and/or T1-FSE and/or T2-STIR) were positive in 209 patients (71%), while native T1 was abnormal in 154(52%) of patients (p<0.0001). As evident in figure 1, mapping and conventional techniques we concordant in 208 patients (64%); in 76 patients (24%) conventional imaging was positive in presence of normal T1 values; in 39 patients T1 values were abnormal despite negative findings in conventional CMR (12%).
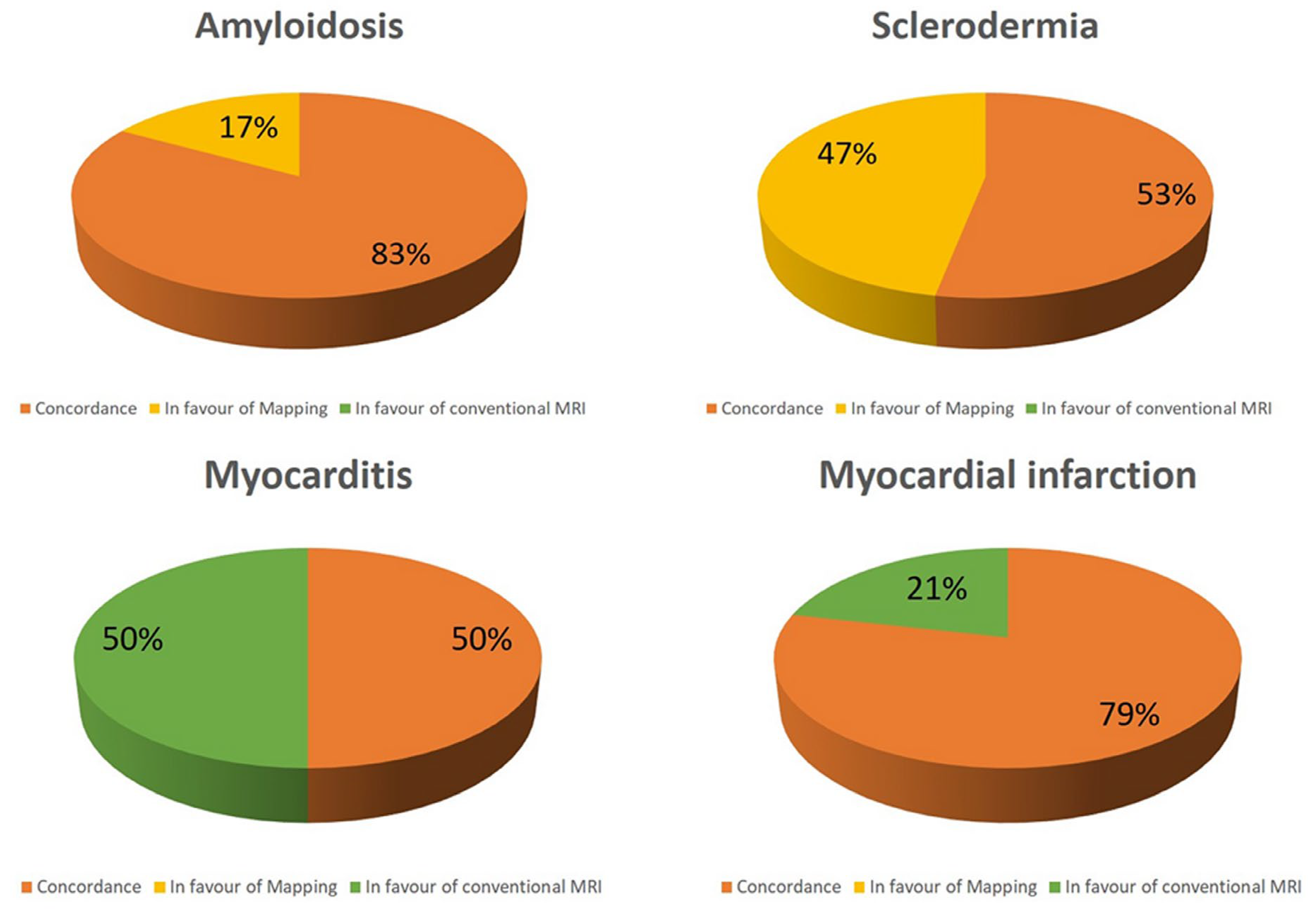
Interestingly, in the suspicion of myocarditis conventional techniques a concordance between conventional techniques and T1 mapping was found in 50% of patients, whereas all the remaining patients had positive conventional CMR but negative T1 mapping (figure 3). In patients with myocarditis conventional techniques were more frequently positive than T1 mapping (p<0.0001). Similar results were found in case of myocardial infarction, where a concordance was showed in 79%, whereas positive conventional techniques with negative mapping was in 21%. In both of these two conditions, mapping had no additive role over conventional techniques.
On the contrary, opposite results were found about amyloidosis and scleroderma where native T1 was abnormal in all the patients (figure 2). Particularly abnormal T1 was the only finding in respectively 17% and 47% of patients. T1 mapping was more frequently positive in scleroderma than conventional techniques (p<0.0001).
Figure 2.
Concordance between conventional technique and T1 mapping in different subgroups.
Figure 2.
Concordance between conventional technique and T1 mapping in different subgroups.
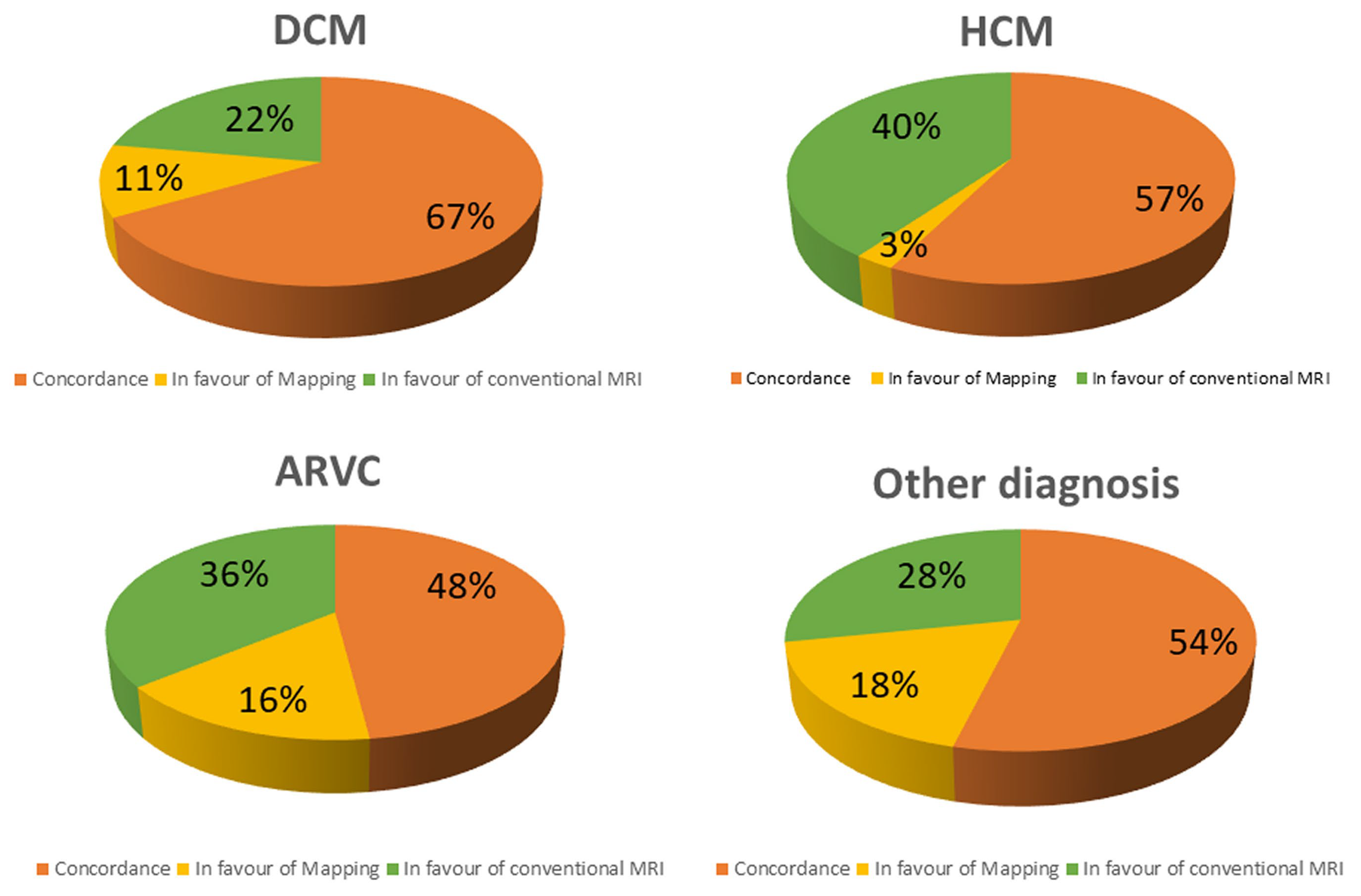
Figure 3.
Concordance between conventional technique and T1 mapping in different subgroups.
Figure 3.
Concordance between conventional technique and T1 mapping in different subgroups.
Abnormal T1 mapping was the only CMR abnormality in 11% of DCM, in 3% of HCM, 16% of ARC and in 18% of other conditions (figure 3). On contrast, native T1 was within the range of normality despite a positive LGE and/or T2-STIR in 22% of DCM, 40% of HCM, 36% of ARC and in 28% of other conditions.
In HCM, myocardial abnormalities were more frequently detected using conventional techniques than with T1 mapping (p =0.0006).
3.3. Diffuse vs regional myocardial damage
T1 mapping was more able to give additive information than conventional techniques in cardiac diseases with diffuse myocardial damage as scleroderma, amyloidosis and Fabry disease. In these conditions, T1 mapping was abnormal in 24 out of 24 patients, demonstrating an additive role over the conventional techniques in 10 patients (42%).
On contrast, T1 mapping was less effective in cardiac conditions with a regional\segmental distribution of myocardial damage as myocardial infarction, myocarditis and HCM. In these conditions T1 was abnormal in 52 out of 88 patients (58%) but only in one of them (1%) demonstrated and additive role. Conventional techniques had an additive role in 36 patients (41%).
Explanation of the low effectiveness of T1 mapping in regional myocardial damage could refer to the limited coverage of LV myocardium. Indeed, T1 mapping was acquired in 3 short axis slices, covering an average of 17±4% of LV mass.
Table 4.
CMR findings of conventional sequences and T1 mapping.
Table 4.
CMR findings of conventional sequences and T1 mapping.
| Indication |
T2-STIR+ |
LGE+ |
T1 Regional abnormality |
Mean T1 |
| DCM |
1 (1.6) |
44 (73.3) |
37 (60.7%) |
1050±44 |
| HCM |
2 (3.9) |
37 (74) |
19 (37.3%) |
1029±48 |
| ARVC |
2 (3.2) |
31 (50.8) |
19 (30.2%) |
1012±54 |
| Myocarditis |
27 (61.4) |
33 (75.0) |
25 (56.8%) |
1053±65 |
| Scleroderma |
4 (19.0) |
10 (50.0) |
18 (85.7%) |
1092±41 |
| Amyloidosis |
0 |
12 (70.6) |
11 (64.7%) |
1063±78 |
| Myocardial infarction (acute/chronic) |
3 (20.0) |
15 (100) |
11 (73.3%) |
1063±87 |
|
Dystrophy/Miasteny/Mitochondrial disease
|
0 (0.0) |
6 (66.7) |
6 (66.7%) |
1058±65 |
| Pericarditis-Pericardial effusion |
1 (16.7) |
1 (16.7) |
3 (50.0%) |
1105±102 |
| LV Non-compaction |
0 (0.0) |
4 (100) |
2 (50.0%) |
1059±76 |
| Systemic sarcoidosis |
1 (33.3) |
2 (66.7) |
2 (66.7%) |
1049±33 |
| Fabry |
0 |
1(100) |
1(100) |
827 |
| Pulmonary hypertension |
0 |
1 (100) |
0 (0.0%) |
1026 |
| Valvular disease |
0 |
1 (100) |
0 (0.0%) |
1018 |
| Healthy controls |
0 (0.0) |
9 (40.9) |
7 (25.9%) |
1034±29 |
Table 5.
CMR findings divided by the initial suspicion.
Table 5.
CMR findings divided by the initial suspicion.
| Initial suspicion |
Specific findingsN (%) |
Alternative diagnosisN (%) |
Non-specific findingsN (%) |
Negative CMRN (%) |
| DCM |
34 (55.7) |
7 (11.5) |
17 (27.9) |
3 (4.9) |
| HCM |
33 (64.7) |
2 (3.9) |
16 (31,4) |
0 |
| ARVC |
12 (19.0) |
11 (17.5) |
22 (34.9) |
18 (28.6) |
| Myocarditis |
22 (50) |
14 (31.8) |
6 (13.6) |
2 (4.6) |
| Scleroderma |
17 (80.9) |
0 |
3 (14.3) |
1 (4.8) |
| Amyloidosis |
5 (29.4) |
4 (23.5) |
6 (35.3) |
2 (11.8) |
| Myocardial infarction (acute/chronic) |
10 (66.7) |
1 (6.6) |
4 (26.7) |
0 |
| Dystrophy/Miasteny/ Mithocondrial disease |
8 (88.9) |
0 |
0 |
1 (11.1) |
| Pericarditis-Pericardial effusion |
4 (66.6) |
1 (16.7) |
1 (16,7) |
0 |
| LV Non-compaction |
1 (25) |
1 (25) |
2 (50) |
0 |
| Systemic sarcoidosis |
1 (33.3) |
0 |
1 (33.3) |
1 (33.3) |
| Fabry |
1(100) |
0 |
0 |
0 |
| Pulmonary hypertension |
1 (100) |
0 |
0 |
0 |
| Valvular disease |
1 (100) |
0 |
0 |
0 |
| Total |
149 (50.3) |
41 (13.8) |
78 (26.4) |
28 (9.5) |
4. Discussion
In the present study we evaluated the clinical impact of T1 mapping as additive imaging tool in a cohort of non-selected patients undergoing CMR. The main results may be summarised as follows: 1) Conventional CMR for tissue characterization (LGE, T1-FSE and T2-STIR) and T1 mapping are complementary techniques in most of the cardiac conditions: they provide concordant findings in 64%; 2) native T1 mapping has an additive diagnostic role over conventional techniques in a range of 6-12% of cases; 3) in 24% of cases, conventional CMR techniques detected myocardial abnormalities despite normal native T1; 4) the role of T1 mapping is different in cardiac diseases: we found that T1 mapping is superior than conventional approach in cardiac conditions as amyloidosis, Fabry and scleroderma, characterized by a diffuse myocardial involvement; 5) conventional techniques are superior to mapping for the evaluation of myocardial disease with segmental distribution as myocardial infarction, hypertrophic cardiomyopathy and myocarditis.
T1 mapping is a quantitative CMR technique allowing voxel-wise quantification of myocardial native T1 [
4]. Native T1 may be abnormal in different cardiac conditions based on the changes of myocardial content of water, proteins and fat [
5]. Water is characterized by the greatest T1 values because of a fast “tumbling” rate of small, rapidly rotating molecules [
1]. Then, myocardial T1 is mostly increased in presence of augment of free-water content as in myocardial edema. Myocardial edema may be found in myocarditis, acute/subacute myocardial infarction and in every case of recent myocardial damage [
5,
8,
9]. Indeed, it was described also in hypertrophic cardiomyopathy, in cocaine-induced myocardial damage, in scleroderma and in many other conditions [
1,
10].
Increased native T1 may also be found in myocardial fibrosis because of augmented water content of interstitial space enlarged by the collagen matrix of scar [
4].
Myocardial T1 is also severely increased in amyloid deposition [
5,
11], whereas it is decreased in presence of intramyocardial fat infiltration or in Fabry disease because of lysosomal sphingolipids accumulation [
12,
13].
By these premises, T1 mapping and conventional techniques, as LGE and T2-STIR, are overlapping in many cardiac conditions, as confirmed by the results of the present study where the concordance between these techniques is seen in 64% of patients. However, T1 mapping has some advantage over conventional techniques. Being a quantitative technique T1 mapping may allow identification of myocardial disease characterized by a diffuse and homogeneous damage, as evident in early stages of DCM, amyloidosis, Fabry disease and in scleroderma [
5,
11,
12,
13,
14,
15].
In the matter of fact, T1 mapping demonstrated and additive diagnostic role in 12% of patients who presented a negative LGE and T2-STIR but abnormal native T1.
We found that the value of T1 mapping is particularly relevant in some conditions as cardiac amyloidosis where it was positive in 17% more patients than LGE. In cardiac amyloidosis LGE has a very specific pattern, characterized by a diffuse subendocardial enhancement, an early darkening of signal of blood cavity and a null defect of myocardium. The specificity of this pattern is near to 100% but it may be absent in early stage of this disease [
11]. Amyloid deposit is associated with a great increase of myocardial native T1 also in early stages. Then, the presence of diffuse increase of native T1, summed to concentric hypertrophy and other morphological signs, as thickening of atrial septal walls and pericardial effusion, and associated to clinical presentation, may allow diagnosis of amyloidosis even in absence of the specific LGE pattern.
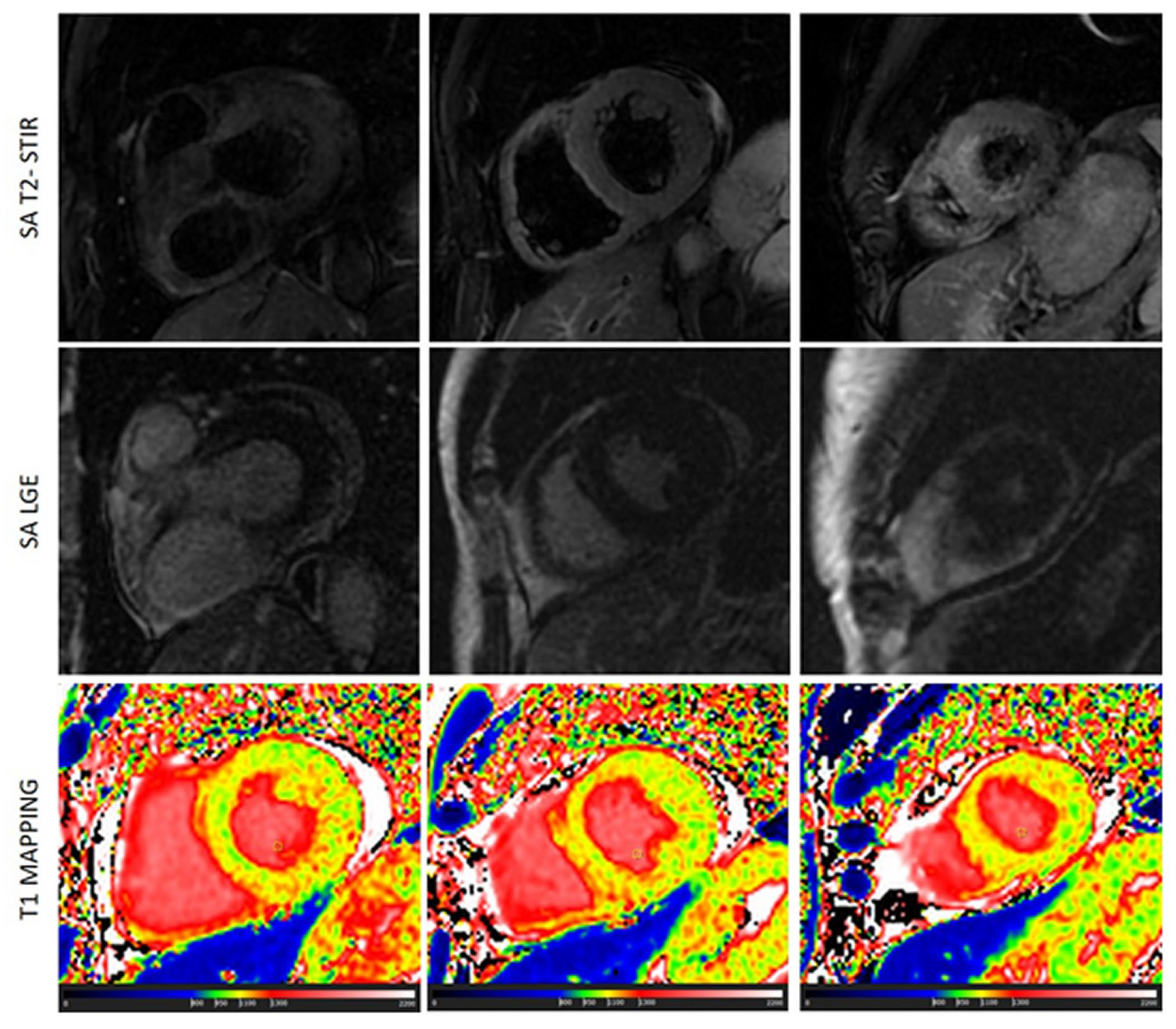
Scleroderma is usually associated with edema and microscopic fibrosis (figure 4). Both of them may present with a diffuse, non-regional, distribution. Microscopic fibrosis could be not detected by LGE [
5,
23]. The identification of diffuse edema, as well, is very challenging using conventional, qualitative, T2-STIR pulse sequence because of lack of comparison with “normal” myocardium. In this setting, native T1 and ECV evaluation could be very useful. Native T1 was abnormal in all the patients with these conditions and an additive role of T1 mapping over conventional techniques was found in 47% of cases of scleroderma. T1 was found positive in a significant higher percentage of cases of scleroderma than LGE\T2-STIR.
Figure 4.
a case of scleroderma with negative LGE and T2-STIR image but with a diffuse increase of myocardial native T1 (normal range of T1 in green).
Figure 4.
a case of scleroderma with negative LGE and T2-STIR image but with a diffuse increase of myocardial native T1 (normal range of T1 in green).
T1 mapping was also instrumental for the diagnosis of the case of Fabry disease where conventional technique where completely normal (figure 5), with only a mild concentric hypertrophy but areas of low T1 were detected. In this case, alpha-galactosidase test confirmed the diagnosis [
18,
19,
20].
Figure 5.
a case of Fabry disease with mild concentric hypertrophy and low T1 (blue region) with negative conventional techniques.
Figure 5.
a case of Fabry disease with mild concentric hypertrophy and low T1 (blue region) with negative conventional techniques.
An advantage of T1 mapping is the absence of contrast injection that is required for LGE technique. The nephrogenic systemic sclerosis, a rare complication of gadolinium-based contrast agents, is associated with severe kidney disease. Contrast injection in patients a severe reduction of glomerular filtration rate is potentially dangerous. The lack of LGE image make CMR less effective in rule-out cardiac disease and this is particularly relevant is subject with ventricular arrhythmias (as frequent PVC) with normal cardiac structure and function. In such conditions, the identification of myocardial fibrosis is very important because the prognosis depends on the presence of a structural myocardial disease [
5,
16]. T1 mapping may help to rule-out structural disease when LGE is not acquired because of renal condition or in case of refuse of injection by the patients.
On contrast, T1 mapping resulted less effective to identify abnormalities in conditions as myocarditis, small myocardial infarction or in case of non-ischemic fibrosis. Indeed, abnormal native T1 was found in 50% of patients with myocarditis, whereas LGE and/or T2-STIR were positive in all of these patients. Similarly, T1 native was abnormal 71% of acute\chronic myocardial infarction, while LGE was positive in all of these patients and T2-STIR in all of the patients with acute myocardial infarction.
A possible explanation of these findings may be found in some technical aspects of T1 mapping acquisition. T1 mapping images usually don’t cover the entire left ventricle but the SCMR position paper [
8] suggest to acquire only 3 short axis views. In the present study we calculated the % of LV mass covered by these 3 short axis slices of T1 mapping and found an average coverage of 17±4% which is a big limitation of this technique particularly in cardiac conditions characterized by regional or focal myocardial damage. For instance, myocardial infarction has obviously a regional distribution which is confined to the vascularization territory of the culprit coronary artery [
9]. Also, myocarditis could affect the whole myocardium, but the signs of myocardial damage may be focal or regionally distributed [
8]. Thus, the 3-slices approach of T1 mapping may be inaccurate and less effective than other technique for which a complete coverage of LV is usually performed.
Recent modified Lake Louise criteria [
24], included T1 and T2 mapping as diagnostic criteria and changed the original criteria by using a 2-out-of-2 approach: to diagnose myocarditis a “T2-based criterion”, as edema at T2-STIR or increased native T2, should be summed to a “T1-based criterion”, as LGE or ECV or increased native T1.
However, results of our study demonstrated that T1 mapping with the 3-short axis approach was not able to detect signal abnormalities associated with myocarditis because in most of cases the signs of myocardial damage were focal. Then, the effectiveness of the new Lake Louise criteria should be assessed by further larger population study.
The regional distribution of LGE and T2-STIR is also seen in HCM where hypertrophy is usually asymmetric and LGE and edema are mostly located in hypertrophic segments [
10]. Then, it is not surprising that we found a significant difference in the prevalence of positive LGE and/or T2-STIR and abnormal T1 in HCM.
The main results of the present study were that T1 mapping had and additive diagnostic role in only 12% of patients and was ineffective in 24% of them. To interpret these results, other limitations of T1 mapping should be considered. Indeed, T1 mapping technique is far from being standardized because it is well known that difference of magnetic field shimming, of pulse sequence and parameters, of patient’s heart rate and of the algorithm of the post-processing software could modify the result of this technique.
Our results suggest that a complete CMR protocol cannot exempt anymore from T1 mapping technique. However, T1 mapping cannot substitute conventional approach based on LGE and T2-STIR techniques. T1 mapping could be used as substitute LGE only in presence of contraindication for contrast media.
4.1. Limitations
T1 mapping was acquired using only 3 short axis slice and, as discussed above, this approach intrinsically limited the effectiveness of T1 mapping to identify focal and/or regional myocardial tissue abnormalities. However, this was method indicated by the most recent SCMR consensus document [
8].
We did not include T2- T2*- and ECV-mapping in the present study. T2-mapping was not available in our laboratory at the time we started the enrolment and in order to have homogeneous population we decided not to include T2 mapping data in the present study. T2* mapping was used in selected indications as in cardiac hemochromatosis or in the suspicion of hemorrhagic infarction, and we follow these indications and not acquired T2* in all the patients. Finally, ECV mapping required hematocrit obtained the day of CMR, we had this in only a minority of patients (60 patients) and we preferred not to include that data.
Finally, cardiac tumors and congenital heart disease, that are frequent indications for CMR, were not included in our population. However, we aimed to evaluate the effectiveness of T1 mapping in different cardiac diseases based on previous evidences. On contrast, the role of T1 mapping for the evaluation of cardiac tumors and congenital disease is still under evaluation.