Submitted:
06 June 2023
Posted:
06 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Etiology and Pathogenesis of EC in Fertile Women: Clinical and Endocrinological Characteristics of EC
2.1. Challenges Arising from Fertility-Sparing Approaches in EC Patients
3. Discussion
3.1. Role of Circulating miRNA in EC: miR, ceRNET and Cancer Biology
3.2. Using Circulating miR for EC Diagnosis
3.3. Profiling the miR Transcriptome for the Evaluation of Endometrial Receptivity
3.4. RNA-Based Diagnostics and Therapeutics: Are Innovations Set to Outpace Bioethics Precepts?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schenker, J.G. Ethical Dilemmas in Assisted Reproductive Technologies, Berlin, Boston: De Gruyter, 2011. [CrossRef]
- Knez, J.; Al Mahdawi, L.; Takač, I.; Sobočan, M. The Perspectives of Fertility Preservation in Women with Endometrial Cancer. Cancers 2021, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Odicino, F.; Maisonneuve, P.; Beller, U.; Benedet, J.L.; Heintz, A.P.; Ngan, H.Y.; Pecorelli, S. Carcinoma of the Corpus Uteri. Int J Gynaecol Obstet 2003, 83 (Suppl 1), 79–118. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial Cancer. Nat Rev Dis Primers 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N Engl J Med 2020, 383, 2053–2064. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Sidorkiewicz, I.; Jóźwik, M.; Niemira, M.; Krętowski, A. Insulin Resistance and Endometrial Cancer: Emerging Role for microRNA. Cancers (Basel) 2020, 12, 2559. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial Cancer. The Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J Clin 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.-B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.M.; et al. Type I and II Endometrial Cancers: Have They Different Risk Factors? J Clin Oncol 2013, 31, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis: Use of FIGO Grading and Genomic Subcategories in Clinical Practice: Recommendations of the International Society of Gynecological Pathologists. Int J Gynecol Pathol 2019, 38 (Suppl 1), S64–S74. [Google Scholar] [CrossRef] [PubMed]
- Andreano, A.; Rechichi, G.; Rebora, P.; Sironi, S.; Valsecchi, M.G.; Galimberti, S. MR Diffusion Imaging for Preoperative Staging of Myometrial Invasion in Patients with Endometrial Cancer: A Systematic Review and Meta-Analysis. Eur Radiol 2014, 24, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Beddy, P.; Moyle, P.; Kataoka, M.; Yamamoto, A.K.; Joubert, I.; Lomas, D.; Crawford, R.; Sala, E. Evaluation of Depth of Myometrial Invasion and Overall Staging in Endometrial Cancer: Comparison of Diffusion-Weighted and Dynamic Contrast-Enhanced MR Imaging. Radiology 2012, 262, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Gullo, G.; Cucinella, G.; Chiantera, V.; Dellino, M.; Cascardi, E.; Török, P.; Herman, T.; Garzon, S.; Uccella, S.; Laganà, A.S. Fertility-Sparing Strategies for Early-Stage Endometrial Cancer: Stepping towards Precision Medicine Based on the Molecular Fingerprint. Int J Mol Sci 2023, 24, 811. [Google Scholar] [CrossRef]
- Merickel, C.R.; Dennison, E.; Moghadamfalahi, M. The Significance of Lower Uterine Segment Involvement in the Surgical Management of Endometrial Carcinoma. Am J Clin Pathol 2012, 138, A155–A155. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Lobo, R.A. Cancer Risk and PCOS. Steroids 2013, 78, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Tanos, P.; Dimitriou, S.; Gullo, G.; Tanos, V. Biomolecular and Genetic Prognostic Factors That Can Facilitate Fertility-Sparing Treatment (FST) Decision Making in Early Stage Endometrial Cancer (ES-EC): A Systematic Review. Int J Mol Sci 2022, 23, 2653. [Google Scholar] [CrossRef]
- Mahdy, H.; Casey, M.J.; Crotzer, D. Endometrial Cancer. [Updated 2022 Sep 26]. In StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer, Ed.; National Cancer Institute: Bethesda, MD, USA, 2021. [Google Scholar]
- Mutlu, L.; Manavella, D.D.; Gullo, G.; McNamara, B.; Santin, A.D.; Patrizio, P. Endometrial Cancer in Reproductive Age: Fertility-Sparing Approach and Reproductive Outcomes. Cancers 2022, 14, 5187. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Cafasso, V.; Boccia, D.; Ascione, M.; Mercorio, A.; Viciglione, F.; Palumbo, M.; Serafino, P.; Buonfantino, C.; De Angelis, M.C.; et al. Fertility-Sparing Approach in Patients with Endometrioid Endometrial Cancer Grade 2 Stage IA (FIGO): A Qualitative Systematic Review. Biomed Res Int 2022, 2022, 4070368. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, W.; Feng, L.; Gao, W. Comparison of Fertility-Sparing Treatments in Patients with Early Endometrial Cancer and Atypical Complex Hyperplasia: A Meta-Analysis and Systematic Review. Medicine (Baltimore) 2017, 96, e8034. [Google Scholar] [CrossRef]
- Obermair, A.; Baxter, E.; Brennan, D.J.; McAlpine, J.N.; Muellerer, J.J.; Amant, F.; Van Gent, M.D.J.M.; Coleman, R.L.; Westin, S.N.; Yates, M.S.; et al. Fertility-Sparing Treatment in Early Endometrial Cancer: Current State and Future Strategies. Obstet Gynecol Sci 2020, 63, 417–431. [Google Scholar] [CrossRef]
- Gonthier, C.; Douhnai, D.; Koskas, M. Lymph Node Metastasis Probability in Young Patients Eligible for Conservative Management of Endometrial Cancer. Gynecol Oncol 2020, 157, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Rodolakis, A.; Scambia, G.; Planchamp, F.; Acien, M.; Di Spiezio Sardo, A.; Farrugia, M.; Grynberg, M.; Pakiž, M.; Pavlakis, K.; Vermeulen, N.; et al. ESGO/ESHRE/ESGE Guidelines for the Fertility-Sparing Treatment of Patients with Endometrial Carcinoma. Facts Views Vis ObGyn 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.-X. Fertility-Preserving Treatment in Women with Early Endometrial Cancer: The Chinese Experience. Cancer Manag Res 2018, 10, 6803–6813. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yu, Z.; Yang, J.; Cao, D.; Yu, M.; Wang, Y.; Shen, K. Oral Progestin Treatment for Early-Stage Endometrial Cancer: A Systematic Review and Meta-Analysis. Int J Gynecol Cancer 2016, 26, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Falcone, F.; Laurelli, G.; Losito, S.; Di Napoli, M.; Granata, V.; Greggi, S. Fertility Preserving Treatment with Hysteroscopic Resection Followed by Progestin Therapy in Young Women with Early Endometrial Cancer. J Gynecol Oncol 2017, 28, e2. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Obermair, A.; Gebski, V.; Janda, M. Efficacy of Oral or Intrauterine Device-Delivered Progestin in Patients with Complex Endometrial Hyperplasia with Atypia or Early Endometrial Adenocarcinoma: A Meta-Analysis and Systematic Review of the Literature. Gynecologic Oncology 2012, 125, 263–270. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up. Ann Oncol 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Gallos, I.D.; Yap, J.; Rajkhowa, M.; Luesley, D.M.; Coomarasamy, A.; Gupta, J.K. Regression, Relapse, and Live Birth Rates with Fertility-Sparing Therapy for Endometrial Cancer and Atypical Complex Endometrial Hyperplasia: A Systematic Review and Metaanalysis. Am J Obstet Gynecol 2012, 207, 266.e1–266.e12. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Chiantera, V.; Perino, A.; Greco, M.E.; Laganà, A.S.; Marinelli, E.; Basile, G.; Zaami, S. Neonatal Outcomes and Long-Term Follow-Up of Children Born from Frozen Embryo, a Narrative Review of Latest Research Findings. Medicina (Kaunas) 2022, 58, 1218. [Google Scholar] [CrossRef]
- Gullo, G.; Perino, A.; Cucinella, G. Open vs. Closed Vitrification System: Which One Is Safer? Eur Rev Med Pharmacol Sci 2022, 26, 1065–1067. [Google Scholar] [CrossRef]
- Zaami, S.; Stark, M.; Signore, F.; Gullo, G.; Marinelli, E. Fertility Preservation in Female Cancer Sufferers: (Only) a Moral Obligation? The European Journal of Contraception & Reproductive Health Care 2022, 27, 335–340. [Google Scholar] [CrossRef]
- Fertility Preservation and Reproduction in Patients Facing Gonadotoxic Therapies: An Ethics Committee Opinion. Fertil Steril 2018, 110, 380–386. [CrossRef] [PubMed]
- Robertson, J.A. Cancer and Fertility: Ethical and Legal Challenges. Journal of the National Cancer Institute Monographs 2005, 2005, 104–106. [Google Scholar] [CrossRef]
- Riolo, G.; Cantara, S.; Marzocchi, C.; Ricci, C. MiRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc 2021, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The Intricate Balance between MicroRNA-Induced MRNA Decay and Translational Repression. FEBS J 2022. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res 2009, 19, 92. [Google Scholar] [CrossRef]
- Shu, J.; Resende E Silva, B.V.; Gao, T.; Xu, Z.; Cui, J. Dynamic and Modularized MicroRNA Regulation and Its Implication in Human Cancers. Scientific Reports 2017 7:1 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Kumar, S.; Gonzalez, E.A.; Rameshwar, P.; Etchegaray, J.-P. Non-Coding RNAs as Mediators of Epigenetic Changes in Malignancies. Cancers (Basel) 2020, 12, 3657. [Google Scholar] [CrossRef]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; Piergentili, R.; Mattei, A.; Vizzielli, G.; Scambia, G.; Straface, G.; Restaino, S.; Signore, F. Towards Personalized Medicine: Non-Coding Rnas and Endometrial Cancer. Healthcare (Switzerland) 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Zaami, S.; Cavaliere, A.F.; Signore, F.; Scambia, G.; Mattei, A.; Marinelli, E.; Gulia, C.; Perelli, F. Non-Coding Rnas as Prognostic Markers for Endometrial Cancer. Int J Mol Sci 2021, 22, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Klicka, K.; Grzywa, T.M.; Klinke, A.; Mielniczuk, A.; Włodarski, P.K. The Role of MiRNAs in the Regulation of Endometrial Cancer Invasiveness and Metastasis—A Systematic Review. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Donkers, H.; Bekkers, R.; Galaal, K.; Donkers, H.; Bekkers, R.; Galaal, K. Diagnostic Value of MicroRNA Panel in Endometrial Cancer: A Systematic Review. Oncotarget 2020, 11, 2010–2023. [Google Scholar] [CrossRef]
- Sayed, S.R. El; Cristante, J.; Guyon, L.; Denis, J.; Chabre, O.; Cherradi, N. MicroRNA Therapeutics in Cancer: Current Advances and Challenges. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Piergentili, R.; Basile, G.; Nocella, C.; Carnevale, R.; Marinelli, E.; Patrone, R.; Zaami, S. Using NcRNAs as Tools in Cancer Diagnosis and Treatment—The Way towards Personalized Medicine to Improve Patients’ Health. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A CeRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Karreth, F.A.; Pandolfi, P.P. CeRNA Cross-Talk in Cancer: When Ce-Bling Rivalries Go Awry. Cancer Discov 2013, 3, 1113–1121. [Google Scholar] [CrossRef]
- Moreno-García, L.; López-Royo, T.; Calvo, A.C.; Toivonen, J.M.; de la Torre, M.; Moreno-Martínez, L.; Molina, N.; Aparicio, P.; Zaragoza, P.; Manzano, R.; et al. Competing Endogenous Rna Networks as Biomarkers in Neurodegenerative Diseases. Int J Mol Sci 2020, 21, 1–42. [Google Scholar] [CrossRef]
- Nuzziello, N.; Liguori, M. The MicroRNA Centrism in the Orchestration of Neuroinflammation in Neurodegenerative Diseases. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Mao, Q.; Chen, B.; Wang, L.; Ma, W.; Liang, Y.; Zhang, T.; Dong, G.; Xu, L.; Jiang, F. The TWIST1-Centered Competing Endogenous RNA Network Promotes Proliferation, Invasion, and Migration of Lung Adenocarcinoma. Oncogenesis 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, S.; Xiong, J.; Long, J.; Zheng, Y.; Sang, X. CeRNA Regulatory Network-Based Analysis to Study the Roles of Noncoding RNAs in the Pathogenesis of Intrahepatic Cholangiocellular Carcinoma. Aging (Albany NY) 2020, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, J. Bin; Deng, J.; Zou, D.Z.; Wu, J.J.; Cao, Y.H.; Yin, J.; Ma, Y.S.; Da, F.; Li, W. The Role of CeRNA-Mediated Diagnosis and Therapy in Hepatocellular Carcinoma. Hereditas 2021, 158. [Google Scholar] [CrossRef]
- Cen, L.; Liu, R.; Liu, W.; Li, Q.; Cui, H. Competing Endogenous RNA Networks in Glioma. Front Genet 2021, 12. [Google Scholar] [CrossRef]
- Morovat, P.; Morovat, S.; Hosseinpour, M.; Moslabeh, F.G.Z.; Kamali, M.J.; Samadani, A.A. Survival-Based Bioinformatics Analysis to Identify Hub Long Non-Coding RNAs along with LncRNA-MiRNA-MRNA Network for Potential Diagnosis/Prognosis of Thyroid Cancer. J Cell Commun Signal 2022. [Google Scholar] [CrossRef]
- Yang, S.; Wang, X.; Zhou, X.; Hou, L.; Wu, J.; Zhang, W.; Li, H.; Gao, C.; Sun, C. NcRNA-Mediated CeRNA Regulatory Network: Transcriptomic Insights into Breast Cancer Progression and Treatment Strategies. Biomed Pharmacother 2023, 162. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, R.; Daraei, A.; Mansoori, Y.; Sepahvand, M.; Amoli, M.M.; Tavakkoly-Bazzaz, J. Competing Endogenous RNA (CeRNA) Cross Talk and Language in CeRNA Regulatory Networks: A New Look at Hallmarks of Breast Cancer. J Cell Physiol 2019, 234, 10080–10100. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, C.; Di Palo, A.; Russo, A.; Potenza, N. The LncRNAs at X Chromosome Inactivation Center: Not Just a Matter of Sex Dosage Compensation. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Shetty, A.; Venkatesh, T.; Kabbekodu, S.P.; Tsutsumi, R.; Suresh, P.S. LncRNA-MiRNA-MRNA Regulatory Axes in Endometrial Cancer: A Comprehensive Overview. Arch Gynecol Obstet 2022, 306, 1431–1447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ren, C.; Yao, Y.; Wang, Q.; Li, F.; Li, Y.; Jiang, A.; Wang, G. Identifying Prognostic Biomarkers in Endometrial Carcinoma Based on CeRNA Network. J Cell Biochem 2020, 121, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Cui, J.; Wang, Z.; Wu, H. Comprehensive Bioinformatic Analyses of LncRNA-Mediated CeRNA Network for Uterine Corpus Endometrial Carcinoma. Transl Cancer Res 2022, 11, 1994–2012. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chu, P.; Li, P.; Li, F. Construction of Endometrial Carcinoma CeRNA Network and Screening of Key Genes Based on TCGA Database. Comput Math Methods Med 2022, 2022. [Google Scholar] [CrossRef]
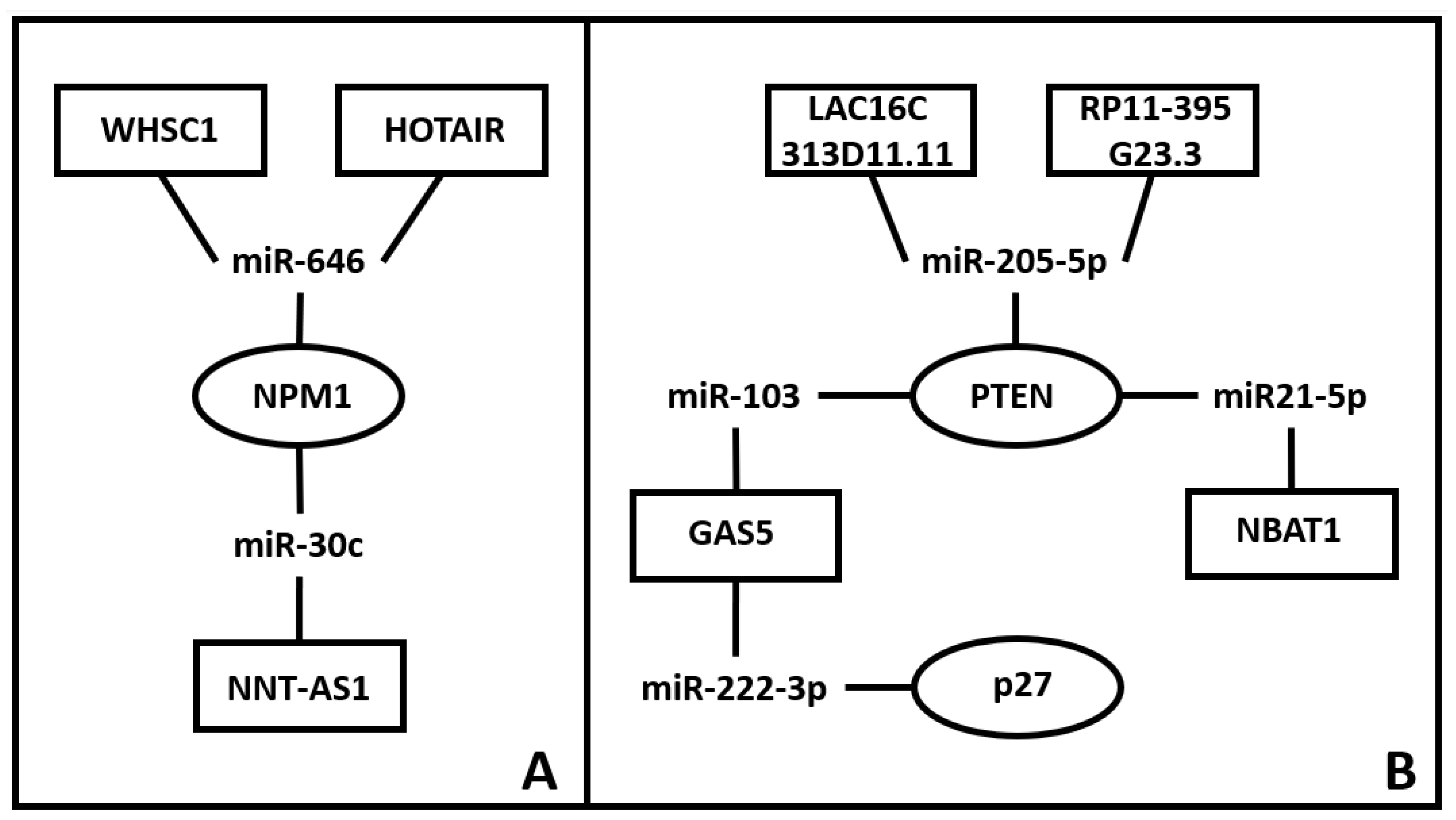
- Guo, C.; Song, W.Q.; Sun, P.; Jin, L.; Dai, H.Y. LncRNA-GAS5 Induces PTEN Expression through Inhibiting MiR-103 in Endometrial Cancer Cells. J Biomed Sci 2015, 22. [Google Scholar] [CrossRef]
- Xin, W.; Gao, X.; Zhao, S.; Zhao, P.; Yu, H.; Wu, Q.; Hua, K. LncRNA RP11-395G23.3 Suppresses the Endometrial Cancer Progression via Regulating MicroRNA-205-5p/PTEN Axis. Am J Transl Res 2020, 12, 4422. [Google Scholar]
- Xin, W.; Zhao, S.; Han, X.; Zhao, P.; Yu, H.; Gao, X.; Li, P.; Wu, Q.; Ding, J.; Hua, K. LncRNA LA16c-313D11.11 Modulates the Development of Endometrial Cancer by Binding to and Inhibiting MicroRNA-205-5p Function and Indirectly Increasing PTEN Activity. Int J Oncol 2020, 57, 355–363. [Google Scholar] [CrossRef]
- Tian, C.; Su, J.; Ma, Z.; Wu, Y.; Ma, H. LncRNA NBAT1 Inhibits Cell Metastasis and Promotes Apoptosis in Endometrial Cancer by Sponging MiR-21-5p to Regulate PTEN. Comput Math Methods Med 2022, 2022. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Z.; Meng, X.; Zhou, S.; Xiao, S.; Li, X.; Liu, S.; Yu, P. Long Noncoding RNA GAS5 Impairs the Proliferation and Invasion of Endometrial Carcinoma Induced by High Glucose via Targeting MiR-222-3p/P27. Am J Transl Res 2019, 11, 2413. [Google Scholar]
- Shen, J.; Feng, X.; Wang, H.; Wang, Y.; Zhou, Y. Long Non-Coding RNA NNT-AS1 Positively Regulates NPM1 Expression to Affect the Proliferation of Estrogen-Mediated Endometrial Carcinoma by Interacting. J Cancer 2022, 13, 112–123. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Wang, C.; Mao, L.W.; Wang, Y.L.; Xia, L.Q.; Zhao, W.; Shen, J.; Chen, J. Long Noncoding RNA HOTAIR Mediates the Estrogen-Induced Metastasis of Endometrial Cancer Cells via the MiR-646/NPM1 Axis. Am J Physiol Cell Physiol 2018, 314, C690–C701. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zong, Z.H.; Guan, X.; Zhao, Y. CircRNA WHSC1 Targets the MiR-646/NPM1 Pathway to Promote the Development of Endometrial Cancer. J Cell Mol Med 2020, 24, 6898–6907. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A Clinically Applicable Molecular-Based Classification for Endometrial Cancers. Br J Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Paleari, L.; Pesce, S.; Rutigliani, M.; Greppi, M.; Obino, V.; Gorlero, F.; Vellone, V.G.; Marcenaro, E. New Insights into Endometrial Cancer. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Njoku, K.; Barr, C.E.; Crosbie, E.J. Current and Emerging Prognostic Biomarkers in Endometrial Cancer. Front Oncol 2022, 12. [Google Scholar] [CrossRef]
- Ravegnini, G.; Gorini, F.; De Crescenzo, E.; De Leo, A.; De Biase, D.; Di Stanislao, M.; Hrelia, P.; Angelini, S.; De Iaco, P.; Perrone, A.M. Can MiRNAs Be Useful Biomarkers in Improving Prognostic Stratification in Endometrial Cancer Patients? An Update Review. Int J Cancer 2022, 150, 1077. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. International Journal of Gynecologic Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Aimagambetova, G.; Terzic, S.; Laganà, A.S.; Bapayeva, G.; la Fleur, P.; Terzic, M. Contemporary Fertility-Sparing Management Options of Early Stage Endometrioid Endometrial Cancer in Young Nulliparous Patients. J Clin Med 2022, 11, 196. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne) 2018, 9. [Google Scholar] [CrossRef]
- Javidi, M.A.; Ahmadi, A.H.; Bakhshinejad, B.; Nouraee, N.; Babashah, S.; Sadeghizadeh, M. Cell-Free MicroRNAs as Cancer Biomarkers: The Odyssey of MiRNAs through Body Fluids. Medical Oncology 2014, 31, 1–11. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc Natl Acad Sci U S A 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Delangle, R.; De Foucher, T.; Larsen, A.K.; Sabbah, M.; Azaïs, H.; Bendifallah, S.; Daraï, E.; Ballester, M.; Mehats, C.; Uzan, C.; et al. The Use of MicroRNAs in the Management of Endometrial Cancer: A Meta-Analysis. Cancers (Basel) 2019, 11. [Google Scholar] [CrossRef]
- Bloomfield, J.; Sabbah, M.; Castela, M.; Mehats, C.; Uzan, C.; Canlorbe, G. Clinical Value and Molecular Function of Circulating MicroRNAs in Endometrial Cancer Regulation: A Systematic Review. Cells 2022, 11. [Google Scholar] [CrossRef]
- Gao, J.; Fan, Y.Z.; Gao, S.S.; Zhang, W.T. Circulating MicroRNAs as Potential Biomarkers for the Diagnosis of Endometrial Cancer: A Meta-Analysis. Reproductive Sciences 2023, 30, 464–472. [Google Scholar] [CrossRef]
- Eismann, J.; Hirschfeld, M.; Erbes, T.; Rücker, G.; Jäger, M.; Ritter, A.; Weiss, D.; Gitsch, G.; Mayer, S. Hypoxia-and Acidosis-Driven Aberrations of Secreted MicroRNAs in Endometrial Cancer in Vitro. Oncol Rep 2017, 38, 993–1004. [Google Scholar] [CrossRef]
- Altmäe, S.; Koel, M.; Võsa, U.; Adler, P.; Suhorutšenko, M.; Laisk-Podar, T.; Kukushkina, V.; Saare, M.; Velthut-Meikas, A.; Krjutškov, K.; et al. Meta-Signature of Human Endometrial Receptivity: A Meta-Analysis and Validation Study of Transcriptomic Biomarkers. Sci Rep 2017, 7. [Google Scholar] [CrossRef]
- Kolde, R.; Laur, S.; Adler, P.; Vilo, J. Robust Rank Aggregation for Gene List Integration and Meta-Analysis. Bioinformatics 2012, 28, 573–580. [Google Scholar] [CrossRef]
- Wu, W.; Lin, Z.; Zhuang, Z.; Liang, X. Expression Profile of Mammalian MicroRNAs in Endometrioid Adenocarcinoma. Eur J Cancer Prev 2009, 18, 50–55. [Google Scholar] [CrossRef]
- Chung, T.K.H.; Cheung, T.H.; Huen, N.Y.; Wong, K.W.Y.; Lo, K.W.K.; Yim, S.F.; Siu, N.S.S.; Wong, Y.M.; Tsang, P.T.; Pang, M.W.; et al. Dysregulated MicroRNAs and Their Predicted Targets Associated with Endometrioid Endometrial Adenocarcinoma in Hong Kong Women. Int J Cancer 2009, 124, 1358–1365. [Google Scholar] [CrossRef]
- Boren, T.; Xiong, Y.; Hakam, A.; Wenham, R.; Apte, S.; Wei, Z.Z.; Kamath, S.; Chen, D.T.; Dressman, H.; Lancaster, J.M. MicroRNAs and Their Target Messenger RNAs Associated with Endometrial Carcinogenesis. Gynecol Oncol 2008, 110, 206–215. [Google Scholar] [CrossRef]
- Drissennek, L.; Baron, C.; Brouillet, S.; Entezami, F.; Hamamah, S.; Haouzi, D. Endometrial MiRNome Profile According to the Receptivity Status and Implantation Failure. Hum Fertil (Camb) 2022, 25, 356–368. [Google Scholar] [CrossRef]
- Riyanti, A.; Febri, R.R.; Zakirah, S.C.; Harzif, A.K.; Rajuddin, R.; Muharam, R.; Asmarinah, A.; Wiweko, B. Suppressing HOXA-10 Gene Expression by MicroRNA 135b During the Window of Implantation in Infertile Women. J Reprod Infertil 2020, 21, 217. [Google Scholar]
- Li, Q.; Liu, W.; Chiu, P.C.N.; Yeung, W.S.B. Mir-Let-7a/g Enhances Uterine Receptivity via Suppressing Wnt/β-Catenin Under the Modulation of Ovarian Hormones. Reproductive Sciences 2020, 27, 1164–1174. [Google Scholar] [CrossRef]
- Yan, Q.; Yan, G.; Zhang, C.; Wang, Z.; Huang, C.; Wang, J.; Zhou, J.; Liu, Y.; Ding, L.; Zhang, Q.; et al. MiR-21 Reverses Impaired Decidualization through Modulation of KLF12 and NR4A1 Expression in Human Endometrial Stromal Cells. Biol Reprod 2019, 100, 1395–1405. [Google Scholar] [CrossRef]
- Ma, H.L.; Gong, F.; Tang, Y.; Li, X.; Li, X.; Yang, X.; Lu, G. Inhibition of Endometrial Tiam1/Rac1 Signals Induced by MiR-22 up-Regulation Leads to the Failure of Embryo Implantation during the Implantation Window in Pregnant Mice. Biol Reprod 2015, 92, 152–153. [Google Scholar] [CrossRef]
- Revel, A.; Achache, H.; Stevens, J.; Smith, Y.; Reich, R. MicroRNAs Are Associated with Human Embryo Implantation Defects. Human Reproduction 2011, 26, 2830–2840. [Google Scholar] [CrossRef]
- Moreno-Moya, J.M.; Vilella, F.; Martínez, S.; Pellicer, A.; Simón, C. The Transcriptomic and Proteomic Effects of Ectopic Overexpression of MiR-30d in Human Endometrial Epithelial Cells. Mol Hum Reprod 2014, 20, 550–566. [Google Scholar] [CrossRef]
- Altmäe, S.; Martinez-Conejero, J.A.; Esteban, F.J.; Ruiz-Alonso, M.; Stavreus-Evers, A.; Horcajadas, J.A.; Salumets, A. MicroRNAs MiR-30b, MiR-30d, and MiR-494 Regulate Human Endometrial Receptivity. Reprod Sci 2013, 20, 308–317. [Google Scholar] [CrossRef]
- Zhang, Q.; Ni, T.; Dang, Y.; Ding, L.; Jiang, J.; Li, J.; Xia, M.; Yu, N.; Ma, J.; Yan, J.; et al. MiR-148a-3p May Contribute to Flawed Decidualization in Recurrent Implantation Failure by Modulating HOXC8. J Assist Reprod Genet 2020, 37, 2535–2544. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Jiang, Y.; Xue, B.; Diao, Z.; Ding, L.; Zhen, X.; Sun, H.; Yan, G.; Hu, Y. MicroRNA-181a Is Involved in the Regulation of Human Endometrial Stromal Cell Decidualization by Inhibiting Krüppel-like Factor 12. Reproductive Biology and Endocrinology 2015, 13, 1–9. [Google Scholar] [CrossRef]
- Graham, A.; Holbert, J.; Nothnick, W.B. MiR-181b-5p Modulates Cell Migratory Proteins, Tissue Inhibitor of Metalloproteinase 3, and Annexin A2 during in Vitro Decidualization in a Human Endometrial Stromal Cell Line. Reproductive Sciences 2017, 24, 1264–1274. [Google Scholar] [CrossRef]
- Pei, T.; Liu, C.; Liu, T.; Xiao, L.; Luo, B.; Tan, J.; Li, X.; Zhou, G.; Duan, C.; Huang, W. MiR-194-3p Represses the Progesterone Receptor and Decidualization in Eutopic Endometrium From Women With Endometriosis. Endocrinology 2018, 159, 2554–2562. [Google Scholar] [CrossRef]
- Jimenez, P.T.; Mainigi, M.A.; Word, R.A.; Kraus, W. Le; Mendelson, C.R. MiR-200 Regulates Endometrial Development During Early Pregnancy. Molecular Endocrinology 2016, 30, 977–987. [Google Scholar] [CrossRef]
- Haraguchi, H.; Saito-Fujita, T.; Hirota, Y.; Egashira, M.; Matsumoto, L.; Matsuo, M.; Hiraoka, T.; Koga, K.; Yamauchi, N.; Fukayama, M.; et al. MicroRNA-200a Locally Attenuates Progesterone Signaling in the Cervix, Preventing Embryo Implantation. Molecular Endocrinology 2014, 28, 1108–1117. [Google Scholar] [CrossRef]
- Shi, C.; Shen, H.; Fan, L.J.; Guan, J.; Zheng, X.B.; Chen, X.; Liang, R.; Zhang, X.W.; Cui, Q.H.; Sun, K.K.; et al. Endometrial MicroRNA Signature during the Window of Implantation Changed in Patients with Repeated Implantation Failure. Chin Med J (Engl) 2017, 130, 566–573. [Google Scholar] [CrossRef]
- Shekibi, M.; Heng, S.; Nie, G. MicroRNAs in the Regulation of Endometrial Receptivity for Embryo Implantation. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Sonu, R.J.; Jonas, B.A.; Dwyre, D.M.; Gregg, J.P.; Rashidi, H.H. Optimal Molecular Methods in Detecting P190 (BCR-ABL) Fusion Variants in Hematologic Malignancies: A Case Report and Review of the Literature. Case Rep Hematol 2015, 2015, 458052. [Google Scholar] [CrossRef]
- Byron, S.A.; Van Keuren-Jensen, K.R.; Engelthaler, D.M.; Carpten, J.D.; Craig, D.W. Translating RNA Sequencing into Clinical Diagnostics: Opportunities and Challenges. Nat Rev Genet 2016, 17, 257–271. [Google Scholar] [CrossRef]
- Rocheleau, C.E.; Downs, W.D.; Lin, R.; Wittmann, C.; Bei, Y.; Cha, Y.H.; Ali, M.; Priess, J.R.; Mello, C.C. Wnt Signaling and an APC-Related Gene Specify Endoderm in Early C. Elegans Embryos. Cell 1997, 90, 707–716. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.-P. The Current State and Future Directions of RNAi-Based Therapeutics. Nat Rev Drug Discov 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Ebbesen, M.; Jensen, T.G.; Andersen, S.; Pedersen, F.S. Ethical Perspectives on RNA Interference Therapeutics. Int J Med Sci 2008, 5, 159–168. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.-H.; Sailor, M.J. Rekindling RNAi Therapy: Materials Design Requirements for In Vivo siRNA Delivery. Adv Mater 2019, 31, e1903637. [Google Scholar] [CrossRef]
- Green, E.D.; Watson, J.D.; Collins, F.S. Human Genome Project: Twenty-Five Years of Big Biology. Nature 2015, 526, 29–31. [Google Scholar] [CrossRef]
- Morris, P.J. From Mendel to the Human Genome Project. N C Med J 2013, 74, 477. [Google Scholar]
- Friedmann, T. A Brief History of Gene Therapy. Nat Genet 1992, 2, 93–98. [Google Scholar] [CrossRef]
- Jackson, D.A.; Symons, R.H.; Berg, P. Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia Coli. Proc Natl Acad Sci U S A 1972, 69, 2904–2909. [Google Scholar] [CrossRef]
- Goeddel, D.V.; Kleid, D.G.; Bolivar, F.; Heyneker, H.L.; Yansura, D.G.; Crea, R.; Hirose, T.; Kraszewski, A.; Itakura, K.; Riggs, A.D. Expression in Escherichia Coli of Chemically Synthesized Genes for Human Insulin. Proc Natl Acad Sci U S A 1979, 76, 106–110. [Google Scholar] [CrossRef]
- Dimitriadis, G.J. Translation of Rabbit Globin mRNA Introduced by Liposomes into Mouse Lymphocytes. Nature 1978, 274, 923–924. [Google Scholar] [CrossRef]
- Schoch, K.M.; Miller, T.M. Antisense Oligonucleotides: Translation from Mouse Models to Human Neurodegenerative Diseases. Neuron 2017, 94, 1056–1070. [Google Scholar] [CrossRef]
- Chan, J.J.; Tay, Y. Noncoding RNA:RNA Regulatory Networks in Cancer. Int J Mol Sci 2018, 19, 1310. [Google Scholar] [CrossRef]
- Tian, Z.; Liang, G.; Cui, K.; Liang, Y.; Wang, Q.; Lv, S.; Cheng, X.; Zhang, L. Insight Into the Prospects for RNAi Therapy of Cancer. Front Pharmacol 2021, 12, 644718. [Google Scholar] [CrossRef]
- Montanari Vergallo, G.; Zaami, S.; Bruti, V.; Signore, F.; Marinelli, E. How the legislation in medically assisted procreation has evolved in Italy. Med Law 2017, 36, 5–28. [Google Scholar]
- Ethics Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org Cross-Border Reproductive Care: An Ethics Committee Opinion. Fertil Steril 2022, 117, 954–962. [Google Scholar] [CrossRef]
- Marinelli, S.; Cucinella, G.; Basile, G. COVID-19 and Female Fertility: The Flaws of Italian Law 40/2004 on Assisted Procreation in Pandemic Times. Acta Biomed 2022, 93, e2022316. [Google Scholar] [CrossRef]
- Ghoshal, R. Assisted Reproductive Technologies: Conundrums and Challenges. Indian J Med Ethics 2018, 3, 95–98. [Google Scholar] [CrossRef]
- Frith, L.; Blyth, E. Assisted Reproductive Technology in the USA: Is More Regulation Needed? Reprod Biomed Online 2014, 29, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.N.; Ke, R.W. Ethical Considerations in the Field of Assisted Reproductive Technology. Minerva Endocrinol 2018, 43, 80–86. [Google Scholar] [CrossRef]
| miR name | extr. | intr. |
|---|---|---|
| 9 | ↓ | ↑ |
| 15b | ↑ | |
| 20b-5p | ↑ | |
| 21 | ↑↓ | ↓ |
| 27a | ↑ | ↑ |
| 29b | ↓ | |
| 30a-5p | ↓ | ↓ |
| 92a | ↑ | |
| 99a | ↑ | ↓ |
| 100 | ↑ | ↓ |
| 135b | ↑ | ↑ |
| 141 | ↑ | ↑ |
| 142-3p | ↑ | ↑↓ |
| 143-3p | ↑ | |
| 146a-5p | ↑ | |
| 150-5p | ↑ | |
| 151a-5p | ↑ | |
| 186 | ↑ | |
| 195-5p | ↑ | |
| 199b | ↑ | ↓ |
| 200a | ↑ | ↑ |
| 203 | ↑ | ↑ |
| 204 | ↑↓ | ↓ |
| 205 | ↑ | ↑ |
| 222 | ↑ | |
| 223 | ↑ | ↑ |
| 301b | ↓ | |
| 423-3p | ↑ | |
| 449 | ↑ | ↑ |
| 484 | ↑ | |
| 887-5p | ↑ | |
| 1228 | ↑ | |
| 1290 | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





