Submitted:
06 June 2023
Posted:
07 June 2023
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Search strategy
2.2. Selection criteria
2.3. Data analysis & Outcome measure
2.4. Risk of bias
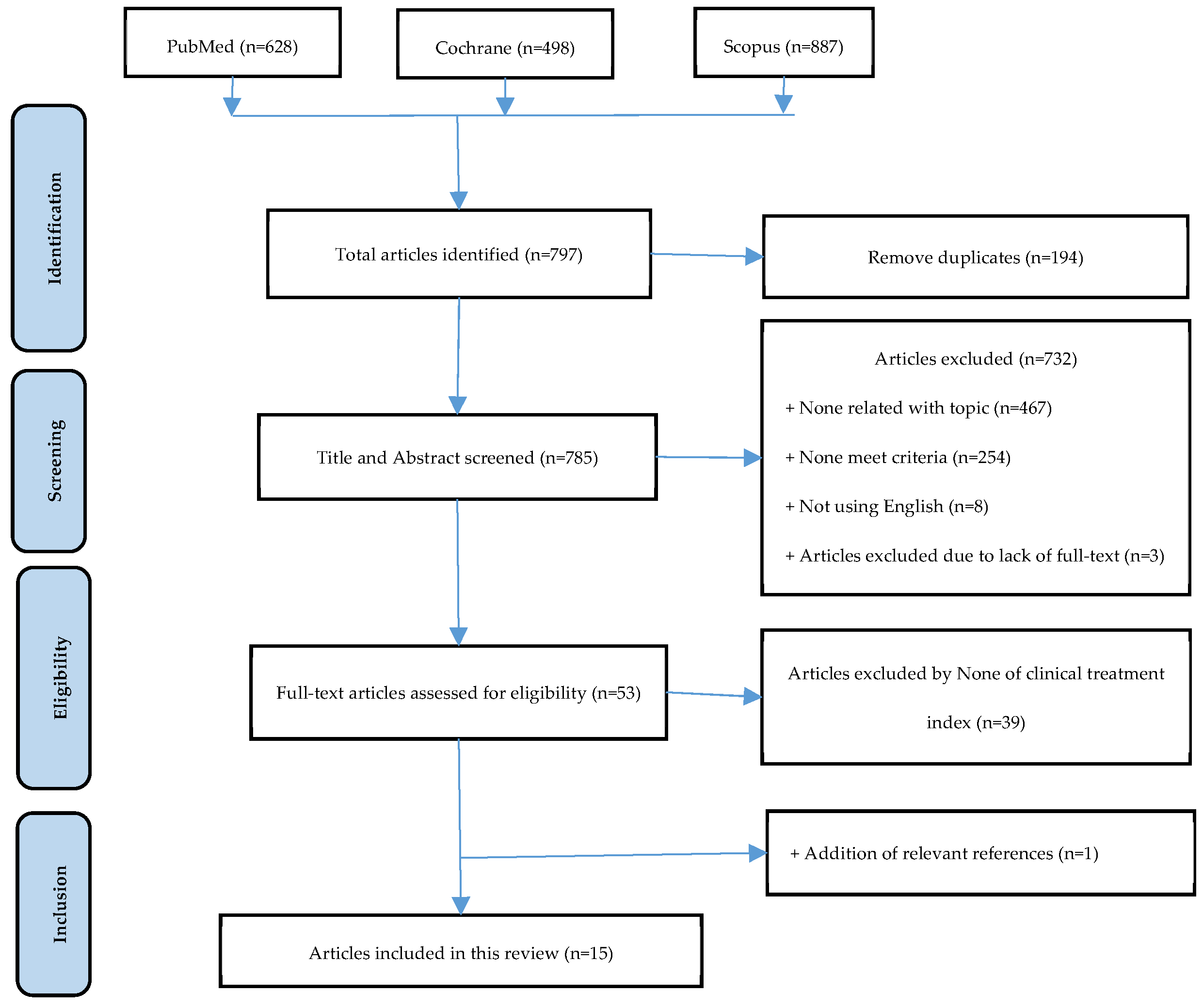
3. Results
3.1. Selection of study process
| References | Study design |
OA site | Intervention | N in control group |
N in glucosamine group |
Follow-up (months) | *Age | Outcome |
|---|---|---|---|---|---|---|---|---|
| Giordano et al., 2009 [13] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS vs P | 30 | 30 | 3 | 57.2 ± 7.2 58.0 ± 8.3 |
WOMAC, VAS |
| Rozendaal et al., 2008 [14] | Randomized, placebo-controlled, blinded trial |
Hip | GS vs P | 111 | 111 | 24 | 63.1 ± 9.5 63.7 ± 8.5 |
WOMAC, VAS |
| Herrero-Beaumont et al., 2007 [15] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS vs P | 104 | 106 | 6 | 63.4 ± 6.9 64.5 ± 7.2 |
WOMAC |
| Fransen et al., 2015 [16] | Randomized, placebo-controlled, double-blind trial |
Knee | GS vs GS+CS vs CS vs P |
151 | 152 | 24 | 61.2 ± 7.7 60.6 ± 8.1 |
WOMAC |
| Hughes et al., 2002 [17] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS vs P | 40 | 40 | 6 | **62.28 ± 9.12 | WOMAC,VAS, McGill pain questionnaire |
| Pavelka et al., 2002 [18] | Randomized, placebo-controlled, double-blind trial |
Knee | GS vs P | 101 | 101 | 36 | 61.2 ± 7.2 63.5 ± 6.9 |
WOMAC |
| Sawitzke et al., 2010 [19] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS vs CS vs GS+CS vs celecoxib vs P |
134 | 131 | 24 | 56.7 ± 10.5 56.9 ±9.8 |
WOMAC |
| Reginster et al., 2001 [20] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS vs P | 106 | 106 | 36 | 66.0 ± 8.1 65.5 ± 7.5 |
WOMAC |
| Rindone et al., 2000 [21] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS vs P | 49 | 49 | 2 | 63 ± 12 64 ± 11 |
VAS |
| Kwoh et al., 2014 [22] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS + P | 103 | 98 | 6 | 52.17±6.05 52.29±6.72 |
WOMAC |
| McAlindon et al., 2004 [23] |
Randomized, placebo-controlled, double-blind trial |
Knee | GH vs P | 104 | 101 | 3 | ND ND |
WOMAC |
| Madhu et al., 2013 [24] |
Randomized, placebo-controlled, single-blind trial |
Knee | GS vs P vs NR-INF-02 vs NR-INF-02 + GS |
30 | 30 | 1,5 (42 days) | 56.80±7.99 56.77±9.98 |
WOMAC, VAS, CGIC |
| Petersen et al., 2011 [25] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS vs ibuprofen vs P |
12 | 12 | 3 | 62.2±3.4 63.1±4.7 | VAS |
| Frestedt et al., 2008 [26] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS vs P vs Aquamin vs GS + aquamin |
16 | 19 | 3 | 59.2 ± 8.3 58.9 ± 7.4 |
WOMAC, 6-MWD |
| Clegg et al., 2006 [27] |
Randomized, placebo-controlled, double-blind trial |
Knee | GH vs CS vs GH+CS vs P vs Celecoxib | 313 | 317 | 6 | 58.6 ± 10.2 58.2 ± 9.8 |
WOMAC |
| Cibere et al., 2004 [28] |
Randomized, placebo-controlled, double-blind trial |
Knee | GS vs P | 66 | 71 | 6 | 64(40–83)a 65(43–88)a |
WOMAC, EQ-5D |
| Nieman et al., 2013 [29] |
Randomized, placebo-controlled, double-blind trial |
KneeHip Ankles Shoulders Hand | GS vs P | 51 | 101 | 2 | 57.6 ± 0.9 58.3 ± 0.8 |
WOMAC, VAS, SF-36, 6-MWD |
3.2. Risk of bias assessment
3.3. Efficacy of Glucosamine on Knee osteoarthritis
| Study and year | Glucosamine | Placebo | Std. Mean Difference |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Total | Mean | SD | Total | Weight | IV, Random, 95% CI | |
| Clegg et al., 2006 | -16 | 26.9 | 317 | -16.6 | 25.2 | 313 | 4.5% | 0.60 [-3.47, 4.67] |
| Fransen et al., 2015 | -8.6 | 24.5 | 152 | -7.2 | 33.8 | 151 | 4.1% | -1.40 [-8.05 , 5.25 ] |
| Giodarno et al., 2009 | -16.6 | 22.4 | 30 | 0.3 | 10.8 | 30 | 3.7% | -16.90 [-25.80,-8.00] |
| Madhu et al., 2013 | -31.7 | 19 | 24 | -15.5 | 18.3 | 29 | 3.5% | -16.20 [-26.31, -6.09] |
| Petersen et al., 2011 | -16.8 | 17.3 | 12 | -1.9 | 10.7 | 12 | 3.3% | -14.90 [-26.41, -3.39] |
| Rindone et al., 2000 | -15 | 26.6 | 49 | -15 | 23.4 | 49 | 3.6% | 0.00 [ -9.92, 9.92] |
| Subtotal (95% Cl) | 584 | 584 | 22.6% |
-7.41 [-14.31, -0.51] |
||||
| Study and year | Glucosamine | Placebo | Std. Mean Difference |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Total | Mean | SD | Total | Weight | IV, Random, 95% CI | |
| Cibere et al., 2004 | 3.2 | 15.5 | 71 | 3.4 | 18.1 | 66 | 9.7% | -0.20 [-5.86, 5.46] |
| Frestedt et al., 2008 | -10.5 | 15 | 14 | -5.9 | 16.9 | 9 | 3.8% | -4.60 [-18.15, 8.95] |
| Herrero-Beaumont et al., 2007 | -17.3 | 13.3 | 78 | -11.7 | 14.3 | 70 | 11.0% | -5.60 [-10.06, -1.14] |
| Kwoh et al., 2014 | -15.1 | 19.3 | 98 | -19.1 | 20.1 | 103 | 9.9% | 4.00 [-1.45, 9.45] |
| Madhu et al., 2013 | -23.4 | 17.1 | 24 | -9.3 | 11.4 | 29 | 7.3% | -14.10 [-22.0, -6.10] |
| McAlindon et al., 2004 | 7.8 | 13.1 | 101 | 7.8 | 13.5 | 104 | 11.9% | 0.00 [-3.64, 3.64] |
| Pavelka et al., 2002 | -7.7 | 7.1 | 66 | -4.7 | 5.9 | 55 | 13.2% | -3.00 [-5.32, -0.68] |
| Regisnter et al., 2001 | -0.2 | 19.2 | 68 | -0.6 | 19.6 | 71 | 8.8% | 0.40 [-6.05, 6.85] |
| Subtotal (95% Cl) | 520 | 507 | 75.6% |
-2.27 [-5.21, 0.66] |
||||
| Study and year | Glucosamine | Placebo | Std. Mean Difference |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Total | Mean | SD | Total | Weight | IV, Fixed, 95% CI | |
| McAlindon et al., 2004 | -2 | 3.4 | 101 | -2.5 | 3.8 | 104 | 12.7% | 0.14 [-0.14, 0.41] |
| Cibere et al., 2004 | -25 | 98 | 71 | -28 | 104 | 66 | 8.5% | 0.03 [-0.31, 0.36] |
| Clegg et al., 2006 | -82.9 | 115.4 | 317 | -86.1 | 114.2 | 313 | 39.1% | 0.03 [-0.13, 0.18] |
| Herreo-Beaumont et al., 2007 | -2.7 | 3.15 | 106 | -1.8 | 4.16 | 104 | 12.9% | -0.24 [-0.51, 0.03] |
| Frestedt et al., 2008 | -12.3 | 16.26 | 19 | -2.9 | 22.16 | 16 | 2.1% | -0.48 [-1.15, 0.02] |
| Nieman et al., 2013 | -2.4 | 2.8 | 49 | -0.9 | 2.86 | 51 | 6.0% | -0.53 [-0.92, -0.13] |
| Fransen et al., 2015 | -22 | 3.55 | 152 | -2.1 | 3.45 | 151 | 18.8% | 0.03 [-0.20, 0.25] |
| Total | 815 | 805 | 100.0% | -0.04 [-0.13, 0.06] |
||||
|
Heterogeneity: Chi2 = 12.33, df = 6 (P = 0.05); I2 = 51% Test for overall effect: Z = 0.74 (P=0.46) | ||||||||
| Study and year | Glucosamine | Placebo | Std. Mean Difference |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Total | Mean | SD | Total | Weight | IV, Fixed, 95% CI | |
| McAlindon et al., 2004 | -5.2 | 9.5 | 101 | -4.6 | 9.6 | 104 | 12.9% | -0.06 [-0.34, 0.21] |
| Cibere et al., 2004 | -58 | 270 | 71 | -63 | 318 | 66 | 8.6% | 0.02 [-0.32, 0.35] |
| Clegg et al., 2006 | -222.3 | 388.3 | 317 | -227.4 | 362.7 | 313 | 39.7% | 0.01 [-0.14, 0.17] |
| Herreo-Beaumont et al., 2007 | -9.2 | 10.51 | 106 | -5.5 | 11.45 | 104 | 13.1% | -0.34 [-0.61, -0.06] |
| Frestedt et al., 2008 | -10.6 | 16.31 | 19 | -7 | 23.55 | 16 | 2.2% | -0.18 [-0.84, 0.49] |
| Nieman et al., 2013 | -8.2 | 9.34 | 36 | -3 | 9.34 | 36 | 4.4% | -0.55 [-1.02, -0.08] |
| Fransen et al., 2015 | -3.9 | 12.68 | 152 | -3.9 | 12.85 | 151 | 19.1% | 0.00 [-0.23, 0.23] |
| Total | 802 | 790 | 100.0% | -0.07 [-0.17, 0.03] |
||||
|
Heterogeneity: Chi2 = 9.48, df = 6 (P=0.15); I2 = 51% Test for overall effect: Z = 1.45 (P =0.15) | ||||||||
| Study and year | Glucosamine | Placebo | Std. Mean Difference |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Total | Mean | SD | Total | Weight | IV, Fixed, 95% CI | |
| Clegg et al., 2006 | -1.4 | 2.1 | 317 | -1.5 | 2.1 | 313 | 19.3% | 0.10 [-0.23, 0.43] |
| Frestedt et al., 2008 | -0.8 | 1.9 | 14 | -0.5 | 1.5 | 9 | 8.6% | -0.30 [-1.70, 1.10] |
| Giordano et al., 2009 | -1 | 0.3 | 30 | 0 | 0.3 | 30 | 20.4% | -1.00 [-1.15, -0.85] |
| McAlindon et al., 2004 | 0.7 | 1.6 | 101 | 0.8 | 1.5 | 104 | 18.4% | -0.10 [-0.52, 0.32] |
| Pavelka et al., 2002 | -0.3 | 1.5 | 66 | 0.1 | 0.7 | 55 | 18.5% | -0.40 [-0.81, 0.01] |
| Regisnter et al., 2001 | 0 | 2.3 | 68 | 0 | 2.2 | 71 | 14.8% | 0.00 [-0.75, 0.75] |
| Total | 596 | 582 | 100.0 % | -0.30 [-0.82, 0.21] |
||||
|
Heterogeneity: Tau2 = 0.33; Chi2 = 51.22, df = 5 (P<0.00001); I2 = 90% Test for overall effect: Z = 1.15 (P = 0.25) | ||||||||
3.4. Safety
3.4.1. Adverse events
3.4.2. Drug interactions
4. Discussion
5. Conclusions
References
- Colletti, A.; Cicero, A.F.G. Nutraceutical Approach to Chronic Osteoarthritis: From Molecular Research to Clinical Evidence. Int J Mol Sci 2021, 22, 12920. [Google Scholar] [CrossRef] [PubMed]
- Conrozier, T.; Lohse, T. Glucosamine as a Treatment for Osteoarthritis: What If It’s True? Front Pharmacol 2022, 13, 820971. [Google Scholar] [CrossRef] [PubMed]
- Gregori, D.; Giacovelli, G.; Minto, C.; Barbetta, B.; Gualtieri, F.; Azzolina, D.; Vaghi, P.; Rovati, L.C. Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis. JAMA 2018, 320, 2564–2579. [Google Scholar] [CrossRef]
- Ogata, T.; Ideno, Y.; Akai, M.; Seichi, A.; Hagino, H.; et al. Effects of glucosamine in patients with osteoarthritis of the knee: a systematic review and meta-analysis. Clin Rheumatol 2018, 37, 2479–2487. [Google Scholar] [CrossRef]
- Simental-Mendía, M.; Sánchez-García, A.; Vilchez-Cavazos, F.; Acosta-Olivo, C.A.; Peña-Martínez, V.M.; Simental-Mendía, L.E. Effect of glucosamine and chondroitin sulfate in symptomatic knee osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Rheumatol Int 2018, 38, 1413–1428. [Google Scholar] [CrossRef]
- Smedslund, G.; Kjeken, I.; Musial, F.; Sexton, J.; Østeråsa, N. Interventions for osteoarthritis pain: A systematic review with network meta-analysis of existing Cochrane reviews. Osteoarthr Cartil Open 2022, 4, 100242. [Google Scholar] [CrossRef]
- Ghouri, A.; Conaghan, P.G. Update on novel pharmacological therapies for osteoarthritis. Ther Adv Musculoskelet Dis 2019, 11, 1759720X19864492. [Google Scholar] [CrossRef]
- Zhu, X.; Sang, L.; Wu, D.; Rong, J.; Jiang, L. Effectiveness and safety of glucosamine and chondroitin for the treatment of osteoarthritis: a meta-analysis of randomized controlled trials. J Orthop Surg Res 2018, 13, 170. [Google Scholar] [CrossRef]
- Bruyère, O.; Altman, R.D.; Reginster, J.-Y. Efficacy and safety of glucosamine sulfate in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum 2016, 45, S12–S17. [Google Scholar] [CrossRef]
- Notes need to know about glucosamine. Available online: http://bachmai.gov.vn/tin-tuc-va-su-kien/thong-tin-thuoc-menuleft-124/6734-mot-so-dieu-can-biet-ve-glucosamin.html (accessed on 31 March 2023).
- MOH. Circular No. 20/2022/TT-BYT. 2022. Ha Noi.
- Runhaar, J.; Rozendaal, R.M.; Middelkoop, M.v.; Bijlsma, H.J.W.; Doherty, M.; et al. Subgroup analyses of the effectiveness of oral glucosamine for knee and hip osteoarthritis: a systematic review and individual patient data meta-analysis from the OA trial bank. Ann Rheum Dis 2017, 76, 1862–1869. [Google Scholar] [CrossRef]
- Giordano, N.; Fioravanti, A.; Papakostas, P.; Montella, A.; Giorgi, G.; Nuti, R. The efficacy and tolerability of glucosamine sulfate in the treatment of knee osteoarthritis: A randomized, double-blind, placebo-controlled trial. Curr Ther Res Clin Exp 2009, 70, 185–196. [Google Scholar] [CrossRef]
- Rozendaal, R.M.; Koes, B.W.; Osch, G.J.V.M.v.; Uitterlinden, E.J.; Garling, E.H.; et al. Effect of glucosamine sulfate on hip osteoarthritis: a randomized trial. Ann Intern Med 2008, 148, 268–277. [Google Scholar] [CrossRef]
- Herrero-Beaumont, G.; Ivorra, J.A.R.; Trabado, M.D.C.; Blanco, F.J.; Benito, P.; et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: a randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum 2007, 56, 555–567. [Google Scholar] [CrossRef]
- Fransen, M.; Agaliotis, M.; Nairn, L.; Votrubec, M.; Bridgett, L.; et al. Glucosamine and chondroitin for knee osteoarthritis: a double-blind randomised placebo-controlled clinical trial evaluating single and combination regimens. Ann Rheum Dis 2015, 74, 851–858. [Google Scholar] [CrossRef]
- Hughes, R.; Carr, A. A randomized, double-blind, placebo-controlled trial of glucosamine sulphate as an analgesic in osteoarthritis of the knee. Rheumatology (Oxford) 2002, 41, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Pavelká, K.; Gatterová, J.; Olejarová, M.; Machacek, S.; Giacovelli, G.; Rovati, L.C. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med 2002, 162, 2113–2123. [Google Scholar] [CrossRef]
- Sawitzke, A.D.; Shi, H.; Finco, M.F.; Dunlop, D.D.; Harris, C.L.; et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis 2010, 69, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Deroisy, R.; Rovati, L.C.; Lee, R.L.; Lejeune, E.; et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet 2001, 357, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Rindone, J.P.; Hiller, D.; Collacott, E.; Nordhaugen, N.; Arriola, G. Randomized, controlled trial of glucosamine for treating osteoarthritis of the knee. West J Med 2000, 172, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Kwoh, C.K.; Roemer, F.W.; Hannon, M.J.; Moore, C.E.; Jakicic, J.M.; Guermazi, A.; Green, S.M.; Evans, R.W.; Boudreau, R. Effect of oral glucosamine on joint structure in individuals with chronic knee pain: a randomized, placebo-controlled clinical trial. Arthritis Rheumatol 2014, 66, 930–939. [Google Scholar] [CrossRef]
- McAlindon, T.; Formica, M.; LaValley, M.; Lehmer, M.; Kabbara, K. Effectiveness of glucosamine for symptoms of knee osteoarthritis: results from an internet-based randomized double-blind controlled trial. Am J Med 2004, 117, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Madhu, K.; K Chanda, M.J.S. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: a randomized placebo-controlled trial. Inflammopharmacology 2013, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.G.; Beyer, N.; Hansen, M.; Holm, L.; Aagaard, P.; Mackey, A.L.; Kjaer, M. Nonsteroidal anti-inflammatory drug or glucosamine reduced pain and improved muscle strength with resistance training in a randomized controlled trial of knee osteoarthritis patients. Arch Phys Med Rehabil 2011, 92, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Frestedt, J.L.; Walsh, M.; Kuskowski, M.A.; Zenk, J.L. A natural mineral supplement provides relief from knee osteoarthritis symptoms: a randomized controlled pilot trial. Nutr J 2008, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.O.; Reda, D.J.; Harris, C.L.; Klein, M.A.; O’Dell, J.R.; et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006, 354, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Cibere, J.; Kopec, J.A.; Thorne, A.; Singer, J.; Canvin, J.; et al. Randomized, double-blind, placebo-controlled glucosamine discontinuation trial in knee osteoarthritis. Arthritis Rheum 2004, 51, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Shanely, R.A.; Luo, B.; Dew, D.; Meaney, M.P.; Sha, W. A commercialized dietary supplement alleviates joint pain in community adults: a double-blind, placebo-controlled community trial. Nutr J 2013, 12, 154. [Google Scholar] [CrossRef]
- Noack, W.; Fischer, M.; Förster, K.K.; Rovati, L.C.; Setnikar, I. Glucosamine sulfate in osteoarthritis of the knee. Osteoarthritis Cartilage 1994, 2, 51–59. [Google Scholar] [CrossRef]
- MOH. Drugbank. .
- Williams, C.; Ampat, G. Glucosamine Sulfate; StatPearls: Treasure Island (FL), 2023. [Google Scholar]
- Drug interaction report Glucosamine and Warfarin. Available online: https://www.drugs.com/interactions-check.php?drug_list=1182-0,2311-0 (accessed on 31 March 2023).
- Dahmer, S.; Schiller, R.M. Glucosamine. Am Fam Physician 2008, 78, 471–476. [Google Scholar]
- Knudsen, J.F.; Sokol, G.H. Potential glucosamine-warfarin interaction resulting in increased international normalized ratio: case report and review of the literature and MedWatch database. Pharmacotherapy 2008, 28, 540–548. [Google Scholar] [CrossRef]
- Costa, B.R.d.; Saadat, P.; Basciani, R.; Agarwal, A.; Johnston, B.C.; Jüni, P. Visual Analogue Scale has higher assay sensitivity than WOMAC pain in detecting between-group differences in treatment effects: a meta-epidemiological study. Osteoarthritis Cartilage 2021, 29, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Largo, R. Glucosamine and O-GlcNAcylation: a novel immunometabolic therapeutic target for OA and chronic, low-grade systemic inflammation? Ann Rheum Dis 2020, 79, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, G.; Nedeljkovic, B.; Trajkovic, G.; Rasic, D.; Mirkovic, Z.; Pajovic, S.; Grbic, R.; Sipetic, S.; Vujcic, I. Pain, Physical Function, Radiographic Features, and Quality of Life in Knee Osteoarthritis Agricultural Workers Living in Rural Population. Pain Res Manag 2019, 7684762. [Google Scholar] [CrossRef]
- Lee, Y.H.; Woo, J.-H.; Choi, S.J.; Ji, J.D.; Song, G.G. Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis. Rheumatol Int 2010, 30, 357–363. [Google Scholar] [CrossRef] [PubMed]
| No. | References | Low / High |
|---|---|---|
| 1 | Clegg et al., 2006 [27] | Low |
| 2 | Fransen et al., 2015 [16] | Low |
| 3 | Giodarno et al., 2009 [13] | Low |
| 4 | Madhu et al., 2013 [24] | Low |
| 5 | Petersen et al., 2011 [25] | Low |
| 6 | Rindone et al., 2000 [21] | Low |
| 7 | Cibere et al., 2004 [28] | Low |
| 8 | Frestedt et al., 2008 [26] | Low |
| 9 | Herrero-Beaumont et al.,2007 [15] | Low |
| 10 | Kwoh et al., 2014 [22] | Low |
| 11 | McAlindon et al., 2004 [23] | Low |
| 12 | Pavelka et al., 2002 [18] | Low |
| 13 | Regisnter et al., 2001 [20] | Low |
| Study ID | Relative risk (RR) 95% CI |
Weight % |
|---|---|---|
| Reginster et al., 2001 | 1.07 [0.70, 1.65] | 14.26 |
| Pavelka et al., 2002 | 1.05 [0.46, 2.39] | 6.57 |
| McAlindon et al., 2004 | 0.50 [0.07, 3.75] | 1.83 |
| Clegg et al., 2006 | 0.71 [0.31, 1.60] | 8.97 |
| Herrero-Beaumont et al., 2007 | 0.54 [0.19, 1.57] | 6.18 |
| Rozendaal et al., 2008 | 2.29 [0.26, 20.13] | 0.71 |
| Fransen et al., 2015 | 7.33 [0.96, 56.00] | 0.33 |
| Kwoh 2014 | 0.39 [0.13, 1.20] | 3.53 |
| Subtotal (I 2 = 24.3%, p = 0.236) | 0.90 [0.66, 1.23] | 42.37 |
| Study ID | Glucosamine sulphate (N) | Adverse events | ||||||
|---|---|---|---|---|---|---|---|---|
| GI | CV | CNS | MU | Skin | Infections | Others | ||
|
Pavelkaet al., 2002 [18] |
101 | 25 | 23 | ND | 30 | 10 | 29 | 14 |
| Noack et al., 1994 [30] |
126 | 5 | 0 | 2 | 0 | 1 | 0 | 0 |
| Reginster et al., 2001 [20] | 106 | 27 | 21 | 31 | ND | ND | ND | 4 |
| McAlindon et al., 2004 [23] | 101 | 4 | ND | 2 | 5 | ND | ND | 7 |
| Herrero-Beaumont et al., 2007 [15] |
106 | 11 | ND | 3 | 10 | ND | 12 | 8 |
| Rozendaal et al., 2008 [14] | 111 | 58 | 21 | 66 | ND | ND | ND | 7 |
| Hughes et al., 2002[17] | 39 | 0 | ND | 1 | 9 | 0 | 1 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





