Submitted:
06 June 2023
Posted:
07 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. A Ketogenic Diet, Alone or in Combination with Gemcitabine, Preserves Muscle Strength in KPC Mice
2.2. Effect of a Ketogenic Diet Alone and in Combination with Gemcitabine on Food Intake, Body Weight and Composition in KPC Mice
2.3. A Ketogenic Diet in Combination with Gemcitabine Induces Metabolic Changes in KPC Mice
2.4. A Ketogenic Diet Does Not Affect Inflammatory Cytokine Levels in KPC Mice Treated with Gemcitabine
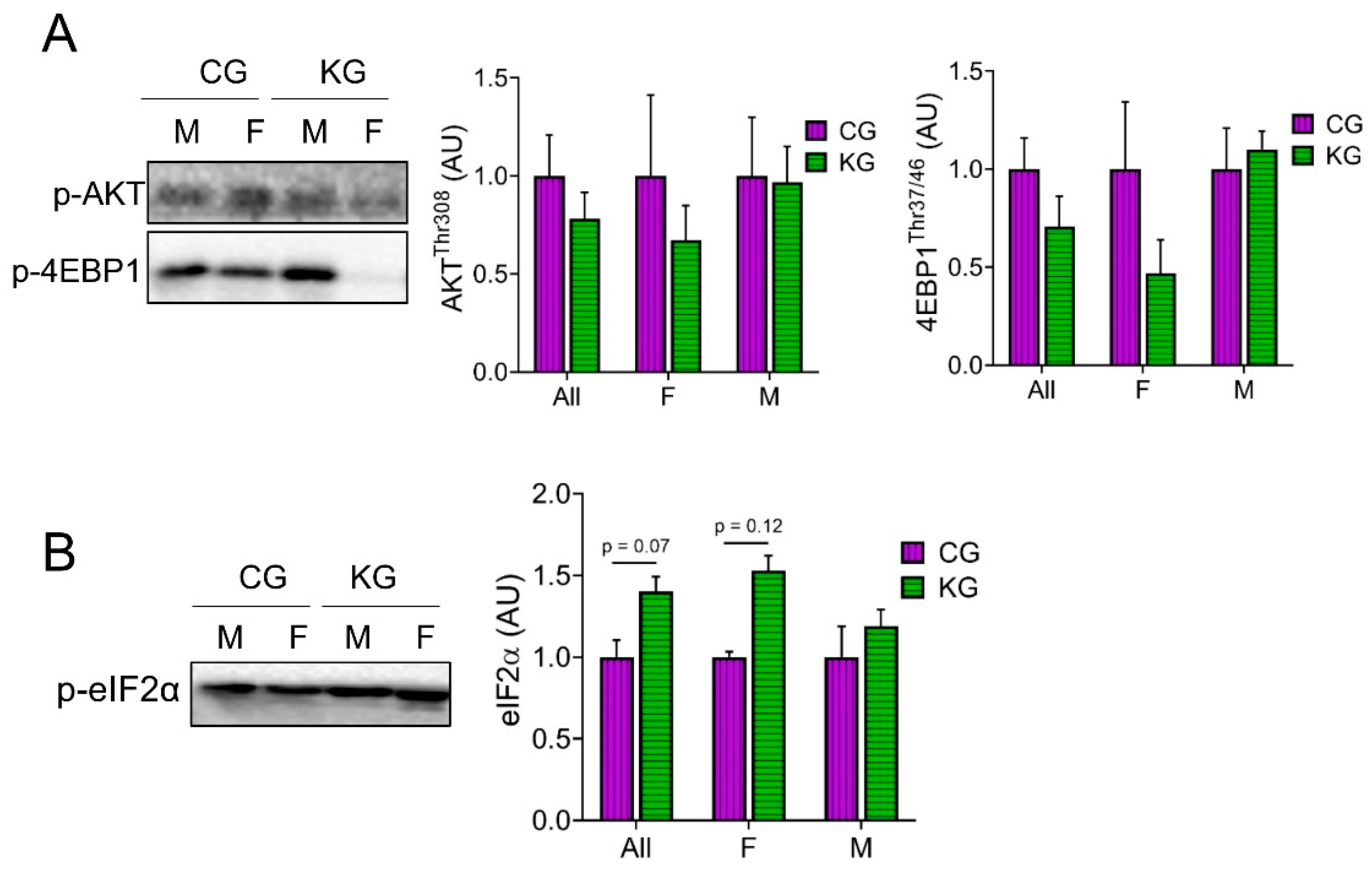
2.5. A Ketogenic Diet Does Not Change Anabolic Signaling in the Gastrocnemius of KPC Mice Treated with Gemcitabine
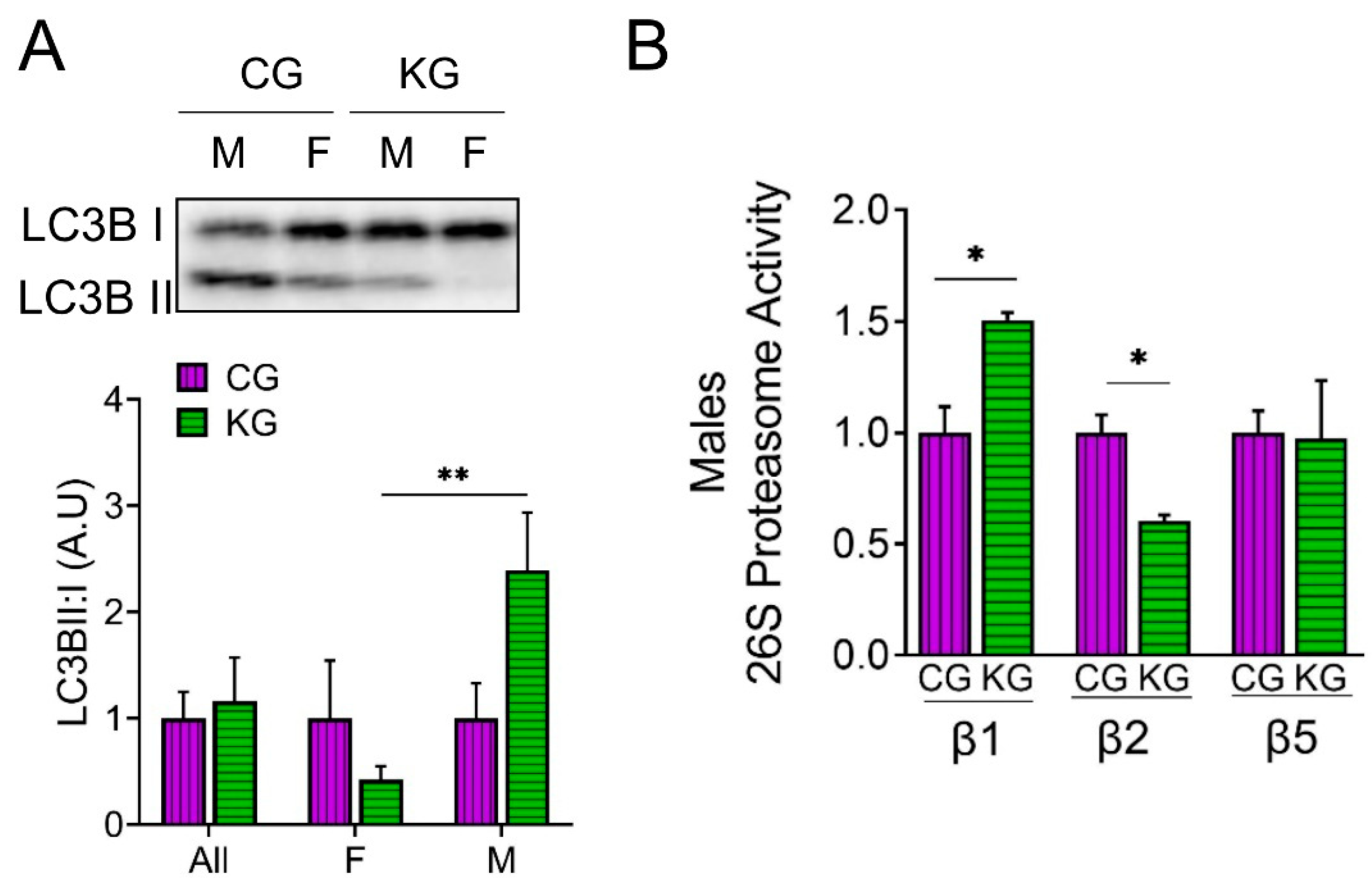
2.6. A Ketogenic Diet Alters Proteosome Activity and Autophagy in the Gastrocnemius of KPC Mice Treated with Gemcitabine
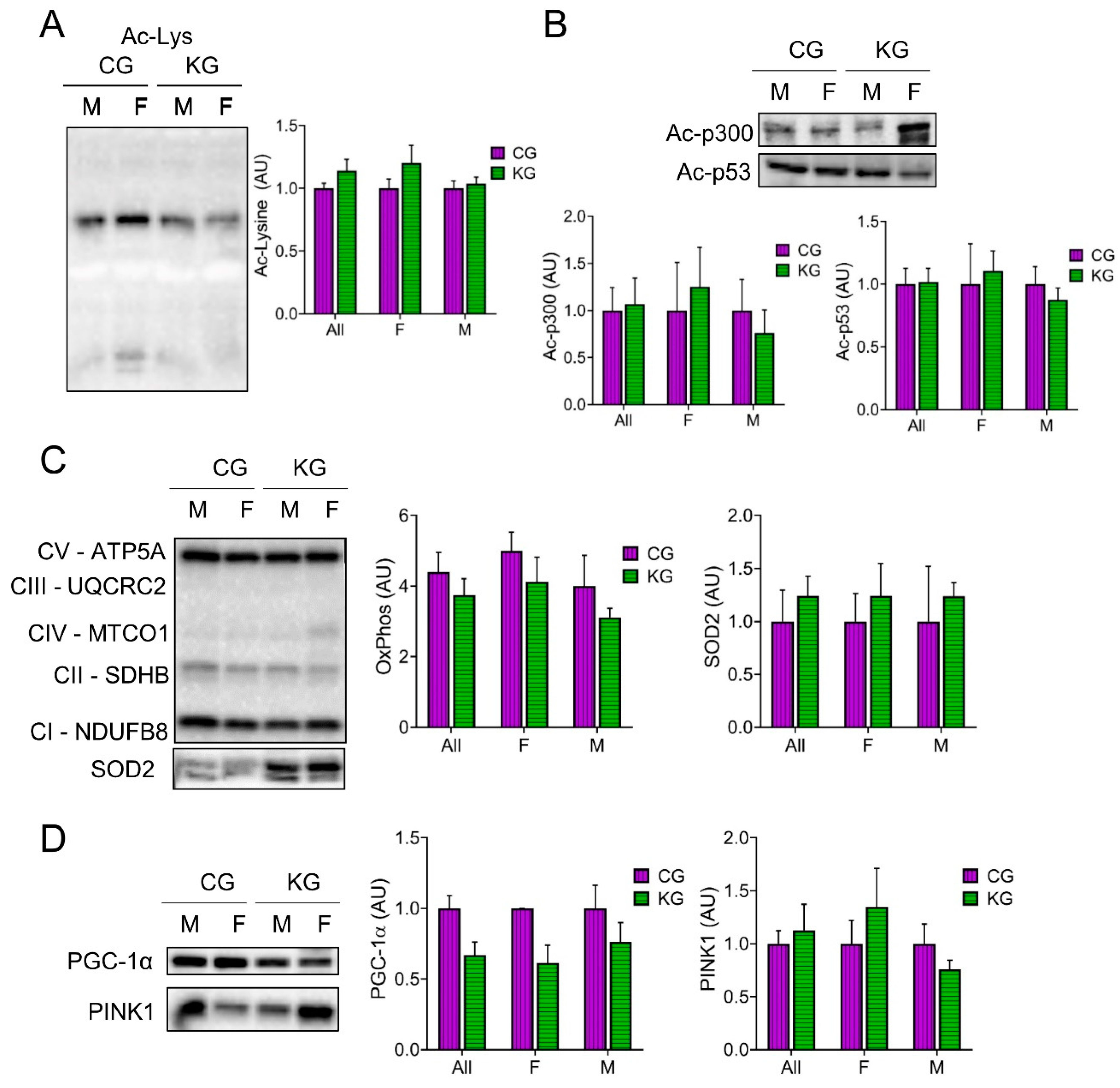
2.7. Effect of a Ketogenic Diet on Protein Acetylation, Antioxidant Levels and Mitochondrial Proteins in the Gastrocnemius of KPC Mice Treated with Gemcitabine
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. Genetically Engineered Transgenic Mice
4.3. Survival Study
4.4. Mechanistic Study
4.5. Forelimb Grip Strength Test
4.6. Blood Glucose and Ketones
4.7. Body Weight and Composition
4.8. Metabolic Measurements
4.9. Tissue Homogenization and Western Blotting
4.10. 26S Proteasome Activity Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: an international consensus. The Lancet Oncology 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Dolan, R.D.; Skipworth, R.J.; Laird, B.J.; McMillan, D.C. Cancer cachexia: a nutritional or a systemic inflammatory syndrome? Br J Cancer, 1038; -2. [Google Scholar] [CrossRef]
- Mueller, T.C.; Burmeister, M.A.; Bachmann, J.; Martignoni, M.E. Cachexia and pancreatic cancer: Are there treatment options? World J Gastroenterol 2014, 20, 9361–9373. [Google Scholar] [CrossRef] [PubMed]
- Henderson, S.E.; Makhijani, N.; Mace, T.A. Pancreatic Cancer-Induced Cachexia and Relevant Mouse Models. Pancreas 2018, 47, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Yoo, W.; Choi, H.; Son, Y.H.; Lee, J.; Jo, S.; Jung, D.; Kim, Y.J.; Koh, S.S.; Yang, Y.R.; Kwon, E.S.; et al. Pancreatic cancer induces muscle wasting by promoting the release of pancreatic adenocarcinoma upregulated factor. Exp Mol Med 2021, 53, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Mueller, T.C.; Bachmann, J.; Prokopchuk, O.; Friess, H.; Martignoni, M.E. Molecular pathways leading to loss of skeletal muscle mass in cancer cachexia – can findings from animal models be translated to humans? BMC Cancer 2016, 16. [Google Scholar] [CrossRef]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr Opin Support Palliat Care 2018, 12, 420–426. [Google Scholar] [CrossRef]
- Miki, M.; Lee, L.; Hisano, T.; Sugimoto, R.; Furukawa, M. Loss of adipose tissue or skeletal muscle during first-line gemcitabine/nab-paclitaxel therapy is associated with worse survival after second-line therapy of advanced pancreatic cancer. Asia Pac J Clin Oncol, 1111. [Google Scholar] [CrossRef]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J Clin Oncol 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Gresham, G.; Placencio-Hickok, V.R.; Lauzon, M.; Nguyen, T.; Kim, H.; Mehta, S.; Paski, S.; Pandol, S.J.; Osipov, A.; Gong, J.; et al. Feasibility and efficacy of enteral tube feeding on weight stability, lean body mass, and patient-reported outcomes in pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle 2021, 12, 1959–1968. [Google Scholar] [CrossRef]
- Paoli, A.; Cancellara, P.; Pompei, P.; Moro, T. Ketogenic Diet and Skeletal Muscle Hypertrophy: A Frenemy Relationship? J Hum Kinet 2019, 68, 233–247. [Google Scholar] [CrossRef]
- Roberts, M.N.; Wallace, M.A.; Tomilov, A.A.; Zhou, Z.; Marcotte, G.R.; Tran, D.; Perez, G.; Gutierrez-Casado, E.; Koike, S.; Knotts, T.A.; et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metabolism 2017, 26, 539–546. [Google Scholar] [CrossRef]
- Poff, A.M.; Ari, C.; Seyfried, T.N.; D’Agostino, D.P. The Ketogenic Diet and Hyperbaric Oxygen Therapy Prolong Survival in Mice with Systemic Metastatic Cancer. PLOS ONE 2013, 8, e65522. [Google Scholar] [CrossRef]
- Yakovenko, A.; Cameron, M.; Trevino, J.G. Molecular therapeutic strategies targeting pancreatic cancer induced cachexia. World J Gastrointest Surg 2018, 10, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Gebregiworgis, T.; Purohit, V.; Chaika, N.V.; Gunda, V.; Radhakrishnan, P.; Mehla, K.; Pipinos, I.I.; Powers, R.; Yu, F.; et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer & Metabolism 2014, 2, 18. [Google Scholar] [CrossRef]
- Cortez, N.E.; Rodriguez Lanzi, C.; Hong, B.V.; Xu, J.; Wang, F.; Chen, S.; Ramsey, J.J.; Pontifex, M.G.; Muller, M.; Vauzour, D.; et al. A ketogenic diet in combination with gemcitabine increases survival in pancreatic cancer KPC mice. Cancer Res Commun 2022, 2, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Olive, K.P.; Politi, K. Translational therapeutics in genetically engineered mouse models of cancer. Cold Spring Harbor protocols 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Narasimhan, A.; Silverman, L.M.; Young, A.R.; Shahda, S.; Liu, S.; Wan, J.; Liu, Y.; Koniaris, L.G.; Zimmers, T.A. Sex specificity of pancreatic cancer cachexia phenotypes, mechanisms, and treatment in mice and humans: role of Activin. J Cachexia Sarcopenia Muscle, 1002. [Google Scholar] [CrossRef]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol 2015, 7, 17–29. [Google Scholar] [CrossRef]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 2004, 279, 41114–41123. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.A.; Sandesara, P.B.; Senf, S.M.; Judge, A.R. Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2012, 26, 987–1000. [Google Scholar] [CrossRef]
- White, J.P.; Puppa, M.J.; Gao, S.; Sato, S.; Welle, S.L.; Carson, J.A. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab 2013, 304, E1042–1052. [Google Scholar] [CrossRef]
- Nader, G.A.; McLoughlin, T.J.; Esser, K.A. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol 2005, 289, C1457–1465. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Pothuraju, R.; Jain, M.; Batra, S.K.; Nasser, M.W. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim Biophys Acta Rev Cancer 2020, 1873, 188359. [Google Scholar] [CrossRef] [PubMed]
- Gaur, V.; Connor, T.; Sanigorski, A.; Martin, S.D.; Bruce, C.R.; Henstridge, D.C.; Bond, S.T.; McEwen, K.A.; Kerr-Bayles, L.; Ashton, T.D.; et al. Disruption of the Class IIa HDAC Corepressor Complex Increases Energy Expenditure and Lipid Oxidation. Cell Rep 2016, 16, 2802–2810. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.A.; Aguirre, N.W.; Marcotte, G.R.; Marshall, A.G.; Baehr, L.M.; Hughes, D.C.; Hamilton, K.L.; Roberts, M.N.; Lopez-Dominguez, J.A.; Miller, B.F.; et al. The ketogenic diet preserves skeletal muscle with aging in mice. Aging Cell 2021, 20, e13322. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.J.; Zhou, Z.; Steffen, D.; Tran, T.; Ad, Y.; Ramsey, J.J.; Rutkowsky, J.M.; Baar, K. 2-month ketogenic diet preferentially alters skeletal muscle and augments cognitive function in middle aged female mice. Aging Cell 2022, 21, e13706. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Peri, C.; Cricrì, D.; Coppi, L.; Caruso, D.; Mitro, N.; De Fabiani, E.; Crestani, M. Ketogenic Diet: A New Light Shining on Old but Gold Biochemistry. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Mulder, S.E.; Dasgupta, A.; King, R.J.; Abrego, J.; Attri, K.S.; Murthy, D.; Shukla, S.K.; Singh, P.K. JNK signaling contributes to skeletal muscle wasting and protein turnover in pancreatic cancer cachexia. Cancer Lett 2020, 491, 70–77. [Google Scholar] [CrossRef]
- Michaelis, K.A.; Zhu, X.; Burfeind, K.G.; Krasnow, S.M.; Levasseur, P.R.; Morgan, T.K.; Marks, D.L. Establishment and characterization of a novel murine model of pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle 2017, 8, 824–838. [Google Scholar] [CrossRef]
- Wallace, M.A.; Aguirre, N.W.; Marcotte, G.R.; Marshall, A.G.; Baehr, L.M.; Hughes, D.C.; Hamilton, K.L.; Roberts, M.N.; Lopez-Dominguez, J.A.; Miller, B.F.; et al. The ketogenic diet preserves skeletal muscle with aging in mice. Aging Cell, 1111. [Google Scholar] [CrossRef]
- Nakamura, K.; Tonouchi, H.; Sasayama, A.; Ashida, K. A Ketogenic Formula Prevents Tumor Progression and Cancer Cachexia by Attenuating Systemic Inflammation in Colon 26 Tumor-Bearing Mice. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Baehr, L.M.; West, D.W.; Marcotte, G.; Marshall, A.G.; De Sousa, L.G.; Baar, K.; Bodine, S.C. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 2016, 8, 127–146. [Google Scholar] [CrossRef]
- Hughes, D.C.; Marcotte, G.R.; Marshall, A.G.; West, D.W.D.; Baehr, L.M.; Wallace, M.A.; Saleh, P.M.; Bodine, S.C.; Baar, K. Age-related Differences in Dystrophin: Impact on Force Transfer Proteins, Membrane Integrity, and Neuromuscular Junction Stability. J Gerontol A Biol Sci Med Sci 2017, 72, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.; Butchbach, M.E.; Sahenk, Z.; Wang, H.; Saji, M.; Carathers, M.; Ringel, M.D.; Skipworth, R.J.; Fearon, K.C.; Hollingsworth, M.A.; et al. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 2005, 8, 421–432. [Google Scholar] [CrossRef]
- Fujikura, Y.; Yamanouchi, K.; Sugihara, H.; Hatakeyama, M.; Abe, T.; Ato, S.; Oishi, K. Ketogenic diet containing medium-chain triglyceride ameliorates transcriptome disruption in skeletal muscles of rat models of duchenne muscular dystrophy. Biochem Biophys Rep 2022, 32, 101378. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.; Zhu, X.; Norgard, M.A.; Levasseur, P.R.; Butler, J.T.; Buenafe, A.; Burfeind, K.G.; Michaelis, K.A.; Pelz, K.R.; Mendez, H.; et al. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat Commun 2021, 12, 2057. [Google Scholar] [CrossRef] [PubMed]
- Koutnik, A.P.; Poff, A.M.; Ward, N.P.; DeBlasi, J.M.; Soliven, M.A.; Romero, M.A.; Roberson, P.A.; Fox, C.D.; Roberts, M.D.; D'Agostino, D.P. Ketone Bodies Attenuate Wasting in Models of Atrophy. J Cachexia Sarcopenia Muscle 2020, 11, 973–996. [Google Scholar] [CrossRef] [PubMed]
- Banh, T.; Snoke, D.; Cole, R.M.; Angelotti, A.; Schnell, P.M.; Belury, M.A. Higher tumor mass and lower adipose mass are associated with colon-26 adenocarcinoma-induced cachexia in male, female and ovariectomized mice. Oncol Rep 2019, 41, 2909–2918. [Google Scholar] [CrossRef]
- Koutnik, A.P.; D’Agostino, D.P.; Egan, B. Anticatabolic Effects of Ketone Bodies in Skeletal Muscle. Trends in Endocrinology & Metabolism 2019, 30, 227–229. [Google Scholar] [CrossRef]
- Greenman, A.C.; Albrecht, D.M.; Halberg, R.B.; Diffee, G.M. Sex differences in skeletal muscle alterations in a model of colorectal cancer. Physiol Rep 2020, 8, e14391. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: let's talk about sex. Biol Sex Differ 2019, 10, 43. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D'Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nature medicine 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Attenuation of muscle atrophy in a murine model of cachexia by inhibition of the dsRNA-dependent protein kinase. Br J Cancer 2007, 96, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Bonetto, A.; Muscaritoli, M.; Costamagna, D.; Minero, V.G.; Bonelli, G.; Rossi Fanelli, F.; Baccino, F.M.; Costelli, P. Muscle atrophy in experimental cancer cachexia: is the IGF-1 signaling pathway involved? Int J Cancer 2010, 127, 1706–1717. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Ballarò, R.; Martinez-Cristobal, P.; Sala, D.; Sebastian, D.; Busquets, S.; Muscaritoli, M.; Argilés, J.M.; Costelli, P.; Zorzano, A. Autophagy Exacerbates Muscle Wasting in Cancer Cachexia and Impairs Mitochondrial Function. J Mol Biol 2019, 431, 2674–2686. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Abe, T.; Yamamoto, S.; Oishi, K. Ketogenic diet induces skeletal muscle atrophy via reducing muscle protein synthesis and possibly activating proteolysis in mice. Scientific reports 2019, 9, 19652. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Costamagna, D.; Pin, F.; Camperi, A.; Fanzani, A.; Chiarpotto, E.M.; Cavallini, G.; Bonelli, G.; Baccino, F.M.; Costelli, P. Autophagic degradation contributes to muscle wasting in cancer cachexia. Am J Pathol 2013, 182, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y.; Yoshioka, K.; Suzuki, N. The ubiquitin-proteasome system in regulation of the skeletal muscle homeostasis and atrophy: from basic science to disorders. J Physiol Sci 2020, 70, 40. [Google Scholar] [CrossRef]
- Carr, R.M.; Enriquez-Hesles, E.; Olson, R.L.; Jatoi, A.; Doles, J.; Fernandez-Zapico, M.E. Epigenetics of cancer-associated muscle catabolism. Epigenomics 2017, 9, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Wang, L.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef]
- Goetze, R.G.; Buchholz, S.M.; Patil, S.; Petzold, G.; Ellenrieder, V.; Hessmann, E.; Neesse, A. Utilizing High Resolution Ultrasound to Monitor Tumor Onset and Growth in Genetically Engineered Pancreatic Cancer Models. J Vis Exp, 3791. [Google Scholar] [CrossRef]
- Sastra, S.A.; Olive, K.P. Quantification of Murine Pancreatic Tumors by High Resolution Ultrasound. Methods Mol Biol 2013, 980. [Google Scholar] [CrossRef]
- Cui, Z.; Gilda, J.E.; Gomes, A.V. Crude and purified proteasome activity assays are affected by type of microplate. Anal Biochem 2014, 446, 44–52. [Google Scholar] [CrossRef]
- Gomes, A.V.; Waddell, D.S.; Siu, R.; Stein, M.; Dewey, S.; Furlow, J.D.; Bodine, S.C. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. Faseb j 2012, 26, 2986–2999. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.V.; Zong, C.; Edmondson, R.D.; Li, X.; Stefani, E.; Zhang, J.; Jones, R.C.; Thyparambil, S.; Wang, G.W.; Qiao, X.; et al. Mapping the murine cardiac 26S proteasome complexes. Circ Res 2006, 99, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Mishra, M.; Salemi, M.R.; Phinney, B.S.; Newens, J.L.; Gomes, A.V. Gender-specific changes in energy metabolism and protein degradation as major pathways affected in livers of mice treated with ibuprofen. Sci Rep 2020, 10, 3386. [Google Scholar] [CrossRef] [PubMed]
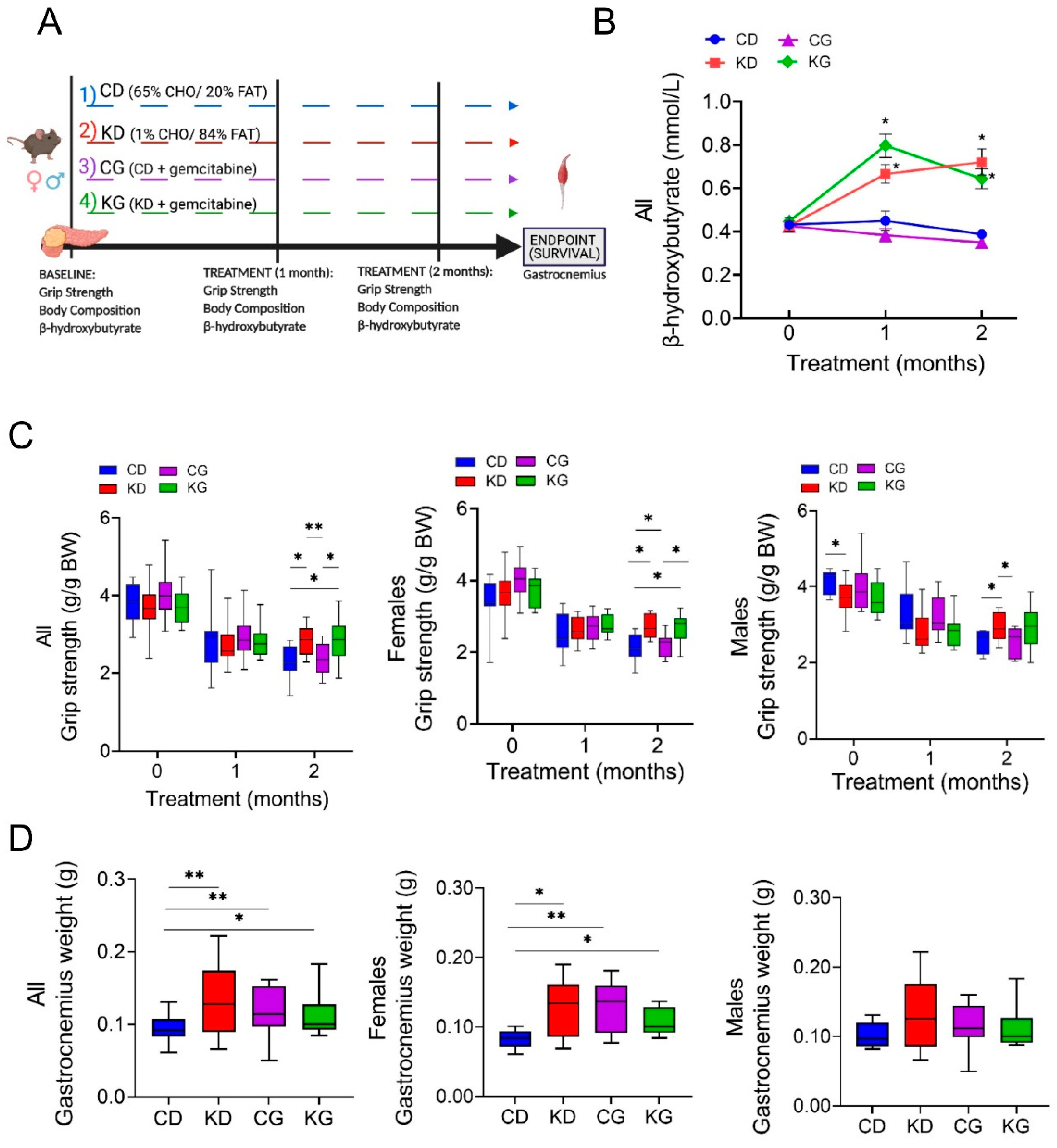
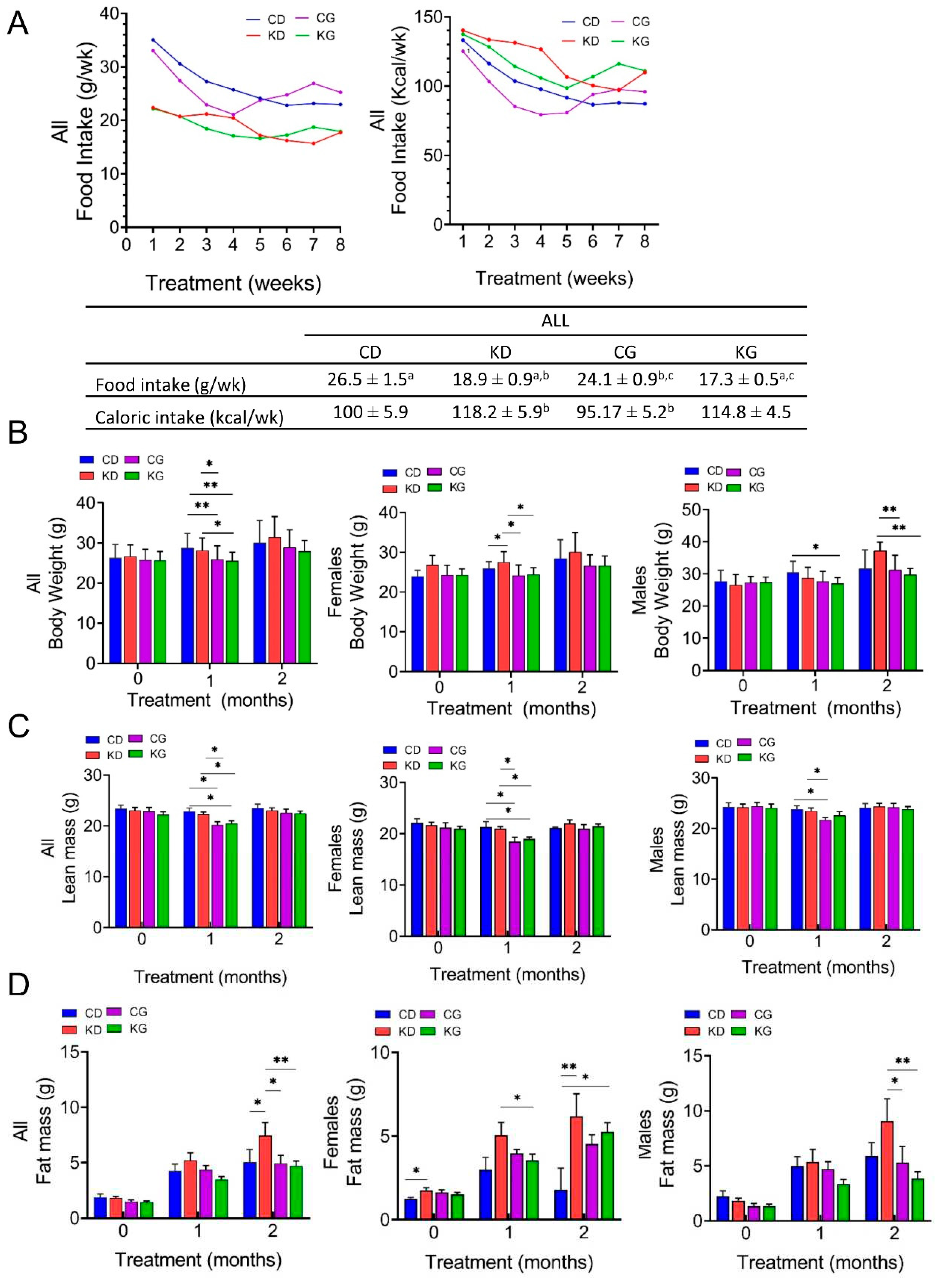
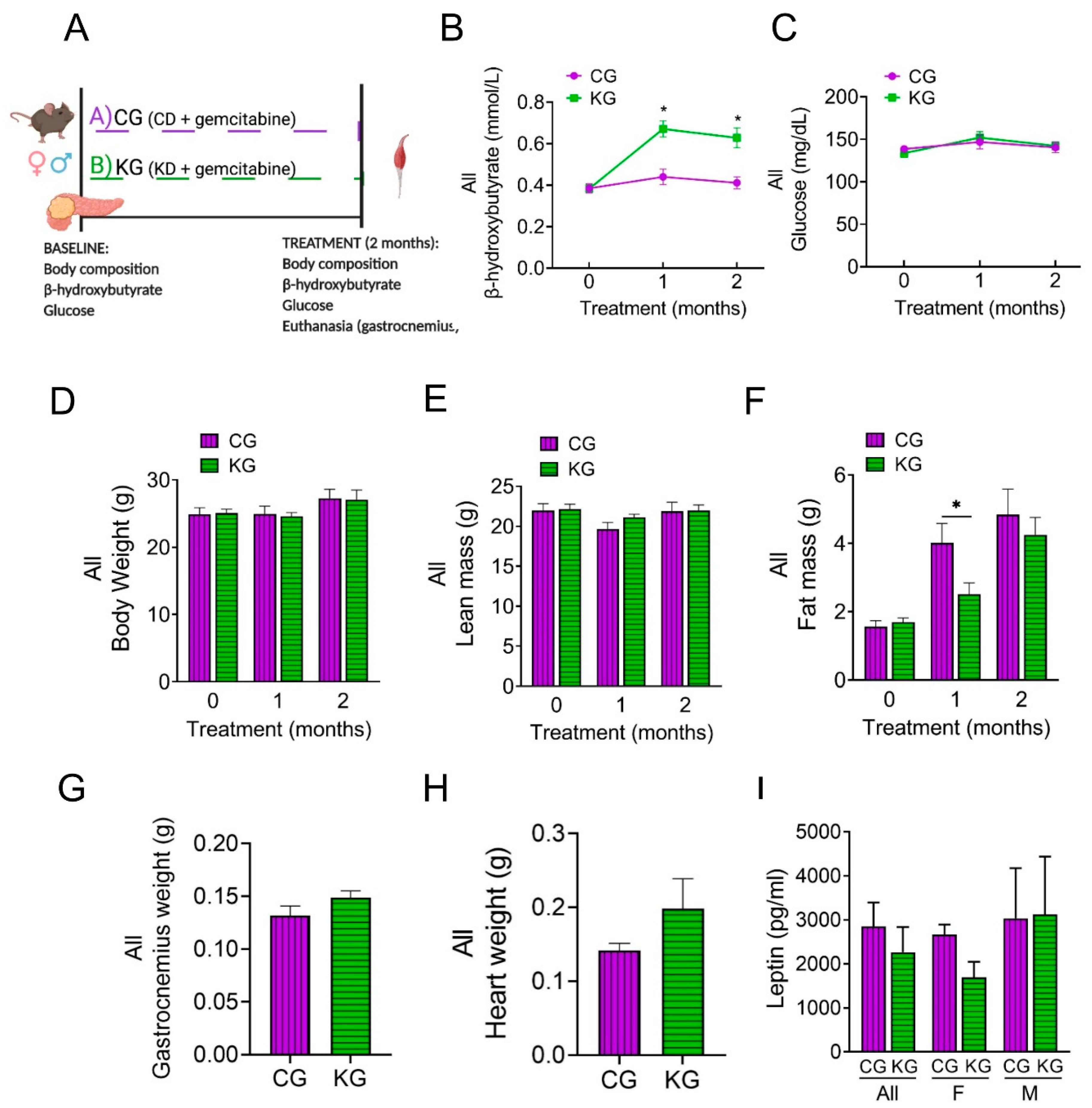
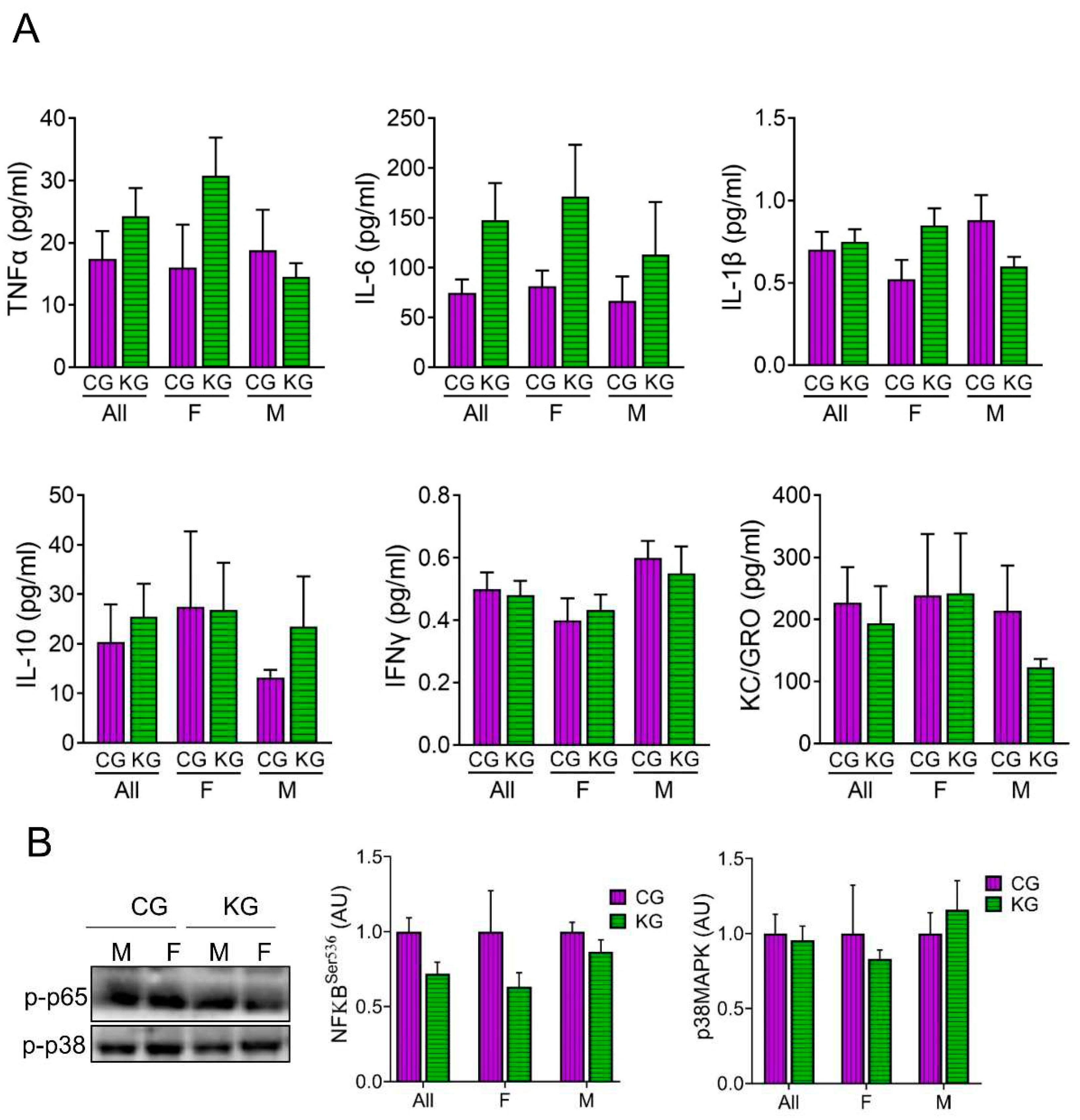
| Outcome | ||||
|---|---|---|---|---|
| Model Term | Grip Strength (per Unit of Weight) g/g BW |
Ketone Bodies (mmol/L) | ||
| Estimate (SE) | P-value | Estimate | P-value | |
| Intercept | 3.04 (0.11) | < 0.001 | 0.487 (0.067) | < 0.001 |
| Effect of KD vs. CD (without GEM, at day 30) | -0.17 (0.11) | 0.121 | 0.128 (0.08) | 0.115 |
| Effect of GEM vs. No GEM (with CD, at day 30) | 0.07 (0.093) | 0.436 | -0.148 (0.094) | 0.121 |
| Effect of Time since intervention (in days) (with CD and no GEM) |
-0.02 (0.0039) | < 0.001 | -0.002 (0.003) | 0.402 |
| Effect of baseline age (in days) | -0.0042 (0.0036) | 0.247 | 0.001 (0.002) | 0.654 |
| Effect of Male vs. Female | -0.35 (0.085) | < 0.001 | - | - |
| Effect of baseline outcome | 0.14 (0.073) | 0.066 | 0.016 (0.196) | 0.935 |
| Interaction between KD and GEM | - | - | 0.355 (0.124) | 0.006 |
| Interaction between KD and Time | 0.021 (0.0053) | < 0.001 | 0.008 (0.004) | 0.024 |
| Interaction between GEM and Time | - | - | 0.005 (0.004) | 0.222 |
| Interaction between KD, GEM, and Time | - | - | -0.018 (0.005) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





