Submitted:
06 June 2023
Posted:
07 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Fabrication of PtNP/SWCNT/PET network film electrode
2.4. Cell culture and imaging
3. Results
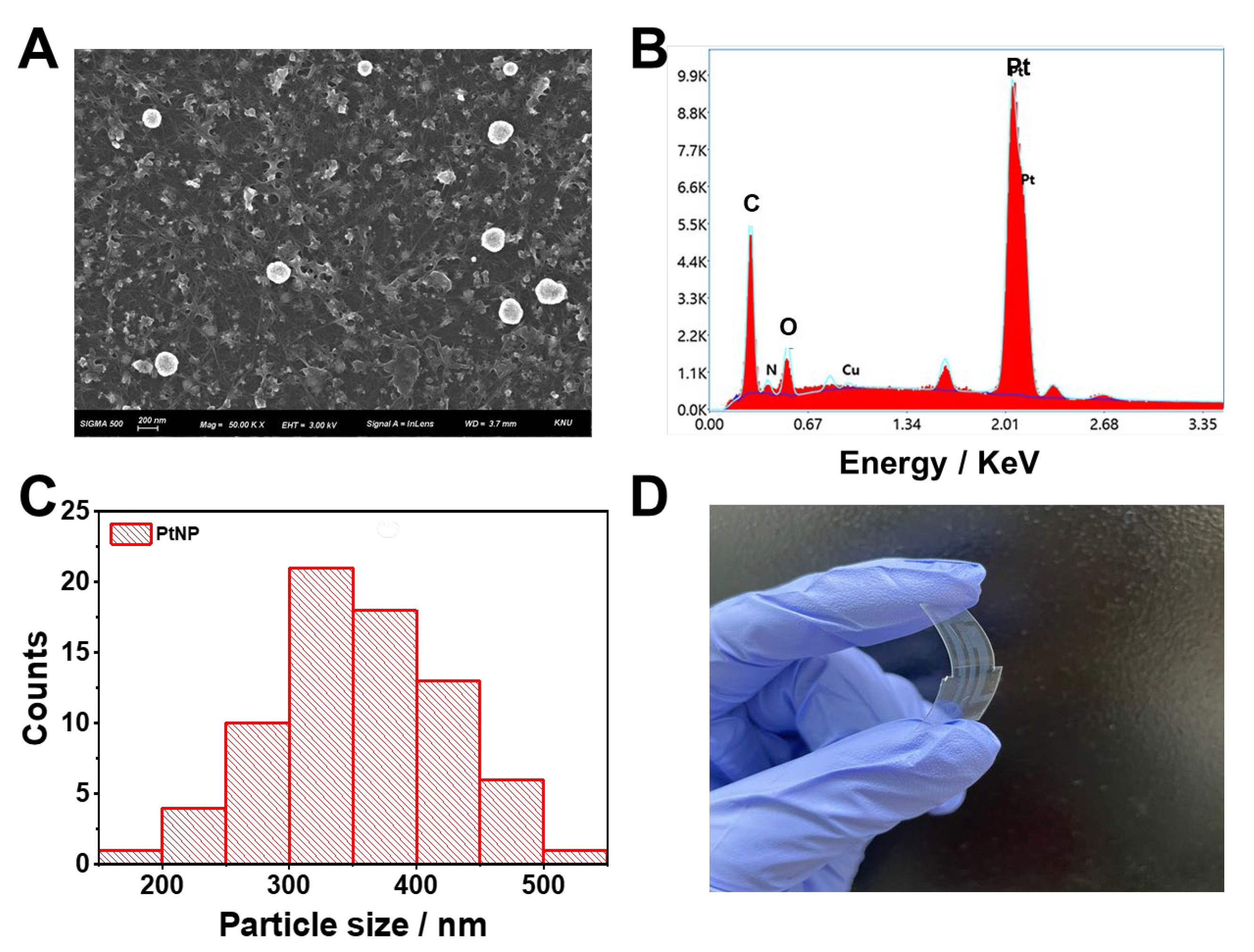
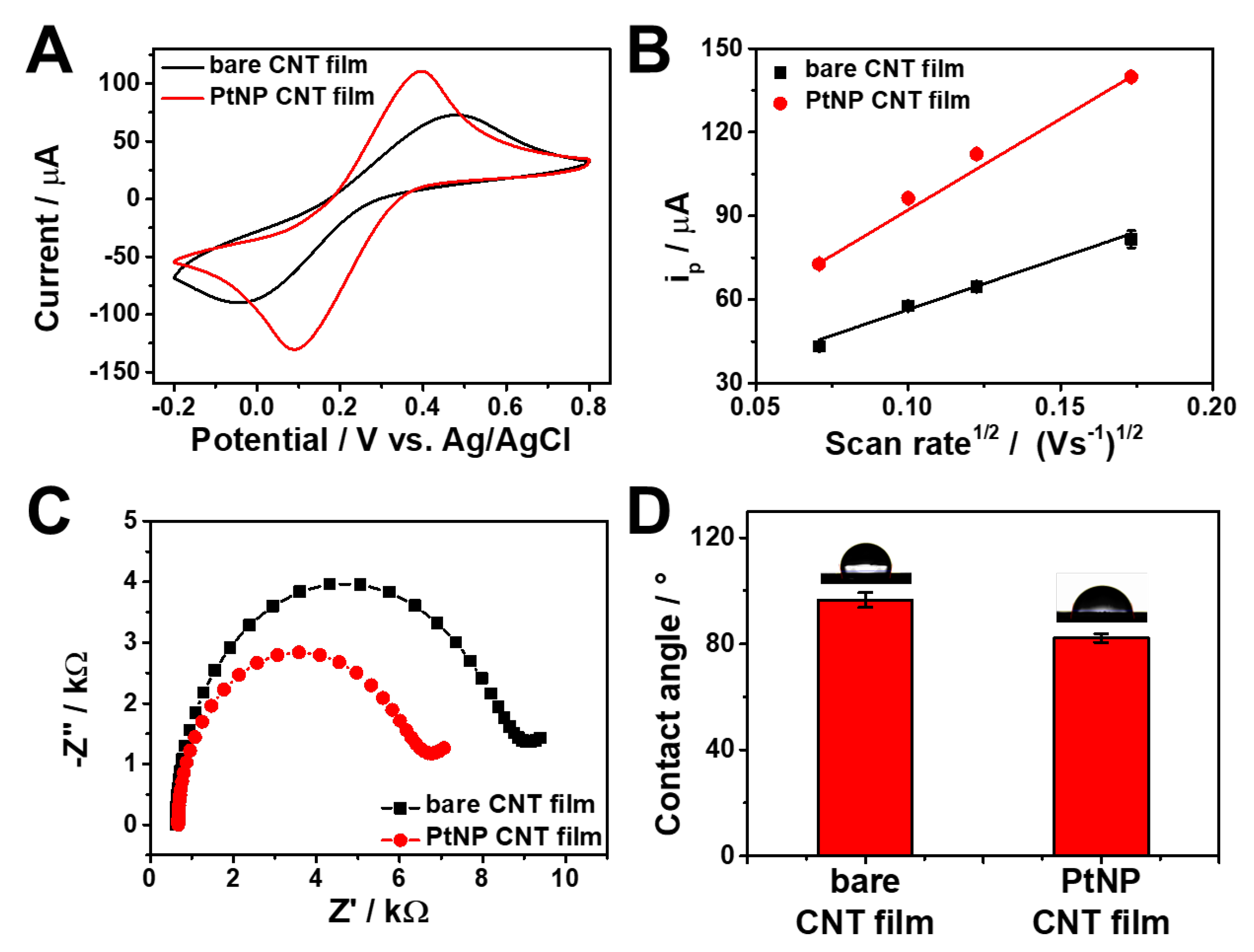
3.1. Preparation and characterization of PtNP/SWCNT network film
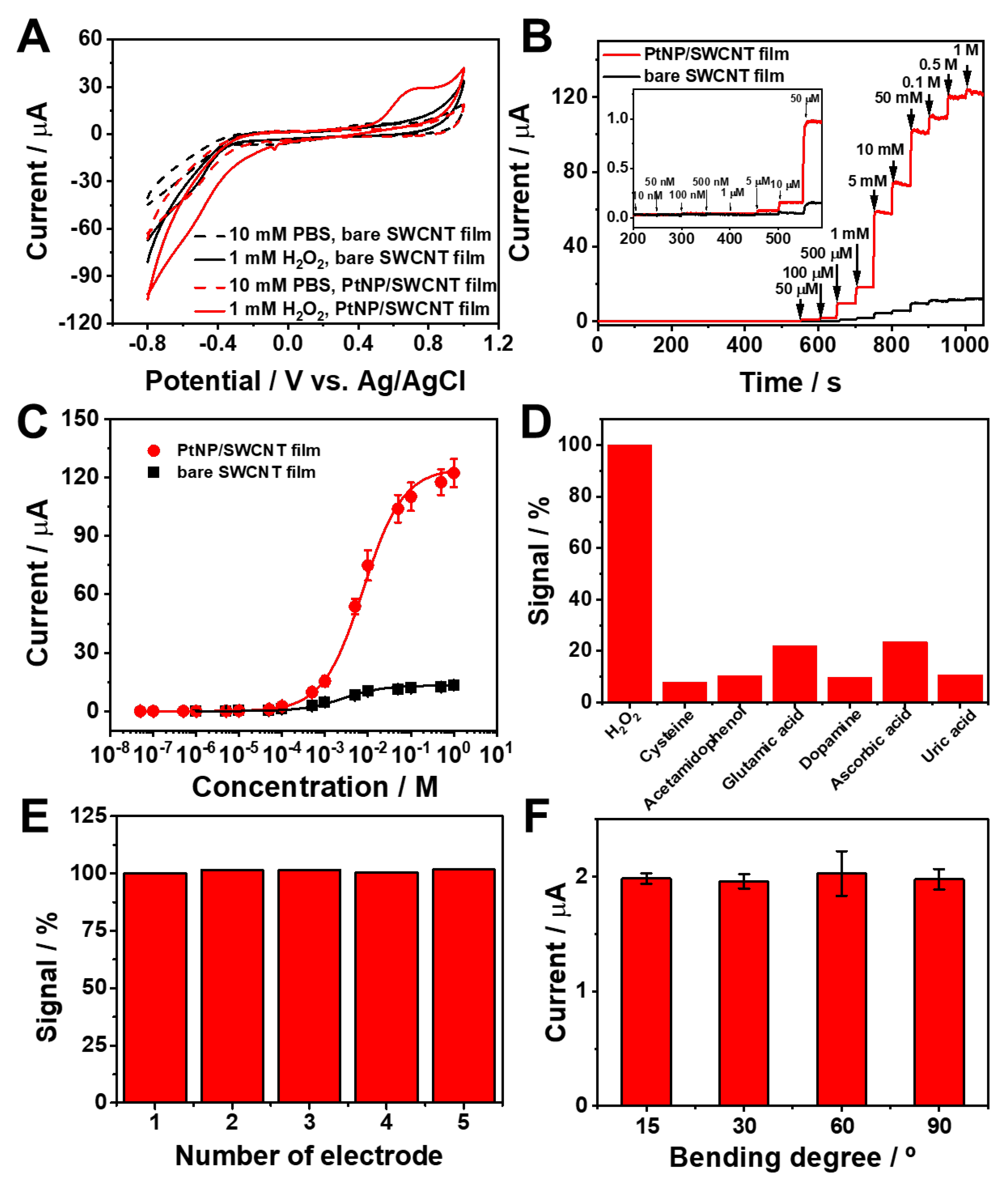
3.2. Electrochemical sensing performance toward H2O2
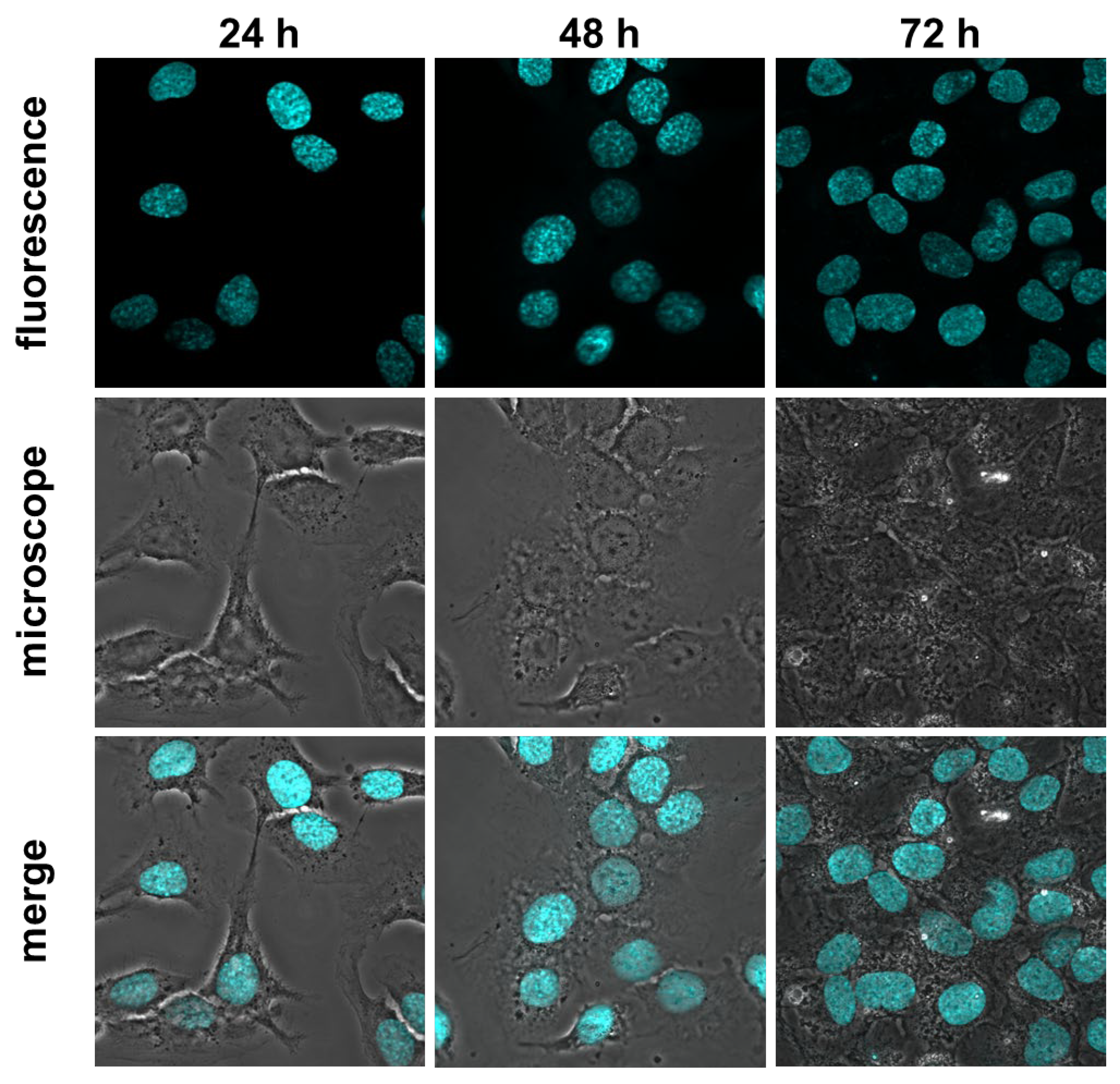
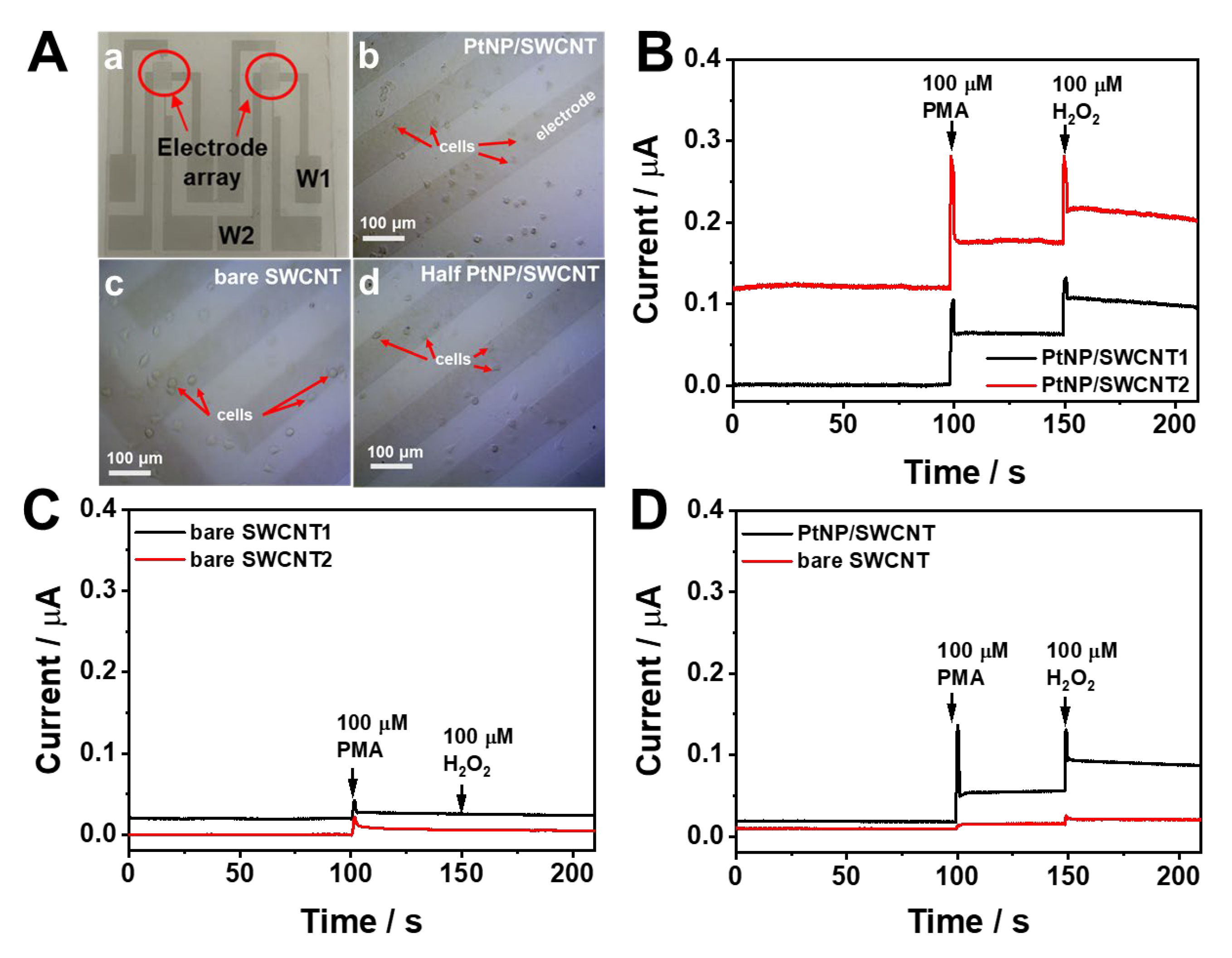
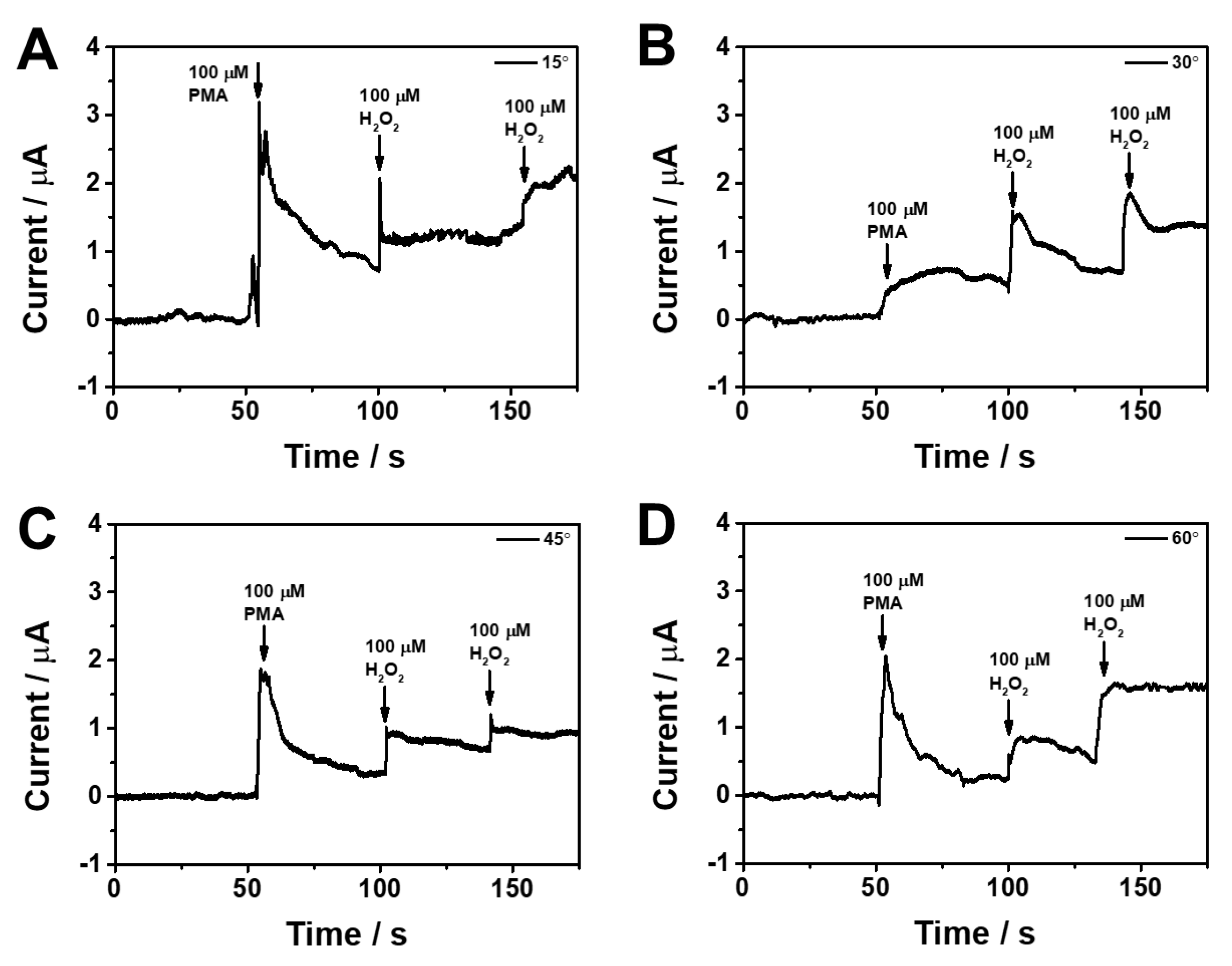
3.3. Real-time monitoring of H2O2 release from living cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.-L.; Huang, W.-H. Stretchable Electrochemical Sensors for Cell and Tissue Detection. Angew. Chem. 2021, 133, 2789–2799. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Cao, D.; Zhang, G.; Li, J.; Li, K.; Yang, Y.; Wang, W.; Jin, Y.; Sun, R.; et al. Recent Advancements in Flexible and Stretchable Electrodes for Electromechanical Sensors: Strategies, Materials, and Features. ACS Appl. Mater. Interfaces 2017, 9, 12147–12164. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-Invasive Wearable Electrochemical Sensors: A Review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-Y.; Lee, H.-B.; Lee, N.-E. A Durable, Stretchable, and Disposable Electrochemical Biosensor on Three-Dimensional Micro-Patterned Stretchable Substrate. Sens. Actuators B Chem. 2019, 283, 312–320. [Google Scholar] [CrossRef]
- Flexible Sensors for Biomedical Technology - Lab on a Chip (RSC Publishing) DOI:10.1039/C5LC90136G Available online: https://pubs.rsc.org/en/content/articlehtml/2016/lc/c5lc90136g (accessed on 30 May 2023).
- Chen, C.-H.; Lin, C.-T.; Hsu, W.-L.; Chang, Y.-C.; Yeh, S.-R.; Li, L.-J.; Yao, D.-J. A Flexible Hydrophilic-Modified Graphene Microprobe for Neural and Cardiac Recording. Nanomedicine Nanotechnol. Biol. Med. 2013, 9, 600–604. [Google Scholar] [CrossRef]
- Lou, C.; Li, R.; Li, Z.; Liang, T.; Wei, Z.; Run, M.; Yan, X.; Liu, X. Flexible Graphene Electrodes for Prolonged Dynamic ECG Monitoring. Sensors 2016, 16, 1833. [Google Scholar] [CrossRef]
- Xu, S.; Yang, D.; Zhang, F.; Liu, J.; Guo, A.; Hou, F. Fabrication of NiCo2O4 and Carbon Nanotube Nanocomposite Films as a High-Performance Flexible Electrode of Supercapacitors. RSC Adv. 2015, 5, 74032–74039. [Google Scholar] [CrossRef]
- Dou, B.; Li, J.; Jiang, B.; Yuan, R.; Xiang, Y. DNA-Templated In Situ Synthesis of Highly Dispersed AuNPs on Nitrogen-Doped Graphene for Real-Time Electrochemical Monitoring of Nitric Oxide Released from Live Cancer Cells. Anal. Chem. 2019, 91, 2273–2278. [Google Scholar] [CrossRef]
- Yang, X.-K.; Tang, Y.; Qiu, Q.-F.; Wu, W.-T.; Zhang, F.-L.; Liu, Y.-L.; Huang, W.-H. Aβ1–42 Oligomers Induced a Short-Term Increase of Glutamate Release Prior to Its Depletion As Measured by Amperometry on Single Varicosities. Anal. Chem. 2019, 91, 15123–15129. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Qiu, Q.-F.; Jiang, H.; Zhang, F.-L.; Liu, Y.-L.; Amatore, C.; Huang, W.-H. Real-Time Intracellular Measurements of ROS and RNS in Living Cells with Single Core–Shell Nanowire Electrodes. Angew. Chem. Int. Ed. 2017, 56, 12997–13000. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Chen, K.; Shin, W.-J.; Lu, C.-J.; Kong, G.-W.; Patnaik, D.; Lee, S.-H.; Cortes, J.F.; Rogers, J.A. Stretchable, Wireless Sensors and Functional Substrates for Epidermal Characterization of Sweat. Small 2014, 10, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-L.; Jung, C.-W.; Oh, Y.-J.; Kim, D.-E. A Highly Flexible Transparent Conductive Electrode Based on Nanomaterials. NPG Asia Mater. 2017, 9, e438–e438. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Lien, D.-H.; Kao, Z.-K.; Huang, T.-H.; Liao, Y.-C.; Lee, S.-C.; He, J.-H. All-Printed Paper Memory. ACS Nano 2014, 8, 7613–7619. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Hwang, J.C.; Kim, G.G.; Park, J.-U. Flexible Electronics Based on One-Dimensional and Two-Dimensional Hybrid Nanomaterials. InfoMat 2020, 2, 33–56. [Google Scholar] [CrossRef]
- Pavel, I.-A.; Lakard, S.; Lakard, B. Flexible Sensors Based on Conductive Polymers. Chemosensors 2022, 10, 97. [Google Scholar] [CrossRef]
- Wang, B.-Y.; Yoo, T.-H.; Lim, J.W.; Sang, B.-I.; Lim, D.-S.; Choi, W.K.; Hwang, D.K.; Oh, Y.-J. Enhanced Light Scattering and Trapping Effect of Ag Nanowire Mesh Electrode for High Efficient Flexible Organic Solar Cell. Small 2015, 11, 1905–1911. [Google Scholar] [CrossRef]
- Xue, T.; Sheng, Y.; Xu, J.; Li, Y.; Lu, X.; Zhu, Y.; Duan, X.; Wen, Y. In-Situ Reduction of Ag+ on Black Phosphorene and Its NH2-MWCNT Nanohybrid with High Stability and Dispersibility as Nanozyme Sensor for Three ATP Metabolites. Biosens. Bioelectron. 2019, 145, 111716. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, L.; Wu, R.; Zhu, Y.; Sheng, Y.; Nie, P.; Liu, P.; Xu, L.; Wen, Y. Portable Wireless Intelligent Sensing of Ultra-Trace Phytoregulator α-Naphthalene Acetic Acid Using Self-Assembled Phosphorene/Ti3C2-MXene Nanohybrid with High Ambient Stability on Laser Induced Porous Graphene as Nanozyme Flexible Electrode. Biosens. Bioelectron. 2021, 179, 113062. [Google Scholar] [CrossRef]
- Lee, C.-S.; Ju, Y.; Kim, J.; Kim, T.H. Electrochemical Functionalization of Single-Walled Carbon Nanotubes with Amine-Terminated Dendrimers Encapsulating Pt Nanoparticles: Toward Facile Field-Effect Transistor-Based Sensing Platforms. Sens. Actuators B Chem. 2018, 275, 367–372. [Google Scholar] [CrossRef]
- Oh, J.-W.; Heo, J.; Kim, T.H. An Electrochemically Modulated Single-Walled Carbon Nanotube Network for the Development of a Transparent Flexible Sensor for Dopamine. Sens. Actuators B Chem. 2018, 267, 438–447. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive Oxygen Species and Cancer Paradox: To Promote or to Suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Reactive Oxygen Species in Cancer: A Dance with the Devil. Cancer Cell 2015, 27, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Brewer, A.C.; Mustafi, S.B.; Murray, T.V.A.; Rajasekaran, N.S.; Benjamin, I.J. Reductive Stress Linked to Small HSPs, G6PD, and Nrf2 Pathways in Heart Disease. Antioxid. Redox Signal. 2013, 18, 1114–1127. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Skiada, V.; Barbouti, A. Redox Signaling and Cancer: The Role of “Labile” Iron. Cancer Lett. 2008, 266, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xin, Y.; Wu, W.; Fu, B.; Zhang, Z. Topotactic Conversion of Copper(I) Phosphide Nanowires for Sensitive Electrochemical Detection of H2O2 Release from Living Cells. Anal. Chem. 2016, 88, 7724–7729. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Pramanik, D.; Dey, S.G. Active Site Environment of Heme-Bound Amyloid β Peptide Associated with Alzheimer’s Disease. J. Am. Chem. Soc. 2011, 133, 81–87. [Google Scholar] [CrossRef]
- Genç, F.; Milcheva, N.P.; Hristov, D.G.; Gavazov, K.B. A Simple Cloud Point Extraction-Spectrophotometric Method for Total Vanadium Determination Using 4-(2-Thiazolylazo)Resorcinol and H2O2. Chem. Pap. 2020, 74, 1891–1901. [Google Scholar] [CrossRef]
- Gupta, V.; Mahbub, P.; Nesterenko, P.N.; Paull, B. A New 3D Printed Radial Flow-Cell for Chemiluminescence Detection: Application in Ion Chromatographic Determination of Hydrogen Peroxide in Urine and Coffee Extracts. Anal. Chim. Acta 2018, 1005, 81–92. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, S.; Cui, X.; Wang, X.; Yang, L. Enzyme-like Catalysis of Polyoxometalates for Chemiluminescence: Application in Ultrasensitive Detection of H2O2 and Blood Glucose. Talanta 2019, 205, 120139. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-W.; Luo, Y.; Wang, Y.-M.; Duan, L.-Y.; Jiang, J.-H.; Yu, R.-Q. Graphitic Carbon Nitride Nanosheets-Based Ratiometric Fluorescent Probe for Highly Sensitive Detection of H2O2 and Glucose. ACS Appl. Mater. Interfaces 2016, 8, 33439–33445. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cen, Y.; Sohail, M.; Xu, G.; Wei, F.; Shi, M.; Xu, X.; Song, Y.; Ma, Y.; Hu, Q. A Ratiometric Fluorescence Universal Platform Based on N, Cu Codoped Carbon Dots to Detect Metabolites Participating in H2O2-Generation Reactions. ACS Appl. Mater. Interfaces 2017, 9, 33011–33019. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Liu, P.; Zhang, Y.; Yu, Z.; Chen, X.; Zhou, L.; Nie, B.; Żaczek, A.; Chen, J.; Liu, J. Sensitive Detection of Single-Cell Secreted H2O2 by Integrating a Microfluidic Droplet Sensor and Au Nanoclusters. Anal. Chem. 2018, 90, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Annalakshmi, M.; Chen, S.-M.; Sathesh, T.; Peng, T.-K.; Balamurugan, T.S.T. Facile Solvothermal Preparation of Mn2CuO4 Microspheres: Excellent Electrocatalyst for Real-Time Detection of H2O2 Released from Live Cells. ACS Appl. Mater. Interfaces 2018, 10, 43543–43551. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.H.; Ko, E.; Kim, M.K.; Tran, V.-K.; Son, S.E.; Geng, Y.; Hur, W.; Seong, G.H. Graphene Oxide-Gold Nanozyme for Highly Sensitive Electrochemical Detection of Hydrogen Peroxide. Sens. Actuators B Chem. 2018, 274, 201–209. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhu, A.; Luo, Y.; Deng, Z.; Tian, Y. Real-Time Electrochemical Monitoring of Cellular H2O2 Integrated with In Situ Selective Cultivation of Living Cells Based on Dual Functional Protein Microarrays at Au−TiO2 Surfaces. Anal. Chem. 2010, 82, 6512–6518. [Google Scholar] [CrossRef]
- Li, Y.; Huan, K.; Deng, D.; Tang, L.; Wang, J.; Luo, L. Facile Synthesis of ZnMn2O4@rGO Microspheres for Ultrasensitive Electrochemical Detection of Hydrogen Peroxide from Human Breast Cancer Cells. ACS Appl. Mater. Interfaces 2020, 12, 3430–3437. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, M.; Meng, X.; Xiang, J.; Wang, L.; Ren, Q.; Guo, S. Graphene/Intermetallic PtPb Nanoplates Composites for Boosting Electrochemical Detection of H2O2 Released from Cells. Anal. Chem. 2017, 89, 3761–3767. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, H.; Zhuo, J.; Zhu, Z.; Papakonstantinou, P.; Lubarsky, G.; Lin, J.; Li, M. Biosensor Based on Ultrasmall MoS2 Nanoparticles for Electrochemical Detection of H2O2 Released by Cells at the Nanomolar Level. Anal. Chem. 2013, 85, 10289–10295. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, M.; Peng, J.; Hu, D.; Hao, Y.; Qian, Z. A Review on Recent Advances in Hydrogen Peroxide Electrochemical Sensors for Applications in Cell Detection. Chin. Chem. Lett. 2022, 33, 4133–4145. [Google Scholar] [CrossRef]
- Zhang, T.; Gu, Y.; Li, C.; Yan, X.; Lu, N.; Liu, H.; Zhang, Z.; Zhang, H. Fabrication of Novel Electrochemical Biosensor Based on Graphene Nanohybrid to Detect H2O2 Released from Living Cells with Ultrahigh Performance. ACS Appl. Mater. Interfaces 2017, 9, 37991–37999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, X.; Wang, X.; Shiu, K.-K.; Zhu, Y.; Jiang, H. Highly Sensitive Graphene–Pt Nanocomposites Amperometric Biosensor and Its Application in Living Cell H2O2 Detection. Anal. Chem. 2014, 86, 9459–9465. [Google Scholar] [CrossRef] [PubMed]
- Choe, Y.; Yu, J.-Y.; Son, Y.-O.; Park, S.-M.; Kim, J.-G.; Shi, X.; Lee, J.-C. Continuously Generated H2O2 Stimulates the Proliferation and Osteoblastic Differentiation of Human Periodontal Ligament Fibroblasts. J. Cell. Biochem. 2012, 113, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-J.; Liu, C.-A.; Huang, B.; Tseng, A.H.; Wang, D.L. Shear-Induced Endothelial Mechanotransduction: The Interplay between Reactive Oxygen Species (ROS) and Nitric Oxide (NO) and the Pathophysiological Implications. J. Biomed. Sci. 2014, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M. Reactive Oxygen Species in Mechanotransduction. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L484–L485. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Lee, J.-J.; Rahman, M.A. Electrochemical Sensors Based on Carbon Nanotubes. Sensors 2009, 9, 2289–2319. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, S.; Luque, R.; Han, S.; Hu, L.; Xu, G. Recent Development of Carbon Electrode Materials and Their Bioanalytical and Environmental Applications. Chem. Soc. Rev. 2016, 45, 715–752. [Google Scholar] [CrossRef]
- Abera, B.D.; Falco, A.; Ibba, P.; Cantarella, G.; Petti, L.; Lugli, P. Development of Flexible Dispense-Printed Electrochemical Immunosensor for Aflatoxin M1 Detection in Milk. Sensors 2019, 19, 3912. [Google Scholar] [CrossRef]
- Zhou, Y.; Azumi, R. Carbon Nanotube Based Transparent Conductive Films: Progress, Challenges, and Perspectives. Sci. Technol. Adv. Mater. 2016, 17, 493–516. [Google Scholar] [CrossRef]
- Agrisuelas, J.; González-Sánchez, M.-I.; Valero, E. Hydrogen Peroxide Sensor Based on in Situ Grown Pt Nanoparticles from Waste Screen-Printed Electrodes. Sens. Actuators B Chem. 2017, 249, 499–505. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Cai, Z.; Oyama, M.; Chen, X. Advances in Enzyme-Free Electrochemical Sensors for Hydrogen Peroxide, Glucose, and Uric Acid. Microchim. Acta 2014, 181, 689–705. [Google Scholar] [CrossRef]
- Jiménez-Pérez, R.; González-Rodríguez, J.; González-Sánchez, M.-I.; Gómez-Monedero, B.; Valero, E. Highly Sensitive H2O2 Sensor Based on Poly(Azure A)-Platinum Nanoparticles Deposited on Activated Screen Printed Carbon Electrodes. Sens. Actuators B Chem. 2019, 298, 126878. [Google Scholar] [CrossRef]
- Rahman, M.M.; Jeon, I.C. Studies of Electrochemical Behavior of SWNT-Film Electrodes. J. Braz. Chem. Soc. 2007, 18, 1150–1157. [Google Scholar] [CrossRef]
- Heinemann, A.; Koenen, S.; Schwabe, K.; Rehbock, C.; Barcikowski, S. How Electrophoretic Deposition with Ligand-Free Platinum Nanoparticles Affects Contact Angle. Key Eng. Mater. 2015, 654, 218–223. [Google Scholar] [CrossRef]
- Goran, J.M.; Phan, E.N.H.; Favela, C.A.; Stevenson, K.J. H2O2 Detection at Carbon Nanotubes and Nitrogen-Doped Carbon Nanotubes: Oxidation, Reduction, or Disproportionation? Anal. Chem. 2015, 87, 5989–5996. [Google Scholar] [CrossRef]
- Sitta, E.; Gómez-Marín, A.M.; Aldaz, A.; Feliu, J.M. Electrocatalysis of H2O2 Reduction/Oxidation at Model Platinum Surfaces. Electrochem. Commun. 2013, 33, 39–42. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Y.; Zhang, H.; Wang, Z. Electrochemiluminescence Biosensor for Nucleolin Imaging in a Single Tumor Cell Combined with Synergetic Therapy of Tumor. ACS Sens. 2020, 5, 1216–1222. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, W.; Liu, Y.; Sun, Y.; Jiang, Y.; Zhang, S. Electrochemiluminescence-Microscopy for MicroRNA Imaging in Single Cancer Cell Combined with Chemotherapy-Photothermal Therapy. Anal. Chem. 2019, 91, 12581–12586. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




