1. Introduction
Understanding the responses of virions to ambient biological, chemical, and physical factors is critical not only for basic biological viewpoints, but also for defining optimum and/or most harmful conditions for the industrial, medical, and agricultural use of virions [
1,
2]. Among these factors, chemism has been studied for a long time [
3,
4,
5,
6,
7,
8]. It is generally accepted that divalent cations, such as Ca
2+ and Mg
2+, affect the lytic cycle of many bacteriophages, acting on either adsorption, penetration, or intracellular development of the virus [
2,
6,
9]. The adsorption on bacterial cells of some phages has been also accelerated by univalent cations, such as Na
+ [
4,
8]. Phages can generally show infection capabilities at a pH range of 5-11 [
1,
10,
11]. The reported results, however, were obtained in solutions of mixed ionic compounds, e.g., media and buffers. Prior to using the multi-ionic conditions for studying the chemical processes of the lytic cycle of bacteriophages, viral responses to individual ion solutions should be elucidated.
To understand the effects of sole-ionic compound solutions on the infectivity of T4 virions, T4 virions need to be immersed in pure ionic solutions. However, the inoculant of virions contains certain number of ionic solutes, because virions will be inactivated immediately in pure water [2, 4, 8, this study]. Therefore, we control the amount of the solutes in the inoculant at the minimum level. This is the reason why we call the sole-ionic compound solutions as quasi-pure.
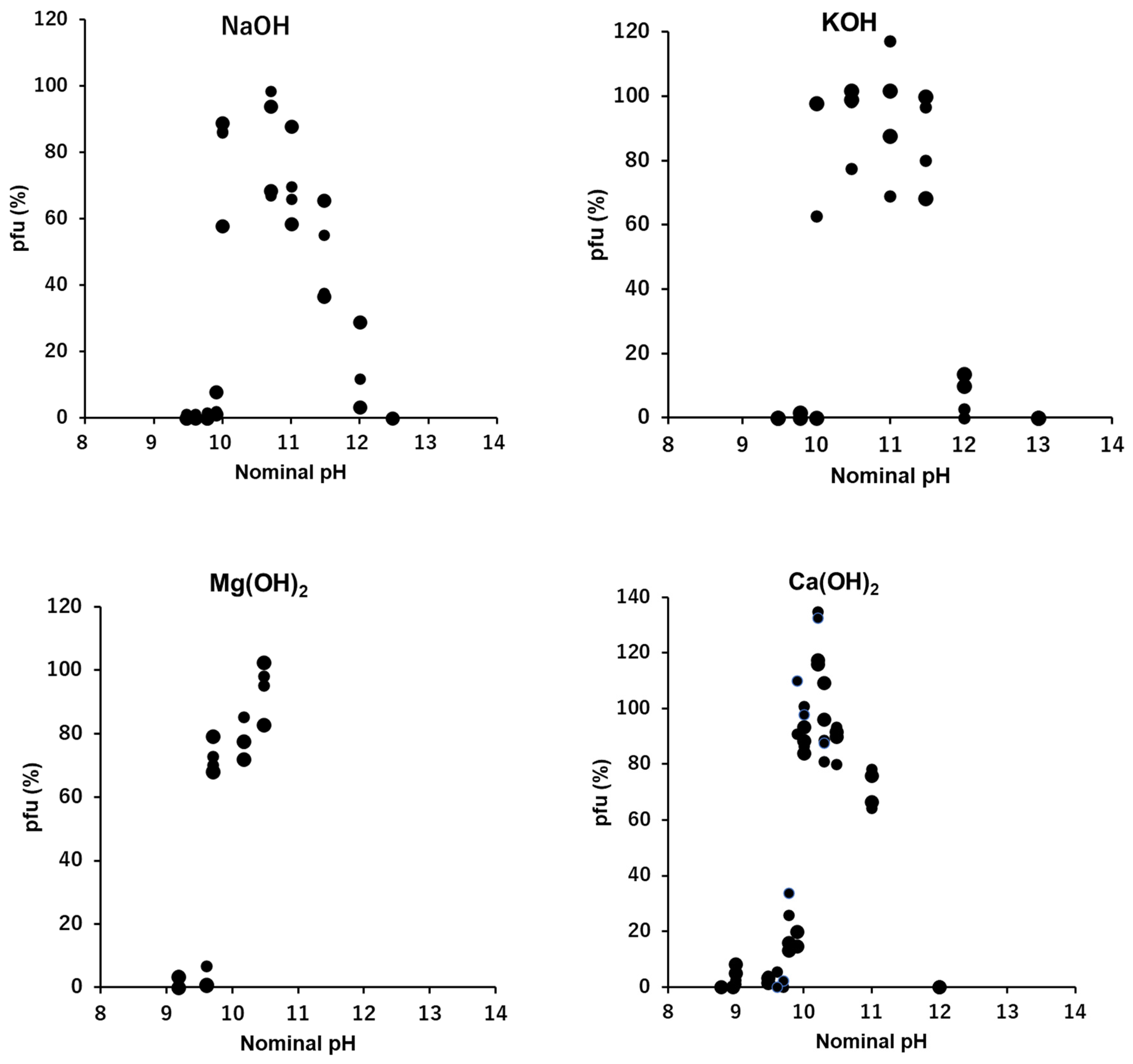
The pfu spectra indicates the viral infectivity is irreversibly changed by soaking the virions in quasi-pure ionic solutions prior to the adsorption process. Our results generally followed the trends in previous reports in neutral conditions [
2,
6], e.g., the optimal divalent cation levels ranged between 0.01 and 10 mM, and one order higher for monovalent cations. One of the major findings of our study was the effect of H
+ and OH
- on phage stability. When T4 virions were directly exposed to acid or alkali solutions, T4 virions were stable in over 10
-1 mM OH
- solution, equivalent to alkaline pH 10, although infectivity was lost below 10
-1 mM OH
- and higher than 10 mM OH
-. No infectious activity was observed in acid to neutral and even in weak alkaline solutions. Hydrogen ion, H
+, clearly shows negative effect on phage stability, while hydroxide ion, OH
-, stabilized phages at around 10
-1 - 10 mM OH
-.
The activation-inactivation is switched on/off at 10-4 N OH-, pH 10 equivalent, which is corresponding to the pKa of the deprotonation of the DNA bases G and T. The results implicate the maintaining of infectivity of a virion may depend on the flexibility of the intra-capsid DNA by deprotonation.
2. Materials and Methods
Strains:
The bacteriophage studied was T4 (ATCC 11303-B4), and its host bacteria were Escherichia coli (ATCC 11303).
Preparation of T4 virions:
Peptone broth was used for culturing the host bacteria,
E. coli. T4 suspension was obtained by the plate lysate method and the small-scale liquid culture [
12]. In the plate lysate method, the virions were suspended in 2-3 ml of an electrolyte solution including 1.8 mM NaCl, 0.12 mM MgSO
4, 0.12 mM MgCl
2, 0.034 mM CaCl
2 and 0.05 mM KCl. The extracted suspension was sterilized by filtration of the eluent with 0.2 μm filter (Advantec AS020). To exclude the effects of anonymous ions, the suspensions of T4 virions were purified with ultracentrifugation and dialysis. For ultracentrifugation, crude bacteriophage particles were purified by isopycnic centrifugation through CsCl gradients [12, Beckman XPN-90, SW32 rotor, 4℃, 24 h]. Following ultracentrifugation, T4 suspensions were dialysed against T-buffer (modified TM buffer [
12] 0.1 M NaCl, 2 mM MgSO
4, 0.5 mM phosphate buffer, pH 7.5) for one week replacing the outside T-buffer five times. The chemicals used were special grade products from Wako Pure Chemical Ind. Ltd.
Preparation of suspension of T4 virions in quasi-pure solution:
It is well known that the infectious ability of virions is instantly lost when they are immersed in pure water [2, 4, 8, this study], with the result that the active virions are practically stored in relevant ionic solutions. When the stored virions are used for experiments, aliquots of stored suspensions are inoculated in target solvents. Accordingly, the ions included in the aliquots of the suspensions are added into the target solutions. It is critical to maintain the concentrations of these ions introduced by the inoculations for studying the connections between the ionic concentrations and the viral activity. In these experiments, the ultra-centrifuged T4 virions were dialysed against T-buffer and stored in T-buffer. After the stored virions were appropriately diluted with T-buffer, these T-buffer suspensions were diluted twice. First, dilution with Milli-Q water 100-fold and acclimated to this condition for five minutes. At this point, the ionic concentrations of the viral suspensions were 0.01 T-buffer. Second, this Milli-Q water dilutions were diluted again 100-fold with the test solutions. As a whole, the final concentrations of T-buffer in the test solutions were 0.0001 T-buffer, i.e., 0.01 mM NaCl, 0.2 μM MgSO
4, 0.05 μM phosphate buffer. In these diluted ionic conditions, virions gradually lost their infectious ability by the time of their manipulation. The time course measurements of viral activities in these diluted conditions indicating the decrease of the activity were expressed as:
Where Vt: density of active virions at t, V0: density of active virions at the initial moment, -k: inactivation coefficient, t: time.
For counting plaque forming units, virions were plated after the acclimation at the first dilution for 5min. and followed by incubation in the second dilution for 15 min.
Ionic solutions:
In this paper, we are concerned with the concentration of a solute. To prepare the acid and alkali solutions of the testing concentrations, 1 M solutions of acid and alkali were diluted to the target concentrations. According to the solubilities, the original solution of Ca(OH)2 was 10 mM and it was 0.15 mM for Mg(OH)2. The pH value of the dilution was not measured each time, and pH was not adjusted. However, the pH values measured by Whatman pH-indicator paper were close to the expected values, e.g., the pH value of 1 mM NaOH was ca. pH 11. The pH values reported here, nominal pH, were not the measured values, but the calculated values from concentrations of H+ and OH-. On the other hand, the pH values of the solutions of neutral ionic compounds, i.e., NaCl, Na2SO4, KCl, K2SO4, CaCl2, CaSO4, MgCl2, MgSO4, fell within the range of pH 6 – 6.5.
4. Discussion
The effects of a quasi-pure solution of a sole ionic compound on the survival of T4 virions are examined. Previous studies of ionic effects on virion activities were mainly focused on the integrated effects of ions on the virus-host adsorption during pre- and on-adsorption processes in multi-ionic media or buffers [
2,
4,
8,
13,
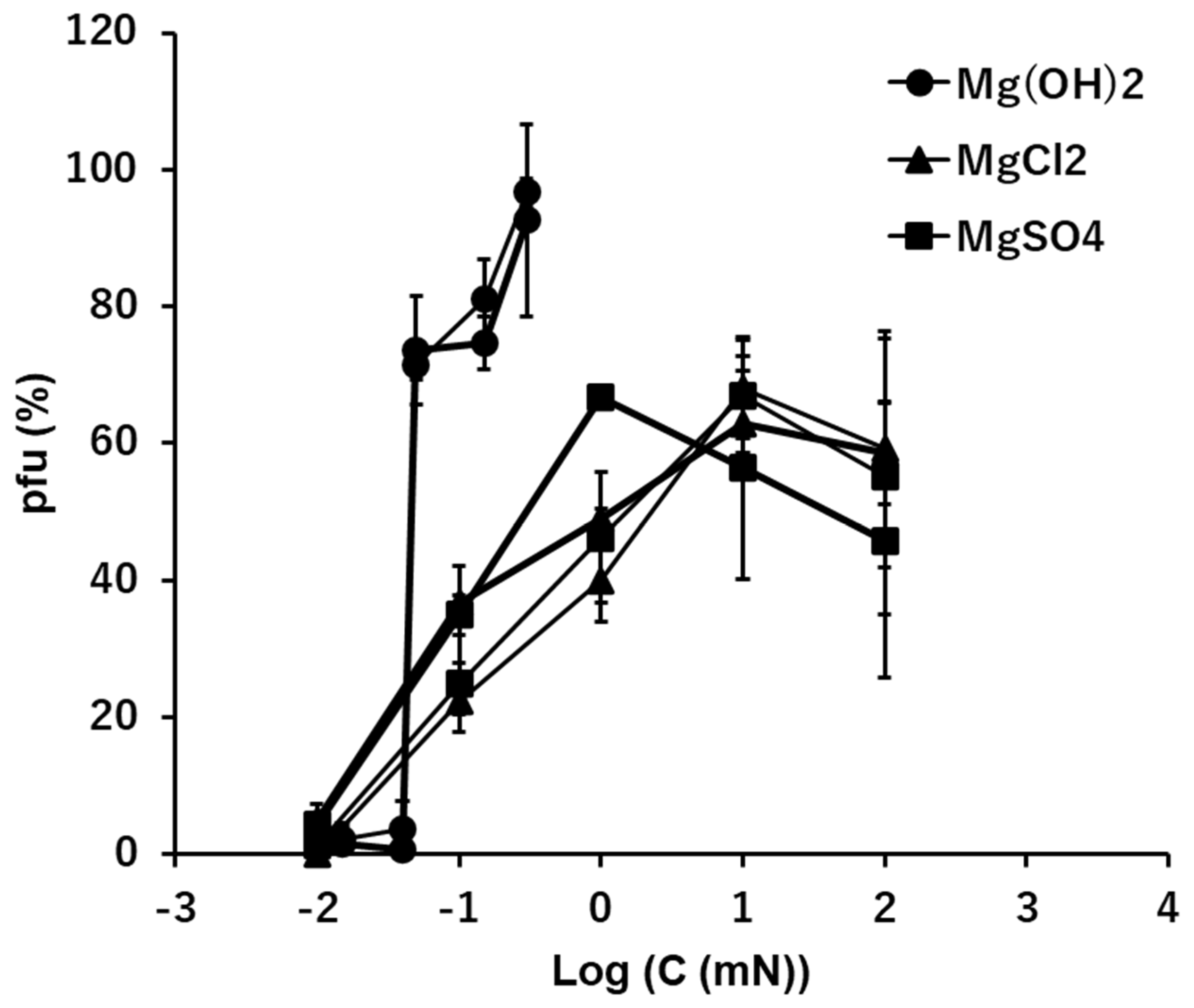
14]. In this study, the pre-adsorption processes are separated from on-adsorption processes and the effect of a sole ionic compound on the pre-adsorption process are examined. Virions experienced the sole ionic compound were plated and adsorbed to host bacteria following the conventional plating method. Accordingly, in this study the conditions of host bacteria and the adsorption were practically identical at whole cases and the numbers of pfu were the reflections of the irreversible alternations of virions produced during the 15 min. immersion in solutions of the sole ionic compound prior to the adsorption processes. Virions in our study were not exposed to pure solution of an ionic compound, but quasi-pure solution, i.e., when an aliquot of viral suspension was inoculated, a trace amount of ions included by the inoculants of virion suspensions were introduced to the test solution. However, the inoculated amounts of ions, i.e., 0.0001 T-buffer: 0.01 mM NaCl, 0.2 μM MgSO
4, 0.05 μM phosphate buffer, were far below the minimum concentrations of these ions necessary for pfu formation in the conditions tested (
Figure 2,
Figure 3,
Figure 4 and
Figure 5). Indeed, if there were no supplementary ions, the virions lost their activity immediately and no pfu was observed at the tested conditions (
Table 1). Therefore, even though the solutions tested were quasi-pure, it was practically pure solution from the viewpoint of viral responses to ionic conditions.
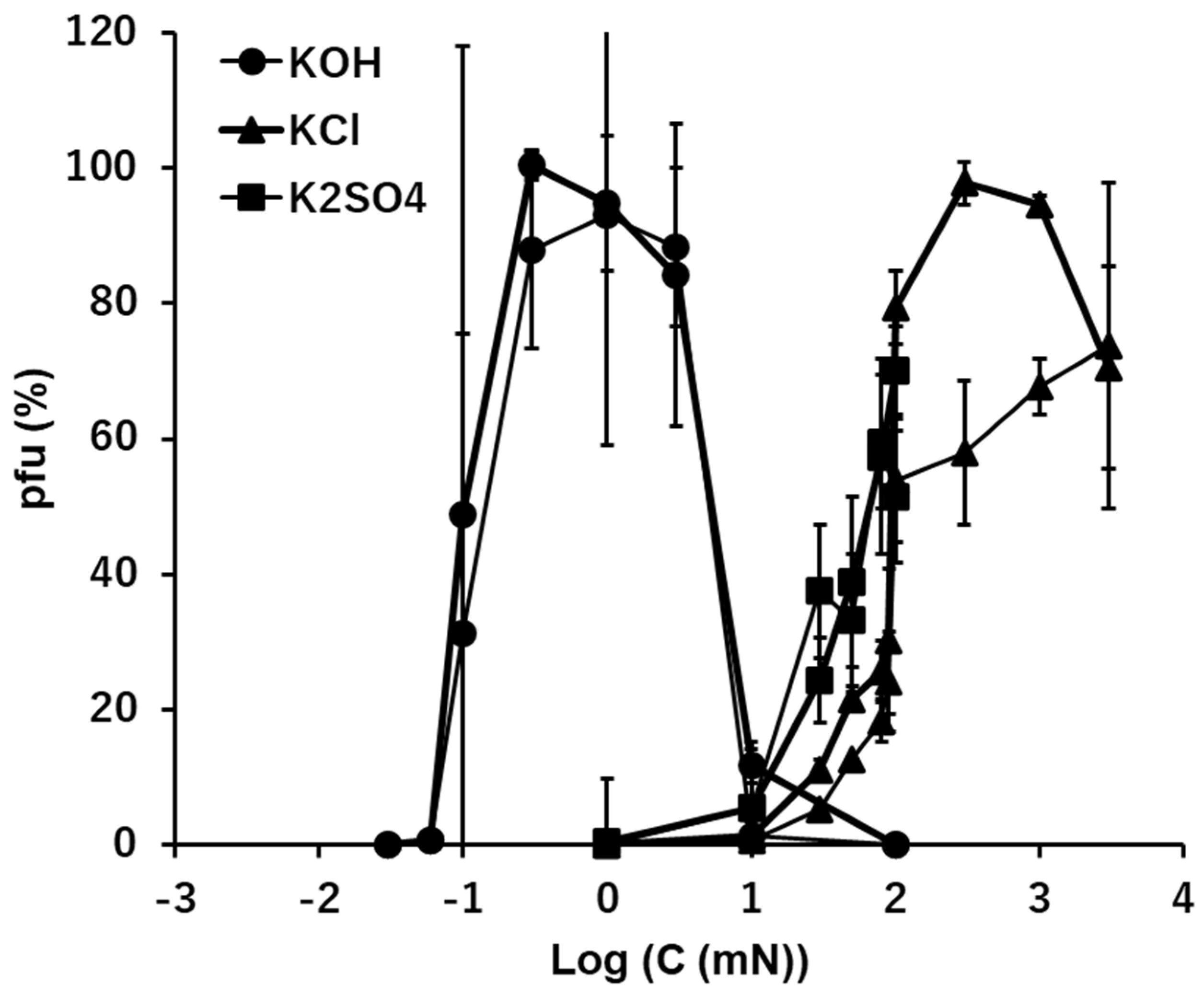
The relatively short time exposure to a solution of one ionic compound defines the survivals of following infection and multiplication processes of T4 virions. It has been known that the optimal divalent cation levels ranged between 10
-2 and 10 mM [
2,
6] and phages such as T2 and T4 having a requirement of NaCl for adsorption at the level of 100 mM which is ca. one order of magnitude higher than divalent cations [
4,
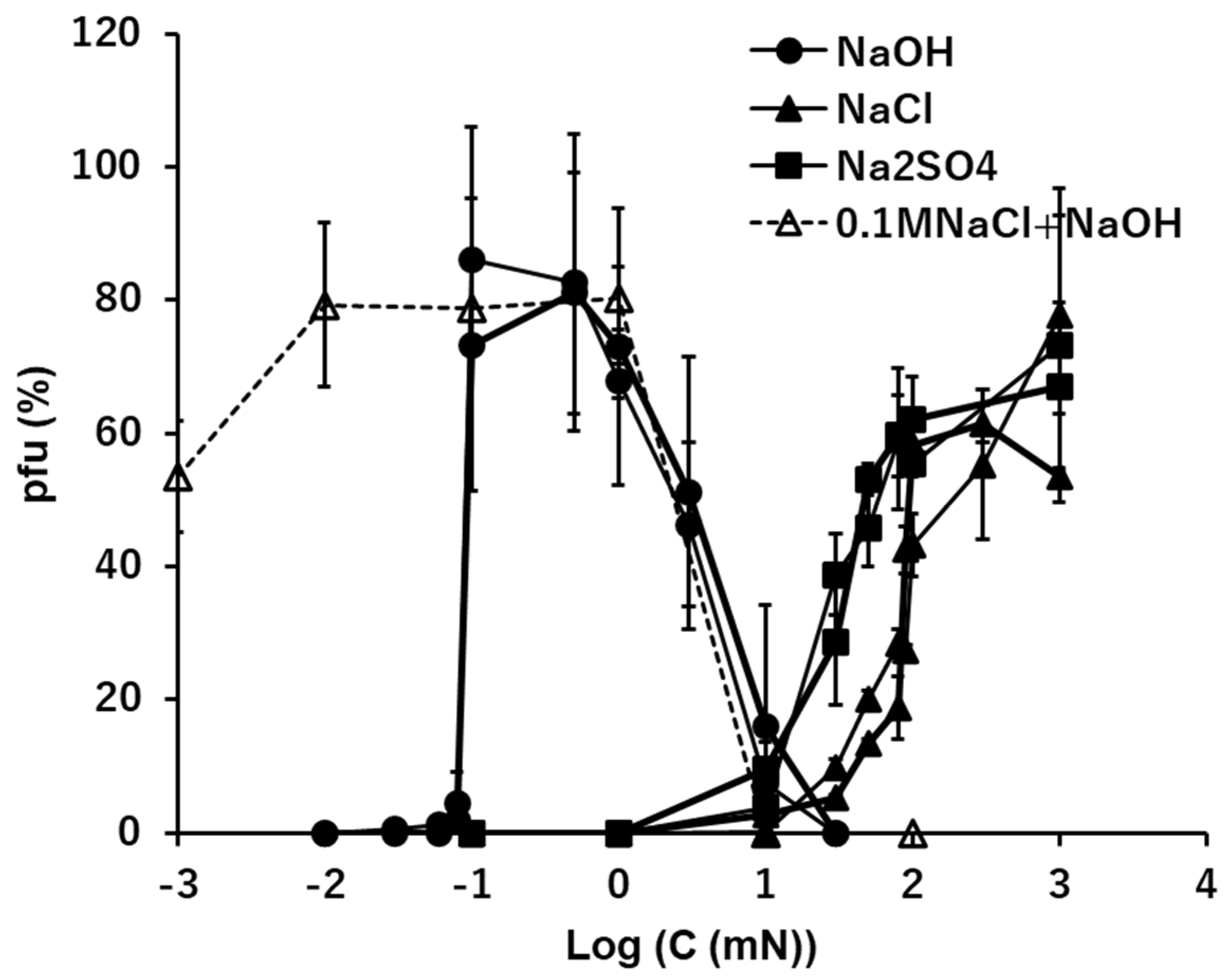
8]. In this study, the results produced in quasi-pure neutral ionic solutions include results equivalent to the previous studies which were gained by controlling the target ions in multiple ionic solutions. The increasing curves of the preservabilities of infectivity along with the increase of the concentrations of quasi-pure neutral ionic solutions were linear to the concentrations of ions in univalent cations (data not shown). However, they were linear in the semi-log plot in divalent cations (
Figure 4 and
Figure 5) or the preservabilities increase logarithmically for divalent cations, i.e., the slopes of pfu to the concentrations of the divalent cations were in reverse proportion to the concentrations of the ions, i.e., the preservation of the viral infectivity increased rapidly at the threshold concentration of the divalent cation, afterwards, the slope of the increase turned gentle rapidly. The threshold concentration of the divalent cations to preserve infectivity was ca. 10
-5 M, which is 10
-2 - 10
-3 times lower than the univalent cations [
4]. This implicates the maintenance of the infectivity, or deprotonation of DNA in the viral head (see below), may require small amounts of divalent cations which may supply the deficient divalent cations to the pre-existed divalent cations in the viral heads.
Phages generally can show infectious abilities in culture media or in buffers the pH ranges of which are adjusted to 5 - 11 [
1,
10,
11,
15,
16]. In this study, virions were exposed directly to “pure” acid or “pure” alkali solutions. Without additional coexisting media or buffers, T4 virions showed no ability to form pfu in any acid to neutral conditions (
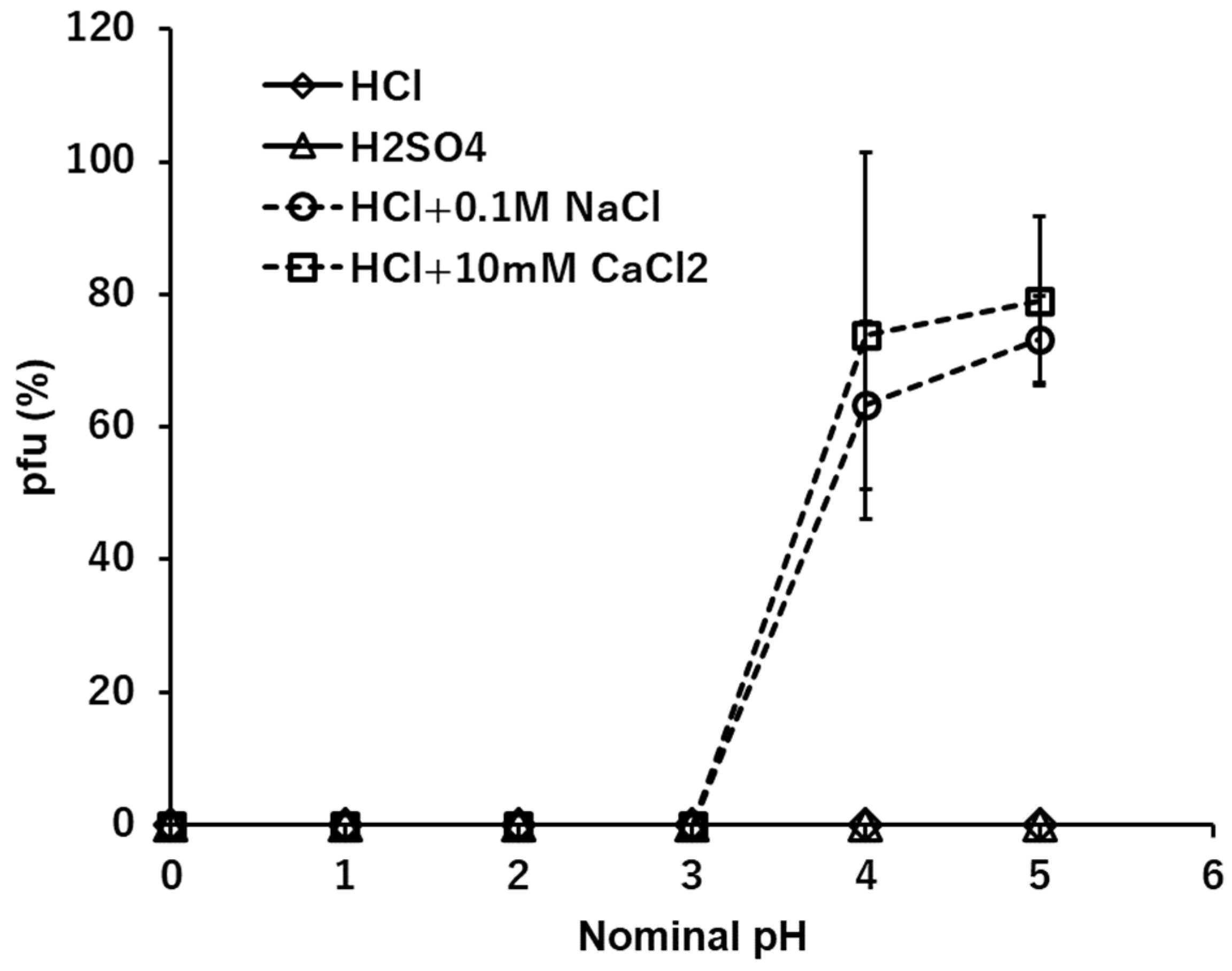
Figure 1). Because virions were inactivated instantly in pure water [2, 4, 8, this study], it is not clear the inactivation in the acidic condition was formed by a passive effect derived from no-preservative agent of infectivity or by an active inactivation of acidic ions. Coexistence of neutral ions, e.g., 0.1 M NaCl or 10 mM CaCl
2, which had abilities to preserve the activities of virions in ca. 50%, protect virions from the inactivation by H+ only when the concentration of H
+ was lower than 10
-4 N, or higher than nominal pH 4 (
Figure 1). Accordingly, hydrogen ion actively inactivated the T4 virions, and without neutral ions, T4 virions has no tolerance to sole acid to neutral condition to maintain their infectivity. On the contrary, it has been reported that virions were more stable in suitably high enough concentrations of alkaline ranging from pH 7 to pH 11 [
10,
11,
15,
16]. In this study, after fifteen minutes incubation, 70 – 90% of viral survival was maintained in 0.1 – 1 mM OH
- solutions, that was equivalent to a range between pH 10 and pH 11. This indicates that in quasi-pure alkaline solutions T4 virions cannot sustain their infectivity below pH 10, i.e., T4 virions need alkaline condition, higher than pH 10, to sustain their activity (
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6). Not only in the short time sustainability, the time-course changes of the viral survivals in these solutions indicated the inactivation coefficients, -k in the equation (1), were compatible in 0.1 mM NaOH, 100 mM NaCl and 10 mM CaCl
2 and also -k of the sole 0.1 mM Ca(OH)
2 and 0.1 mM Mg(OH)
2 were equivalent or smaller than the full strength T-buffer (
Table 1). Pure relevant alkaline solutions can preserve the viral activity considerably long time. At around pH 10, the bases of DNA, guanine and thymine start the deprotonation, pKa of deprotonation of guanine deoxyribose-5’-phosphate and thymidine-5’-phosphate are 9.7 and 10, respectively [
17]. In the environment of pH higher than 12, the viral DNA becomes denatured [
18]. This indicates a condition; the bases of the nucleic acids are deprotonated, but the DNA is not denatured; is a required condition for the maintaining of the infectivity of phage virions. The addition of neutral ions, e.g., 0.1 M NaCl to NaOH and 10 mM CaCl
2 to Ca(OH)
2, expanded the infectivity toward the lower alkaline concentration range. At 0.01 mN of OH
-, at which hydroxy ion had no potential to sustain the infectivity, addition of neutral ions showed equivalent sustainability with the infectivity at 0.1 mN hydroxy ion (
Figure 2 and
Figure 5). This implicates these neutral ions may maintain the pre-existed deprotonation of the DNA bases in a viral head (see below).
In the acidic buffers, virions can maintain the infectivity over wider acidic ranges [
1,
10,
11,
19], however, in the quasi-pure acidic condition, T4 virions immediately lose their infectivity (Figure1). Alcohol-based disinfectants appear to have a minimal effect on non-enveloped viruses, while low-pH alcohols exhibit strong virucidal effects against them [
19]. Virions tend to irreversibly lose their infectivity in the low-pH range. Contrarily, T4 virions maintain their infectivity in quasi-pure alkaline conditions in the pH range between pH 10 and pH 11 (12) (
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6). When neutral salts coexist in the alkaline solutions, the sustainable range of pH expands wider to the lower alkaline concentrations (
Figure 2 and
Figure 5). Calcium hydroxide is used as virucide at the pH 13 in farms [
20,
21]. Our results also indicate the loss of infectivity of T4 virions at the pH range higher than 12. However, alkaline, including calcium hydroxide, show protective actions to the viral infectivity at the dilute ranges (
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6). If other ions coexist, the range of protection can expand wider. Around the area where the alkaline virucidal agent is applied at higher concentration, zones of lower concentration surrounding the area always exist, and some types of virions may be able to maintain their infectivity at these lower concentration zones. While care may be required for using alkaline agents as the virucidal uses, the results of a bacteriophage, T4, cannot directly apply to the pathogenic virions. Understanding the activities of virions in ionic solutions is essential for the viral dynamics [
3,
4,
5,
6,
7,
8,
9]. Prior to investigating the behaviors of virions in the solutions of the mixtures of multiple ions, like buffers, effects of individual ions on the viral behavior should be elucidated first. Our methodology provides a tool to elucidate the basic effects of individual ions, as well as mixed and combined ions, on the infectivity of viruses.
The solvent for the thermal denaturation of DNA used in Marmur and Doty (1962) was 0.15 M NaCl plus 0.015 M sodium citrate. The 15 mM of sodium citrate preserved DNA in the coil form, not the compact composition [
23]. They emphasized that the solvent of DNA containing the univalent cation as concentration of 0.15 - 0.2 M Na
+ ensure the DNA in the coil composition will not to be denatured. This implicates univalent cations may support the infectivity of virions by preventing the denaturation of DNA in the deprotonation condition in the viral head. In addition, Rao and Black (2010) claimed that T4 DNA is packed in the head with ~1000 molecules of imbedded and mobile internal proteins, and the IPI*, the major of them, distributed high density of basic residues on the surfaces pf them that may allow rapid DNA ejection through the portal and tail without unfolding-refolding. If this state of DNA is derived from the deprotonation of the DNA bases and the IPI* is the major agent of the deprotonation in the neutral ambient condition, the neutral ions may support the deprotonation by the IPI*, because without suitable concentrations of neutral ions virions lost the ability of infection [
1,
2,
4,
6,
8,
9,
10,
11]. Further studies to elucidate the specific roles of neutral ions and chelates on the preservation of the viral infectivity is anticipated.
5. Conclusions
Clear differences were identified in the minimum required concentrations to maintain the infectivity of virions; around 10
-1 mM for hydroxide ions, 10
-2 mM for divalent cations, and 1 mM for univalent cations; and the pfu-ion concentration curves of viral preservabilities; a switch on/off type for hydroxide ions, direct correlation for univalent cations and logarithmic curve for divalent cations (
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6). The minimum required concentrations and pfu-ion concentration curves may be two facets for one process, while the mechanism how pre-adsorption exposure of virions to ionic solutions causes irreversible changes of infectivity in the following multiplication processes of them is unknown. The viral DNA in capsids maintain their compactions by ions like Ca
2+, and immersion of virions in ionic solution will exchange ions between in heads and outer solution [
23,
25]. This conformational condition of DNA in the head, in addition to the ionic alternation on proteins [
2,
6], can affect the following multiplication processes of the virions. We proposed here the deprotonation of the bases of DNA, guanine and thymine, is essential for the infectivity of T4 virions. Higher than ca. pH 10 is the critical alkaline condition for the deprotonation [
17], which reduces the hydrogen bond energy between the double helix DNA chains [
26]. The viral DNA becomes denatured when the environmental pH is higher than 12 [
18]. In the pH range between 10 to 12, the DNA is not yet denatured but at the state of lower hydrogen bond energy between the DNA chains. This may be the condition where the viral infectivity is preserved in alkaline solutions. However, the viral infectivity is preserved in the neutral condition when the solvent of the viral suspension contains relevant amounts of neutral ions as discussed above and shown in
Figure 2,
Figure 3,
Figure 4 and
Figure 5. These neutral ions are not the acceptor of protons of the bases but may protect the state of the pre-existed deprotonation of DNA. The maintenance of the deprotonation of DNA in the viral head can be another factor which affect the multiplication of the viruses. Further studies are required for elucidating the mechanism of these processes.