Submitted:
07 June 2023
Posted:
07 June 2023
You are already at the latest version
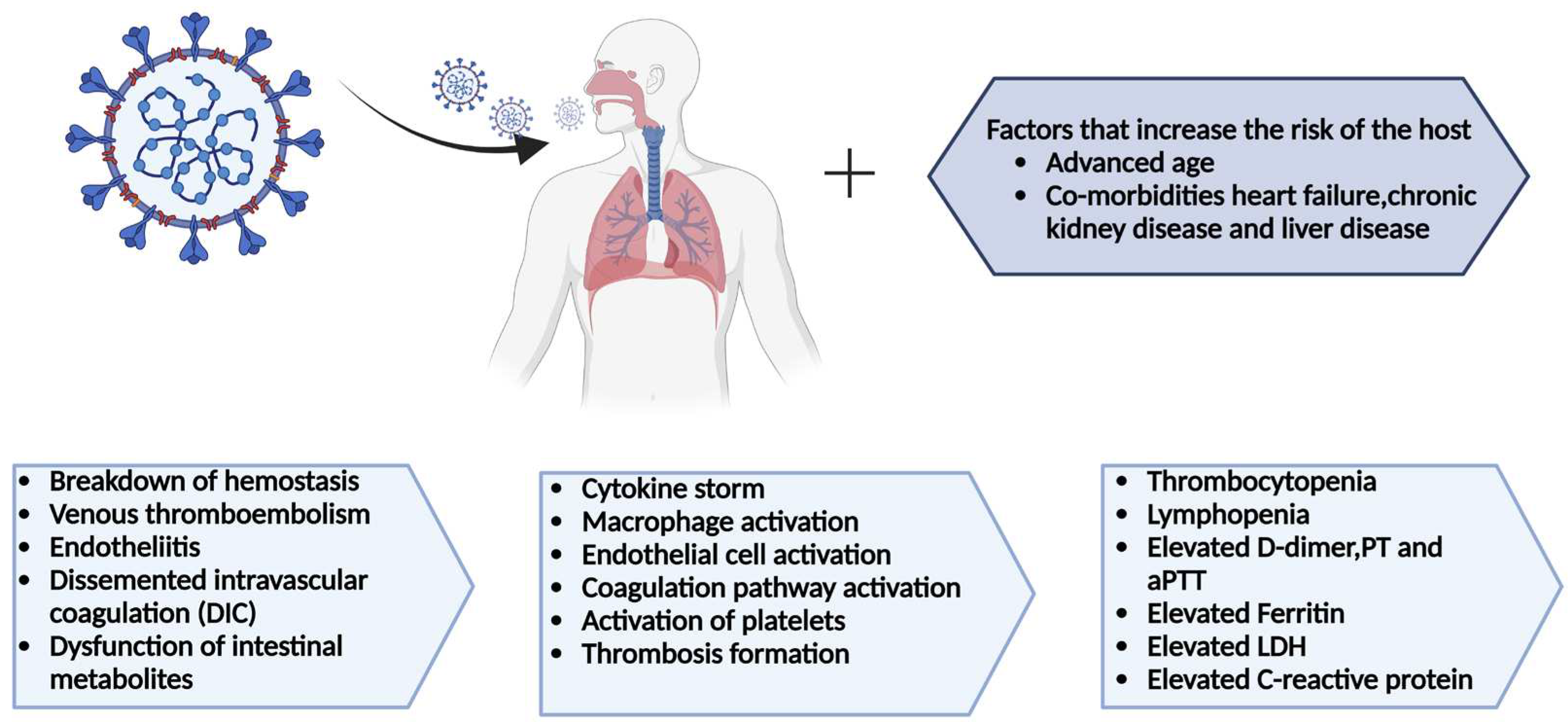
Abstract
Keywords:
1. Introduction
2. COVID-19 and the hematological abnormalities
3. Hematological system and their complications
3.1. Complication in the hematological system and their mechanism
4. COVID-19 and pre-existing hematological complication
4.1. Coagulation manifestations
4.2. Thrombocytopenia
4.3. Lymphopenia
4.4. Neutrophilia
5. Biomarkers
6. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Pandemic. World Health Organization (WHO). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. (Coronaviridae Study Group of the International Committee on Taxonomy of Viruses). The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-Ncov and Naming it SARS-CoV-2. Nat Microbiol (2020) 5(4):536–44. [CrossRef]
- Kim, K. S. , Ejima, K., Iwanami, S., Fujita, Y., Ohashi, H., Koizumi, Y.,... & Iwami, S. (2021). A quantitative model used to compare within-host SARS-CoV-2, MERS-CoV, and SARS-CoV dynamics provides insights into the pathogenesis and treatment of SARS-CoV-2. PLoS biology, 19(3), e3001128. [CrossRef]
- Standl, F.; Jöckel, K.-H.; Brune, B.; Schmidt, B.; Stang, A. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020, 21, e77–E77. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Li, Q. et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 1199. [Google Scholar]
- Shah, H.; Khan, S.H.; Dhurandhar, N.V.; Hegde, V. The triumvirate: why hypertension, obesity, and diabetes are risk factors for adverse effects in patients with COVID-19. Acta Diabetol. 2021, 58, 831–843. [Google Scholar] [CrossRef]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef]
- Shahri, M.K.; Niazkar, H.R.; Rad, F. COVID-19 and hematology findings based on the current evidences: A puzzle with many missing pieces. Int. J. Lab. Hematol. 2020, 43, 160–168. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Guo, D.; Wei, S.; Geng, Y.; Wang, E.; Wang, Z.; Zhao, X.; Su, M.; Liu, Q.; et al. Co-Circulation of Canine Coronavirus I and IIa/b with High Prevalence and Genetic Diversity in Heilongjiang Province, Northeast China. PLOS ONE 2016, 11, e0146975–e0146975. [Google Scholar] [CrossRef]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.; To, K.; et al. A Major Outbreak of Severe Acute Respiratory Syndrome in Hong Kong. New Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef]
- Lu, L.; Zhong, W.; Bian, Z.; Li, Z.; Zhang, K.; Liang, B.; Zhong, Y.; Hu, M.; Lin, L.; Liu, J.; et al. A comparison of mortality-related risk factors of COVID-19, SARS, and MERS: A systematic review and meta-analysis. J. Infect. 2020, 81, e18–e25. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ng, M.H.; Li, C.K. Thrombocytopenia in patients with severe acute respiratory syndrome (review). Hematology 2005, 10, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.M.; Wu, A.; To, K.F.; Lee, N.; Lam, C.W.K.; Wong, C.K.; Chan, P.K.S.; Ng, M.H.L.; Yu, L.M.; Hui, D.S.; et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ 2003, 326, 1358–1362. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. 4: Lancet 395(10223), 1022.
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. 5: Lancet 395(10223), 1022.
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.E. Hematologic parameters in patients with COVID-19 infection: a reply. Am. J. Hematol. 2020, 95, E215–E215. [Google Scholar] [CrossRef]
- Liao, D.; Zhou, F.; Luo, L.; Xu, M.; Wang, H.; Xia, J.; Gao, Y.; Cai, L.; Wang, Z.; Yin, P.; et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020, 7, e671–e678. [Google Scholar] [CrossRef]
- Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Thrombolysis. 1: 2020;50(3), 2020.
- Gholizadeh P, Safari H, Rostami M, Daneshmandi S, Yousefi M, Gholizadeh A. Hematological findings in COVID-19 patients with and without pneumonia: a case-control study. J Clin Pathol. 7: 2020;73(11), 2020.
- Passamonti, F.; Cattaneo, C.; Arcaini, L.; Bruna, R.; Cavo, M.; Merli, F.; Angelucci, E.; Krampera, M.; Cairoli, R.; Della Porta, M.G.; et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020, 7, e737–e745. [Google Scholar] [CrossRef]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The science underlying Covid-19: Implications for the cardiovascular system. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, U.; Nair, S.; Goel, A.; Balasubramanian, K.; Mackie, I.; Elias, E.; Eapen, C. Targeting raised von Willebrand factor levels and macrophage activation in severe COVID-19: Consider low volume plasma exchange and low dose steroid. Thromb. Res. 2020, 192, 2–2. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Pinney, S.P.; Lala, A.; Reddy, V.Y.; Johnston-Cox, H.A.; Mechanick, J.I.; Halperin, J.L.; Fuster, V. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia. J. Am. Coll. Cardiol. 2020, 76, 2011–2023. [Google Scholar] [CrossRef]
- Gajendra, S. Spectrum of hematological changes in COVID-19. . 2022, 12, 43–53. [Google Scholar] [PubMed]
- Hamming, I.; Cooper, M.; Haagmans, B.; Hooper, N.; Korstanje, R.; Osterhaus, A.; Timens, W.; Turner, A.; Navis, G.; van Goor, H. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007, 212, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.S.; Siqueira, S.; Soares, W.R.d.A.; Rangel, F.d.S.; Santos, N.O.; Freitas, A.d.S.; da Silveira, P.R.; Tiwari, S.; Alzahrani, K.J.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensinconverting enzyme 2: Sars-cov-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ace2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Kassiri, Z.; Jiang, C.; Liu, P.P.; Poutanen, S.M.; Penninger, J.M.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625. [Google Scholar] [CrossRef]
- Escher, R.; Breakey, N.; Lämmle, B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020, 190, 62–62. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: Ii. Representative vascular beds. Circ. Res. 2007, 100, 174–190. [Google Scholar]
- Boisrame-Helms, J.; Kremer, H.; Schini-Kerth, V.; Meziani, F. Endothelial dysfunction in sepsis. Curr. Vasc. Pharmacol. 2013, 11, 150–160. [Google Scholar]
- Lax, S. F. , Skok, K., Zechner, P., Kessler, H. H., Kaufmann, N., Koelblinger, C.,... & Trauner, M. (2020). Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series.
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; Heide, R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Girmenia C, Bertaina A, Piciocchi A, et al. COVID-19 in patients with hematological malignancies: a retrospective case series from eight Italian referral hospitals.J Hematol Oncol. 2020;13(1):119. [CrossRef]
- Martin-Romano EA, Sanchez-Lopez M, Saez MC, et al. Thrombocytopenia and lymphopenia in patients with cancer and COVID-19. Br J Cancer. 2021;124(2):320-325. [CrossRef]
- Hasan SS, Radford S, Kow CS, Zaidi STR (2020) Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: a systematic review and meta-analysis. 8: J Thromb Thrombolysis 50(4).
- Previtali E, Bucciarelli P, Passamonti SM, Martinelli I (2011) Risk factors for venous and arterial thrombosis. Blood Transfus 9(2):120–138.
- Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, Kwan JM, Krause DS, Lee AI, Halene S, Martin KA, Chun HJ, Hwa J (2020) Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation.
- Papageorgiou C, Jourdi G, Adjambri E, Walborn A, Patel P, Fareed J, Elalamy I, Hoppensteadt D, Gerotziafas GT (2018) Disseminated intravascular coagulation: an update on pathogenesis, diagnosis, and therapeutic strategies. 8: Clin Appl Thromb Hemost 24(9_suppl).
- Iba T, Levy JH, Raj A, Warkentin TE (2019) Advance in the management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Clin Med 8(5).
- Levi M, Toh CH, Thachil J, Watson HG (2009) Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 145(1):24–33.
- Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M (2020) The unique characteristics of COVID-19 coagulopathy. Crit Care 24(1):360.
- Campbell CM, Kahwash R (2020) Will complement inhibition be the new target in treating COVID-19–related systemic thrombosis? 1: Circulation 141(22), 1739.
- Hess DC, Eldahshan W, Rutkowski E (2020) COVID-19-related stroke. Transl Stroke Res 11(3):322–325.
- Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H (2020) Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191:145–147.
- Gunasekaran K, Amoah K, Rajasurya V, Buscher MG (2020) Stroke in a young COVID-19 patient. Qjm 113(8):573–574.
- Cui S, Chen S, Li X, Liu S, Wang F (2020) Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. 1: J Thromb Haemost 18(6), 1421.
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Alexia B, Sandri MT, Barco S (2020) Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. 9: Thromb Res 191.
- Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A (2020) Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. 1: Int J Lab Hematol 42(Suppl 1).
- Giannis, D.; Allen, S.L.; Tsang, J.; Flint, S.; Pinhasov, T.; Williams, S.; Tan, G.; Thakur, R.; Leung, C.; Snyder, M.; et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood 2021, 137, 2838–2847. [Google Scholar] [CrossRef]
- Bunch, C.M.; Thomas, A.V.; Stillson, J.E.; Gillespie, L.; Khan, R.Z.; Zackariya, N.; Shariff, F.; Al-Fadhl, M.; Mjaess, N.; Miller, P.D.; et al. Preventing Thrombohemorrhagic Complications of Heparinized COVID-19 Patients Using Adjunctive Thromboelastography: A Retrospective Study. J. Clin. Med. 2021, 10, 3097. [Google Scholar] [CrossRef]
- Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S (2020) Autopsy findings and venous thromboembolism in patients with COVID-19. 2: Ann Intern Med 173.
- Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY (2020) Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. 7: J Thorac Oncol 15(5).
- Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A (2020) Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 1: 77.
- Aggarwal M, Dass J, Mahapatra M (2020) Hemostatic abnormalities in COVID-19: an update. 1: Indian J Hematol Blood Transfus 36(4).
- Wool GD, Miller JL (2020) The impact of COVID-19 disease on platelets and coagulation. Pathobiology.
- Long H, Nie L, Xiang X, Li H, Zhang X, Fu X, Ren H, Liu W, Wang Q, Wu Q (2020) D-dimer and prothrombin time are the significant indicators of severe COVID-19 and poor prognosis. Biomed Res Int 2020:6159720–6159710.
- Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, Liu L, Shan H, Lei C-l, Hui DSC, Du B, Li L-j, Zeng G, Yuen K-Y, Chen R-c, Tang C-l, Wang T, Chen P-y, Xiang J, Li S-y, Wang J-l, Liang Z-j, Peng Y-x, Wei L, Liu Y, Hu Y-h, Peng P, Wang J-m, Liu J-y, Chen Z, Li G, Zheng Z-j, Qiu S-q, Luo J, Ye C-j, Zhu S-y, Zhong N-s (2020) Clinical characteristics of coronavirus disease 2019 in China. 1: N Engl J Med 382(18), 1708.
- Li Q, Cao Y, Chen L, Wu D, Yu J, Wang H, He W, Chen L, Dong F, Chen W (2020) Hematological features of persons with COVID-19.
- Seyit, M.; Avci, E.; Nar, R.; Senol, H.; Yilmaz, A.; Ozen, M.; Oskay, A.; Aybek, H. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am. J. Emerg. Med. 2020, 40, 110–114. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Emerson, S.G.; Punt, J.; Goff, W.D. Decreased naïve T-cell production leading to cytokine storm as cause of increased COVID-19 severity with comorbidities. Aging Dis. 2020, 11, 742–745. [Google Scholar] [CrossRef]
- Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G (2020) Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. 1: Clin Chim Acta 507.
- Zhang C, Wang XM, Li SR, Twelkmeyer T, Wang WH, Zhang SY, Wang SF, Chen JZ, Jin X, Wu YZ, Chen XW, Wang SD, Niu JQ, Chen HR, Tang H (2019) NKG2A is a NK cell exhaustion checkpoint for HCV persistence. 1: Nat Commun 10(1), 1507.
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.-Q.; Wang, Q.; Miao, H. Correction: Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target. Ther. 2020, 5, 1–1. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.-E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef]
- McClain, M.T.; Park, L.P.; Nicholson, B.; Veldman, T.; Zaas, A.K.; Turner, R.; Lambkin-Williams, R.; Gilbert, A.S.; Ginsburg, G.S.; Woods, C.W. Longitudinal analysis of leukocyte differentials in peripheral blood of patients with acute respiratory viral infections. J. Clin. Virol. 2013, 58, 689–695. [Google Scholar] [CrossRef]
- Liu, K.; Fang, Y.-Y.; Deng, Y.; Liu, W.; Wang, M.-F.; Ma, J.-P.; Xiao, W.; Wang, Y.-N.; Zhong, M.-H.; Li, C.-H.; et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med J. 2020, 133, 1025–1031. [Google Scholar] [CrossRef]
- Ashrafi, F.; Nematollahi, P.; Salmasi, M.; Hedayat, A.; Amra, B. Association of lymphocyte subsets with mortality in severe COVID-19 pneumonia patients. J. Clin. Lab. Anal. 2021, 35, e24046. [Google Scholar] [CrossRef]
- Ganji, A.; Farahani, I.; Khansarinejad, B.; Ghazavi, A.; Mosayebi, G. Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cells, Mol. Dis. 2020, 83, 102437–102437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-Y.; Zhang, M.; Yang, C.-X.; Zhang, N.; Wang, X.-C.; Yang, X.-P.; Dong, X.-Q.; Zheng, Y.-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Mazzoni, A.; Salvati, L.; Maggi, L.; Capone, M.; Vanni, A.; Spinicci, M.; Mencarini, J.; Caporale, R.; Peruzzi, B.; Antonelli, A.; et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Investig. 2020, 130, 4694–4703. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Schanz, O.; Garbers, C.; Zaremba, A.; Hegenbarth, S.; Kurts, C.; Beyer, M.; Schultze, J.L.; Kastenmüller, W.; Rose-John, S.; et al. IL-6 trans-Signaling-Dependent Rapid Development of Cytotoxic CD8+ T Cell Function. Cell Rep. 2014, 8, 1318–1327. [Google Scholar] [CrossRef]
- Liu, Y.; Du, X.; Chen, J.; Jin, Y.; Peng, L.; Wang, H.H.; Luo, M.; Chen, L.; Zhao, Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020, 81, e6–e12. [Google Scholar] [CrossRef]
- Ma, Y. et al. Predictive value of the neutrophil-to-lymphocyte ratio (NLR) for diagnosis and worse clinical course of the COVID-19: findings from ten provinces in China. [CrossRef]
- Surh, C.D.; Sprent, J. Homeostasis of Naive and Memory T Cells. Immunity 2008, 29, 848–862. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Huang, F.; Yang, Y.; Wang, F.; Yuan, J.; Zhang, Z.; Qin, Y.; Li, X.; Zhao, D.; et al. Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury. Natl. Sci. Rev. 2020, 7, 1003–1011. [Google Scholar] [CrossRef]
- Thierry, A.R.; Pastor, B.; Abraham, J.-D.; Pisareva, E.; Mazard, T. Abstract P11: The elevated level of the main markers of neutrophil extracellular traps in metastatic colorectal cancer plasma highlights the enhanced risk of severe forms of COVID-19 in cancer patients. Clin. Cancer Res. 2021, 27, P11–P11. [Google Scholar] [CrossRef]
- Yao, X.H.; Li, T.Y.; He, Z.C.; Ping, Y.F.; Liu, H.W.; Yu, S.C.; Mou, H.M.; Wang, L.H.; Zhang, H.R.; Fu, W.J.; et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020, 49, E009. [Google Scholar]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Yaqinuddin, A.; Kvietys, P.; Kashir, J. COVID-19: Role of neutrophil extracellular traps in acute lung injury. Respir. Investig. 2020, 58, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, M.; Dúbrava, M.; Celec, P. On the Origin of Neutrophil Extracellular Traps in COVID-19. Front. Immunol. 2022, 13, 821007. [Google Scholar] [CrossRef]
- Oury, C.; Marichal, T. Neutrophil extracellular traps: key drivers of severe Covid-19. 27. [CrossRef]
- Middleton EA, He X-Y, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil extracellular traps (NETs) contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood [Internet]. 2020.
- Al-Sharwey, H.M.A.; Helmy, M.W.; Mostafa, K.S.; Aiad, K. NEUTROPHIL - LYMPHOCYTE RATIO AS AN ANTICIPATORY FACTOR IN THE PROGNOSIS OF MORTALITY AND SEVERITY IN COVID-19 PATIENTS. ALEXMED ePosters 2023, 5, 18–19. [Google Scholar] [CrossRef]
- Ding, X.; Yu, Y.; Lu, B.; Huo, J.; Chen, M.; Kang, Y.; Lou, J.; Liu, Z. Dynamic profile and clinical implications of hematological parameters in hospitalized patients with coronavirus disease 2019. cclm 2020, 58, 1365–1371. [Google Scholar] [CrossRef]
- Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R, Song M, Wang L, Zhang W, Han B, Yang L, Wang X, Zhou G, Zhang T, Li B, Wang Y, Chen Z, Wang X (2020) Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med 18(1):206.
- Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, Chen L, Zhao Y (2020) Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. e: J Infect 81(1).
- Simadibrata, D.M.; Calvin, J.; Wijaya, A.D.; Ibrahim, N.A.A. Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: A meta-analysis. Am. J. Emerg. Med. 2021, 42, 60–69. [Google Scholar] [CrossRef]
- Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A (2020) The role of biomarkers in diagnosis of COVID-19 – a systematic review. 1: Life Sci 254, 1177.
- Wang L (2020) C-reactive protein levels in the early stage of COVID-19. Med Mal Infect 50(4):332–334.
- Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, Li B, Song X, Zhou X (2020) Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. 1: J Clin Virol 127, 1043.
- Wang L (2020) C-reactive protein levels in the early stage of COVID-19. Med Mal Infect 50(4):332–334.
- Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, Jiang X, Li X (2020) C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. 8: J Med Virol 92(7).
- Thakur, B.; Bora, K.; Chakraborty, M. Association of Serum Lactate Dehydrogenase and Qualitative C-Reactive Protein with the Severity of COVID-19 Disease. J. Clin. Diagn. Res. 2022, 16, BC1–BC4. [Google Scholar] [CrossRef]
- Mesa, A.M.; César, E.C.; Martín-Montañez, E.; Alvarez, E.S.; Lopez, P.M.; Romero-Zerbo, Y.; Garcia-Fernandez, M.; Garrido, J.L.V. Acute Lung Injury Biomarkers in the Prediction of COVID-19 Severity: Total Thiol, Ferritin and Lactate Dehydrogenase. Antioxidants 2021, 10, 1221. [Google Scholar] [CrossRef]
- Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18(4):844–847.
- Kaftan, A.; Hussain, M.; Algenabi, A.; Naser, F.; Enaya, M. Predictive Value of C-reactive Protein, Lactate Dehydrogenase, Ferritin and D-dimer Levels in Diagnosing COVID-19 Patients: a Retrospective Study. Acta Inform. Medica 2021, 29, 45–50. [Google Scholar] [CrossRef]
- Dahan, S.; Segal, G.; Katz, I.; Hellou, T.; Tietel, M.; Bryk, G.; Amital, H.; Shoenfeld, Y.; Dagan, A. Ferritin as a Marker of Severity in COVID-19 Patients: A Fatal Correlation. . 2020, 22, 494–500. [Google Scholar]
- Chen, J.; He, Z.-X.; Wang, F.-K. RETRACTED ARTICLE: Evaluation of ferritin level in COVID-19 patients and its inflammatory response. Appl. Nanosci. 2022, 13, 3121–3121. [Google Scholar] [CrossRef] [PubMed]
- Para, O.; Caruso, L.; Pestelli, G.; Tangianu, F.; Carrara, D.; Maddaluni, L.; Tamburello, A.; Castelnovo, L.; Fedi, G.; Guidi, S.; et al. Ferritin as prognostic marker in COVID-19: the FerVid study. Postgrad. Med. 2021, 134, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Beni, F. , Vahedian-Azimi, A., Shojaei, S., Rahimi-Bashar, F., Shahriary, A., Johnston, T. P., & Sahebkar, A. (2021). The level of procalcitonin in severe COVID-19 patients: a systematic review and meta-analysis. Clinical, Biological and Molecular Aspects of COVID-19, 277-286.
- Waris, A.; Din, M.; Iqbal, N.; Yar, L.; Khalid, A.; Nawaz, M.; Baset, A.; Ali, M. Evaluation of serum procalcitonin level as a biomarker for disease severity in COVID-19 patients. New Microbes New Infect. 2021, 43, 100922. [Google Scholar] [CrossRef]
- Xu, J.-B.; Xu, C.; Zhang, R.-B.; Wu, M.; Pan, C.-K.; Li, X.-J.; Wang, Q.; Zeng, F.-F.; Zhu, S. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evidence-Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



