1. Introduction
Harbor porpoise (
Phocoena phocoena vomerina) were considered to be a commonly observed cetacean in the waters of Puget Sound during the 1940s[
1], but by the 1970s their numbers were greatly reduced throughout the Washington State (hereafter, Washington or WA) waters of the Salish Sea and were completely absent from Puget Sound [
2,
3]. Harbor porpoise data from British Columbia, Canada, (hereafter British Columbia, or BC) prior to the mid-1990s are sparse [
4,
5] (Hall unpub. data). Several systematic studies spanned the late 1990’s and early 2000’s that included the inland waters of southern British Columbia [
6,
7]. Aerial surveys documented their numbers increasing in Washington waters through the 1990s, and reentering Puget Sound beginning in 2000 [
8,
9]. The first sighting of a small group in South Puget Sound, the southernmost area within the Salish Sea, was in September 2005 by two of the authors (Shuster and Anderson), with regular sightings of more and larger groups beginning in 2008 (Anderson, unpub. data). Today, harbor porpoise are once again the most common cetacean found throughout most of the Salish Sea. This recovery has led to an interest in gaining a better understanding of harbor porpoise habitat usage and behavior in recent years [
10].
Salish Sea harbor porpoise are most often seen singly or in small groups, averaging less than 3 animals [
9,
11,
12], which is typical throughout their global range [
13]. However, several smaller groups of harbor porpoise occasionally come together in larger aggregations, where many smaller groups are in close proximity to each other. These aggregations can spread over several kilometers, possibly consisting of distinct subgroups that are more densely packed [
14,
15,
16]. Some aggregations are dense in structure, with all animals closely associated in a small area, while others are more sparse, yet close enough together for the subgroups to regularly interact with other subgroups.
In other parts of the world, some of these aggregations are thought to be related to seasonal migrations in areas such as in the Bay of Fundy [
17] and in relation to the icing up of the fjords or following migrating herring in the Baltic Sea [
18]. Mostly these occurrences are thought to be feeding aggregations that occur when there is a large amount of food in an area and are often considered to be spurious and rare occurrences [
14]. Similarly, in the Salish Sea of Washington and British Columbia, their prevalence and importance are often dismissed or treated as rare events. In their seminal work on marine mammals in Washington State, Scheffer and Slipp (1948) make no mention of larger aggregations, with the observations, “usually in groups of 2 to 5, occasionally 10 to 12”, though they do note that, “[r]arely are more than 3 of a group in sight at one time, although several groups may gather in favored waters”. Recent observations, however, suggest that these large aggregations may be much more common in the Salish Sea than previously documented. Harbor porpoise aggregations in these waters are not related to migration or icing up, as harbor porpoise are known to remain year round, with long-term photo-identification (photo-ID) [
12], genetic data [
19], and tag data [
20] suggesting the possibility of high site fidelity among this population. Long-term sighting data analyses (1991-2008) from British Columbia, determined harbor porpoise high density aggregation data are associated with foraging and reproductive behaviors, specific habitats, and oceanographic variables related to tidal phase and mixing [
16]. On-going photo-ID studies in British Columbia are also noting positive identifications of individuals on an inter-annual basis (Porpoise Conservation Society, unpublished data).
In this study we compared data from several sources throughout the Salish Sea, including small boat surveys, whale watch vessels, marine mammal monitoring field efforts, and community/citizen scientist observers. We quantify the occurrence of these large aggregations, their relation to environmental patterns (like season, tide, and bathymetric features), and the prevalence of social behaviors (like mating, fission/fusion of subgroups, coordinated feeding behavior, and willingness to approach vessels) during these groupings. We hypothesize that these aggregations occur more commonly than previously thought and provide important feeding and socializing opportunities for Salish Sea harbor porpoise.
2. Materials and Methods
2.1. Study Location
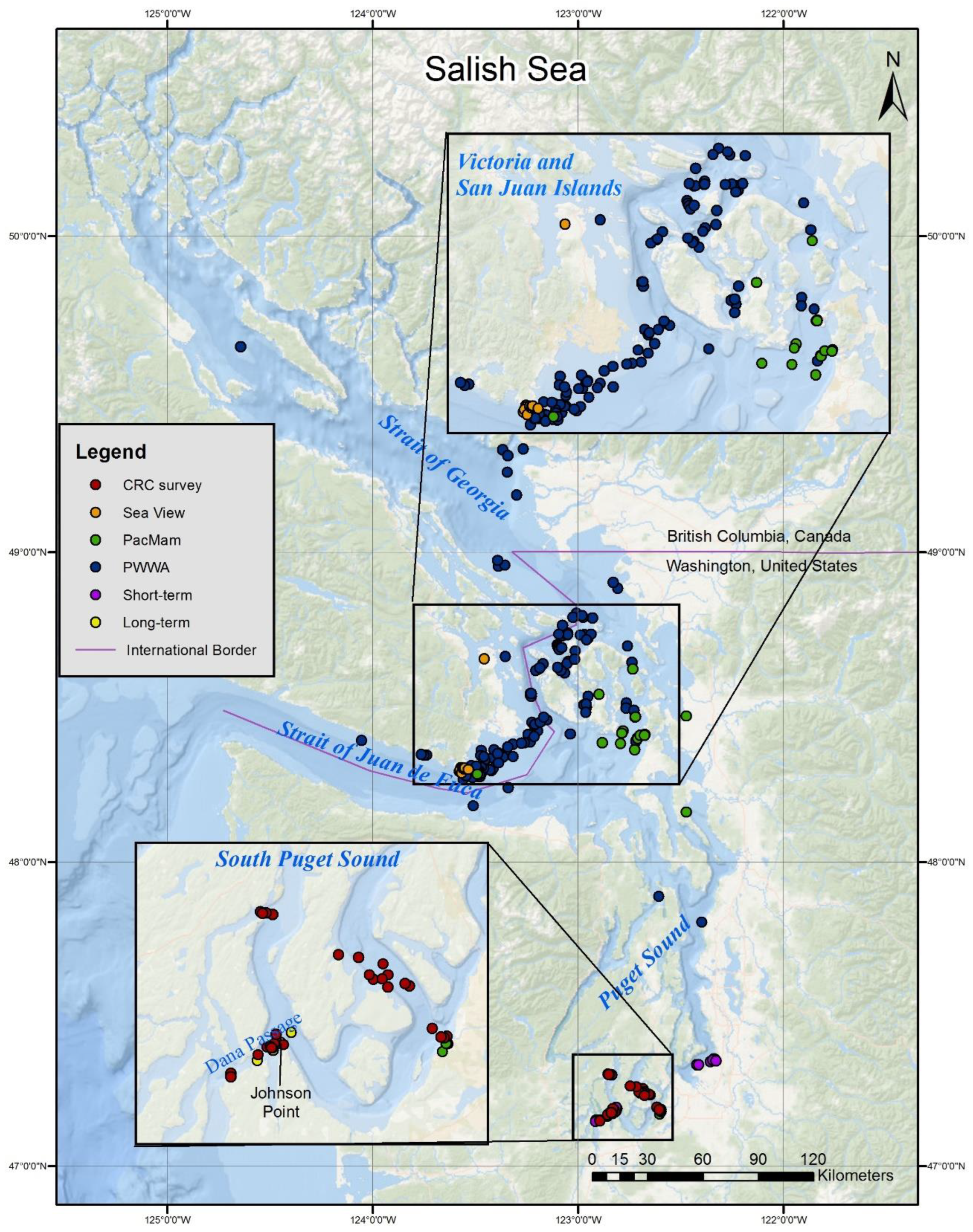
The Salish Sea is an inland fjord-like body of water composed of many inlets, passages and bays in Washington State, USA and British Columbia, Canada (
Figure 1). The major basins include the Strait of Juan de Fuca (Juan de Fuca Strait in Canada), connecting to the Pacific Ocean; the San Juan Islands, northeast of the Strait of Juan de Fuca in Washington; the Gulf Islands, in Canada north of the San Juan Islands; the Strait of Georgia, between mainland BC and Vancouver Island; and Puget Sound, south of the east end of the Strait of Juan de Fuca.
2.2. Data collection
Cascadia Research Collective (CRC) has conducted year-round regular small boat based (4.2 m Zodiac) surveys in South Puget Sound since summer 2016. Sightings of all marine mammals are recorded in Google Sheets. Porpoise counts are estimates of the number of animals within good sighting distance from the boat, usually around 300 m. For larger aggregations, several sightings are recorded while passing through the area. Additionally, reports are collected from fishers and community scientist residents living on banks overlooking various locations of Puget Sound. Only reports from experienced observers, or those that supplied photographs or video were included.
Pacific Mammal Research (PacMam), based in Anacortes, WA, is a research organization studying harbor porpoises and harbor seals through land-based, long-term photo-ID and behavioral surveys. In March of 2021, a custom opportunistic sighting project (PacMam harbor porpoise project) was created using the Epicollect5 app platform through a collaboration with Kwiáht (Center for the Historical Ecology of the Salish Sea). This app allows the public to easily document opportunistic harbor porpoise sightings throughout the Salish Sea. The majority of sightings are from the general public, though there are some from local researchers. Information on total group size, number of calves, Global Positioning System (GPS) location, weather, tidal phase, boat presence, gull presence, behavior, length of time watching the porpoises, expertise of the observer, and any extra notes can be documented. Data entry is not required for every field and observer expertise varies, therefore, some sighting records do not contain information about each of these factors. To date, users of this app have documented over 300 harbor porpoise sightings throughout the Salish Sea, from South Puget Sound, north to the San Juan Islands, and out the Strait of Juan de Fuca. These sightings are not restricted to large aggregations and range from 1 - 100+ harbor porpoises. Thus for this study a subset of the data was used (group sizes ≥ 20, and observer expertise level of experienced or expert).
The Pacific Whale Watch Association (PWWA) is a professional association of ecotourism operators in Washington State and British Columbia. As of 2023, the PWWA comprises 30 member companies departing from 23 ports ranging as far south as Seattle, WA, as far north as Telegraph Cove, BC, and as far west as Port Renfrew, BC. PWWA members utilize the private PWWA App, developed by Johannes Krieger in 2018, to record wildlife sightings throughout the Salish Sea. Sightings of harbor porpoise in the region are fairly common and not routinely reported by whale watchers, but for this study, PWWA captains and naturalists were asked to document "large aggregations" of harbor porpoise, groups of 10 or more individuals, beginning in April 2021. Sightings records in the PWWA App include species, group size, travel direction (if known), time, date, and GPS location of the sighting.
Some sightings may be duplicated across platforms, therefore care was taken to remove these from the data. Sighting reports from PWWA vessels, which were duplicated in the PacMam data, using criteria of same day, location and reporting party, were deleted from the PacMam data. Reports to CRC that matched PacMam data were deleted from CRC data.
Sea View Marine Sciences (Sea View) specializes in marine mammal research, monitoring and mitigation. Sea View is on Vancouver Island near Victoria, British Columbia and has worked extensively in southern BC waters conducting numerous field assessments and research projects with professional biologists and observers. From 2017 to 2023, harbor porpoise group size and behavioral data were collected by Sea View as part of a larger Marine Mammal Monitoring Program of the Canadian Department of National Defence training operations in the Salish Sea. Field efforts and data collection were conducted entirely in Canadian waters.
All contributing groups recorded porpoise behavior, paying particular attention to those rarely seen outside of these aggregations, especially social and unique foraging behaviors not possible in smaller groups.
The timing of these aggregations can vary, and we differentiate between long and short-term events. Long-term aggregations are defined as harbor porpoise remaining in the same area, in large numbers (20+), lasting at least one week. Short-term aggregations are defined as large numbers of harbor porpoise (20+), usually lasting for a few hours, or up to a few days at most.
All coordinates of sightings should be considered to be estimates. None of the shore-based sightings were monitored with a theodolite, so they are estimates of the location by necessity, either generated by the reporting party, or by the authors given location data included in the report. Vessel GPS locations can be taken from within a larger aggregation, but are likely to be a couple hundred meters from smaller aggregations. Large aggregations can cover several square kilometers, so even accurate GPS locations do not represent the extent of the entire aggregation. Map of sightings was generated using ArcMAP 10.8.2. (
Figure 1).
4. Discussion
The recovery of the harbor porpoise population in the Salish Sea documented by Evenson et al. (2016), has likely been a driver behind increased occurrence of aggregations. Even so, these aggregations would likely go largely undocumented without a coordinated effort to monitor this behavior. Local harbor porpoise researchers promoted the need to collect data on harbor porpoises to various people and organizations in an effort to better understand this poorly known species. The use of cell phone apps not only allows for the collection of reports from the community but encourages community scientists to spend more time looking for the animals and recording their behavior. Whale watching boats are primarily focused on dolphins and whales, don’t often log porpoise sightings, but when local harbor porpoise researchers specifically requested that larger (10+) groupings of harbor porpoise be logged in their app, they were happy to assist. This led to a majority of reports from 2021-22 coming from whale watching boats. These examples show that people are eager to contribute, but only if they know what information is wanted, and have a platform (like sightings apps) that makes it easy to do so. The results of this study show how working together (researchers, community scientists and whale watching crews) can provide valuable scientific data, much more than could be obtained from one organization alone, that increases the scope of the data collected and information that can be derived from it. This type of collaborative research also demonstrates the importance of data collection irrespective of the socio-political boundaries such as the US-Canadian border, as this type of administrative boundary is unrelated to the ecological and social connections of harbor porpoise (and other marine species).
While reports of harbor porpoise aggregations in the Salish Sea have previously been treated as rare events, by collecting data from multiple sources, we have shown that aggregations occur more commonly than previously thought. During the year 2022, the first full year that included reporting by PWWA, there were a total of 160 aggregations documented (10 by CRC, 22 by PacMam, 111 by PWWA, and 17 by Sea View). Some of these encounters are short-lived, lasting for only part of a tidal cycle, while others appear to last for days up to months, and can recur annually. The spatial distribution of these aggregations over time is important for the identification and recognition of important habitats for harbor porpoise in the Salish Sea.
Food supply is likely one of the primary drivers for these aggregations, as evidenced by documented harbor porpoise foraging behaviors, along with the presence of other marine mammals and birds in many of these locations. Most individuals in the aggregations, even while traveling, exhibit regular foraging dives or evident surface foraging behavior (like surface chases). Fusion/fission behavior is quite common during foraging, with subgroups coming together and synchronizing their dives in groups of 10 or more, splitting up again after a series of dives. The larger numbers also allow them to participate in foraging behavior that is rarely seen outside the groups, such as synchronized feeding on surface bait balls, either in a line abreast, or in a line head-to-tail [
16](Anderson and Shuster, pers. obs.). On a few occasions, individual porpoise were seen swimming in a circle on their side at the surface. This could be a way to condense a bait ball before making a feeding pass, as has been documented in other cetacean species [
22,
23,
24]. Given the high metabolic rate of harbor porpoise [
25,
26,
27], consideration should be given to the amount of food necessary to feed 100+ harbor porpoise over a period of several months during the long-term aggregations. The large number of short and long-term aggregations of harbor porpoise in the Salish Sea documented in this study indicates there are also significant amounts of prey available in these locations over short and/or long periods of time.
Harbor porpoise are opportunistic feeders, with the majority of their diet made up of small forage fish, along with some mollusks, crustaceans and arthropods [
28,
29]. Occasionally larger fish are also consumed [
30]. There are two sorts of events that tend to concentrate enough food to support a large number of harbor porpoise. First, there are shoals and other fronts that concentrate plankton and forage fish during the high tidal flows (tidal changes >6 m in some areas). Second is spawning events, of which there are many in the Salish Sea. There are a wide variety of forage fish that are either resident in the Salish Sea, or come in to breed. Pacific herring (
Chupea pallasi) has traditionally been the dominant Salish Sea commercial market forage fish, with most stocks breeding January through April, though the Cherry Point stock, near Bellingham, WA, is the largest, running April through June31. Pacific sand lance (
Ammodytes hexapterus) breed November through February on Salish Sea beaches [
31]. Surf smelt (
Hypomesus pretiosus) is present in these waters year round, with most spawning occurring during the summer or fall months, though in some areas they spawn year round [
31,
32]. Northern anchovy (
Engraulis mordax) is not traditionally viewed as one of the most abundant species of forage fish within the Salish Sea. The high ocean temperatures offshore during The Blob event, 2014-2016 [
33,
34] has led to much greater abundance of anchovies in recent years, especially in South Puget Sound [
35,
36], which has been sufficient to feed several hundred California sea lions (
Zalophus californianus) for several months in Case Inlet (Jefferies, personal communication). Eulachon (
Thaleichthys pacificus) is commonly called “candlefish” because of its high fat content, which also makes it an ideal high-calorie food for harbor porpoise. It is a common forage fish in the northern Salish Sea, though it can be found throughout the waters [
36]. These species have been found to be important to harbor porpoise in the Salish Sea [
6,
28]. Additionally, salmon and steelhead runs are common in the many rivers entering the Salish Sea [
37]. Though salmonids are not considered to be a significant portion of the harbor porpoise’s diet, as opportunistic feeders, they are likely to eat smolts when they come across them, as was shown by a stranded harbor porpoise on Washington’s outer coast with a Chinook smolt transponder found in its stomach [
38], and have been observed taking adult salmon in some locations [
30]. Market squid (
Doryteuthis opalescens) enter the Strait of Juan de Fuca in the summer months, and enter South Puget Sound by December, and stay through February [
36,
39]. Thus, there are a variety of prey species that may concentrate in large enough numbers to support short and longer-term harbor porpoise aggregations. More research is needed to understand which forage species are more important for driving these events.
There is also evidence that social interactions are important during aggregations. Social behaviors, such as mating attempts and a variety of other non-foraging group activities, are much more common in many of the aggregations. Mating attempts are seen year-round in small groups as well [
40] (Elliser unpublished data, Anderson unpublished data, Hall unpublished data), but usually only one or two attempts are observed (compared to the dozens sometimes observed in the aggregations). During aggregation events, porpoises are also more likely to interact with slow moving or stopped vessels, and are less likely to make major moves to avoid fast moving vessels. In several encounters, harbor porpoises have approached the research vessel in a small group, diving under the boat, and reemerging on the other side at high speed, porpoising away from the boat. Harbor porpoise have also approached whale watch vessels during these encounters. Researchers and whale watchers have observed an increased likelihood of porpoise wake riding or following the prop wash of slow moving vessels, though this behavior is also seen by individuals in smaller groups in Burrows Pass, WA (Elliser, unpublished data). This increase in more rarely observed behaviors during aggregations was also noted in the more northern Salish Sea waters in BC [
16]. While harbor porpoise are generally considered relatively solitary, formation into larger groups may allow them to have more diverse social interactions. As these aggregations are likely attributable to an abundance of food in the area, porpoise may need to spend less time foraging, freeing up time for more social activities. It may also be that aggregating for food provides the opportunity to interact with many more individuals than is normal for this small grouping species. This can facilitate the occurrence and increased amount of these behaviors observed during these aggregations.
The social structure of harbor porpoises has not been well investigated and is unknown at this time. Due to their vocalization patterns (e.g. lack of whistles normally attributed to communicative calls) and tendency for very small groups (1-3 individuals), it has been thought that they do not have very strong social ties. However, there is evidence suggesting that there is more to their sociality than previously thought. Flaherty and Stark (1982) [
41] attributed breaching and splashing in wild harbor porpoise as social behaviors and concluded that strong evidence exists that individual and group relationships amongst harbor porpoise exists. A previous review of harbor porpoise social behaviors, from wild and captive settings, noted these to be well developed and set within a context of individual and group relationships [
16].
Harbor porpoise have been observed using complex cooperative foraging behaviors with role specialization that is rarely seen in animals [
42,
43]. A common dolphin has been found to change vocalization to match local harbor porpoise [
44], and harbor porpoise clicks have been shown to be used in communicative contexts, not just foraging [
45]. Although little is known about their associations, there is early evidence through photo-ID that shows at least some individuals are often sighted together often over weeks to months at a time (Elliser unpublished data). It is likely that social interactions are more important to this species than what is observed in the limited social encounters observed at the surface [
45]. These large aggregations may be important aspects of their social structure. The importance of larger groups is seen in other species, such as the Southern Resident killer whales (SRKW). In the Salish Sea SRKW are normally found in tight matrilineal pods but periodically join to form superpods where the individuals mix and socialize with members of other pods [
46]. Large aggregations may provide similar opportunities for individual harbor porpoises to socialize with others in their community or population, and also facilitate genetic diversity. Further research is needed to determine the role of these aggregations in harbor porpoise society.
5. Conclusions
It is clear from the results of this study, that large harbor porpoise aggregations are now more common in the Salish Sea than previously realized. In all likelihood, the aggregations documented here are a small portion of the ones actually happening throughout these waters. Due to the behaviors observed, these are likely both important foraging and socialization opportunities for harbor porpoises. These events may also play a vital role in the reproduction of the species as noted by the long-term habitat selection and occurrence of mating behavior commonly observed in southern BC [
16]. Understanding when and why these aggregations are occurring can help us better understand the foraging ecology, behavioral ecology, and social structure of this enigmatic species. Moreover, this may also assist in the identification of important habitats that are vital for the long-term survival of the Salish Sea harbor porpoise population(s).
Monitoring their populations can provide critical data on ecosystem health. The decline and recovery of harbor porpoise in the Salish Sea is not isolated, and is mirrored in the population in San Francisco Bay [
47] around the same time. This reminds us that harbor porpoise are a good sentinel species for the health of local ecosystems, and how important it is to better understand their behavioral and foraging ecology, for their conservation and that of their ecosystem.
This study is an example of the value of recruiting community scientists and on the water professionals to help contribute to knowledge about these and many other animals. Researchers cannot cover such a wide area on their own, with limited funding, time, and resources available for the purpose. Additional groups of potential community science collaborators are already being identified to contribute to these data.
Further study could focus on determining what food sources are involved in attracting these aggregations, behavioral analyses, as well as determining if there are patterns in the locations and timing of repeated aggregations.