1. Background
Parkinson’s disease (PD) is one of the most severe neurodegenerative disorders worldwide [
1]. PD affects 3% of population over the age of 65 and more effective treatment strategies are still needed [
2]. The diagnosis of PD is currently dependent on the presence of motor deficits including bradykinesia, rigidity and resting tremor, unilaterally or asymmetrically [
3,
4]. In fact, non-motor symptoms (NMS) such as cognitive deficits, sleep disorders, anxiety etc., co-occur or even precede the onset of motor symptoms [
4]. Remarkably, NMS is also the key factor that contribute to low quality of life and progression of overall disability [
5,
6,
7].
In the past decades, neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced non-human primate (NHP) PD model has been recognized as a reliable preclinical animal model [
8]. MPTP-lesioned monkeys exhibit neuroanatomical and behavioral signs simulating symptoms in PD patients, particularly the motor deficits [
9]. However, non-motor symptoms in MPTP-induced PD monkeys have not been fully investigated [
4]. Most studies only involved one or two specific non-motor symptoms [
10]. Compared to rodents, the NHPs are more suitable for comprehensive assessment of the non-motor symptoms [
11]. In this study, we investigated a battery of the non-motor symptoms in MPTP-induced PD cynomolgus monkeys. The non-motor assessment includes cognitive function, sleep and psychiatric behaviors. One of the reasons that non-motor behaviors were not well studied is that these behaviors often rely on normal motor function. The present data were collected eight years after the MPTP administration, thus the monkeys had eighter years of adaption to motor dysfunction.
Pramipexole (PPX) is a non-ergot D2/D3 dopamine receptors agonist [
12]. The neuroprotective effects of PPX have been reported in several studies [
13]. For example, PPX improved motor symptoms, psychiatric symptoms and unified Parkinson’s disease rating scale (UPDRS) scores in PD patients [
14,
15,
16,
17,
18]. In the current study, we tested the effects of PPX on non-motor symptoms in NHP PD models.
2. Materials and Methods
2.1. Animals
Ten male cynomolgus monkeys (age: 15±1.5years, weight: 8.5±1kg) were used in this study. All experiments were conducted at Wincon TheraCells Biotechnologies Co., Ltd., Nanning, China. A group of five monkeys were injected with MPTP unilaterally through left internal carotid eight years ago. Another group of five age-matched naive monkeys were injected with vehicle. All animals were individually housed in standard laboratory condition (room temperature of 23~27℃, humidity 40%~75%) in a 12hr light/12hr dark cycle (lights on from 7 AM to 7 PM) with ad libitum access to water. The primate diet [China standard (GB) 14924. 8-2001] was provided twice daily and fresh fruit/vegetables were given once daily at noon. The health condition was monitored daily, and the environment was enriched with various rubber toys. All efforts were made to limit animals’ stress. All experimental protocols were approved by the Institutional Animal Care and Use Committee.
2.2. Drugs
Pramipexol hydrochloride (Booehringer Ingelheim Pharma GmbH & Co KG, Germany) was purchased from Nanning Hospital (Nanning, Guangxi, China). Doses used in the current study were 0.375mg for 3 days, 0.75mg for 3 days, 2.25mg for 5 days and 3.375mg for 7 days. The treatment plan was modified from the directions of using this drug in human patients.
2.3. Methods
The behavioral tests in the current study include Delayed Matching-To-Sample (DMTS), Physical Activity Monitor (PAM), Apathy Feeding Task (AFT), Human Intruder Test (HIT), Novel Fruit Test (NFT) and Predator Confrontation Test (PCT).
2.4. Delayed Matching-To-Sample task
The delayed matching-to-sample (DMTS) task was modified from the Wisconsin General Test Apparatus (WGTA) which is often used to test learning and memory in monkeys [
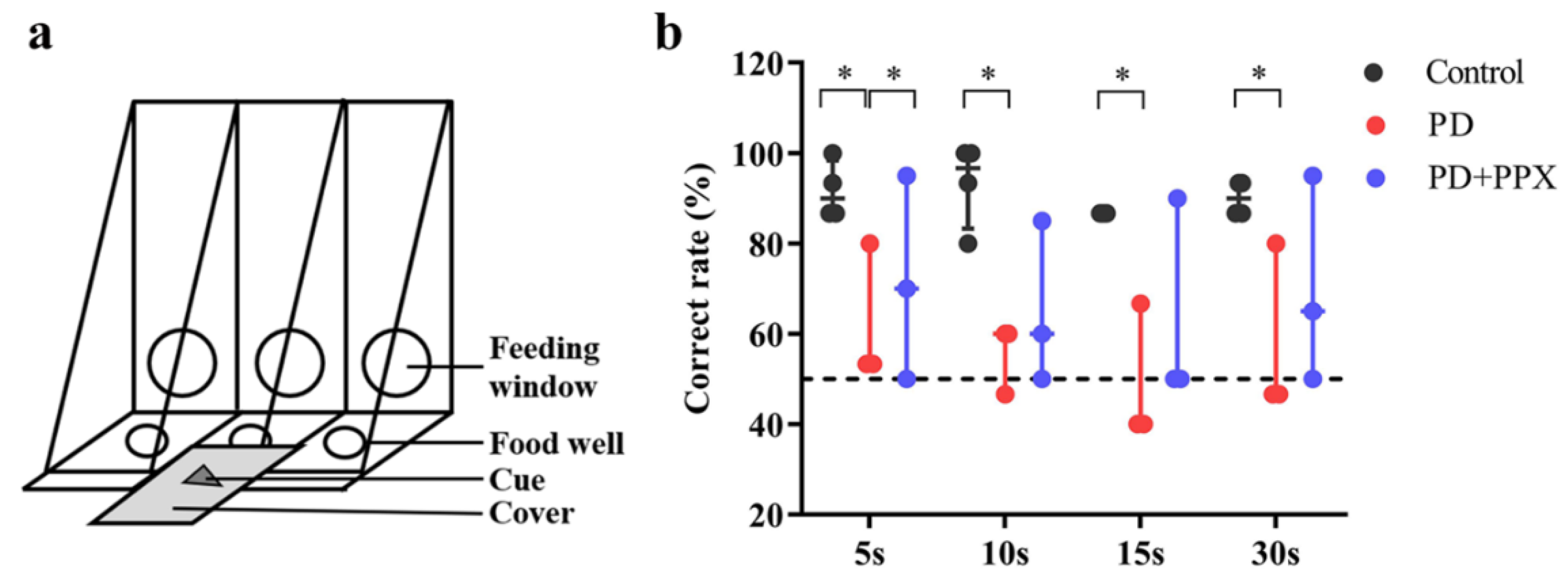
19]. DMTS was performed in a quiet testing room. The apparatus has three food wells with cover for hiding food reward and presenting cues (
Figure 1a). The monkey was transferred into a testing cage equipped with a sliding opaque board. Monkeys were well trained to perform DMTS task (correct rate was ≥ 80%) before the tests. Each trial comprised three phases, cue presentation phase, delay phase and responding phase. During cue presentation phase, a visual cue was presented on the middle cover and the opaque board was lifted for ~5 seconds to make sure that the animal saw the cue. The delay phase started from the drop of the opaque board and lasted for 5, 10, 15, or 30 seconds, randomly. During the delay, food reward (a small piece of fruit or nut) was placed in the left or right well (randomly selected). The previously presented cue was put on top of the food well cover. A distractive cue was placed on top of the other cover. After the delay, the opaque board was lifted. The monkey was able to open the cover and take the food reward. There were 80 trials in the DMTS test (20 trials for each delay). The correct responses were recorded, and correct rate was used to evaluate the performance of DMTS.
2.5. Physical Activity Monitor
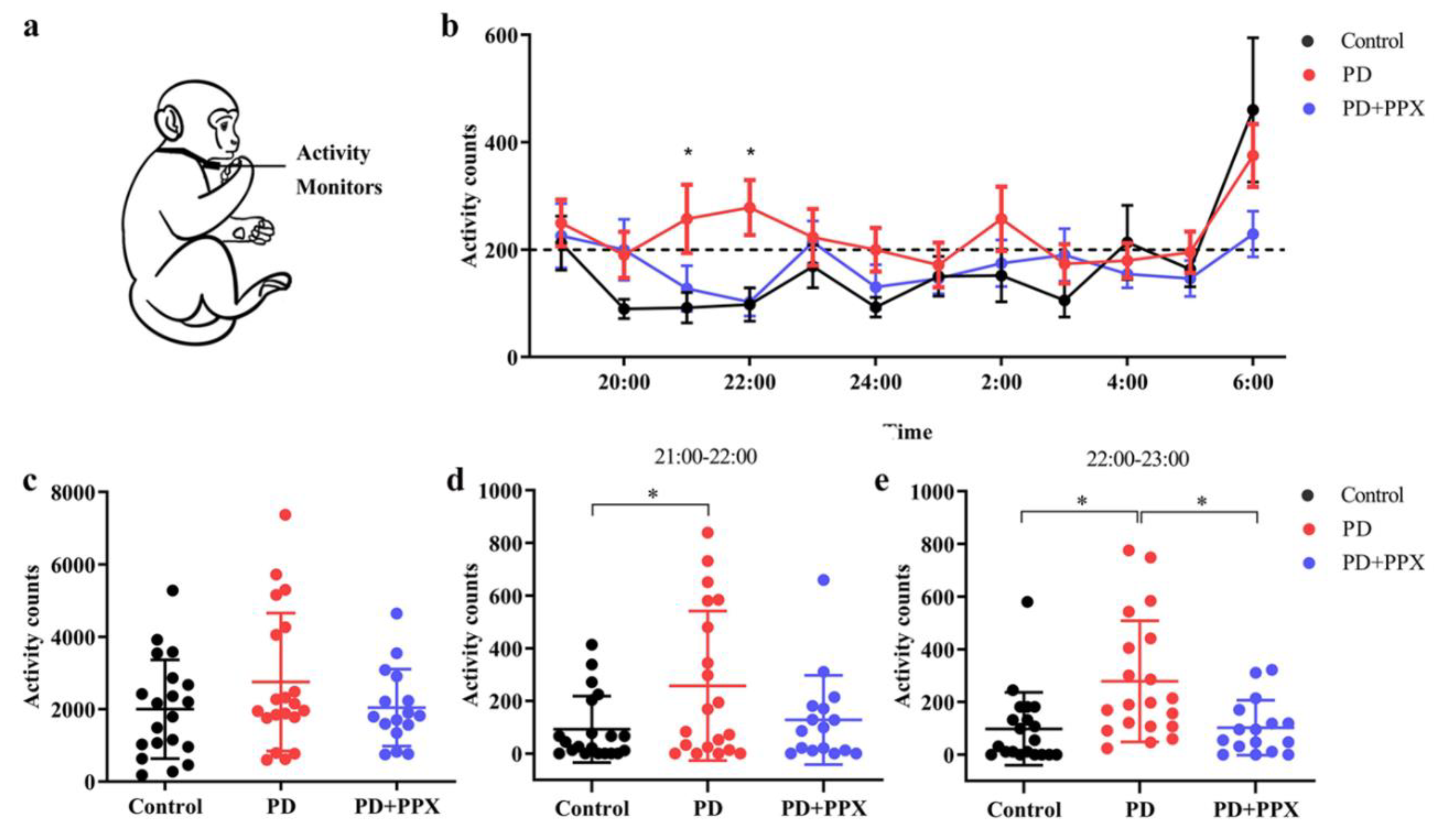
Physical Activity Monitor (PAM) measures the locomotor activity which indicates the sleep status indirectly during night. PAM was recorded using omnidirectional accelerometers (Actical Activity Monitor; MiniMitter Inc., Bend, OR). The accelerometer was placed in a small stainless-steel box, and the box was attached to a loose-fitting collar on the neck (
Figure 2a). The accelerometer was deployed one week before the test. The data in accelerometer was downloaded using ActiReader (MiniMitter Inc., Bend, OR), and three consecutive days of data were analyzed. Locomotor activity count was determined by the total number of moving counts per minute. Nocturnal activity between 7:00 PM and 7:00 AM was analyzed in this study.
2.6. Apathy Feeding Task
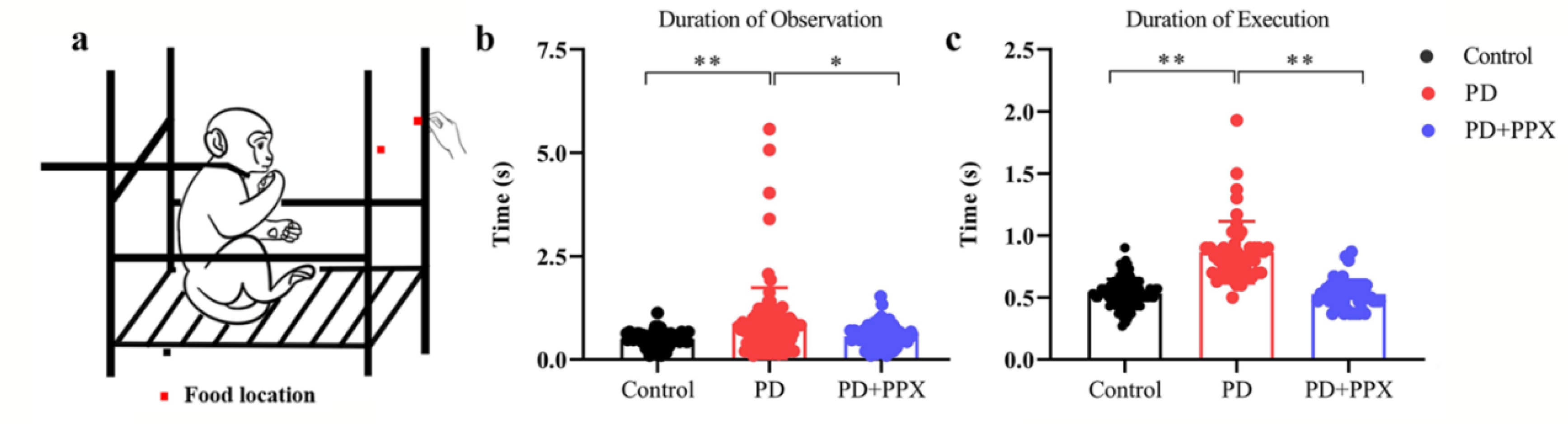
The Apathy Feeding Task (AFT) measures the degree and frequency of coaxing required to motivate the animal to attempt[
20]. The animal was restrained in a primate chair. A piece of food was offered by a research assistant the animal familiar with (
Figure 3a). The food was put on the two sides alternatively. If the monkey failed to reach for the food in 5 seconds, the food was discarded and substituted with another piece of food. If the monkey failed to reach for the fruit again, then the fruit was placed in the monkey’s hand. One successful trial was defined as fetching the food from experimenter’s hand. The tests were videotaped, and the behaviour was scored offline later. The duration of observation was the time from food presentation to the onset of reaching out for the food. Duration of execution was the time from reaching out for the food to the time of putting the food into the mouth.
2.7. Behavioral tests for anxiety
We used Human Intruder Test (HIT), Novel Fruit Test (NFT) and Predator Confrontation Test (PCT) to measure the anxiety level of the animals.
2.8. Human Intruder Test
HIT was modified from Kalin and colleagues’ study to assess behavioral responsiveness to a potential threatening or a nonthreatening social stimulus in rhesus monkeys[
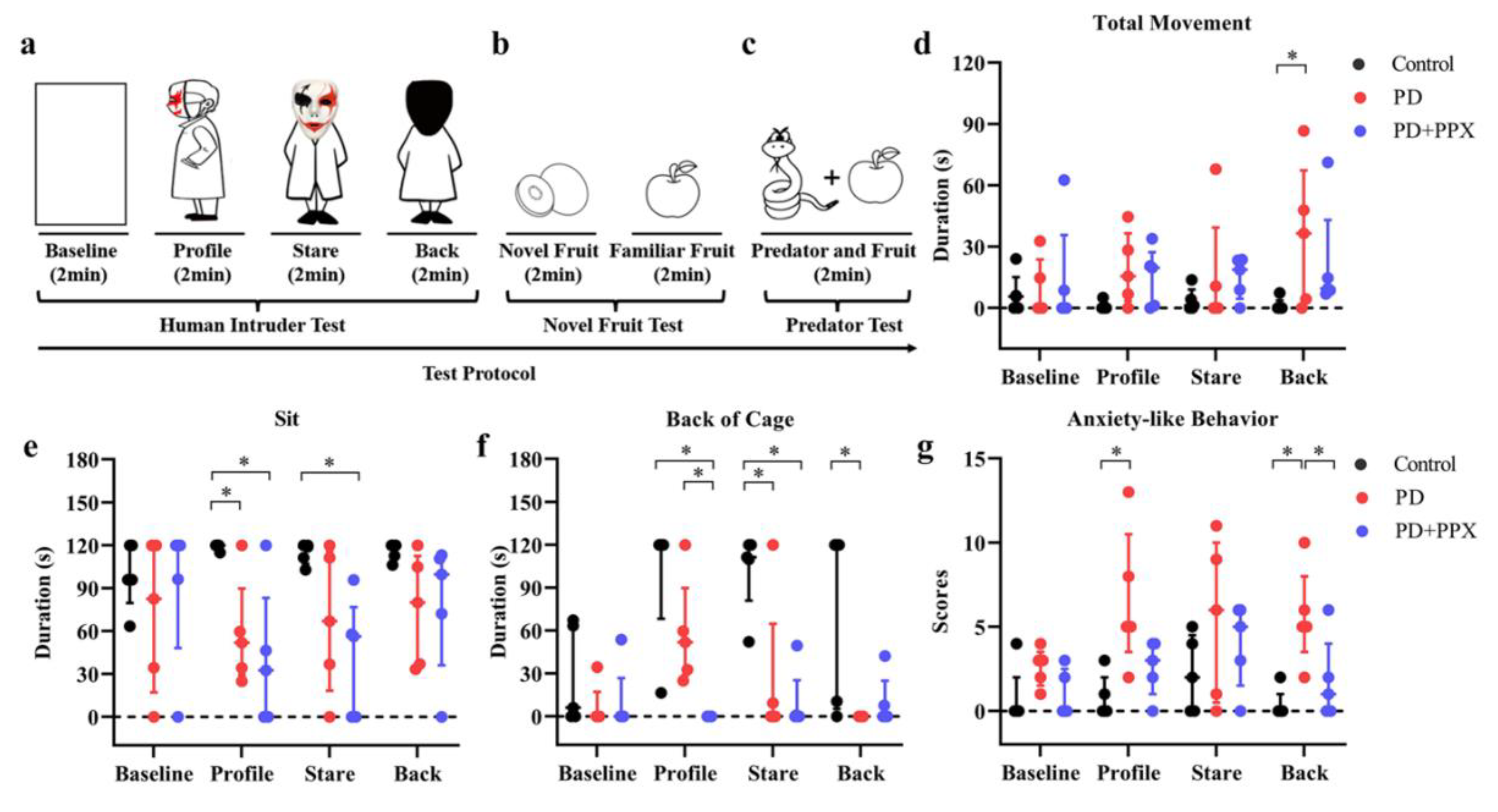
21]. The test consisted of four phases, baseline, profile, stare and back, 2 min for each phase (
Figure 4a). A camera was used to record the behavioral responses. Follow baseline phase, a human intruder wearing masks and cloaks entered the testing room. The human intruder presented obliquely a profile to the monkey (profile), then turned to the monkey (stare). During the back phase, the intruder turned the back to the monkey. The intruder left the testing room after the tests.
Video was processed using Squared5 (MPEG Streamclip software) and compressed to 30 frames/s for behavioral scoring. The HIT induced anxious behavior depends on the orientation of the human intruders and the specific threat level posed by each orientation[
22]. Increased vigilance, excessive fear and other context dependent anxiety-like behaviors were scored to evaluate the anxiety level [
23]. The scored behaviors were movement (the whole body moves from one position to another), sitting up (put the buttocks on the cage, support the body weight), tactile/oral exploration (tactile or oral manipulation of the cage with finger/mouth), self-groom (pick up dirt or brush hair with hands or mouth), lip-smack (pursing and moving the lips together to produce a smacking sound) [
24], backing of cage (subject positioning itself with at least three limbs in the back half of the cage), freezing (remaining immobile for longer than 2sec)[
21,
25], scratching (moving digits quickly through fur), growling (grunt, short, understated vocalization), cage shaking (grab and shake the cage with hands or feet), mouth-opening (open-mouth in an "O" shape stare), screaming (a high-pitched, loud sound), and the total times of fear grimace (a large grin-like facial expression showing the teeth) [
24], grinding teeth (upper and lower teeth moving noisily together), yawn (opening mouth and showing clenched teeth)[
25]. The behaviors scored were shown in Table 1.
2.9. Novel Fruit Test
NFT was performed after HIT to assess of the monkey’s motivation to explore novel objects. The assumption of the test is that animals with lower anxiety will exhibit more explorative behaviors. The paradigm used in this study was modified from Williamson et al (
Figure 4b)[
26]. During each trial, a piece of novel fruit (the animals never had before) was placed in the cage for 2 min. Afterwards, a piece of a familiar fruit (fruit used as food enrichment) was placed in the cage for 2 min. There were five trials in the test. The behaviour of the monkey was videotaped. The consumption rate, observation time and execution time was calculated offline.
2.10. Predator Confrontation Test
PCT was performed right after the NFT test. Barros and colleagues have shown that stimulus with specific features of natural predators induced fear and anxiety-like reactions in non-human primates[
27]. A potential predator model (i.e., snake model) and a familiar object (i.e., fruit) was placed in the front part of the cage, one on the left side the other on the right side (
Figure 4c). There were five testing trials for each monkey. The responses of animal were videotaped and scored. Withdrawal behavior (retreat to the back of the cage) and consumption rate of fruits were used to determine the fear and anxiety level.
2.11. Statistical Analysis
All data were analyzed using SPSS 22. 0, the data of the AFT, PAM, NFT and PCT were log transformed, and passed normal distribution test (Shapiro–Wilk test, p > 0.05), Multi-way ANOVA was used to analyze the duration of observation data in AFT. One-way ANOVA was used to analyze PAM data, and data are expressed as mean ± SD. Nonparametric tests were used to analyze DMTS, the duration of execution data in AFT and HIT data (Shapiro–Wilk test, p <0.05). The effects of PPX treatment on DMTS, HIT, NFT, PCT and the duration of execution in AFT were assessed using the Kruskal-Wallis H Test, and data were expressed as median (interquartile interval). A p-value of less than 0.05 was used to determine the statistical significance.
3. Results
3.1. PD monkeys showed working memory deficits in DMTS task
Four of five healthy monkeys were successfully trained to complete DMTS task, the correct ratios were above 80% without delay. The PD monkeys had noticeable unilateral limb disabilities. Four of five PD monkeys were successfully trained before the tests. Two of them complete the task using only the normal limbs. The correct ratio of control monkeys was significantly higher than PD monkeys for all delays (5s delay: 0.90(0.11) vs 0.53, H = -2.160, p = 0.031; 10s delay: 0.97 (0.17) vs 0.60, H = -2.160, p = 0.031; 15s delay: 0.87(0) vs 0.40, H (0.000) = -2.366, p = 0.018; 30s delay: 0.90(0.06) vs 0.47, H = -2.181, p = 0.029). After PPX treatment, the correct ratio was modestly improved (5s: 0.53 vs 0.75(0.36), H = 0.825, p = 0.031; 10s: 0.60 vs 0.70(0.32), H = 1.101, p = 0.487; 15s: 0.40 vs 0.50(0.37), H = 0.741, p = 0.261; 30s: 0.47 vs 0.65(0.34), H = 1.080, p = 0.268). These data indicated that working memory in PD monkeys was impaired, and PPX treatment did not improve the performance significantly (
Figure 1b).
3.2. PD monkeys showed more nocturnal activity
The activity was continuously monitored in the home cage. The total nocturnal activity in PD monkeys (2752.40 ± 1906.57) was higher than that in control monkeys (2000.55 ± 1363.78), but not to a significant level (F (2,53) = 1.289, p = 0.114,
Figure 2c). From 21:00 to 23:00, the hourly activity in the PD monkeys was significantly higher (21:00 - 22:00: 92.10 ±126.42 vs 257.30 ± 284.26, F (2,53) = 3.482, p = 0.044; 22:00 - 23:00: 98.05 ± 138.42 vs 278.60 ± 230.03, F (2,53) = 7.094, p = 0.004,
Figure 2d-e). After PPX treatment, the total nocturnal activity in PD monkeys decreased (2752.40 ± 1906.57 vs 2042.56±1060.97, F (2,53) = 1.289, p = 0.440,
Figure 2c). Specifically, and the activity 21:00 - 22:00 and 22:00 - 23:00 decreased significantly (257.30 ± 284.26 vs 127.75 ± 169.27, F (2,53) = 3.482, p = 0.202; and 278.60 ± 230.03 vs 102.56 ± 104.06, F (2,53) = 7.094, p = 0.01,
Figure 2d-e).
3.3. PD monkeys showed deficit in AFT task
The duration of observation in the AFT task was significantly longer in PD monkeys (0.99 ±1.13), compared to healthy control monkeys (0.530 ± 0.165, F (2, 142) = 5.954, p = 0.001,
Figure 3b). Similarly, the duration of execution was significantly longer in PD monkeys (0.830 (0.200)) than in control monkeys (0.53 (0.13), H (2) = 87.75, p = 0.000,
Figure 3c). After PPX treatment, the duration of observation and execution were significantly reduced (0.67± 0.25, F (2, 142) = 5.954, p = 0.020,
Figure 3b; 0.53 (0.14), H (2) = 87.747, p = 0.000,
Figure 3c). Note that when food was on one side, PD monkeys only use the normal limb no matter which side the food was. Control monkeys use both limbs to get the food. The monkeys got the food every time.
3.4. PPX reduced anxiety-like behaviors in PD monkeys
The behavioral responses during HIT tests were shown in
Figure 4. Overall, the anxiety-like behaviors were related to the human intruder’s face orientation. Compared to the control monkeys, PD monkeys spent less time sitting during profile, stare, and back phases (profile: 120.00(2.56) vs 51.93(60.27), p = 0.014; stare: 118.87(12.93) vs 66.90(97.30), p = 0.139; and back: 120.00(10.50) vs 80.00(77.22), p = 0.100; H (11) = 21.695, p = 0.027;
Figure 4e). Instead, they had more locomotor movement ((profile: 0.00(2.57) vs 15.53(33.09), p = 0.153; stare: 1.13(8.98) vs 0.00(39.29), p = 0.342; and back: 0.00(3.67) vs 36.47(65.10), p = 0.009; H (11) = 16.350, p = 0.129;
Figure 4d). They spent less time in the back of the cage when facing the human intruder (profile: 120.00(51.77) vs 51.93(61.01), p = 0.455; stare: 111.27(38.94) vs 0.00(64.70), p = 0.007; and back: 120.00(114.69) vs 0.00(0.00), p = 0.007; H (11) = 35.411, p = 0.000;
Figure 4f). After PPX treatment, the total movement, sitting time, or time in the back of the cage in PD monkeys did not change (
Figure 4d-f).
Accumulation score of anxiety-like behaviors was used to evaluate anxiety level in each test phase (see Table 1). PD monkeys showed higher anxiety-related accumulation scores across testing phases, especially during the profile and stare phases (profile: 0.00(2.00) vs 5.00(7.00), p = 0.006; stare: 2.00(5.00) vs 6.00(10.00), p = 0.246; back: 0.00(1.00) vs 5.00(5.00), p =0.003, H (11) = 25.978, p = 0.007;
Figure 4g). PPX treatment didn’t significantly decrease the score (profile: 5.00(7.00) vs 3.00(3.00), p = 0.140; stare: 6.00(10.00) vs 5.00(5.00), p = 0.963; back: 5.00(5.00) vs 1.00(4.00), p = 0.046, H (11) = 25.978, p = 0.007;
Figure 4g).
In the NFT test, the consumption rates of novel fruit in the control monkeys and PD monkeys were 100% and 80%, respectively. The consumption rate of familiar food was 100% in all monkeys. After PPX treatment, the consumption rates of PD monkeys increased to 100%. The observation time and execution time was calculated, no difference was found between the control monkeys and PD monkeys (data not shown).
In the PCT test, when the predator model (a snake model) was presented, the consumption rates for the control monkeys and PD monkeys were 80% and 40%, respectively. The withdrawal rates were 20% and 60% respectively. PPX treatment did not change the consumption rates (data not shown).
4. Discussion
The NHP PD models play important roles in PD drug discovery and development. Although new animal models are emerging during the past years, such as virus-based transgenic techniques or genetic editing monkey models, the MPTP-induced PD model is still the most widely used animal model to mimic the PD symptoms, especially motor deficits. Beside of motor deficit, cognitive or other non-motor deficits have been found here and there. However, it is still not clear MPTP-induced animal model can reliably mimic the non-motor symptoms in PD patients. In the current study, we quantitatively investigated the non-motor symptoms in MPTP-induced monkey PD model. Our results showed that these PD monkeys had various degree of non-motor deficits including working memory, novelty seeking, abnormal sleep and anxiety-like behaviors. After pramipexole (PPX, a dopamine receptor agonist) treatment, these deficits were reversed partially.
The current findings that PD monkeys showed working memory deficits and sleep disorders were consistent with clinical observations in PD patients [
4]. In fact, we reported previously that these monkeys had difficulty to learn DMTS task [
18]. Eight years later, four out of five PD monkeys completed the task. We believe this improvement is due to the years of adaptation to unilateral motor dysfunction.
The present results suggest that the MPTP-tread monkeys display cognitive deficits which mimic some core symptoms in Parkinson's patients. It is difficult to clarify that whether cognitive deficits were due to the motor dysfunction or dopamine systems disruption. Studies have suggested MPTP-induced putamen dopamine depletions is irreversible, and MPTP-induced cognitive deficits are due to disruption of fronto-striatal circuits, which is independent of clinical manifestations of motor dysfunction [
28,
29]. A longitudinal study of Rhesus macaques showed that the animals continued to exhibit spatial deficits ten years after receiving low doses of MPTP, although they displayed no obvious motor impairments [
30].
It has been reported that monkeys with prefrontal cortical ablations displayed varying degrees of apathy, increased distractibility, and poor attentive capacity for relevant stimuli [
28]. The MPTP-treated monkeys in current study showed no obvious apathy. The observation and execution periods in the AFT task were significantly increased in PD monkeys. In addition, the MPTP-treated monkeys did not use their ipsilateral limbs at all, and movement of the normal limbs were slower than the normal monkeys.
In the physical activity monitor test, the nocturnal activity of the MPTP-treated monkeys in each hour was higher, especially at 21:00 hour and 22:00 hour. This is consistent with previous findings which showed that the MPTP-induced monkeys replicated fragmented sleep observed in PD [
31,
32].
We used human intruder test to assess anxiety-like behaviors by presenting the animals with an unfamiliar human intruder [
22]. When the monkeys faced the intruders, they showed defensive or aggressive behaviors. For example, they moved more and spent less time sitting during profile, stare, and back phases. Accumulation score of anxiety-like behaviors indicate that PD monkeys were more anxious across testing phases, especially during the profile and stare phases.
The novel food test showed that MPTP-treated monkeys consumed less fruits. Remarkably, they took significantly less food in the predator confrontation test (40% compared to 80% in control monkeys). Monkeys usually show no fear of rubber snakes. However, most of the time, MPTP-treated monkeys in our study showed fear of rubber snakes and did not take food. These results indicated that the MPTP- treated monkeys were more anxious than control monkeys.
PPX has been shown to be able to prevent MPTP-induced loss of nigrostriatal dopaminergic neurons [
33]. In addition to relieve motor symptoms, PPX was also found to have anti-anxiety, anti-depression and paresthesia effects in PD patients [
34]. However, PPX did not improve working memory in PD patients [
35]. Consistently, our results showed that working memory deficit in DMTS task was not improved significantly after PPX treatment.
Nighttime activity was lowered to the control level after PPX treatment, especially at 21:00 hour and 22:00 hour. This indicates that PPX improved sleep quality in the MPTP-treated monkeys. In the AFT task, PPX significantly decreased the duration of execution in PD monkeys. Lastly, PPX treatment decreased the anxiety accumulation scores modestly.
PD is a complex neurodegenerative disease, and the causative factors are still not well understood [
36]. Non-motor symptoms in PD are known for a long time. But there is still no effective treatment. Most of previous studies using animal PD models have focused on motor deficits. Relatively little attention has been paid to the non-motor symptoms such as cognition decline [
9] or sleep deficits [
37,
38]. Interestingly, studies have shown that anxiety-like behaviors did not appear in MPTP-treated rodents [
10,
39,
40,
41]. MPTP-induced marmoset PD model exhibits abnormal psychotomimetic behaviors [
42], but not mood-related behaviors [
43], or social behaviors [
39,
44]. Our results showed that mood-related disorders can be induced in MPTP-treated cynomolgus monkeys. This may further suggest that MPTP-induced non-human primate models are of great significance for studying non-motor symptoms of PD [
45].
We used unilateral MPTP-induced model in the current study. Because systemic MPTP treatment would result in difficulty of the monkeys to eat, drink and performing the behavioral tasks due to dysphagia. In summary, we quantitatively evaluated the non-motor symptoms in MPTP-treated monkeys. Our results showed that these monkeys expressed non-motor deficits similar to those found in PD patients. We believe this MPTP-induced PD monkey model will play important role in future drug discovery and development targeting non-motor symptoms.
Author Contributions
Y.B. and F.Y. designed the experiments. Y.B. and C.G. executed experiments. Y.B., Z.C. and Z.Q. analyzed data. Y.B. and Z.M. drafted the manuscript, Y.B., Z.M. and F.Y. edited of final version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported in part by the National Key Research and Development Project of China (2018YFA0108503, 2018YFA0108304), National Natural Science Foundation of China (31472056, 31240043, U20A6005), Shenzhen Science and Technology Program (KQTD20210811090117032), National Hainan Key Research and Development Project (ZDYF2021SHFZ049). The funding body did not contribute to the design of the study, analysis, interpretation of data or writing the manuscript.
Ethics approval and consent to participate
All experimental protocols were approved by the Institutional Animal Care and Use Committee.
Consent for publication
Not applicable.
Availability of data and materials
The data and materials are available upon request directly to the corresponding authors’ emails.
Acknowledgments
We thank Binbin Luo, Kang Du and Qing Li for their valuable comments and technical help.
Conflicts of interest
The authors declare that they have no competing interests.
References
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Movement disorders : official journal of the Movement Disorder Society 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Prediger, R.D.; Matheus, F.C.; Schwarzbold, M.L.; Lima, M.M.; Vital, M.A. Anxiety in Parkinson's disease: a critical review of experimental and clinical studies. Neuropharmacology 2012, 62, 115–124. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nature reviews. Neuroscience 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.P.s.D. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018, 17, 939–953. [Google Scholar] [CrossRef]
- van Wamelen, D.J.; Martinez-Martin, P.; Weintraub, D.; Schrag, A.; Antonini, A.; Falup-Pecurariu, C.; Odin, P.; Ray Chaudhuri, K.; International, P.; Movement Disorder Society Parkinson's Disease Non-Motor Study, G. The Non-Motor Symptoms Scale in Parkinson's disease: Validation and use. Acta neurologica Scandinavica 2021, 143, 3–12. [Google Scholar] [CrossRef]
- Hely, M.A.; Morris, J.G.; Reid, W.G.; Trafficante, R. Sydney Multicenter Study of Parkinson's disease: non-L-dopa-responsive problems dominate at 15 years. Movement disorders : official journal of the Movement Disorder Society 2005, 20, 190–199. [Google Scholar] [CrossRef]
- Villalba, R.M.; Smith, Y. Loss and remodeling of striatal dendritic spines in Parkinson's disease: from homeostasis to maladaptive plasticity? Journal of neural transmission 2018, 125, 431–447. [Google Scholar] [CrossRef]
- Vezoli, J.; Fifel, K.; Leviel, V.; Dehay, C.; Kennedy, H.; Cooper, H.M.; Gronfier, C.; Procyk, E. Early presymptomatic and long-term changes of rest activity cycles and cognitive behavior in a MPTP-monkey model of Parkinson's disease. PLoS One 2011, 6, e23952. [Google Scholar] [CrossRef]
- Han, N.R.; Kim, Y.K.; Ahn, S.; Hwang, T.Y.; Lee, H.; Park, H.J. A Comprehensive Phenotype of Non-motor Impairments and Distribution of Alpha-Synuclein Deposition in Parkinsonism-Induced Mice by a Combination Injection of MPTP and Probenecid. Frontiers in aging neuroscience 2020, 12, 599045. [Google Scholar] [CrossRef] [PubMed]
- Tardif, S.D.; Coleman, K.; Hobbs, T.R.; Lutz, C. IACUC review of nonhuman primate research. ILAR journal 2013, 54, 234–245. [Google Scholar] [CrossRef]
- Przedborski, S.; Jackson-Lewis, V.; Yokoyama, R.; Shibata, T.; Dawson, V.L.; Dawson, T.M. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A 1996, 93, 4565–4571. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Zhang, P.; Ma, Y.; Wang, L.; Xu, H.; Sui, D. Neuroprotective effects of pramipexole transdermal patch in the MPTP-induced mouse model of Parkinson's disease. Journal of pharmacological sciences 2018, 138, 31–37. [Google Scholar] [CrossRef]
- Xiang, W.; Sun, Y.Q.; Teoh, H.C. Comparison of nocturnal symptoms in advanced Parkinson's disease patients with sleep disturbances: pramipexole sustained release versus immediate release formulations. Drug design, development and therapy 2018, 12, 2017–2024. [Google Scholar] [CrossRef]
- Shen, T.; Ye, R.; Zhang, B. Efficacy and safety of pramipexole extended-release in Parkinson's disease: a review based on meta-analysis of randomized controlled trials. European journal of neurology 2017, 24, 835–843. [Google Scholar] [CrossRef]
- Mansouri, F.A.; Egner, T.; Buckley, M.J. Monitoring Demands for Executive Control: Shared Functions between Human and Nonhuman Primates. Trends in neurosciences 2017, 40, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: a review. Jama 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Barone, P.; Ceravolo, R.; Fabbrini, G.; Tinazzi, M.; Abbruzzese, G. Role of pramipexole in the management of Parkinson's disease. CNS drugs 2010, 24, 829–841. [Google Scholar] [CrossRef]
- Yue, F.; Chan, P.; Wan, H.; Weeks, R.; Grondin, R.; Than, Z. Similarities and Differences in Cognitive Deficits and Responsiveness to L-Dopa Between Aged and MPTP-Treated Cynomolgus Monkeys Tested on the Same Tasks. Cell Transplant 2014, 23, 790–790. [Google Scholar]
- Brown, C.A.; Campbell, M.C.; Karimi, M.; Tabbal, S.D.; Loftin, S.K.; Tian, L.L.; Moerlein, S.M.; Perlmutter, J.S. Dopamine pathway loss in nucleus accumbens and ventral tegmental area predicts apathetic behavior in MPTP-lesioned monkeys. Experimental neurology 2012, 236, 190–197. [Google Scholar] [CrossRef]
- Kalin, N.H.; Shelton, S.E. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science 1989, 243, 1718–1721. [Google Scholar] [CrossRef]
- Hamel, A.F.; Lutz, C.K.; Coleman, K.; Worlein, J.M.; Peterson, E.J.; Rosenberg, K.L.; Novak, M.A.; Meyer, J.S. Responses to the Human Intruder Test are related to hair cortisol phenotype and sex in rhesus macaques (Macaca mulatta). Am J Primatol 2017, 79, 1–10. [Google Scholar] [CrossRef]
- Celano, C.M.; Daunis, D.J.; Lokko, H.N.; Campbell, K.A.; Huffman, J.C. Anxiety Disorders and Cardiovascular Disease. Curr Psychiatry Rep 2016, 18, 101. [Google Scholar] [CrossRef]
- Maestripieri, D. Gestural communication in three species of macaques ( Macaca mulatta, M. nemestrina, M. arctoides ). 2007.
- Machado, C.J.; Kazama, A.M.; Bachevalier, J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion 2009, 9, 147–163. [Google Scholar] [CrossRef]
- Williamson, D.E.; Coleman, K.; Bacanu, S.A.; Devlin, B.J.; Rogers, J.; Ryan, N.D.; Cameron, J.L. Heritability of fearful-anxious endophenotypes in infant rhesus macaques: a preliminary report. Biological psychiatry 2003, 53, 284–291. [Google Scholar] [CrossRef]
- Barros, M.; Tomaz, C. Non-human primate models for investigating fear and anxiety. Neurosci Biobehav Rev 2002, 26, 187–201. [Google Scholar] [CrossRef]
- Schneider, J.S.; Kovelowski, C.J., 2nd. Chronic exposure to low doses of MPTP. I. Cognitive deficits in motor asymptomatic monkeys. Brain Res 1990, 519, 122–128. [Google Scholar] [CrossRef]
- Schneider, J.S. Chronic exposure to low doses of MPTP. II. Neurochemical and pathological consequences in cognitively-impaired, motor asymptomatic monkeys. Brain Res 1990, 534, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, J.; Doudet, D.J.; Aigner, T.G. Long-term cognitive impairment in MPTP-treated rhesus monkeys. Neuroreport 1995, 7, 102–104. [Google Scholar] [CrossRef]
- Boulet, S.; Mounayar, S.; Poupard, A.; Bertrand, A.; Jan, C.; Pessiglione, M.; Hirsch, E.C.; Feuerstein, C.; Francois, C.; Feger, J.; et al. Behavioral recovery in MPTP-treated monkeys: neurochemical mechanisms studied by intrastriatal microdialysis. J Neurosci 2008, 28, 9575–9584. [Google Scholar] [CrossRef]
- Barraud, Q.; Lambrecq, V.; Forni, C.; McGuire, S.; Hill, M.; Bioulac, B.; Balzamo, E.; Bezard, E.; Tison, F.; Ghorayeb, I. Sleep disorders in Parkinson's disease: the contribution of the MPTP non-human primate model. Experimental neurology 2009, 219, 574–582. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Heng, Y.; Mou, Z.; Huang, J.Y.; Yuan, Y.H.; Chen, N.H. Reassessment of subacute MPTP-treated mice as animal model of Parkinson's disease. Acta pharmacologica Sinica 2017, 38, 1317–1328. [Google Scholar] [CrossRef]
- Seppi, K.; Weintraub, D.; Coelho, M.; Perez-Lloret, S.; Fox, S.H.; Katzenschlager, R.; Hametner, E.M.; Poewe, W.; Rascol, O.; Goetz, C.G.; et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society 2011, 26 Suppl 3, S42-80. [Google Scholar] [CrossRef]
- Costa, A.; Peppe, A.; Dell'Agnello, G.; Caltagirone, C.; Carlesimo, G.A. Dopamine and cognitive functioning in de novo subjects with Parkinson's disease: effects of pramipexole and pergolide on working memory. Neuropsychologia 2009, 47, 1374–1381. [Google Scholar] [CrossRef]
- Delamarre, A.; MacSweeney, C.; Suzuki, R.; Brown, A.J.; Li, Q.; Pioli, E.Y.; Bezard, E. Gastrointestinal and metabolic function in the MPTP-treated macaque model of Parkinson's disease. Heliyon 2020, 6, e05771. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Mao, Y.; Feng, X.L.; Zheng, N.; Lu, L.B.; Ma, Y.Y.; Qin, D.D.; Hu, X.T. Early adversity contributes to chronic stress induced depression-like behavior in adolescent male rhesus monkeys. Behav Brain Res 2016, 306, 154–159. [Google Scholar] [CrossRef]
- Willard, S.L.; Daunais, J.B.; Cline, J.M.; Shively, C.A. Hippocampal volume in postmenopausal cynomolgus macaques with behavioral depression. Menopause 2011, 18, 582–586. [Google Scholar] [CrossRef]
- Konnova, E.A.; Swanberg, M. Animal Models of Parkinson's Disease Brisbane (AU). Parkinson's Disease: Pathogenesis and Clinical Aspects 2018. [Google Scholar]
- Vuckovic, M.G.; Wood, R.I.; Holschneider, D.P.; Abernathy, A.; Togasaki, D.M.; Smith, A.; Petzinger, G.M.; Jakowec, M.W. Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiology of disease 2008, 32, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Titova, N.; Schapira, A.H.V.; Chaudhuri, K.R.; Qamar, M.A.; Katunina, E.; Jenner, P. Nonmotor Symptoms in Experimental Models of Parkinson's Disease. International review of neurobiology 2017, 133, 63–89. [Google Scholar] [CrossRef]
- Visanji, N.P.; Gomez-Ramirez, J.; Johnston, T.H.; Pires, D.; Voon, V.; Brotchie, J.M.; Fox, S.H. Pharmacological characterization of psychosis-like behavior in the MPTP-lesioned nonhuman primate model of Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society 2006, 21, 1879–1891. [Google Scholar] [CrossRef]
- Phillips, K.A.; Ross, C.N.; Spross, J.; Cheng, C.J.; Izquierdo, A.; Biju, K.C.; Chen, C.; Li, S.; Tardif, S.D. Behavioral phenotypes associated with MPTP induction of partial lesions in common marmosets (Callithrix jacchus). Behav Brain Res 2017, 325, 51–62. [Google Scholar] [CrossRef]
- Coleman, K.; Tully, L.A.; McMillan, J.L. Temperament correlates with training success in adult rhesus macaques. Am J Primatol 2005, 65, 63–71. [Google Scholar] [CrossRef]
- Yue, F.; Zeng, S.; Tang, R.; Tao, G.; Chan, P. MPTP Induces Systemic Parkinsonism in Middle-Aged Cynomolgus Monkeys: Clinical Evolution and Outcomes. Neuroscience bulletin 2017, 33, 17–27. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).