Submitted:
05 June 2023
Posted:
08 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
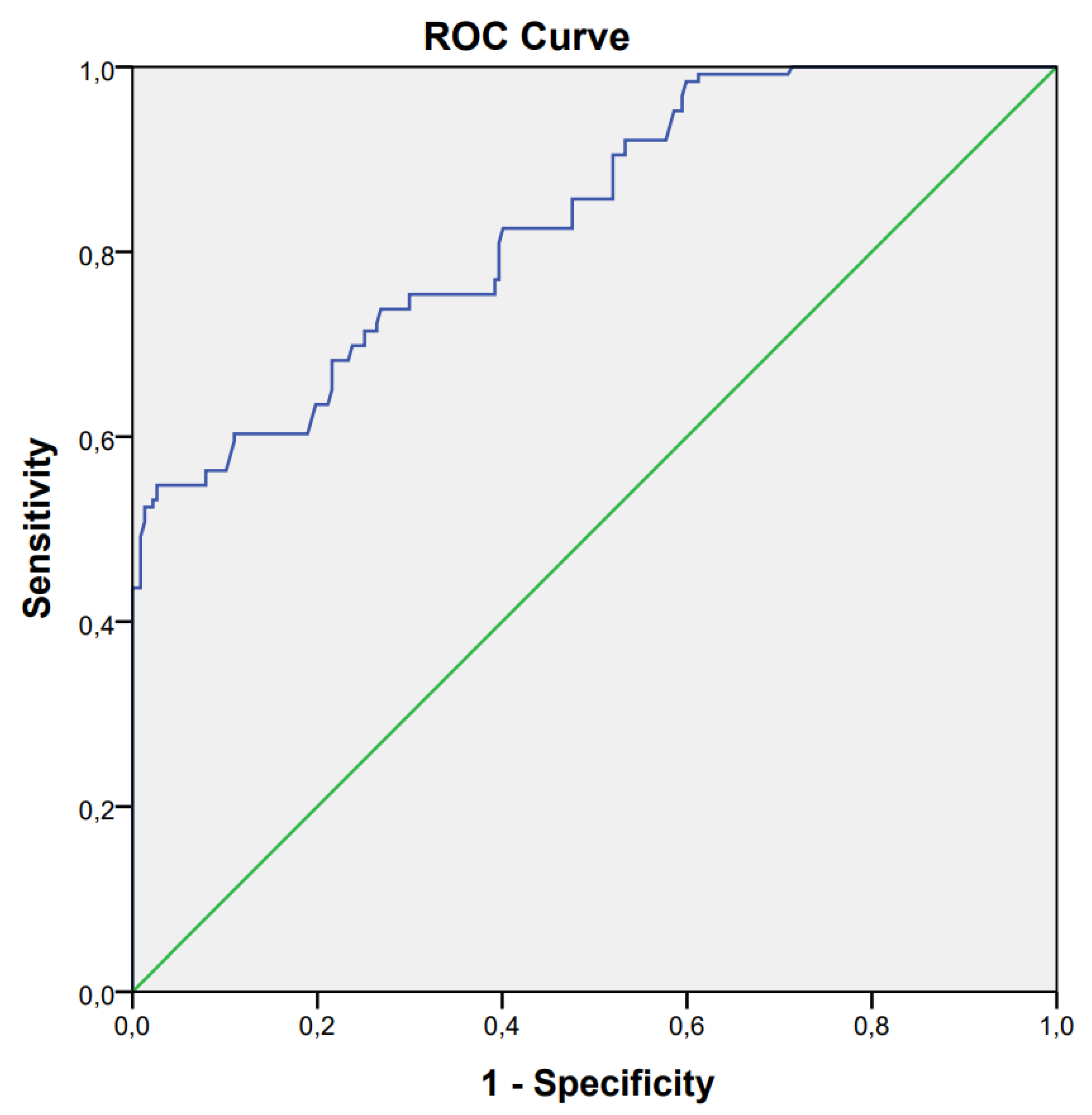
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turkish Endocrinology And Metabolism Association, Diagnosis, Treatment And Follow-Up Guide Of Diabetes Mellitus And Complications. 2022: p. 15-16.
- Guariguata, L.; et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes research and clinical practice 2014, 103, 137–149. [Google Scholar] [CrossRef]
- Murphy, D.; et al. Trends in prevalence of chronic kidney disease in the United States. Annals of internal medicine 2016, 165, 473–481. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Xue, R; et al. Mechanistic Insight and Management of Diabetic Nephropathy: Recent Progress and Future Perspective. J Diabetes Res. 2017, 2017, 1839809. [Google Scholar] [CrossRef]
- Mima, A. Diabetic nephropathy: protective factors and a new therapeutic paradigm. Journal of Diabetes and its Complications 2013, 27, 526–530. [Google Scholar] [CrossRef]
- Zhang, L.; Long, J.; Jiang, W.; et al. Trends in chronic kidney disease in China. New England Journal of Medicine 2016, 375, 905–906. [Google Scholar] [CrossRef]
- Ritz, E.; Stefanski, A. Diabetic nephropathy in type II diabetes. American journal of kidney diseases 1996, 27, 167–194. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, C.; Berl, T. Pathogenesis of diabetic nephropathy. Reviews in Endocrine and Metabolic Disorders 2004, 5, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. European journal of clinical investigation 2004, 34, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Cooper, M.E. Pathogenesis of diabetic nephropathy. Journal of diabetes investigation 2011, 2, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Jha, J.C.; Banal, C.; Chow, B.S.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic kidney disease: challenges, progress, and possibilities. Clinical journal of the American Society of Nephrology 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Tervaert, T.W.; et al. Renal Pathology Society. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010, 21, 556–63. [Google Scholar] [CrossRef]
- Maezawa, Y.; Takemoto, M.; Yokote, K. Cell biology of diabetic nephropathy: roles of endothelial cells, tubulointerstitial cells and podocytes. Journal of Diabetes Investigation 2015, 6, 3–15. [Google Scholar] [CrossRef]
- Najafian, C.; Alpers, E.; Fogo, A.B. Pathology of human diabetic nephropathy. Diabetes and the Kidney 2011, 170, 36–47. [Google Scholar]
- Pourghasem, M.; Shafi, H.; Babazadeh, Z. Histological changes of kidney in diabetic nephropathy. Caspian Journal of Internal Medicine 2015, 6, 120–127. [Google Scholar]
- Cao, Z.; Cooper, M.E. Pathogenesis of diabetic nephropathy. Journal of diabetes investigation 2011, 2, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.E. Interaction ofmetabolic and haemodynamicfactors in mediating experimental diabetic nephropathy. Diabetologia 2001, 44, 1957–1972. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Pickering, R.J.; Tsorotes, D.; et al. Genetic Ace2deficiency accentuates vascular inflammation and athero-sclerosis in the ApoE knockout mouse. Circ Res 2010, 107, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Tikellis, C.; Bialkowski, K.; Pete, J.; et al. ACE2 deficiency modifiesrenoprotection afforded by ACE inhibition in experimentaldiabetes. Diabetes 2008, 57, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Cooper, M.E.; Kawachi, H.; et al. Irbesartan normalisesthe deficiency in glomerular nephrin expression in a modelof diabetes and hypertension. Diabetologia 2001, 44, 874–877. [Google Scholar]
- Bonnet, F.; Tikellis, C.; Kawachi, H.; et al. Nephrin expressionin the post-natal developing kidney in normotensiveand hypertensive rats. Clin Exp Hypertens 2002, 24, 371–381. [Google Scholar] [CrossRef]
- Davis, B.J.; Cao, Z.; de Gasparo, M.; et al. Disparate effects ofangiotensin II antagonists and calcium channel blockers onalbuminuria in experimental diabetes and hypertension: potential role of nephrin. J Hypertens 2003, 21, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Morales, R.E.; et al. Inflammation in diabetic kidney disease. Nephron 2019, 143, 12–16. [Google Scholar] [CrossRef]
- Liu, Q.; et al. The association between high-sensitivity C-reactive protein concentration and diabetic nephropathy: a meta-analysis. Eur Rev Med Pharmacol Sci 2015, 19, 4558–4568. [Google Scholar]
- Tang, M.; et al. Association between high-sensitivity c-reactive protein and diabetic kidney disease in patients with type 2 diabetes mellitus. Frontiers in Endocrinology 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Bilgin S; et al. Does C-reactive protein to serum Albumin Ratio correlate with diabEtic nephropathy in patients with Type 2 dIabetes MEllitus? The CARE TIME study. Prim Care Diabetes 2021, 15, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Atak, B.; et al. Diabetes control could through platelet-to-lymphocyte ratio in hemograms. Revista da Associação Médica Brasileira 2019, 65, 38–42. [Google Scholar] [CrossRef]
- Aktaş, G.; Cakiroglu, B.; Sit, M.; Uyeturk, U.; Alcelik, A.; Savli, H.; Kemahli, E. Mean Platelet Volume: A simple indicator of chronic prostatitis. Acta Medica Mediterranea 2013, 29, 551–554. [Google Scholar]
- Cakır, L.; et al. Are Red Cell Distribution Width and Mean Platelet Volume associated with Rheumatoid Arthritis? Biomedical Research 2016, 27, 292–294. [Google Scholar]
- Kocak, M.Z.; et al. Z.; et al. Mean Platelet Volume to Lymphocyte Ratio as a Novel Marker for Diabetic Nephropathy. J Coll Physicians Surg Pak. 2018, 28, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clinical Cancer Research 2014, 20, 6212–6222. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World journal of gastroenterology 2017, 23, 6261. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; et al. Systemic immune-inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score-matched analysis. Scientific reports 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; et al. Systemic immune-inflammation index, based on platelet counts and neutrophil-lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. The Tohoku journal of experimental medicine 2015, 236, 297–304. [Google Scholar] [CrossRef]
- Huang, H.; et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Scientific reports 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Miao, Y.; et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer Biomarkers 2016, 17, 33–40. [Google Scholar] [CrossRef]
- Wang, J.; et al. Association between systemic immune-inflammation index and diabetic depression. Clinical interventions in aging 2021, 97–105. [Google Scholar] [CrossRef]
- Özata Gündoğdu, K.; et al. Serum inflammatory marker levels in serous macular detachment secondary to diabetic macular edema. European Journal of Ophthalmology 2022, 32, 3637–3643. [Google Scholar] [CrossRef]
- Chung, F.-M.; et al. Peripheral total and differential leukocyte count in diabetic nephropathy: the relationship of plasma leptin to leukocytosis. Diabetes care 2005, 28, 1710–1717. [Google Scholar] [CrossRef]
- Huang, W.; et al. Neutrophil–lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clinical endocrinology 2015, 82, 229–233. [Google Scholar] [CrossRef]
- Liu, J.; et al. The association of neutrophil to lymphocyte ratio, mean platelet volume, and platelet distribution width with diabetic retinopathy and nephropathy: a meta-analysis. Bioscience reports 2018, 38. [Google Scholar] [CrossRef]
- Zhang, R.; et al. Increased neutrophil count Is associated with the development of chronic kidney disease in patients with diabetes. Journal of Diabetes 2022, 14, 442–454. [Google Scholar] [CrossRef]
- Phillipson, M. and P. Kubes, The neutrophil in vascular inflammation. Nature medicine 2011, 17, 1381–1390. [Google Scholar] [CrossRef]
- Shanmugam, N.; et al. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 2003, 52, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; et al. Molecular mechanisms of tumor necrosis factor α gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and-independent pathways. Journal of Biological Chemistry 2000, 275, 17728–17739. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; et al. Insufficient glycemic control increases nuclear factor-κB binding activity in peripheral blood mononuclear cells isolated from patients with type 1 diabetes. Diabetes Care 1998, 21, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Kedziora-Kornatowska, K. Production of Superoxide and Nitric Oxide by Granulocytes in Non-Insulin-Dependent Diabetic Patients with and Without Diabetic Nephropathy. IUBMB life 1999, 48, 359–362. [Google Scholar]
- Korpinen, E.; et al. Increased secretion of TGF-β1 by peripheral blood mononuclear cells from patients with type 1 diabetes mellitus with diabetic nephropathy. Diabetic medicine 2001, 18, 121–125. [Google Scholar] [CrossRef]
- Chow, F.; et al. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney international 2004, 65, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.Z.; et al. Is Uric Acid elevation a random finding or a causative agent of diabetic nephropathy? Rev Assoc Med Bras (1992) 2019, 65, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Duman, T.T.; et al. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. African health sciences 2019, 19, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.Z.; et al. Monocyte lymphocyte ratio As a predictor of Diabetic Kidney Injury in type 2 Diabetes mellitus; The MADKID Study. J Diabetes Metab Disord. 2020, 19, 997–1002. [Google Scholar] [CrossRef] [PubMed]
| DKI Present n (%) | DKI Absent n (%) | Healthy n (%) | p value* | |
|---|---|---|---|---|
| Gender Female Male |
71(56,3%) 55(43,7%) |
134(59,0%) 93(41,0%) |
37(19,9%) 149 (80,1%) |
<0.001 |
| Smoking User Non-user |
25(20,0%) 100(80,0%) |
37 (16,3%) 190(83,7%) |
17(10,7%) 142(89,3%) |
0.088 |
| Alcohol Consumer Non-consumer |
6(4,8%) 120(95,2%) |
2(0,9%) 225(99,1%) |
0(0,0%) 159(100,0%) |
0.003 |
| Retinopathy Present Absent |
17(13,5%) 109(96,5%) |
15(6,6%) 212(93,4%) |
- |
0.031 |
| Neuropathy Present Absent |
86(68,3%) 40(31,7%) |
52(22,9%) 175(77,1%) |
- |
<0.001 |
| DKI Present | DKI Absent | Healthy | p value* | |
|---|---|---|---|---|
| Median (Min-Max) | ||||
| Age (years) | 59(41,86) | 58(29,76) | 53(18,76) | <0.001 |
| Height (cm) | 1,59(1,46-1,81) | 1,61(1,45-1,85) | 1,68(1,52-1,87) | <0.001 |
| Weight (kg) | 78(48-106) | 86(59-117) | 187,3(55-136) | <0.001 |
| BMI (kg/m2) | 29,1 (16,6-43,5) | 32 (21,9-46,1) | 27,7 (18,3-49,4) | <0.001 |
| Waist Circumference (cm) | 102 (75-126) | 107 (82-104) | 98 (65-144) | <0.001 |
| Systolic Blood Pressure (mmHg) | 120 (90-180) | 130 (100-180) | 120 (90-180) | 0.02 |
| Diastolic Blood Pressure (mmHg) | 80 (50-110) | 80 (60-100) | 80 (50-105) | 0.867 |
| DKI Present | DKI Absent | Healthy | p value* | |
|---|---|---|---|---|
| Median (Min-Max) | ||||
| WBC (mm3/count) | 6.240 (3.360-10.800) | 5.050 (2.500-13.700) | 5.500 (1.240-14.100) | <0.001 |
| Plt (mm3/count) | 251.000 (92.600-418.000) | 229.000 (150.000-915.000) | 239.000 (151.000-374.000) | <0.001 |
| Neu (mm3count) | 2.085 (356-7.170) | 2.470 (980-109.000) | 3.200 (1.000-8.330) | <0.001 |
| Lym (mm3/count) | 1980(356-3900) | 1950(1120-5010) | 2100(833-4510) | <0.001 |
| Hb (g/dL) | 13,1(9,8-16,2) | 13,3(10,2-17,9) | 14(8,8-17,1) | <0.001 |
| Hct (%) | 38,8(29,7-48,5) | 38,1(31-51,3) | 42(27,3-51,2) | <0.001 |
| Fasting Blood Glucose (mg/dL) | 186(86-565) | 143(92-514) | 93(69-118) | <0.001 |
| HbA1c (mmoL/dL) | 9(6,4-16,5) | 7,6(5,1-16) | 5,4(5,2-6,8) | <0.001 |
| CRP (mg/dL) | 8,1(0,9-45) | 3,4(0,01-22) | 2,4(0,1-11,9) | <0.001 |
| Urea (mg/dL) | 30(17,58) | 32(13-57,8) | 28(13-62) | 0.124 |
| Creatine (mg/dL) | 0,8(0,63-1,38) | 0,78(0,66-1,87) | 0,8(0,4-2,7) | 0.235 |
| GFR (mL/min) | 96,3(39,14-150,8) | 111,5(51,2-187,2) | 115,2(88,4-208) | 0.001 |
| Uric Acid (mg/dL) | 5,5(3,2-10) | 5,5(2,4-9,6) | 5,7(2,5-10,4) | 0.071 |
| Serum Albumin (g/L) | 4,3(3,5-5,1) | 4,4(3,9-4,9) | 4,5(3,8-5,1) | 0.001 |
| AST (U/L) | 16(11-39) | 19(8-39) | 18(9-31) | <0.001 |
| ALT(U/L) | 18(8-64) | 23(6-94) | 19(6-58) | <0.001 |
| Total Cholesterol (mg/dL) | 208(107-318) | 198(92-325) | 200(114-290) | 0.124 |
| Triglyceride (mg/dL) | 176(47-411) | 153(50-1050) | 134(52-680) | 0.013 |
| LDL (mg/dL) | 126(42,3-200) | 119(42-244) | 115(49-192) | 0.352 |
| HDL (mg/dL) | 49,8(26,8-86,6) | 44(25,5-61) | 47(20,6-85,2) | <0.001 |
| SII | 584(178,4-4819) | 282,5(64,3-618,4) | 236(77,4-616,7) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




