Submitted:
08 June 2023
Posted:
08 June 2023
You are already at the latest version
Abstract
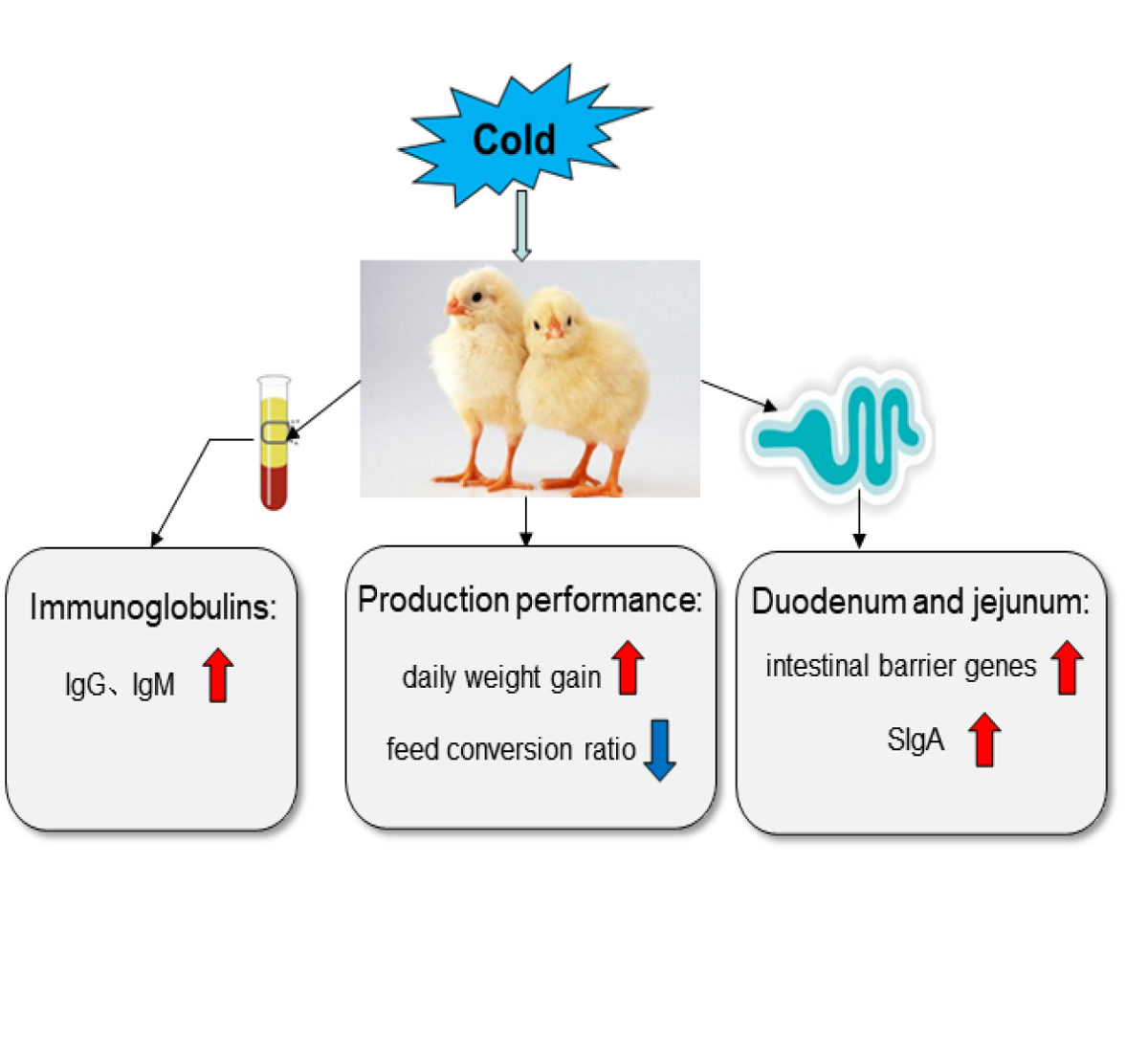
Keywords:
1. Introduction
2. Materials and Methods
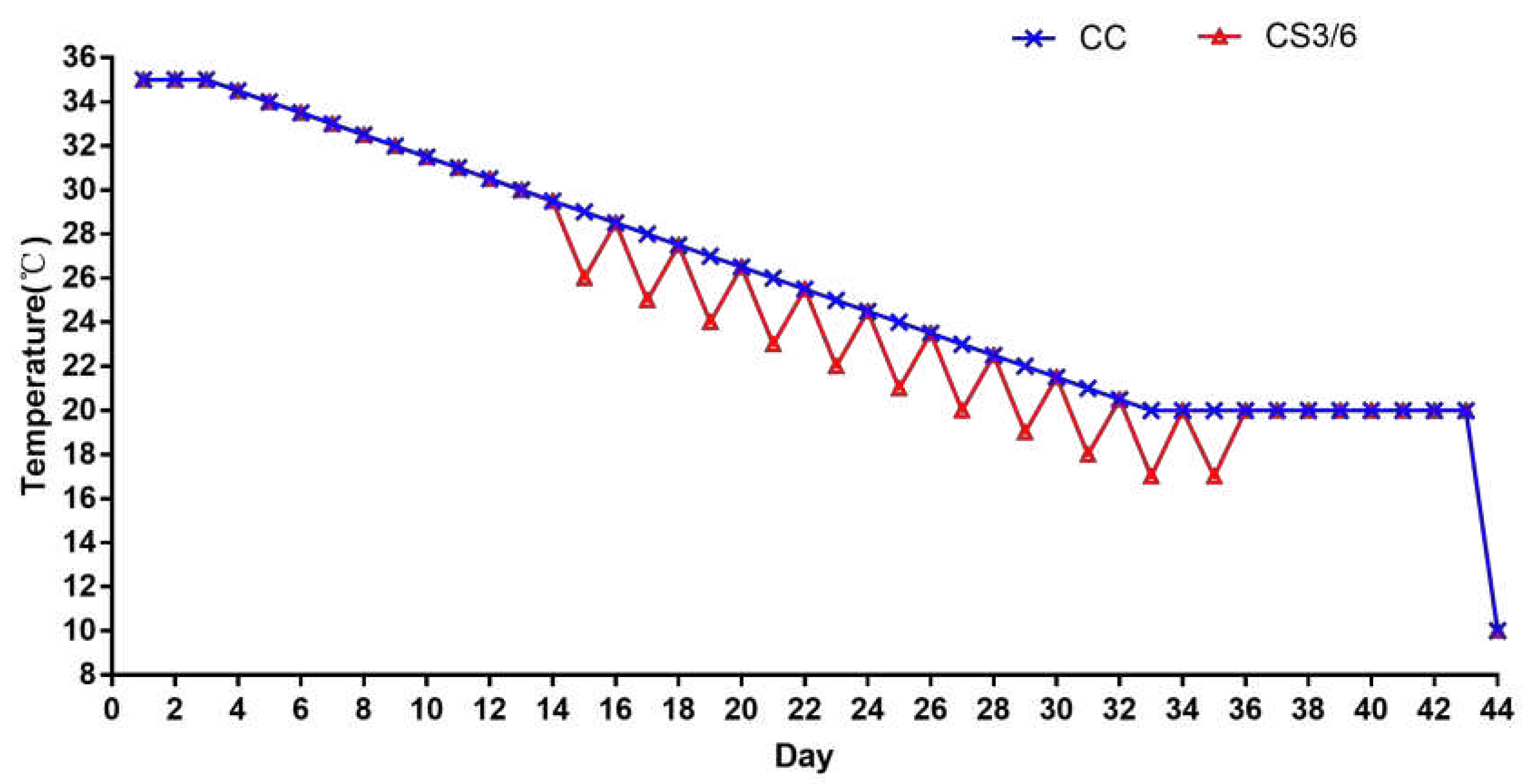
2.1. Animals and Experimental Design
2.2. Sample Collection
2.3. Evaluation of Broiler Production Performance
2.4. RNA Extraction and Reverse Transcription
2.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.6. Western Blot Analysis
2.7. Elisa Detection
2.8. Statistics and Analysis
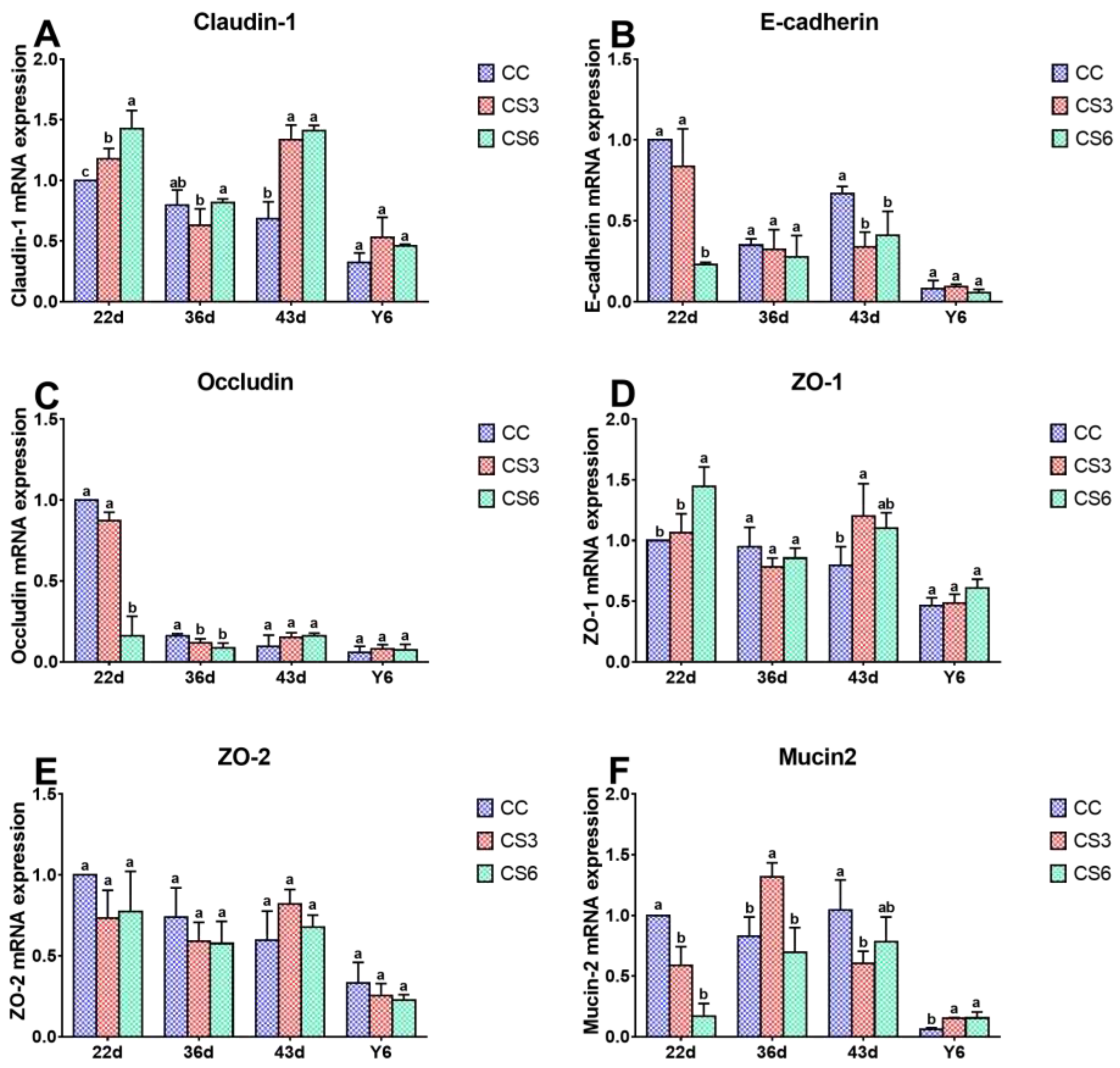
3. Results
3.1. Production Performance of Broilers
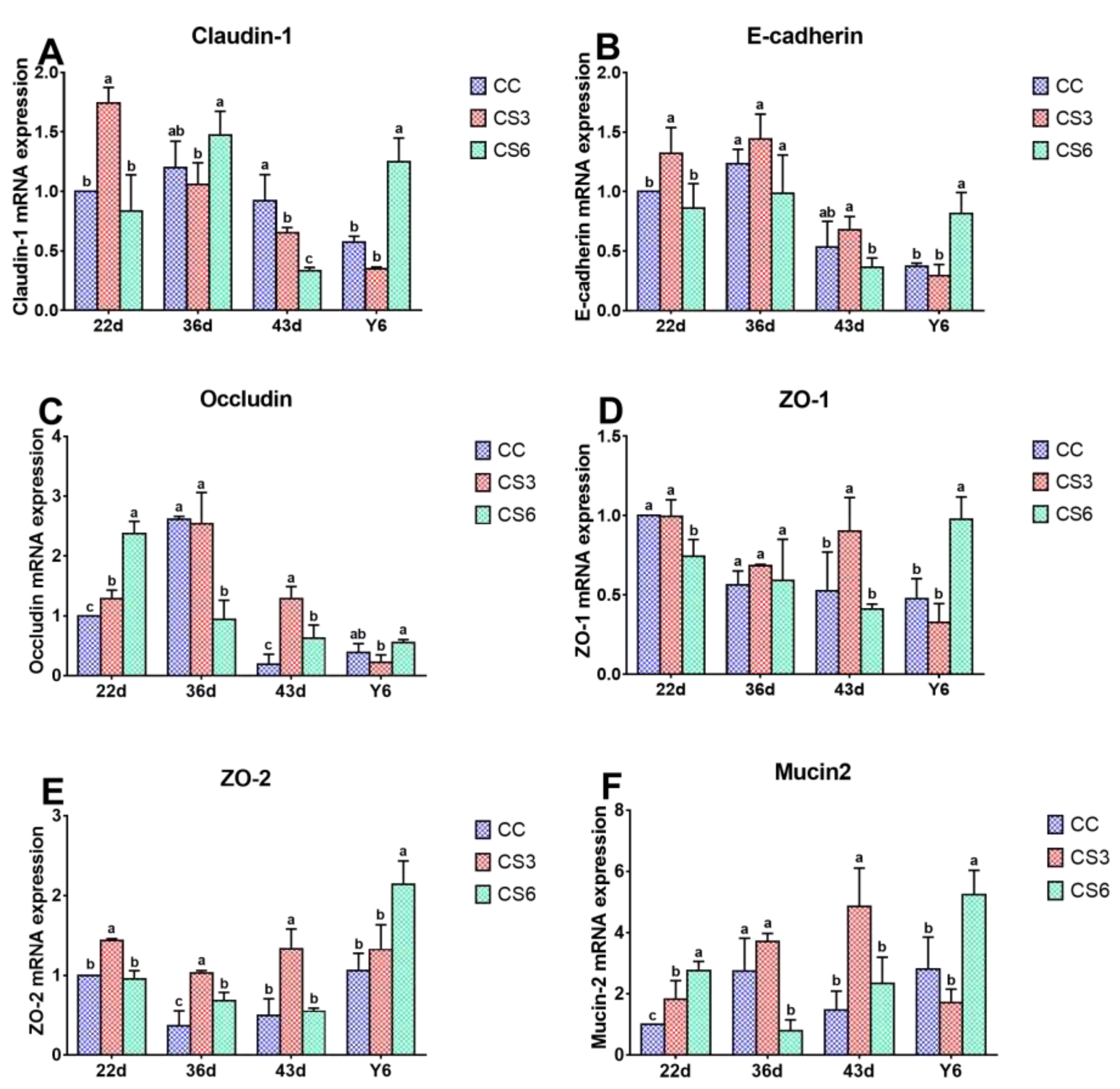
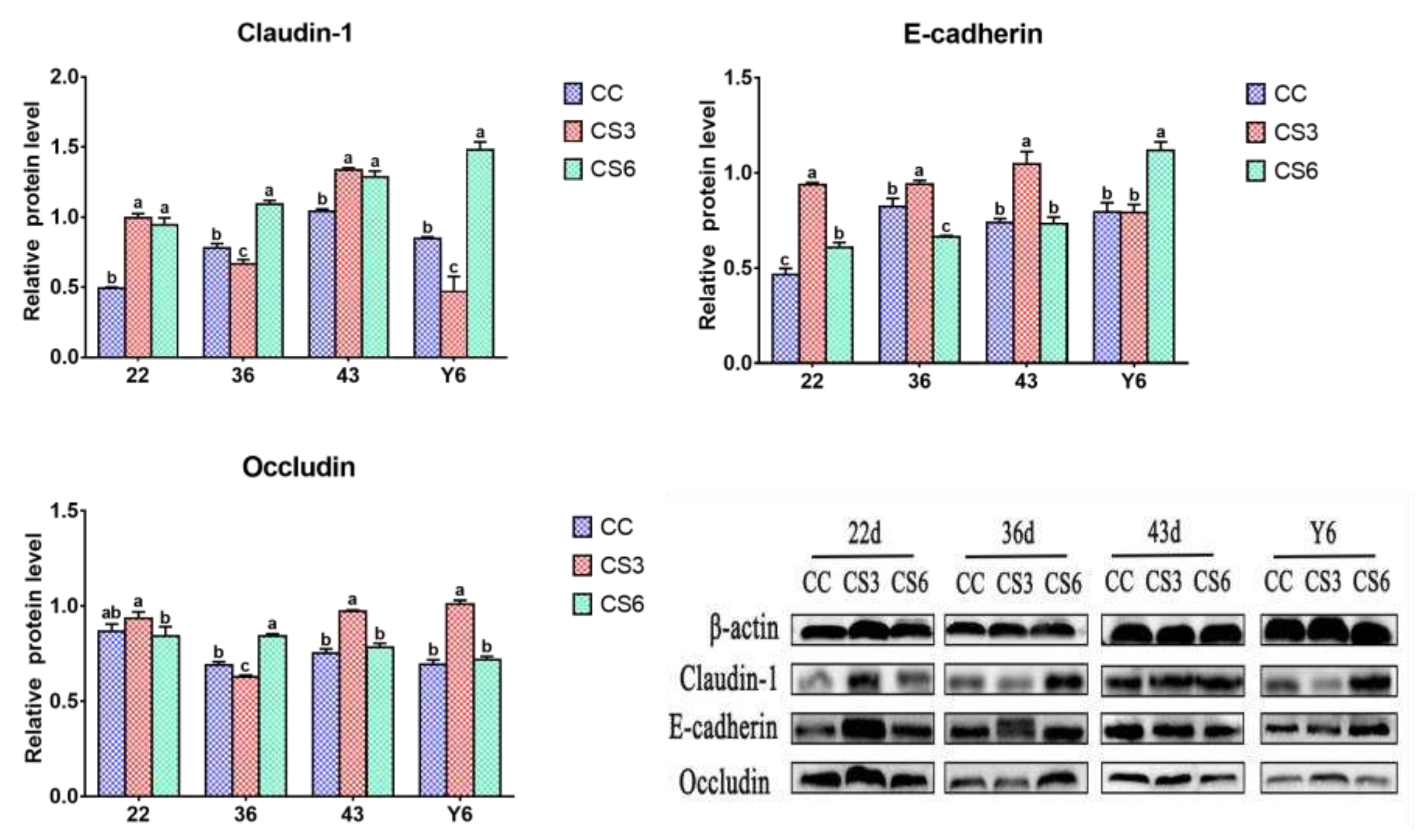
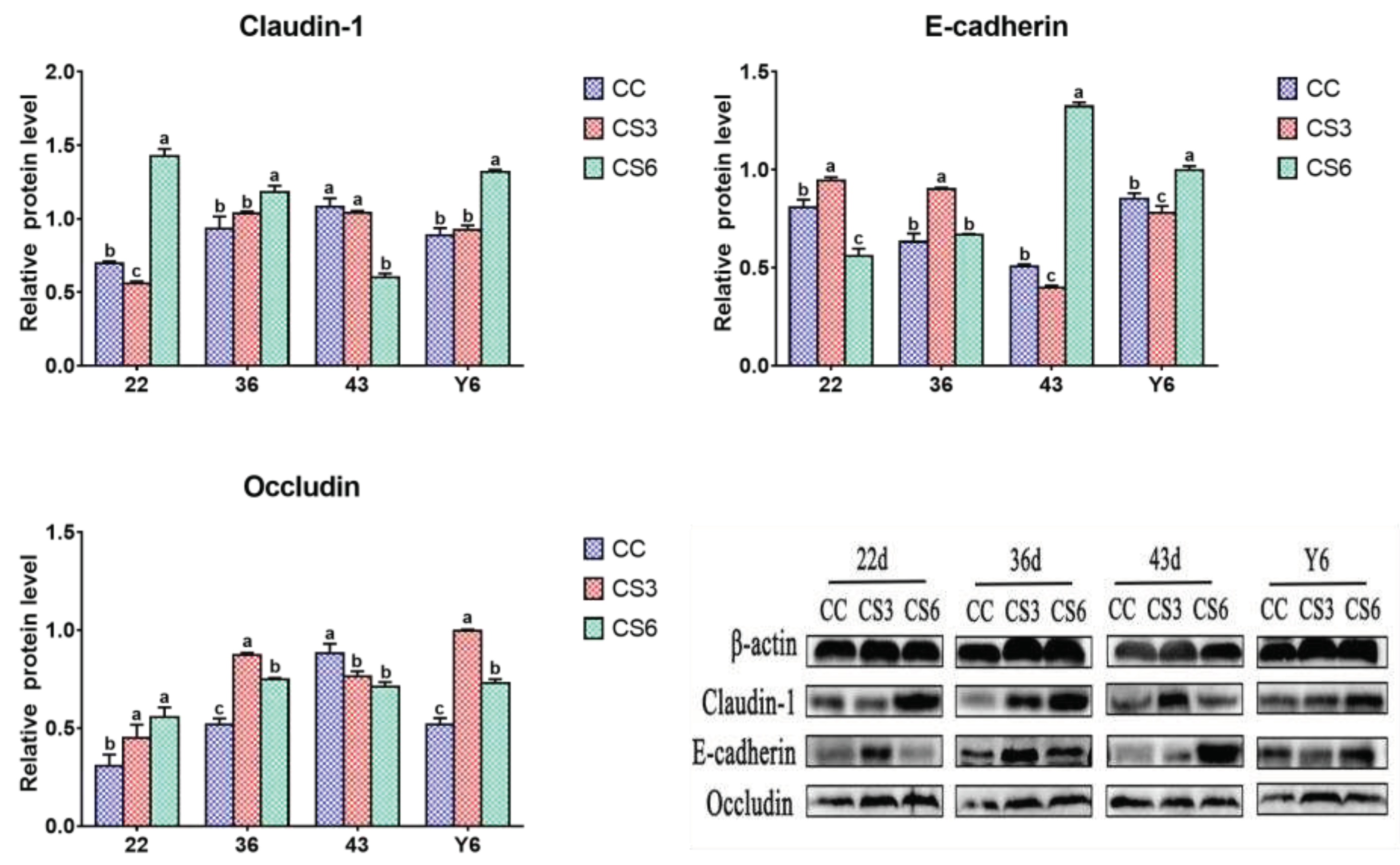
3.3. Protein Levels of Intestinal Barrier Genes in Duodenum
3.5. Protein Levels of Intestinal Barrier Genes in Jejunum
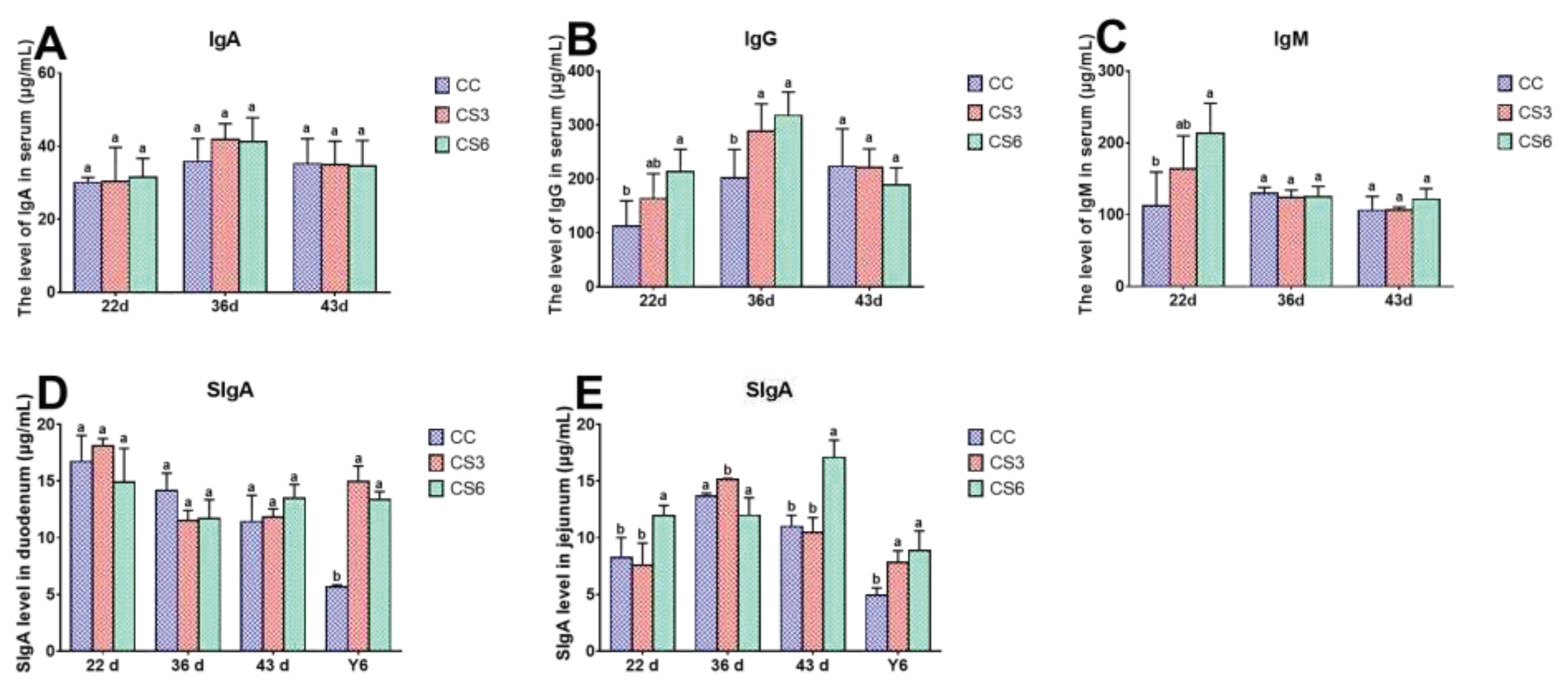
3.6. Levels of Immunoglobulin in Serum, Duodenum and Jejunum
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aarif, O.; Shergojry, S. A.; Dar, S. A.; Khan, N.; Mir, N. A.; Sheikh, A. A. , Impact of Cold Stress on Blood Biochemical and Immune Status in Male and Female Vanaraja Chickens. Indian Journal of Animal Research 2014, 48, 139–142. [Google Scholar] [CrossRef]
- Su, Y. Y.; Zhang, X.; Xin, H. W.; Li, S.; Li, J. F.; Zhang, R. X.; Li, X.; Li, J. H.; Bao, J. , Effects of prior cold stimulation on inflammatory and immune regulation in ileum of cold-stressed broilers. Poult Sci 2018, 97, 4228–4237. [Google Scholar] [CrossRef] [PubMed]
- Hu, J. Y.; Cheng, H. W. , Warm perches: a novel approach for reducing cold stress effect on production, plasma hormones, and immunity in laying hens. Poult Sci 2021, 100, 101294. [Google Scholar] [CrossRef]
- Hirata, Y.; Broquet, A. H.; Menchén, L.; Kagnoff, M. F. , Activation of Innate Immune Defense Mechanisms by Signaling through RIG-I/IPS-1 in Intestinal Epithelial Cells. The Journal of Immunology 2007, 179, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Melo, A. N. F.; Souza, G. T.; Schaffner, D.; Oliveira, T. C. M.; Maciel, J. F.; Souza, E. L.; Magnani, M. , Changes in thermo-tolerance and survival under simulated gastrointestinal conditions of Salmonella Enteritidis PT4 and Salmonella Typhimurium PT4 in chicken breast meat after exposure to sequential stresses. Int J Food Microbiol 2017, 251, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Theerawatanasirikul, S.; Koomkrong, N.; Kayan, A.; Boonkaewwan, C. , Intestinal barrier and mucosal immunity in broilers, Thai Betong, and native Thai Praduhangdum chickens. Turk J Vet Anim Sci 2017, 41, 357–364. [Google Scholar] [CrossRef]
- Brandtzaeg, P. , The gut as communicator between environment and host: Immunological consequences. Eur J Pharmacol 2011, 668, S16–S32. [Google Scholar] [CrossRef] [PubMed]
- Turner, J. R. , Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R. C.; McNabb, W. C.; Moughan, P. J.; Wells, J. M.; Roy, N. C. , Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 2011, 141, 769–76. [Google Scholar] [CrossRef]
- Fanning, A. S.; Jameson, B. J.; Jesaitis, L. A.; Anderson, J. M. , The Tight Junction Protein ZO-1 Establishes a Link between the Transmembrane Protein Occludin and the Actin Cytoskeleton. Journal of Biological Chemistry 1998, 273, 29745–29753. [Google Scholar] [CrossRef]
- Catalioto, R. M.; Maggi, C. A.; Giuliani, S. , Intestinal epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr Med Chem 2011, 18, 398–426. [Google Scholar] [CrossRef]
- Krause, G.; Winkler, L.; Mueller, S. L.; Haseloff, R. F.; Piontek, J.; Blasig, I. E. , Structure and function of claudins. Biochim Biophys Acta 2008, 1778, 631–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tellez, G.; Richards, J. D.; Escobar, J. , Identification of Potential Biomarkers for Gut Barrier Failure in Broiler Chickens. Front Vet Sci 2015, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Guo, Y.; Wang, Z. , beta-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult Sci 2013, 92, 1764–73. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W. M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M. L.; Sakai, M.; Sa, L. R.; Ferreira, A. J.; Palermo-Neto, J. , Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult Sci 2010, 89, 1905–14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y. F.; Chen, Y. P.; Chen, R.; Su, Y.; Zhang, R. Q.; He, Q. F.; Wang, K.; Wen, C.; Zhou, Y. M. , Dietary mannan oligosaccharide ameliorates cyclic heat stress-induced damages on intestinal oxidative status and barrier integrity of broilers. Poult Sci 2019, 98, 4767–4776. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Han, H.; Zhang, L.; Wang, T. , Bisdemethoxycurcumin attenuates lipopolysaccharide-induced intestinal damage through improving barrier integrity, suppressing inflammation, and modulating gut microbiota in broilers. J Anim Sci 2021, 99. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, B.; Wang, Y.; Huang, X.; Yan, Z.; Jiang, Q.; Chen, Q. , Ellagic Acid Improves Antioxidant Capacity and Intestinal Barrier Function of Heat-Stressed Broilers via Regulating Gut Microbiota. Animals (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Zhao, F. Q.; Zhang, Z. W.; Yao, H. D.; Wang, L. L.; Liu, T.; Yu, X. Y.; Li, S.; Xu, S. W. , Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res Vet Sci 2013, 95, 146–55. [Google Scholar] [CrossRef]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. , Differences in Susceptibility to Heat Stress along the Chicken Intestine and the Protective Effects of Galacto-Oligosaccharides. PLoS One 2015, 10, e0138975. [Google Scholar] [CrossRef]
- Liu, X. T.; Li, S.; Zhao, N.; Xing, L.; Gong, R. X.; Li, T. T.; Zhang, S. J.; Li, J. H.; Bao, J. , Effects of Acute Cold Stress after Intermittent Cold Stimulation on Immune-Related Molecules, Intestinal Barrier Genes, and Heat Shock Proteins in Broiler Ileum. Animals (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Z. , The protective effect of gamma-aminobutyric acid on the development of immune function in chickens under heat stress. J Anim Physiol Anim Nutr (Berl) 2016, 100, 768–77. [Google Scholar] [CrossRef]
- Xue, G.; Yin, J.; Zhao, N.; Liu, Y.; Fu, Y.; Zhang, R.; Bao, J.; Li, J. , Intermittent mild cold stimulation improves the immunity and cold resistance of spleens in broilers. Poult Sci 2021, 100, 101492. [Google Scholar] [CrossRef]
- Leeson, S. Nutritional considerations of poultry during heat stress. World's Poultry Science Journal 1986, 42, 69–81. [Google Scholar] [CrossRef]
- Mashaly, M. M.; Hendricks, G. L., 3rd; Kalama, M. A.; Gehad, A. E.; Abbas, A. O.; Patterson, P. H. , Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult Sci 2004, 83, 889–94. [Google Scholar] [CrossRef]
- Sagher, B. M. , The effect of cold stress on muscle growth in young chicks. Growth 1975, 39, 281–8. [Google Scholar] [PubMed]
- Blahová, J.; Dobšíková, R.; Straková, E.; Suchý, P. , Effect of Low Environmental Temperature on Performance and Blood System in Broiler Chickens (Gallus domesticus). Acta Veterinaria Brno 2007, 76, S17–S23. [Google Scholar] [CrossRef]
- Shinder, D. L., D. Rusal, M. Rzepakovsky, V. Bresler, V. Yahav, S., Early age cold conditioning in broiler chickens(Gallus domesticus)_ thermotolerance and growth responses. J Therm Biol 2002, 27, 517–523. [CrossRef]
- Dokladny, K.; Moseley, P. L.; Ma, T. Y. , Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol 2006, 290, G204–12. [Google Scholar] [CrossRef]
- Zhou, H. J.; Kong, L. L.; Zhu, L. X.; Hu, X. Y.; Busye, J.; Song, Z. G. , Effects of cold stress on growth performance, serum biochemistry, intestinal barrier molecules, and adenosine monophosphate-activated protein kinase in broilers. Animal 2021, 15, 100138. [Google Scholar] [CrossRef]
- Umeda, K.; Ikenouchi, J.; Katahira-Tayama, S.; Furuse, K.; Sasaki, H.; Nakayama, M.; Matsui, T.; Tsukita, S.; Furuse, M.; Tsukita, S. , ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006, 126, 741–54. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Perez-Moreno, M. A.; Rodrigo, I.; Locascio, A.; Blanco, M. J.; del Barrio, M. G.; Portillo, F.; Nieto, M. A. , The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L. W.; Artis, D. , Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 2014, 14, 141–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shao, D.; Wu, S.; Song, Z.; Shi, S. , Heat stress-induced intestinal barrier damage and dimethylglycine alleviates via improving the metabolism function of microbiota gut brain axis. Ecotoxicol Environ Saf 2022, 244, 114053. [Google Scholar] [CrossRef] [PubMed]
- Fihn, B. M.; Sjoqvist, A.; Jodal, M. , Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology 2000, 119, 1029–36. [Google Scholar] [CrossRef]
- Woof, J. M.; Kerr, M. A. , The function of immunoglobulin A in immunity. J Pathol 2006, 208, 270–82. [Google Scholar] [CrossRef]
- Carr, D. J.; Woolley, T. W.; Blalock, J. E. , Phentolamine but not propranolol blocks the immunopotentiating effect of cold stress on antigen-specific IgM production in mice orally immunized with sheep red blood cells. Brain Behav Immun 1992, 6, 50–63. [Google Scholar] [CrossRef]
- Choi, S.-O. P. J. H. C.-M. R. B.-S. P. Y.-H., Effects of extreme heat stress on growth performance, lymphoid organ, IgG and cecum microflora of broiler chickens. International Journal of Agriculture & Biology 2013, 15, 120.
- Thaxton, P. , Influence of temperature on the immune response of birds. Poult Sci 1978, 57, 1430–40. [Google Scholar] [CrossRef]
- Chen, W.; Yin, C.; Li, J.; Sun, W.; Li, Y.; Wang, C.; Pi, Y.; Cordero, G.; Li, X.; Jiang, X. , Stimbiotics Supplementation Promotes Growth Performance by Improving Plasma Immunoglobulin and IGF-1 Levels and Regulating Gut Microbiota Composition in Weaned Piglets. Biology (Basel) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lin, J.; Pan, T.; Li, T.; Jiang, H.; Fang, Y.; Wang, Y.; Wu, F.; Huang, J.; Zhang, H.; Chen, D.; Chen, Y. , Polymeric immunoglobulin receptor deficiency exacerbates autoimmune hepatitis by inducing intestinal dysbiosis and barrier dysfunction. Cell Death Dis 2023, 14, 68. [Google Scholar] [CrossRef] [PubMed]
| Reagent | Usage |
| 5×gDNA Eraser Buffer | 2.0µl |
| gDNA Eraser | 1.0µl |
| Total RNA | 1µg |
| RNase Free dH2O up to | 10.0µl |
| 42 °C Water-bath for 2min | |
| 5×PrimeScript Buffer 2 | 4.0µl |
| RT Primer Mix | 1.0µl |
| PrimeScript RT Enzyme Mix I | 1.0µl |
| RNase Free dH2O | 4.0µl |
| 37 °C Water-bath for 15 min, then 85 °C Water-bath for 5 s | |
| Gene | Gene Reference Sequence | Primer Sequences (5’-3’) |
| β-actin | NM_205518.1 | Forward: CACCACAGCCGAGAGAGAAAT |
| Reverse: TGACCATCAGGGAGTTCATAGC | ||
| Claudin-1 | NM_001013611.2 | Forward: TGGAGGATGACCAGGTGAAGA |
| Reverse: CGAGCCACTCTGTTGCCATA | ||
| E-cadherin | NM 001039258.2 | Forward: GACAGGGACATGAGGCAGAA |
| Reverse: GCCGTGACAATGCCATTCTC | ||
| Occludin | NM 205128.1 | Forward: TCATCGCCTCCATCGTCTAC |
| Reverse: TCTTACTGCGCGTCTTCTGG | ||
| ZO-1 | XM 413773.4 | Forward: TGTAGCCACAGCAAGAGGTG |
| Reverse: CTGGAATGGCTCCTTGTGGT | ||
| ZO-2 | XM_025144669.1 | Forward: CGGCAGCTATCAGACCACTC |
| Reverse: CACAGACCAGCAAGCCTACAG | ||
| Mucin2 | XM_421035 | Forward: CAGCACCAACTTCTCAGTTC |
| Reverse: TCTGCAGCCACACATTCTTT |
| CC | CS3 | CS6 | P value | |
| Feed conversion ratio | 2.01±0.07a | 1.84±0.06b | 1.98±0.07a | 0.000 |
| Daily feed intake (kg) | 1.21±0.37a | 1.21±0.36a | 1.24±0.40a | 0.163 |
| Daily weight gain (kg) | 0.60±0.14b | 0.65±0.15a | 0.62±0.14b | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





