1. Introduction
The growth of obesity in recent years has been alarming, and it has become a serious public health concern worldwide. According to the World Health Organization (WHO), 39% of adults over 18 years of age in 2016 were overweight and 13% were obese [
1]. As overweight and obesity increase the risk of chronic and aggressive conditions such as respiratory complications, systemic arterial hypertension (SAH), type 2 diabetes mellitus (T2DM), sleep disorders, cancer, and cardiovascular diseases, these numbers are concerning [
2].
Particularly for the cardiovascular system, obesity initiates both direct effects via structural and functional adaptations owing to excess body weight, and indirect effects through adipokines that induce a pro-inflammatory and pro-thrombotic state mediated by insulin resistance, T2DM, visceral obesity, SAH, and dyslipidemia [
3,
4,
5]. When indirect effects occur in the same patient, it is labeled metabolic syndrome (MetS). More specifically, according to the National Cholesterol Education Program, MetS occurs when the concomitant presence of hyperglycemia/insulin resistance, visceral obesity, dyslipidemia, and SAH is observed [
6,
7,
8]. The prevalence of MetS varies from 20 to 25% in the adult population [
9,
10] and 0 to 19.2% [
11] in children, and has a potential of reaching 80% in patients with T2DM [
12]. Although it has been accepted that obesity leads to the establishment of systemic inflammatory conditions and is closely associated with MetS, the pathophysiology of MetS is still not well established, with some studies suggesting inflammation as an etiology [
13,
14].
It is noteworthy that in obesity, the accumulation of fat in adipocytes plays a marked role in the inflammatory process; as the volume of adipose tissue increases, so does the production of adipocytokines, triggering a series of pathophysiological processes related to inflammation [
15]. This long-term clinical picture of low-grade inflammation is an important risk factor for the emergence of some types of cancer, non-alcoholic fatty liver disease, atherosclerosis, SAH, T2DM, and MetS [
16].
Among the various biomarkers related to obesity and MetS, adiponectin, leptin, C-reactive protein (CRP), and cytokines such as interleukins (ILs) -1 and -6 stand out13. Interestingly, according to the global scientific literature the increase in circulating concentrations of leptin in contrast to a decrease in adiponectin levels is a striking aspect of both obesity and MetS. Thus, the adiponectin/leptin ratio can be considered a biomarker of inflammation in adipose tissue [
17].
Based on this information, it is evident that the management of obesity and MetS through lifestyle changes, regular physical activity, weight loss pharmacotherapy, use of gastric devices, and bariatric surgeries is essential to minimize the deleterious effects of these conditions [
18]. In particular, bariatric surgery (BS) is an effective alternative for reducing excess body weight and leads to a reduction in cardiovascular risk, dyslipidemia, non-alcoholic fatty liver disease, insulin resistance, and T2DM among other obesity-related metabolic conditions. BS is also closely associated with a significant reduction in circulating levels of leptin and an increase in adiponectin levels, as it encourages satiety via hormonal regulation and reduction in stomach volume [
19,
20,
21,
22,
23,
24]. It is noteworthy that patients undergoing BC must also receive adequate nutritional and psychological monitoring to achieve the goals of reducing body mass index (BMI) and maintaining a healthy metabolic state [
25]. The appropriate surgical procedure should be indicated according to the individual factors and comorbidities of each patient as well as confidence and experience of the surgeon [
26].
It has been reported that patients with severe obesity, with or without T2DM, present significant and sustained weight loss with a consequent reduction in several mediators, some inflammatory, with an increase in adiponectin values associated with a reduction in leptin and tumour necrosis factor alpha (TNFα) levels after BS by Roux-en-Y gastric bypass (RYGB) [
27,
28]. Given this background, this study aimed to investigate the adiponectin/leptin ratio in women with severe obesity with and without MetS before and after RYGB and to characterize the biochemical markers of blood total cholesterol, low-density lipoprotein (LDL), high- density lipoprotein (HDL), non-HDL total cholesterol, and glucose.
2. Materials and Methods
2.1. Trial design
This was a randomized controlled clinical trial that enrolled female patients with a clinical diagnosis of severe obesity with or without MetS and who were indicated for bariatric surgery. The study design followed the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) [
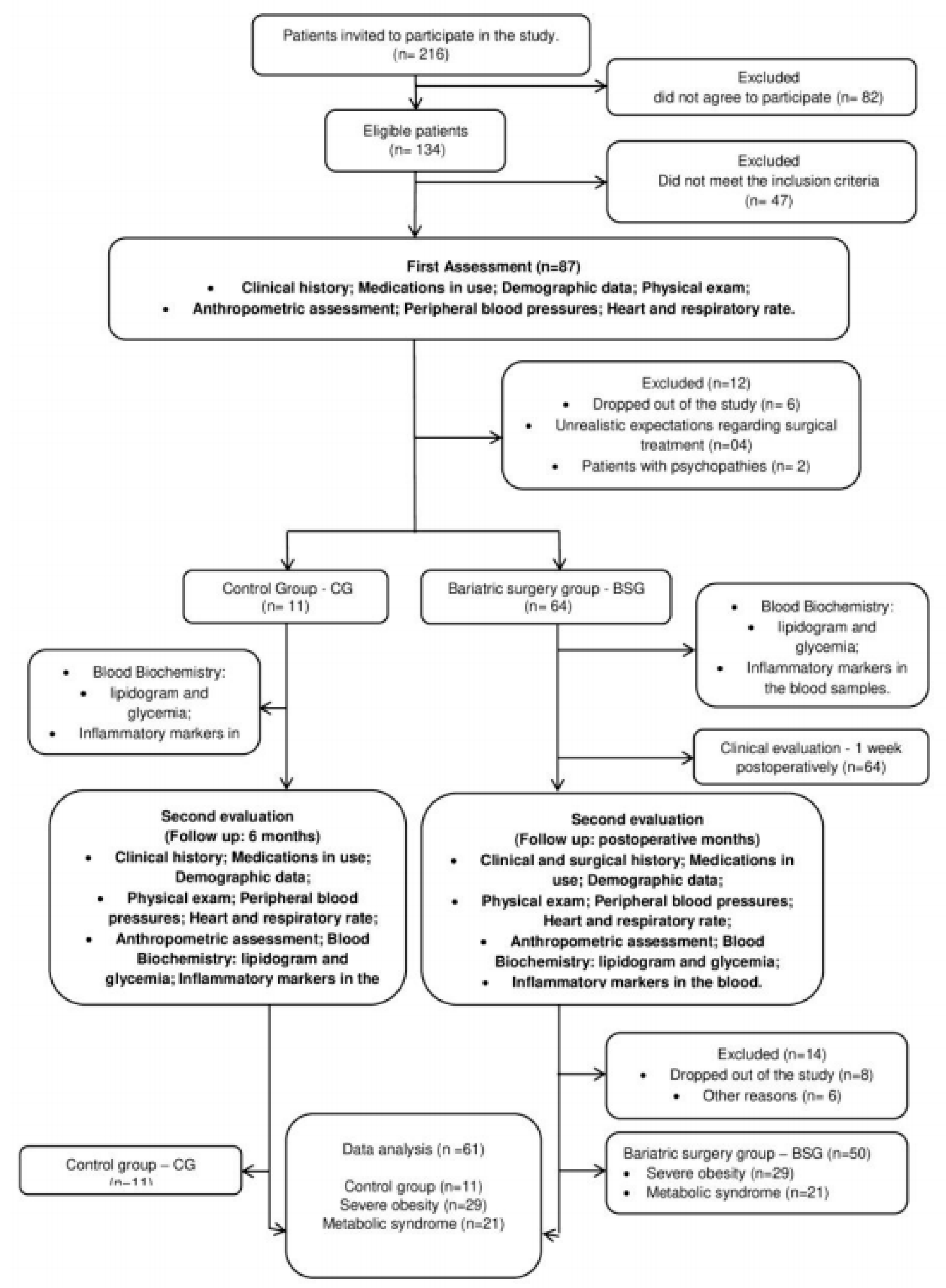
29] and the Regulatory Guidelines and Norms for Research Involving Human Subjects of the National Health Council of the Ministry of Health, December 2012. The study was approved by the Ethics Committee for Research with Human of Santa Casa de Misericórdia (process number 178/2012) and was registered at ClinicalTrials.gov (NCT02409160). All volunteers provided informed consent and were notified of their ability to leave at any time without any cost. The study design is illustrated in
Figure 1.
Obesity was classified according to the WHO established criteria that defines BMI as body weight in kilograms divided by the square of the patient’s height in meters (kg/m2). For this study, obesity was stratified into five distinct classes based on BMI: class I (30 to 34.9 kg/m2), class II (35.0 to 39.9 kg/m2), class III (≥40 kg/m2) [
2,
30,
31], class IV (≥50 kg/m2), and class V (≥60 kg/m2) [
3,
32].
2.2. Study setting
This study was conducted at the Faculty of Medical Sciences of Santa Casa de São Paulo, Brazil between January and December of 2019. The doctors and surgeons of the hospital’s obesity surgery department performed all surgical procedures. Blood samples were collected at the Hospital da Irmandade da Santa Casa de Misericórdia de São Paulo and the Immunology Laboratory of the Federal University of São Paulo, São José dos Campos.
2.1. Participants and eligibility criteria
Clinically stable participants were consecutively recruited from the Surgery Unit of Santa Casa de Misericórdia de São Paulo and selected according to the eligibility criteria established by the study protocol. Our inclusion criteria allowed patients aged between 18 and 65 years with severe obesity level III (BMI ≥40 kg/m2 or ≥35 kg/m2 associated with comorbidities); with or without MetS; with clinical indication for bariatric surgery according to the criteria of the Conselho Federal de Medicina (Brazil); a documented history of failure in conventional weight loss; and the ability to understand, agree, and sign the informed consent. The exclusion criteria included clinical and/or mental instability, BMI> 55 kg/m2, unrealistic postoperative target weight and/or unrealistic expectations regarding surgical treatment, active cancer, pregnancy, breastfeeding, planned pregnancy within 2 years, mental illnesses (schizophrenia or depression), epilepsy, and underlying diseases genetics. Patients with an absence of safe access to the abdominal cavity or gastrointestinal tract, abusive use of alcohol or drugs, and/or any cardiorespiratory and/or medical conditions that contraindicated surgery were also excluded.
We conducted a sample size calculation based on a previous study by Rafey et al. [
33], who identified plasma levels of adiponectin in patients with severe obesity undergoing BS. Considering a margin of error of 0.05 and a significance level of 95%, we determined that 25 patients were required to detect an effect size of 0.95 [
33].
2.1. Recruitment and randomization
Patients who met the inclusion criteria and had an urgent need for surgery were allocated to the bariatric surgery group (BSG), whereas patients who did not meet the inclusion criteria or have an urgent need for surgery were allocated to the control group (CG); that is, patients in the CG did not undergo the surgical procedure for a period of 6 months. All investigators involved in the interpretation of the clinical trials were blinded to the composition of the study groups. The patients were examined preoperatively and again 6 months following each surgical procedure. The CG participants were assessed at baseline when randomization took place and again 6 months following the first assessment. The patients were instructed to maintain their daily routines, especially in terms of physical activity and eating habits. Any change in routine was required to be communicated to the researchers.
2.1. Protocol for clinical and surgical evaluation
Patients in both groups were evaluated for clinical and surgical history, use of medication, demographic data, anthropometric measurements, and biochemical test values. All evaluations were performed before and 6 months after the surgical procedure (BSG) or after the first evaluation (CG) by a consistent team of researchers, physicians, and physical therapists.
Clinical evaluation of the patients was performed at the Bariatric Surgery Outpatient Clinic of Santa Casa de Misericórdia de São Paulo. Weight and height assessments were performed with the patients wearing light clothes, without shoes, and after emptying the bladder using a digital electronic anthropometric scale (model 200/5, Welmy Indústria e Comércio Ltda., São Paulo, Brazil). BMI was calculated by dividing weight (kg) by height squared (m2) according to the WHO classification [
2,
34].
Clinical information regarding signs and symptoms including presence of cough, sputum, dyspnea, fatigue, associated diseases and possible exacerbations, drug use, and comorbidities were collected from the medical records of patients at the Hospital da Brotherhood of the Santa Casa de Misericórdia de São Paulo.
2.1. Blood biochemical analysis
Venous blood samples (5 ml) were obtained preoperatively after 12 h of fasting through cubital venipuncture and collected in vacuum tubes (Vacuette do Brasil Ltda, Campinas, Brazil) containing EDTA anticoagulant (Merck KGaA, Darmstadt, Germany) for plasma preparation. The levels of total cholesterol, HDL, LDL, triglycerides, and glucose were determined using commercial kits (Gold Análise Diagnostica Ltd., MG, Brazil) for the SpectraMax i3 analysis system (Molecular Devices, Sunnyvale, CA, USA). The inflammatory markers adiponectin and leptin were analyzed using ELISA kits (R&D Systems, Minneapolis, MN, USA) and BioLegend (Sellex Inc., Washington, DC, USA), following manufacturer recommendations.
2.1. Surgical procedures
The RYGB technique is the most commonly used surgical procedure for obesity. This procedure creates restrictive and malabsorptive effects based on the combination of the small gastric pouch and total bypass of the duodenum and proximal jejunum. Surgical procedures were performed at the Department of Bariatric Surgery of the Irmandade da Santa Casa de Misericórdia de São Paulo linked to the Faculty of Medical Sciences of Santa Casa de São Paulo by five surgeons who used a standard open RYGB technique [
35,
36]. The clinical success of this procedure can be partly attributed to its alteration of the secretion of hormones that influence glucose regulation and the patient's perception of hunger and satiety. All patients underwent identical anesthetic and surgical protocols.
2.1. Statistical analysis
Initially, a procedure for identifying outlier values was used to avoid possible trends in the results. The data were then submitted to the Shapiro–Wilk and D'Agostino & Pearson normality tests to verify their proximity with a normal curve, and the variance homogeneity was later evaluated by the Levene test. The variables that presented a normal distribution (parametric data) are presented as means and standard deviations, and the differences between the means of the values of these variables was evaluated using the Student’s t-test. A one-way analysis of variance with Dunn's post-test was used to compare control and experimental variables. The Pearson’s correlation test was used to analyze possible trends and product-moment correlations.
In cases for which a normal distribution was not observed (non-parametric data), the data were presented as medians and interquartile ranges, and the Wilcoxon test was used to verify the differences in values of these variables. In addition, the Friedman test was applied to compare the control and experimental variables. The Spearman correlation test was again used to analyze possible trends and product-moment correlations. Categorical data are presented as absolute numbers and percentages of the total. Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS 21.0®, IBM Corp., Armonk, NY, USA). The significance level was set at 5% for all tests (p<0.05).
3. Results
There was a predominance of Caucasian women (88%) followed by brown (8%) and black (4%) women, with a mean age of 44 years (± 12.69) for the CG and 41 (± 9.8) for the BSG.
Table 1 shows the anthropometric and biochemical characteristics of the patients involved in this study, divided into CG (n=11) and BSG with or without MetS (n=50) for the first and second assessments as well as before and after bariatric surgery. The values obtained in the post-surgery BSG showed significant reductions compared to preoperative values.
Table 2 shows the anthropometric characteristics of the 50 patients who received bariatric surgery subdivided into the severe obesity bariatric surgery group (SOBSG) and the metabolic bariatric surgery group (METSBSG). Both groups showed significant reductions in weight and BMI following surgery when compared to pre-surgery values, with no difference observed between the two groups at the time points evaluated.
The values of plasma analysis of glycemic levels and lipid profile (total cholesterol and fractions [LDL and HDL] and triglycerides) of patients divided into SOBSG and METSBSG can be seen in
Table 3. In both groups, the values found after bariatric surgery were significantly different from their preoperative values. However, in the intergroup analysis, while pre-surgery triglyceride values were higher in the SOBSG than in the METSBSG in the post-surgery period, there were no significant differences between the two groups.
The circulating levels in blood plasma of the inflammatory markers adiponectin and leptin and their ratio in patients undergoing bariatric surgery, subdivided into SOBSG and METSBSG are shown in
Table 4. It can be seen that while circulating adiponectin levels increased significantly, leptin levels were significantly reduced after bariatric surgery when compared to pre-surgery values. These data showed a more significant relationship between plasma levels of adiponectin and leptin observed post-surgery than pre-surgery. No differences were observed between the two groups.
4. Discussion
Our results showed that in female patients having severe obesity with or without MetS, weight loss induced by bariatric surgery with RYGB improved biochemical and systemic inflammatory parameters, particularly the adiponectin/leptin ratio. In line with the existing literature, our findings from pre- and post- surgical evaluations showed a relationship between MetS and severe initial obesity resulting from increases in leptin levels and reductions in adipose levels. In one randomized study, patients undergoing serial gastroplasty with unspecified forms of T2DM were selected for IL-6, IL-10, and IL-10. The authors observed significant improvements in leptin (p≤001) and CRP (p=0.003) values at 1 and 6 months following bariatric surgery, as well as a significant reduction in IL-6 at 6 months (p=0.001). However, in contrast to the findings of our study, no significant difference was observed in adiponectin levels between patient groups [
37].
Leptin and adiponectin have opposing effects on subclinical inflammation and insulin resistance, as leptin upregulates pro-inflammatory cytokines, such as TNF-α and IL-6, which are associated with insulin resistance and T2DM; and in contrast, adiponectin exhibits anti-inflammatory properties by reducing the expression and release of several pro-inflammatory immune mediators. Thus, interactions between angiotensin II and adiponectin/leptin imbalances may be important mediators of the T2DM and cardiovascular disease risks associated with abdominal obesity [
38]. Considering this information, the increase in adiponectin concentration and decrease in leptin observed in this study may have been associated with a reduction in subclinical inflammation in patients with severe obesity and MetS.
Confirming the hypothesis of reduced inflammation for our study population, we observed an improvement in the adiponectin/leptin ratio, a biomarker for inflammation in adipose tissue [
39]. This ratio may be used to estimate the cardiometabolic risk associated with obesity and MetS, allowing for a broad identification of at-risk individuals [
17]. Fruhbeck et al. showed a low adiponectin/leptin ratio to be related to increased levels of inflammation markers such as CRP and serum amyloid A (SAA), demonstrating that in severe obesity and MetS, adipose tissue dysfunction characterized by the adiponectin/leptin ratio leads to an increase in pro-inflammatory factors as potential mediators in its etiopathogenesis [
39].
In addition to being good markers of adipose tissue-associated inflammation, both leptin and adiponectin are involved in the regulation of lipolysis, and thus reductions in adiponectin/leptin ratios may also reflect changes in this process, further metabolically contributing to obesity [
40]. Therefore, this emerging biomarker correlates with insulin resistance better than adiponectin or leptin alone and shows a reduction compared to the increasing number of metabolic risk factors [
41]. In this context, the findings of this study suggest that an increase in adiponectin/leptin ratio may be related to the reduction in glucose levels observed after BS.
Similarly, adiponectin/leptin ratio values have been reported as having a high negative correlation with markers of low-grade chronic inflammation, thus they have emerged as an important predictive marker of cardiometabolic risk associated with obesity and MetS. In this sense, an increase in this proportion has been related to a reduction in the risk of atherosclerosis, as well as a reduction in the risk of some types of cancer [
41]. In recent years, adipose tissue has been considered an extremely active endocrine organ that produces several biologically active adipokines such as leptin, adiponectin, TNF-α, and IL-6, that participate in various physiological processes [
42]. These adipokines play an important role in the pathophysiological link between increased adiposity and cardiometabolic changes [
43,
44]. To verify whether the adiponectin/leptin ratio contributes to the pathophysiology of increased systemic inflammation and oxidative stress in patients with MetS, Fruhbeck et al. verified the values of leptin, adiponectin, and other markers of inflammation and oxidative stress in a sample of 140 Caucasian individuals (74 men and 66 women; aged 28-82 years; 60 patients with MetS and 80 without MetS). Total concentrations of adiponectin were significantly lower and the adiponectin/leptin ratio was dramatically reduced in patients with MetS41. According to their study, systemic oxidative stress evidenced by the levels of thiobarbituric acid reactive substances (TBARS) as well as inflammation markers such as SAA, CRP, and osteopontin were significantly increased in subjects with MetS. Further, total adiponectin concentrations were negatively correlated with TBARS and CRP levels, and a negative correlation between adiponectin levels and markers of inflammation and oxidative stress was observed. The authors concluded that MetS is accompanied by a chronic pro-inflammatory state and increased oxidative stress, and that dysfunctional adipose tissue as evidenced by a low adiponectin/leptin ratio may contribute to increased oxidative stress and inflammation, which are characteristics of MetS [
44]. The data of our study corroborate the study from Fruhbeck et al., in which it was possible to observe in a serial manner increases in the adiponectin/leptin ratio as well as a reduction in triglyceride levels, total cholesterol, HDL, and LDL following surgery using the RYGB technique [
39].
Notably, the results of a systematic review conducted by Lopez-Jaramillo et al. reinforces the role of the adiponectin/leptin ratio. The authors showed that abdominal obesity was exclusively related to reduced plasma concentrations of adiponectin and increased levels of leptin in patients with severe coronary artery disease. An adiponectin/leptin ratio imbalance has also been associated with increased waist circumference, reduced vascular response to acetylcholine, and increased vasoconstriction due to angiotensin II [
36]. Therefore, in line with the results of these recent studies, it is believed that the adiponectin/leptin ratio may be an important inflammatory marker that can be used to investigate comorbidities in patients with severe obesity and MetS before and after bariatric surgery.
According to the existing literature, bariatric and metabolic surgery has been effective in maintaining weight loss and metabolic improvement in patients with a BMI > 40 kg/m2 or a BMI > 35 kg/m2 in the presence of comorbidities, especially for those with a history of failure in previous conservative therapeutic approaches for weight reduction [
45]. Similar to the findings of our study, previous authors have demonstrated excellent health outcomes for individuals with obesity or MetS undergoing RYGB including remission of comorbidities, reduction of systemic inflammation, and significant reduction in high CRP levels [
46,
47].
One of the limitations of this study was the fact that it involved only female patients. This fact is justified by the greater demand for bariatric surgery in our hospital by women with severe obesity.
5. Conclusions
Based on our results, it can be concluded that weight loss induced by BS in female patients with severe obesity and or without METs significantly improved anthropometric, biochemical, and systemic inflammatory variables, especially the ratio of adiponectin to leptin. In accordance with these results and with what has been shown by some previous studies, we can draw the attention of the scientific community to the adiponectin to leptin ratio as a considerable inflammatory marker and mainly for cardiometabolic risk. Another detail to be highlighted was the impact caused by RYGB-induced weight loss on the inflammatory profile of severely obese patients with and without MetS.
Author Contributions
Conceptualization, Sandra Moreira, Elias Jirjos, Carlos Malheiros, Sergio Vencio, Giuseppe Insalaco and Wilson Freitas Júnior; Data curation, Elias Jirjos, Vera Alves, Eduardo Perez, Maria Lino, Shayra Souza, Juliana Santos, Miriã Oliveira and Giuseppe Insalaco; Formal analysis, Miriã Oliveira, Adriano Luís Fonseca, Rodolfo Vieira and Wilson Freitas Júnior; Funding acquisition, Luis Oliveira; Investigation, Sandra Moreira, Andre Bachi, Elias Jirjos, Carlos Malheiros, Sergio Vencio, Vera Alves, Alan Sousa, Lucenda Felipe, Eduardo Perez, Maria Lino, Shayra Souza, Juliana Santos, Adriano Luís Fonseca, Carlos Silva, Rodolfo Vieira, Giuseppe Insalaco, Wilson Freitas Júnior and Luis Oliveira; Methodology, Sandra Moreira, Andre Bachi, Vera Alves, Alan Sousa, Lucenda Felipe, Shayra Souza, Miriã Oliveira, Adriano Luís Fonseca, Carlos Silva, Rodolfo Vieira and Luis Oliveira; Project administration, Sergio Vencio and Eduardo Perez; Supervision, Elias Jirjos and Carlos Malheiros; Visualization, Lucenda Felipe and Juliana Santos; Writing – original draft, Sandra Moreira, Vera Alves, Alan Sousa, Maria Lino, Miriã Oliveira, Adriano Luís Fonseca, Carlos Silva, Rodolfo Vieira and Luis Oliveira; Writing – review & editing, Andre Bachi, Elias Jirjos, Carlos Malheiros, Sergio Vencio, Giuseppe Insalaco, Wilson Freitas Júnior and Luis Oliveira. All authors have read and agreed to the published version of the manuscript.
Funding
L.A.F: and S.M.B.P.M. receives grants of Coordenaçao de Apoio ao Pessoal de Nível Superior (CAPES/PROSUP); J.P.R.A., and M.C.O. receives grants of Fundação de Amparo a Pesquisa (FAPEG), Goiás (GO), Brazil; L.V.F.O. receive grants Research Productivity, modality PQII; process no. 310241/2022-7 of Conselho Nacional de Desenvolvimento Cientifco e Tecnologico (local acronym CNPq), Brazil. R.P.V. receive grants Research Productivity, modality PQII; process no. 313299/2018-8 of Conselho Nacional de Desenvolvimento Cientifco e Tecnologico (local acronym CNPq), Brazil. G.I. is a senior researcher at the Institute for Biomedical Research and Innovation, National Research Council—CNR, Palermo (SI), Italy.
Institutional Review Board Statement
This protocol was previously approved by the National Ethics Committee in Research with Human Beings of Santa Casa de Misericordia (Process number 178/2012) and registered in ClinicalTrials.gov NCT02409160. The study was conducted in accordance with the Regulatory Norms for Research Involving Human Subjects of the National Health Council of the Ministry of Health, RESOLUTION No. 466, on December 12, 2012.
Informed Consent Statement
All the recruited volunteers signed a free and informed consent.
Data Availability Statement
The data generated by this study will be available to the scientific community upon request.
Acknowledgments
The authors would like to thank the all of the patients who completed the study protocol.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The Obesity Transition: Stages of the Global Epidemic. The lancet Diabetes & endocrinology 2019, 7, 231–240. [Google Scholar]
- World Health Organization. Obesity and overweight. 2021 July 5. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 7 June 2023).
- Piché, M.-E.; Poirier, P.; Lemieux, I.; Després, J.-P. Overview of Epidemiology and Contribution of Obesity and Body Fat Distribution to Cardiovascular Disease: An Update. Progress in cardiovascular diseases 2018, 61, 103–113. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and Cardiovascular Disease: Revisiting an Old Relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Mathew, B.; Francis, L.; Kayalar, A.; Cone, J. Obesity: Effects on Cardiovascular Disease and Its Diagnosis. The Journal of the American Board of Family Medicine 2008, 21, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, E. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Jama 2001, 285, 2486–2497. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.B.; Brewer Jr, H.B.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith Jr, S.C.; Stone, N.J. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Disease models & mechanisms 2009, 2, 231–237. [Google Scholar]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.; Misra, A. Prevalence and Trends of Metabolic Syndrome among Adults in the Asia-Pacific Region: A Systematic Review. BMC public health 2017, 17, 1–9. [Google Scholar] [CrossRef]
- do Vale Moreira, N.C.; Hussain, A.; Bhowmik, B.; Mdala, I.; Siddiquee, T.; Fernandes, V.O.; Júnior, R.M.M.; Meyer, H.E. Prevalence of Metabolic Syndrome by Different Definitions, and Its Association with Type 2 Diabetes, Pre-Diabetes, and Cardiovascular Disease Risk in Brazil. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2020, 14, 1217–1224. [Google Scholar]
- Friend, A.; Craig, L.; Turner, S. The Prevalence of Metabolic Syndrome in Children: A Systematic Review of the Literature. Metabolic syndrome and related disorders 2013, 11, 71–80. [Google Scholar] [CrossRef]
- Tan, M.C.; Ng, O.C.; Wong, T.W.; Joseph, A.; Chan, Y.M.; Hejar, A.R. Prevalence of Metabolic Syndrome in Type 2 Diabetic Patients: A Comparative Study Using WHO, NCEP ATP III, IDF and Harmonized Definitions. Health 2013, 2013. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic Syndrome Is an Inflammatory Disorder: A Conspiracy between Adipose Tissue and Phagocytes. Clinica Chimica Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.B.; Milani, R.V. An Overview and Update on Obesity and the Obesity Paradox in Cardiovascular Diseases. Progress in cardiovascular diseases 2018, 61, 142–150. [Google Scholar] [CrossRef]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms Linking Obesity with Cardiovascular Disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef]
- Rodriguez-Ayala, E.; Gallegos-Cabrales, E.C.; Gonzalez-Lopez, L.; Laviada-Molina, H.A.; Salinas-Osornio, R.A.; Nava-Gonzalez, E.J.; Leal-Berumen, I.; Escudero-Lourdes, C.; Escalante-Araiza, F.; Buenfil-Rello, F.A. Towards Precision Medicine: Defining and Characterizing Adipose Tissue Dysfunction to Identify Early Immunometabolic Risk in Symptom-Free Adults from the GEMM Family Study. Adipocyte 2020, 9, 153–169. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-Leptin Ratio Is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef]
- Tchang, B.G.; Saunders, K.H.; Igel, L.I. Best Practices in the Management of Overweight and Obesity. Medical Clinics 2021, 105, 149–174. [Google Scholar] [CrossRef]
- Gómez, F.I.; Ortega, M.G.; Alonso, A.A.; Soler, I.O.; Tafalla, M.S.A.; Paredes, M.P.; Almela, M.L.L. Obesity, Endothelial Function and Inflammation: The Effects of Weight Loss after Bariatric Surgery. Nutricion hospitalaria 2016, 33, 1340–1346. [Google Scholar]
- Chiappetta, S.; Schaack, H.M.; Wölnerhannsen, B.; Stier, C.; Squillante, S.; Weiner, R.A. The Impact of Obesity and Metabolic Surgery on Chronic Inflammation. Obesity surgery 2018, 28, 3028–3040. [Google Scholar] [CrossRef]
- Villarreal-Calderon, J.R.; Cuellar-Tamez, R.; Castillo, E.C.; Luna-Ceron, E.; García-Rivas, G.; Elizondo-Montemayor, L. Metabolic Shift Precedes the Resolution of Inflammation in a Cohort of Patients Undergoing Bariatric and Metabolic Surgery. Scientific Reports 2021, 11, 12127. [Google Scholar] [CrossRef]
- Look AHEAD Research Group Look AHEAD (Action for Health in Diabetes): Design and Methods for a Clinical Trial of Weight Loss for the Prevention of Cardiovascular Disease in Type 2 Diabetes. Controlled clinical trials 2003, 24, 610–628. [CrossRef]
- Look AHEAD Research Group Eight-year Weight Losses with an Intensive Lifestyle Intervention: The Look AHEAD Study. Obesity 2014, 22, 5–13. [CrossRef]
- Wadden, T.A.; Chao, A.M.; Bahnson, J.L.; Bantle, J.P.; Clark, J.M.; Gaussoin, S.A.; Jakicic, J.M.; Johnson, K.C.; Miller, G.D.; Unick, J.L. End-of-trial Health Outcomes in Look AHEAD Participants Who Elected to Have Bariatric Surgery. Obesity 2019, 27, 581–590. [Google Scholar] [CrossRef]
- Cordero, P.; Li, J.; Oben, J. Bariatric Surgery as a Treatment for Metabolic Syndrome. Journal of the Royal College of Physicians of Edinburgh 2017, 47, 364–368. [Google Scholar] [CrossRef]
- Calvo, B.; Gracia, J.; Bielsa, M.; Martínez, M. Metabolic Effects and Outcomes of Sleeve Gastrectomy and Gastric Bypass: A Cohort Study. Surgical Endoscopy 2020, 34, 5550–5557. [Google Scholar] [CrossRef]
- Yadav, R.; Hama, S.; Liu, Y.; Siahmansur, T.; Schofield, J.; Syed, A.A.; France, M.; Pemberton, P.; Adam, S.; Ho, J.H. Effect of Roux-En-Y Bariatric Surgery on Lipoproteins, Insulin Resistance, and Systemic and Vascular Inflammation in Obesity and Diabetes. Frontiers in immunology 2017, 8, 1512. [Google Scholar] [CrossRef]
- Rao, S.R. Inflammatory Markers and Bariatric Surgery: A Meta-Analysis. Inflammation Research 2012, 61, 789–807. [Google Scholar] [CrossRef]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Dickersin, K.; Moher, D. SPIRIT 2013: New Guidance for Content of Clinical Trial Protocols. The Lancet 2013, 381, 91–92. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss: An Update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- Lavie, C.J.; Arena, R.; Alpert, M.A.; Milani, R.V.; Ventura, H.O. Management of Cardiovascular Diseases in Patients with Obesity. Nature Reviews Cardiology 2018, 15, 45–56. [Google Scholar] [CrossRef]
- Poirier, P.; Alpert, M.A.; Fleisher, L.A.; Thompson, P.D.; Sugerman, H.J.; Burke, L.E.; Marceau, P.; Franklin, B.A. Cardiovascular Evaluation and Management of Severely Obese Patients Undergoing Surgery: A Science Advisory from the American Heart Association. Circulation 2009, 120, 86–95. [Google Scholar] [CrossRef]
- Rafey, M.; Fang, C.; Ioana, I.; Griffin, H.; Hynes, M.; O’Brien, T.; McAnena, O.; O’Shea, P.; Collins, C.; Davenport, C. The Leptin to Adiponectin Ratio (LAR) Is Reduced by Sleeve Gastrectomy in Adults with Severe Obesity: A Prospective Cohort Study. Scientific Reports 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Pesquisa Nacional de Saúde 2019: Atenção Primária à Saúde e Informações Antropométricas. IBGE: Rio de Janeiro, Brasil, 2020; p. 66.
- Stahl, J.M.; Malhotra, S. Obesity Surgery Indications and Contraindications. 2018.
- Salari, N.; Jafari, S.; Darvishi, N.; Valipour, E.; Mohammadi, M.; Mansouri, K.; Shohaimi, S. The Best Drug Supplement for Obesity Treatment: A Systematic Review and Network Meta-Analysis. Diabetology & Metabolic Syndrome 2021, 13, 1–12. [Google Scholar]
- Mallipedhi, A.; Prior, S.L.; Barry, J.D.; Caplin, S.; Baxter, J.N.; Stephens, J.W. Changes in Inflammatory Markers after Sleeve Gastrectomy in Patients with Impaired Glucose Homeostasis and Type 2 Diabetes. Surgery for Obesity and Related Diseases 2014, 10, 1123–1128. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The Role of Leptin/Adiponectin Ratio in Metabolic Syndrome and Diabetes. Hormone molecular biology and clinical investigation 2014, 18, 37–45. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Portincasa, P.; Gómez-Ambrosi, J. Normalization of Adiponectin Concentrations by Leptin Replacement in Ob/Ob Mice Is Accompanied by Reductions in Systemic Oxidative Stress and Inflammation. Scientific Reports 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Frühbeck, G.; Méndez-Giménez, L.; Fernández-Formoso, J.-A.; Fernández, S.; Rodriguez, A. Regulation of Adipocyte Lipolysis. Nutrition research reviews 2014, 27, 63–93. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-Leptin Ratio: A Promising Index to Estimate Adipose Tissue Dysfunction. Relation with Obesity-Associated Cardiometabolic Risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- Milone, M.; Lupoli, R.; Maietta, P.; Di Minno, A.; Bianco, P.; Ambrosino, P.; Coretti, G.; Milone, F.; Di Minno, M.N.D.; Musella, M. Lipid Profile Changes in Patients Undergoing Bariatric Surgery: A Comparative Study between Sleeve Gastrectomy and Mini-Gastric Bypass. International Journal of Surgery 2015, 14, 28–32. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V.; Rodríguez, A.; Andrada, P.; Ramírez, B.; Ibáñez, P.; Vila, N.; Romero, S.; Margall, M.A.; Gil, M.J. Increased Cardiometabolic Risk Factors and Inflammation in Adipose Tissue in Obese Subjects Classified as Metabolically Healthy. Diabetes Care 2014, 37, 2813–2821. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Portincasa, P.; Colina, I.; Gómez-Ambrosi, J. Involvement of the Leptin-Adiponectin Axis in Inflammation and Oxidative Stress in the Metabolic Syndrome. Scientific Reports 2017, 7, 6619. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S. Clinical Practice Guidelines for the Perioperative Nutritional, Metabolic, and Nonsurgical Support of the Bariatric Surgery Patient—2013 Update: Cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surgery for Obesity and Related Diseases 2013, 9, 159–191. [Google Scholar]
- Fernández-Soto, M.L.; Martín-Leyva, A.; González-Jiménez, A.; García-Rubio, J.; Cózar-Ibáñez, A.; Zamora-Camacho, F.J.; Leyva-Martínez, M.S.; Jiménez-Ríos, J.A.; Escobar-Jiménez, F. Remission of Type 2 Diabetes Mellitus after Bariatric Surgery—Comparison between Procedures. Endokrynologia Polska 2017, 68, 18–25. [Google Scholar] [CrossRef]
- Zagorski, S.M.; Papa, N.N.; Chung, M.H. The Effect of Weight Loss after Gastric Bypass on C-Reactive Protein Levels. Surgery for obesity and related diseases 2005, 1, 81–85. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).