Submitted:
08 June 2023
Posted:
09 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
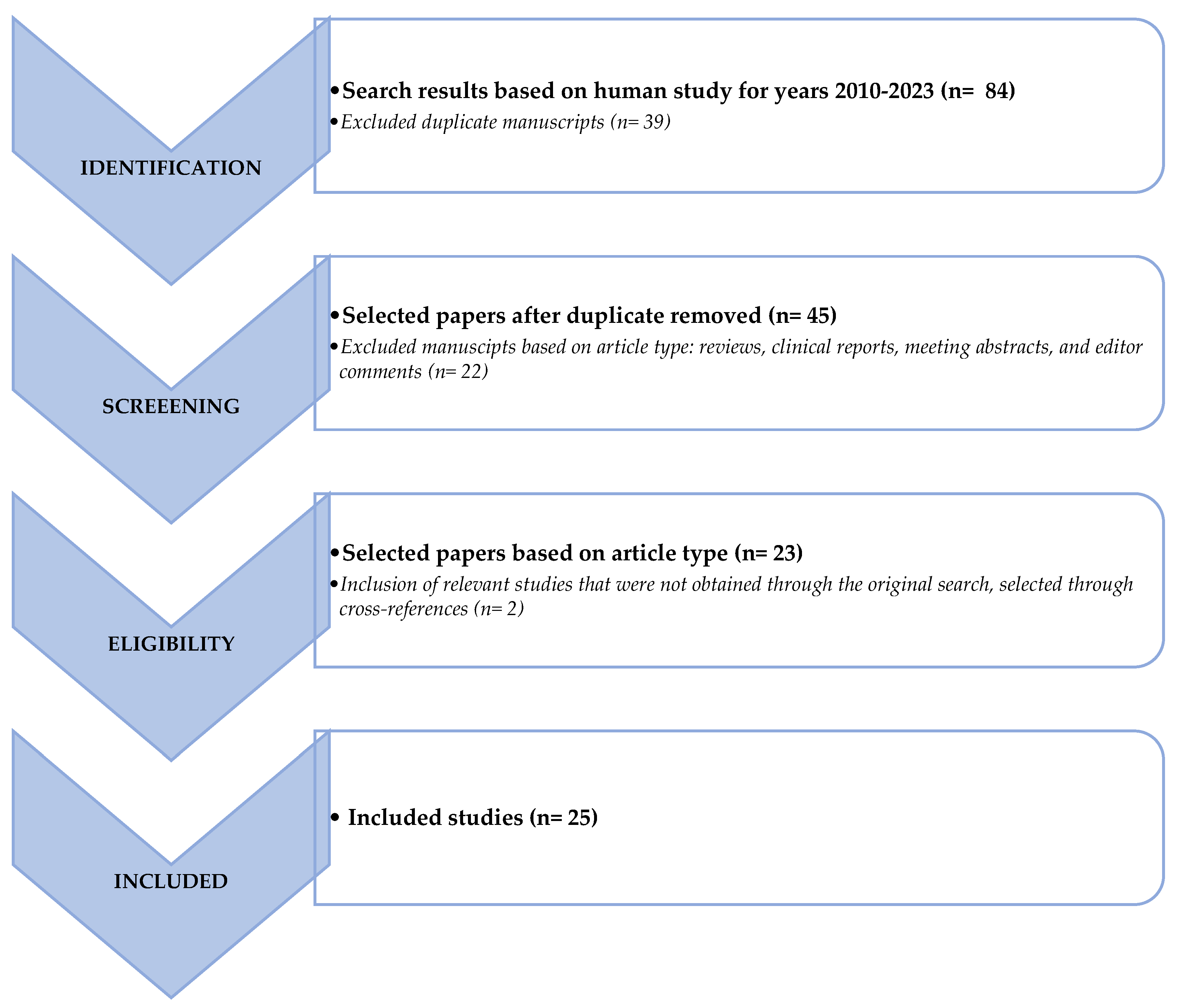
3. Clinical Applications
3.1. Staging
3.2. Restaging
4. Technical Applications
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Mete, O.; Wenig, B.M. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Overview of the 2022 WHO Classification of Head and Neck Neuroendocrine Neoplasms. Head Neck Pathol. 2022, 16, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Bogaards, M.; May, A.M.; Hassan, F.A.; Valk, G.D.; van Leeuwaarde, R.S. Lifestyle factors and development and natural course of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): a review of the literature. Neuroendocrinology 2022. [Google Scholar] [CrossRef]
- Choi, J.H.; Paik, W.H. Risk Stratification of Pancreatic Neuroendocrine Neoplasms Based on Clinical, Pathological, and Molecular Characteristics. J. Clin. Med. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allìa, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.; Bussolati, G. Expression of somatostatin receptor types 1-5 in 81 cases of gastrointestinal and pancreatic endocrine tumors: A correlative immunohistochemical and reverse-transcriptase polymerase chain reaction analysis. Virchows Arch. 2002, 440, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Geijer, H.; Breimer, L.H. Somatostatin receptor PET/CT in neuroendocrine tumours: Update on systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1770–1780. [Google Scholar] [CrossRef]
- Pauwels, E.; Cleeren, F.; Bormans, G.; Deroose, C.M. Somatostatin receptor PET ligands - the next generation for clinical practice. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 311–331. [Google Scholar]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine and Hybrid Imaging. In Proceedings of the Neuroendocrinology; S. Karger AG, 2017; Vol. 105; pp. 212–244. [Google Scholar]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F–DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Virgolini, I.; Ambrosini, V.; Bomanji, J.B.; Baum, R.P.; Fanti, S.; Gabriel, M.; Papathanasiou, N.D.; Pepe, G.; Oyen, W.; De Cristoforo, C.; et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA- conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef]
- Panagiotidis, E.; Alshammari, A.; Michopoulou, S.; Skoura, E.; Naik, K.; Maragkoudakis, E.; Mohmaduvesh, M.; Al-Harbi, M.; Belda, M.; Caplin, M.E.; et al. Comparison of the impact of 68Ga-DOTATATE and 18F-FDG PET/CT on clinical management in patients with Neuroendocrine tumors. J. Nucl. Med. 2017, 58, 91–96. [Google Scholar] [CrossRef]
- Chan, D.L.H.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual somatostatin receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: Proposal for a novel grading scheme with prognostic significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef]
- Hatt, M.; Tixier, F.; Pierce, L.; Kinahan, P.E.; Le Rest, C.C.; Visvikis, D. Characterization of PET/CT images using texture analysis: the past, the present… any future? Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Choi, H.; Paeng, J.C.; Cheon, G.J. Radiomics in Oncological PET/CT: a Methodological Overview. Nucl. Med. Mol. Imaging (2010). 2019, 53, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; Van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images are more than pictures, they are data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Sanduleanu, S.; Woodruff, H.C.; de Jong, E.E.C.; van Timmeren, J.E.; Jochems, A.; Dubois, L.; Lambin, P. Tracking tumor biology with radiomics: A systematic review utilizing a radiomics quality score. Radiother. Oncol. 2018, 127, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image biomarker standardisation initiative. 2016.
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Hatt, M.; Krizsan, A.K.; Rahmim, A.; Bradshaw, T.J.; Costa, P.F.; Forgacs, A.; Seifert, R.; Zwanenburg, A.; El Naqa, I.; Kinahan, P.E.; et al. Joint EANM/SNMMI guideline on radiomics in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging 2022, 1–24. [Google Scholar] [CrossRef]
- Avanzo, M.; Porzio, M.; Lorenzon, L.; Milan, L.; Sghedoni, R.; Russo, G.; Massafra, R.; Fanizzi, A.; Barucci, A.; Ardu, V.; et al. Artificial intelligence applications in medical imaging: A review of the medical physics research in Italy. Phys. Medica 2021, 83, 221–241. [Google Scholar] [CrossRef] [PubMed]
- Yousefirizi, F.; Pierre Decazes; Amyar, A. ; Ruan, S.; Saboury, B.; Rahmim, A. AI-Based Detection, Classification and Prediction/Prognosis in Medical Imaging:: Towards Radiophenomics. PET Clin. 2022, 17, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Hasani, N.; Morris, M.A.; Rhamim, A.; Summers, R.M.; Jones, E.; Siegel, E.; Saboury, B. Trustworthy Artificial Intelligence in Medical Imaging. PET Clin. 2022, 17, 1–12. [Google Scholar] [CrossRef]
- Goldenberg, S.L.; Nir, G.; Salcudean, S.E. A new era: artificial intelligence and machine learning in prostate cancer. Nat. Rev. Urol. 2019, 16, 391–403. [Google Scholar] [CrossRef]
- Panayides, A.S.; Amini, A.; Filipovic, N.D.; Sharma, A.; Tsaftaris, S.A.; Young, A.; Foran, D.; Do, N.; Golemati, S.; Kurc, T.; et al. AI in Medical Imaging Informatics: Current Challenges and Future Directions. IEEE J. Biomed. Heal. Informatics 2020, 24, 1837–1857. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. In Proceedings of the Journal of clinical epidemiology; J Clin Epidemiol, 2009; Vol. 62, pp. e1–e34.
- Oberg, K.; Krenning, E.; Sundin, A.; Bodei, L.; Kidd, M.; Tesselaar, M.; Ambrosini, V.; Baum, R.P.; Kulke, M.; Pavel, M.; et al. A delphic consensus assessment: Imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr. Connect. 2016, 5, 174–187. [Google Scholar] [CrossRef]
- Giesel, F.L.; Schneider, F.; Kratochwil, C.; Rath, D.; Moltz, J.; Holland-Letz, T.; Kauczor, H.U.; Schwartz, L.H.; Haberkorn, U.; Flechsig, P. Correlation between SUVmax and CT radiomic analysis using lymph node density in PET/CT-based lymph node staging. J. Nucl. Med. 2017, 58, 282–287. [Google Scholar] [CrossRef]
- Weber, M.; Kessler, L.; Schaarschmidt, B.; Fendler, W.P.; Lahner, H.; Antoch, G.; Umutlu, L.; Herrmann, K.; Rischpler, C. Textural analysis of hybrid DOTATOC-PET/MRI and its association with histological grading in patients with liver metastases from neuroendocrine tumors. Nucl. Med. Commun. 2020, 41, 363–369. [Google Scholar] [CrossRef]
- Thuillier, P.; Liberini, V.; Rampado, O.; Gallio, E.; De Santi, B.; Ceci, F.; Metovic, J.; Papotti, M.; Volante, M.; Molinari, F.; et al. Diagnostic value of conventional pet parameters and radiomic features extracted from 18f-fdg-pet/ct for histologic subtype classification and characterization of lung neuroendocrine neoplasms. Biomedicines 2021, 9, 1–14. [Google Scholar] [CrossRef]
- Fonti, R.; Panico, M.; Pellegrino, S.; Pulcrano, A.; Vastarella, L.A.; Torbati, A.H.M.; Giuliano, M.; Palmieri, G.; De Placido, S.; Del Vecchio, S. Heterogeneity of SSTR2 Expression Assessed by 68Ga-DOTATOC PET/CT Using Coefficient of Variation in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2022, 63, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Partelli, S.; Salgarello, M.; Doraku, J.; Pasetto, S.; Rancoita, P.M.V.; Muffatti, F.; Bettinardi, V.; Presotto, L.; Andreasi, V.; et al. Dual tracer 68Ga-DOTATOC and 18F-FDG PET/computed tomography radiomics in pancreatic neuroendocrine neoplasms: An endearing tool for preoperative risk assessment. Nucl. Med. Commun. 2020, 41, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Bezzi, C.; Palumbo, D.; Canevari, C.; Ghezzo, S.; Samanes Gajate, A.M.; Catalfamo, B.; Messina, A.; Presotto, L.; Guarnaccia, A.; et al. 68Ga-DOTATOC PET/MR imaging and radiomic parameters in predicting histopathological prognostic factors in patients with pancreatic neuroendocrine well-differentiated tumours. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Calabrò, D.; Malavasi, S.; Ricci, C.; Casadei, R.; Campana, D.; Baiocco, S.; Fanti, S.; Ambrosini, V. A [68ga]ga-dotanoc pet/ct radiomic model for non-invasive prediction of tumour grade in pancreatic neuroendocrine tumours. Diagnostics 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Noortman, W.A.; Vriens, D.; de Geus-Oei, L.F.; Slump, C.H.; Aarntzen, E.H.; van Berkel, A.; Timmers, H.J.L.M.; van Velden, F.H.P. [18F]FDG-PET/CT radiomics for the identification of genetic clusters in pheochromocytomas and paragangliomas. Eur. Radiol. 2022, 32, 7227–7236. [Google Scholar] [CrossRef] [PubMed]
- Schork, N.J. Personalized medicine: Time for one-person trials. Nature 2015, 520, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.J.R.; Azad, G.; Owczarczyk, K.; Siddique, M.; Goh, V. Challenges and Promises of PET Radiomics. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; Huellner, M.W.; Grimaldi, S.; Finessi, M.; Thuillier, P.; Muni, A.; Pellerito, R.E.; Papotti, M.G.; Piovesan, A.; Arvat, E.; et al. The Challenge of Evaluating Response to Peptide Receptor Radionuclide Therapy in Gastroenteropancreatic Neuroendocrine Tumors: The Present and the Future. Diagnostics 2020, 10, 1083. [Google Scholar] [CrossRef]
- Nogueira, M.A.; Abreu, P.H.; Martins, P.; Machado, P.; Duarte, H.; Santos, J. An artificial neural networks approach for assessment treatment response in oncological patients using PET/CT images. BMC Med. Imaging 2017, 17. [Google Scholar] [CrossRef]
- Wetz, C.; Apostolova, I.; Steffen, I.G.; Hofheinz, F.; Furth, C.; Kupitz, D.; Ruf, J.; Venerito, M.; Klose, S.; Amthauer, H. Predictive Value of Asphericity in Pretherapeutic [111In]DTPA-Octreotide SPECT/CT for Response to Peptide Receptor Radionuclide Therapy with [177Lu]DOTATATE. Mol. Imaging Biol. 2017, 19, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Wetz, C.; Rogasch, J.; Genseke, P.; Schatka, I.; Furth, C.; Kreissl, M.; Jann, H.; Venerito, M.; Amthauer, H. Asphericity of somatostatin receptor expression in neuroendocrine tumors: an innovative predictor of outcome in everolimus treatment? Diagnostics 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Kessler, L.; Schaarschmidt, B.; Fendler, W.P.; Lahner, H.; Antoch, G.; Umutlu, L.; Herrmann, K.; Rischpler, C. Treatment-related changes in neuroendocrine tumors as assessed by textural features derived from 68Ga-DOTATOC PET/MRI with simultaneous acquisition of apparent diffusion coefficient. BMC Cancer 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O.; et al. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget 2017, 8, 7039–7049. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.A.; Ilhan, H.; Lehner, S.; Papp, L.; Zsótér, N.; Schatka, I.; Muegge, D.O.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; et al. Pre-therapy Somatostatin Receptor-Based Heterogeneity Predicts Overall Survival in Pancreatic Neuroendocrine Tumor Patients Undergoing Peptide Receptor Radionuclide Therapy. Mol. Imaging Biol. 2019, 21, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Önner, H.; Abdülrezzak, Ü.; Tutuş, A. Could the skewness and kurtosis texture parameters of lesions obtained from pretreatment Ga-68 DOTA-TATE PET/CT images predict receptor radionuclide therapy response in patients with gastroenteropancreatic neuroendocrine tumors? Nucl. Med. Commun. 2020, 41, 1034–1039. [Google Scholar] [CrossRef]
- Ortega, C.; Wong, R.K.S.; Schaefferkoetter, J.; Veit-Haibach, P.; Myrehaug, S.; Juergens, R.; Laidley, D.; Anconina, R.; Liu, A.; Metser, U. Quantitative 68Ga-Dotatate PET/CT parameters for the prediction of therapy response in patients with progressive metastatic neuroendocrine tumors treated with 177Lu-Dotatate. J. Nucl. Med. 2021, 62. [Google Scholar] [CrossRef]
- Liberini, V.; Rampado, O.; Gallio, E.; De Santi, B.; Ceci, F.; Dionisi, B.; Thuillier, P.; Ciuffreda, L.; Piovesan, A.; Fioroni, F.; et al. 68Ga-DOTATOC PET/CT-Based Radiomic Analysis and PRRT Outcome: A Preliminary Evaluation Based on an Exploratory Radiomic Analysis on Two Patients. Front. Med. 2021, 7. [Google Scholar] [CrossRef]
- Atkinson, C.; Ganeshan, B.; Endozo, R.; Wan, S.; Aldridge, M.D.; Groves, A.M.; Bomanji, J.B.; Gaze, M.N. Radiomics-Based Texture Analysis of 68Ga-DOTATATE Positron Emission Tomography and Computed Tomography Images as a Prognostic Biomarker in Adults With Neuroendocrine Cancers Treated With 177Lu-DOTATATE. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Laudicella, R.; Comelli, A.; Liberini, V.; Vento, A.; Stefano, A.; Spataro, A.; Crocè, L.; Baldari, S.; Bambaci, M.; Deandreis, D.; et al. [68 Ga]DOTATOC PET/CT Radiomics to Predict the Response in GEP-NETs Undergoing [177 Lu]DOTATOC PRRT: The “Theragnomics” Concept. Cancers (Basel). 2022, 14. [Google Scholar] [CrossRef]
- Zwanenburg, A. Radiomics in nuclear medicine: robustness, reproducibility, standardization, and how to avoid data analysis traps and replication crisis. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2638–2655. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Bodet-Milin, C.; Couespel, S.; Necib, H.; Kraeber-Bodéré, F.; Ansquer, C.; Carlier, T. Revisiting the Robustness of PET-Based Textural Features in the Context of Multi-Centric Trials. PLoS One 2016, 11, e0159984. [Google Scholar] [CrossRef] [PubMed]
- Liberini, V.; De Santi, B.; Rampado, O.; Gallio, E.; Dionisi, B.; Ceci, F.; Polverari, G.; Thuillier, P.; Molinari, F.; Deandreis, D. Impact of segmentation and discretization on radiomic features in 68Ga-DOTA-TOC PET/CT images of neuroendocrine tumor. EJNMMI Phys. 2021, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuze, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F. erique; Buvat, I. Lifex: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Hou, J.; Yang, Y.; Chen, N.; Chen, D.; Hu, S. Prognostic Value of Volume-Based Parameters Measured by SSTR PET/CT in Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 771912. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.A.; Johnbeck, C.B.; Loft, M.; Pfeifer, A.; Oturai, P.; Langer, S.W.; Knigge, U.; Ladefoged, C.N.; Kjaer, A. Semi-automatic tumor delineation for evaluation of 64Cu-DOTATATE PET/CT in patients with neuroendocrine neoplasms: Prognostication based on lowest lesion uptake and total tumor volume. J. Nucl. Med. 2021, 62. [Google Scholar] [CrossRef] [PubMed]
- Thuillier, P.; Liberini, V.; Grimaldi, S.; Rampado, O.; Gallio, E.; De Santi, B.; Arvat, E.; Piovesan, A.; Filippi, R.; Abgral, R.; et al. Prognostic value of whole-body PET volumetric parameters extracted from 68Ga-DOTATOC-PET/CT in well-differentiated neuroendocrine tumors. J. Nucl. Med. 2022, 63. [Google Scholar] [CrossRef]
- Tirosh, A.; Papadakis, G.Z.; Millo, C.; Hammoud, D.; Sadowski, S.M.; Herscovitch, P.; Pacak, K.; Marx, S.J.; Yang, L.; Nockel, P.; et al. Prognostic Utility of Total 68Ga-DOTATATE-Avid Tumor Volume in Patients With Neuroendocrine Tumors. Gastroenterology 2018, 154, 998–1008. [Google Scholar] [CrossRef]
- Toriihara, A.; Baratto, L.; Nobashi, T.; Park, S.; Hatami, N.; Davidzon, G.; Kunz, P.L.; Iagaru, A. Prognostic value of somatostatin receptor expressing tumor volume calculated from 68Ga-DOTATATE PET/CT in patients with well-differentiated neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2244–2251. [Google Scholar] [CrossRef]
- Kim, Y. il; Yoo, C.; Oh, S.J.; Lee, S.J.; Kang, J.; Hwang, H.S.; Hong, S.M.; Ryoo, B.Y.; Ryu, J.S. Tumour-to-liver ratio determined by [68Ga]Ga-DOTA-TOC PET/CT as a prognostic factor of lanreotide efficacy for patients with well-differentiated gastroenteropancreatic-neuroendocrine tumours. EJNMMI Res. 2020, 10, 63. [Google Scholar] [CrossRef]
- Pauwels, E.; Van Binnebeek, S.; Vandecaveye, V.; Baete, K.; Vanbilloen, H.; Koole, M.; Mottaghy, F.M.; Haustermans, K.; Clement, P.M.; Nackaerts, K.; et al. Inflammation-Based Index and 68Ga-DOTATOC PET-Derived Uptake and Volumetric Parameters Predict Outcome in Neuroendocrine Tumor Patients Treated with 90Y-DOTATOC. J. Nucl. Med. 2020, 61, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Bagci, U.; Yao, J.; Miller-Jaster, K.; Chen, X.; Mollura, D.J. Predicting Future Morphological Changes of Lesions from Radiotracer Uptake in 18F-FDG-PET Images. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Wehrend, J.; Silosky, M.; Xing, F.; Chin, B.B. Automated liver lesion detection in 68Ga DOTATATE PET/CT using a deep fully convolutional neural network. EJNMMI Res. 2021, 11. [Google Scholar] [CrossRef]
- Carlsen, E.A.; Lindholm, K.; Hindsholm, A.; Gæde, M.; Ladefoged, C.N.; Loft, M.; Johnbeck, C.B.; Langer, S.W.; Oturai, P.; Knigge, U.; et al. A convolutional neural network for total tumor segmentation in [64Cu]Cu-DOTATATE PET/CT of patients with neuroendocrine neoplasms. EJNMMI Res. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Rydèn, T.; Van Essen, M.; Svensson, J.; Bernhardt, P. Activity concentration estimation in automated kidney segmentation based on convolution neural network method for 177LU-SPECT/CT kidney dosimetry. Radiat. Prot. Dosimetry 2021, 195, 164–171. [Google Scholar] [CrossRef]
- Dewaraja, Y.K.; Mirando, D.M.; Peterson, A.; Niedbala, J.; Millet, J.D.; Mikell, J.K.; Frey, K.; Wong, K.K.; Wilderman, S.; Nelson, A.S. A pipeline for automated voxel dosimetry: application in patients with multi-SPECT/CT imaging following 177 Lu peptide receptor radionuclide therapy. J. Nucl. Med. 2022, 63, jnumed–121. [Google Scholar] [CrossRef]
- Ding, W.; Yu, J.; Zheng, C.; Fu, P.; Huang, Q.; Feng, D.D.; Yang, Z.; Wahl, R.L.; Zhou, Y. Machine Learning-Based Noninvasive Quantification of Single-Imaging Session Dual-Tracer 18F-FDG and 68Ga-DOTATATE Dynamic PET-CT in Oncology. IEEE Trans. Med. Imaging 2022, 41, 347–359. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
| Lung and thymus | Mitotic Index | Necrosis | Other features | Gastro-intestinal (GI) tract and hepatopancreatobiliary organs | Mitotic Index | Ki67 Index | Other features | Upper aerodigestive tract and salivary glands | Mitotic Index | Ki67 Index | Other features | Thyroid | Mitotic Index | Ki67 Index | Necrosis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Well-differentiated* |
Low grade MTC | |||||||||||||||
| NET, TC | <2/10HPF | No | NET, G1 | <2/10HPF | <3% | NET. G1 | <2/10HPF | <20% | <5/10HPF | <5% | No | |||||
| NET, AC | 2-10/10HPF | Yes (punctate) | NET, G2 | 2–20/10HPF | 3-20% | NET. G2 | 2–10/10HPF | <20% | ||||||||
| Carcinoids/NETs | >10/10HPF | Yes | and/or Ki67 index (>30%) | NET, G3 | >20/10HPF | >20% | NET, G3 | >10/10HPF | >20% | |||||||
| Poorly differentiated* | ||||||||||||||||
| NEC, SCLC | >10/10HPF | Yes | small cell cytomorphology | NEC, SCNEC | >20/10HPF | >20% (often >70%) | small cell cytomorphology | NEC, SCNEC | >20/10HPF | >20% (often >70%) | small cell cytomorphology | High grade MTC | >/10HPF | >5% | Yes | |
| NEC, LCNEC | >10/10HPF | Yes | large cell cytomorphology | NEC, LCNEC | >20/10HPF | >20% (often >70%) | large cell cytomorphology | NEC, LCNEC | >20/10HPF | >20% (often >55%) | large cell cytomorphology | |||||
| Mixed neoplasms | ||||||||||||||||
| MiNENs | NA | >30% | MiNENs | NA | >30% | MiNENs | NA | >30% | ||||||||
| NOTE: AC atypical carcinoid, HPF high-power field, LCNEC large cell neuroendocrine carcinoma, MiNEN mixed neuroendocrine/non-neuroendocrine neoplasm, MTC medullary thyroid carcinomas, NEC neuroendocrine carcinoma, NET neuroendocrine tumor, SCLC small-cell lung carcinoma, SCNEC small cell neuroendocrine carcinoma, TC typical carcinoid ,*Morphologically well-differentiated or poorly differentiated | ||||||||||||||||
| Author | Year of Publication | Study Design | NET Type | Number of patients | Source of data | Software | AI application | Validation | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Giesel et al. [58] | 2017 | retrospective | GEP-NET | 35 | [68Ga]DOTA-peptides PET/CT | software developed at the Fraunhofer Institute for Medical Image Computing | No | No | PET-positive lymph nodes had significantly higher CT densities than PET-negative ones, irrespective of the type of cancer |
| Weber et al. [59] | 2020 | retrospective | all NENs | 100 | [68Ga]DOTA-peptides PET/MRI | LIFEx | No | No | the correlation between imaging parameters (conventional PET parameters, ADC values from MRI, and RFs parameters) and Ki-67-index was weak |
| Thuillier et al. [60] | 2020 | retrospective | Lung-NET | 44 | [18F]FDG PET/CT | LIFEx | No | No | conventional PET parameters resulted to be able to distinguish Lu-NECs from Lu-NETs, but not TC from AC. On the contrary, RFs did not provide additional information |
| Fonti et al. [61] | 2022 | retrospective | all NENs | 38 | [68Ga]DOTA-peptides PET/CT | LIFEx | No | No | The CoVs of malignant lesions were up to 4-fold higher than those of normal tissues (P ≤ 0.0001) |
| Mapelli et al. [62] | 2020 | retrospective | Pan-NENs | 61 | [68Ga]DOTA-peptides and [18F]FDG PET/CT | Chang-Gung Image Texture Analysis software package | No | No | Intensity variability, SZV, homogeneity, SUVmax and MTV were predictive for tumor dimension in [18F]FDG images. All principal components except PC4 significantly predicted tumor dimension (p < 0.0001 for PC1, P = 0.0016 for PC2 and p < 0.0001 for PC3). |
| Mapelli et al. [63] | 2022 | retrospective | Pan-NENs | 16 | [68Ga]DOTA-peptides PET/MRI | Python package Pyradiomics 3.0.1 | No | No | a significant inverse correlation between SUVmax and LN involvement (rho = − 0.58, p = 0.02). Only second-order GLV and HGLZE extracted from T2 MRI demonstrated significant correlations with LN involvement (adjusted p = 0.009) |
| Bevilacqua et al. [64] | 2021 | retrospective | Pan-NENs | 51 | [68Ga]DOTA-peptides PET/TC | ImageJ and MATLAB® | No | Yes | SUVmax values did not significantly differ between G1 and G2 (p-value = 0.60). On the contrary, the primary lesion’s grade was correctly identified when using RFs, second-order normalized homogeneity and entropy (p-value = 0.0002 with AUC = 0.94) |
| Noortman et al. [65] | 2022 | retrospective | PPGLs | 40 | [18F]FDG-PET/CT | Python package Pyradiomics 3.0.1 | No | Yes | the three-factor PET model showed the best classification performance to distinguish cluster 1 from cluster 2 of PPGL (multiclass AUC of 0.88), however comparable to the performance achieved by SUVmax alone (multiclass AUC of 0.85) |
| Author | Year of Publication | Study Design | NET Type | Number of patients | Source of data | Software | AI application | Validation | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Nogueira et al. [69] | 2017 | retrospective | NENs | 34 | [18F]FDG and [68Ga]DOTA-peptides PET/CT | NA | Yes | No | LVQNN assured classification accuracies of 100%, 100%, 96.3%, and 100% regarding the 4 response-to-treatment classes (negative, neutral, positive incomplete and positive complete) |
| Wetz et al. [70] | 2016 | retrospective | GEP-NENs | 20 | [111In]DTPA-octreotide scintigraphy | ROVER version 2.1.20 (ABX, Radeberg, Germany) | No | No | a higher ASP level was associated with poorer response to RLT |
| Wetz et al. [71] | 2020 | retrospective | GEP-NENs | 30 | [111In]DTPA-octreotide scintigraphy | ROVER version 2.1.20 (ABX, Radeberg, Germany) | No | No | ASP > 12.9% (p = 0.024) resulted statistically significant in multivariable Cox analysis to predicte response to everolimus |
| Weber et al. [72] | 2020 | retrospective | All NENs | 18 | [68Ga]DOTA-peptides PET/MRI | LIFEx | No | No | PRRT-responding patients (9 pts) showed a significant decrease in lesion volume on ADC maps and a borderline significant decrease in entropy after RLT, even if non-statistically significant |
| Werner et al. [73] | 2017 | retrospective | All NENs (108 GEP-NET) | 141 | [68Ga]DOTA-peptides PET/CT | Interview Fusion Workstation (Mediso Medical Imaging Systems Ltd., Budapest, Hungary) | No | No | RF entropy predicted both PFS and overall survival (OS) (cut-off = 6.7, AUC = 0.71, p = 0.02), without significant results for conventional PET parameters |
| Werner et al. [74] | 2019 | retrospective | Pan-NET | 31 | [68Ga]DOTA-peptides PET/CT | Interview Fusion Workstation (Mediso Medical Imaging Systems Ltd., Budapest, Hungary) | No | No | entropy was predictive for OS (cutoff = 6.7, AUC= 0.71, p= 0.02); indeed, an increased entropy predicted longer survival (entropy > 6.7, OS = 2.5 years, 17/31), while conventional PET parameters failed to predict patient outcome |
| Önner et al. [75] | 2020 | retrospective | GEP-NET | 22 | [68Ga]DOTA-peptides PET/CT | LIFEx | No | No | The skewness and kurtosis values of the lesions which did not respond to RLT were significantly higher than those with a response (p < 0.001 and p = 0.004, respectively). |
| Ortega et al. [52] | 2021 | retrospective | All NENs | 91 | [68Ga]DOTA-peptides PET/CT | nuclear medicine PACS system with fusion software (Mirada Medical) | No | No | At baseline-PET, from the multivariable analysis, mean SUVmax (p = 0.019), SUVmax T/L (p = 0.018), SUVmax T/S (p = 0.041), SUVmean Liver (p = 0.0052) and skewness (p = 0.048) remained significant predictors of PFS after RLT. On the other hand, interim-PET parameters were not predictive of PFS. |
| Liberini et al. [76] | 2021 | retrospective | GEP-NEC | 2 | [68Ga]DOTA-peptides PET/CT | LIFEx | No | No | 28 RFs extracted from pre-therapy PET/CT showed significant differences between the two patients in the Mann–Whitney test (p < 0.05) and the modifications of tumor burden parameter obtained from pre- and post-PRRT PET/CT correlated with RECIST1.1 response |
| Atkinson et al. [77] | 2021 | retrospective | All NENs | 44 | [68Ga]DOTA-peptides PET/CT | TexRAD research software (TexRAD, part of Feedback Medical Ltd, www.fbkmed.com, Cambridge, UK) | No | No | Multivariate analysis identified that CT-coarse kurtosis (HR = 2.57, 95% CI = 1.22–5.38, p = 0.013) independently predicted PFS, while PET-unfiltered skewness (HR = 9.05, 95% CI = 1.19–68.91, p = 0.033) independently predicted OS |
| Laudicella et al. [78] | 2022 | retrospective | GEP-NET | 38 | [68Ga]DOTA-peptides PET/CT | LIFEx | Yes | Yes | SUVmax could not predict response to RLT (p = 0.49, AUC 0.523), while HISTO_Skewness and HISTO_Kurtosis were able to predict RLT response with AUC, sensitivity, and specificity of 0.745, 80.6%, 67.2% and 0.72, 61.2%, 75.9%, respectively |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





