Submitted:
08 June 2023
Posted:
09 June 2023
You are already at the latest version
Abstract
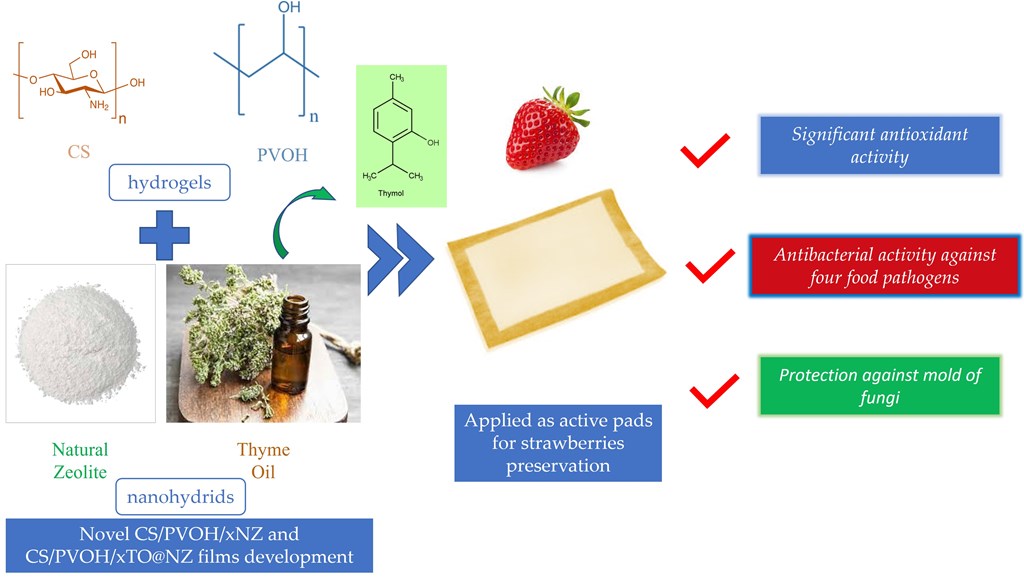
Keywords:
1. Introduction
2. Results
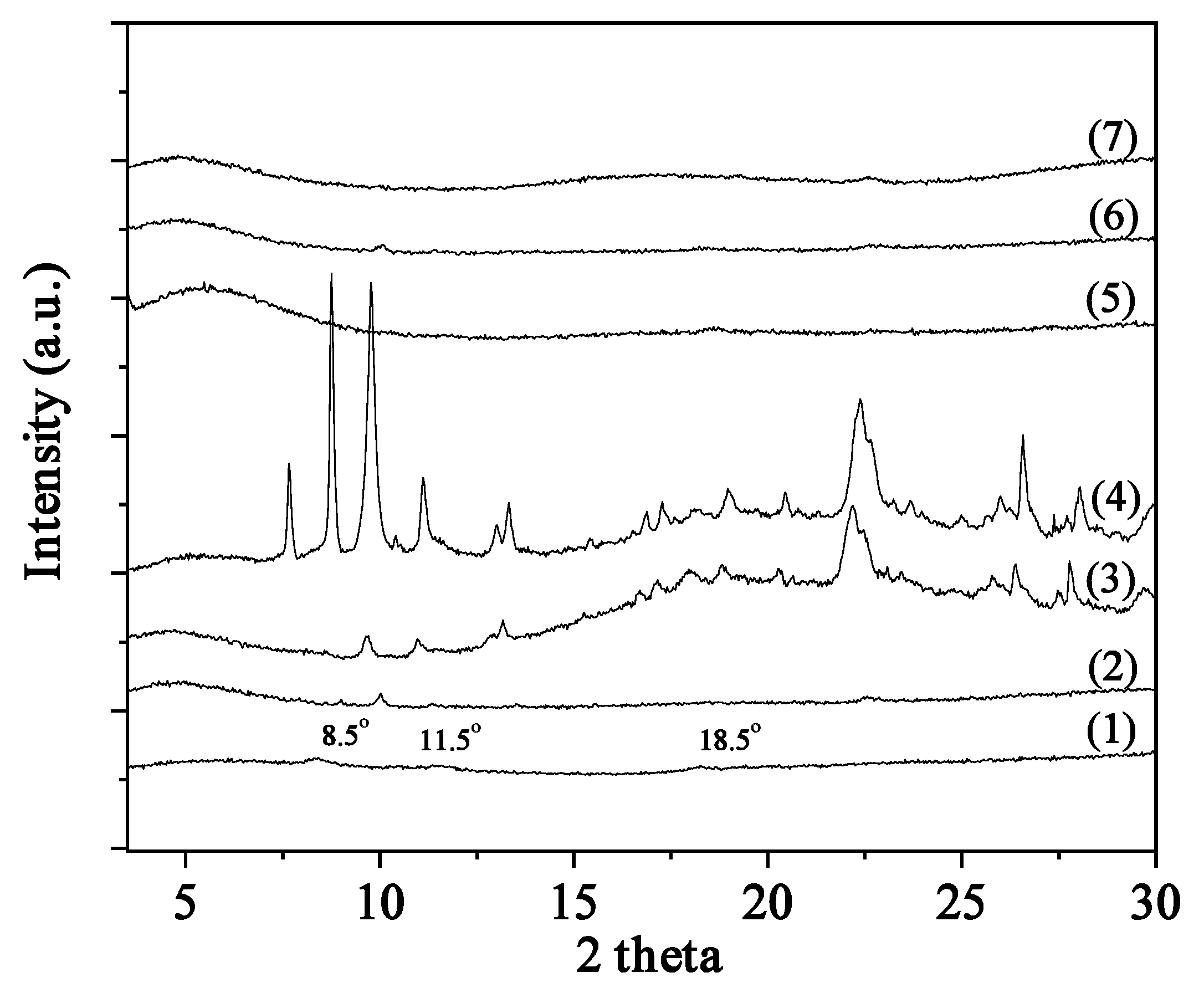
2.1. XRD analysis of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
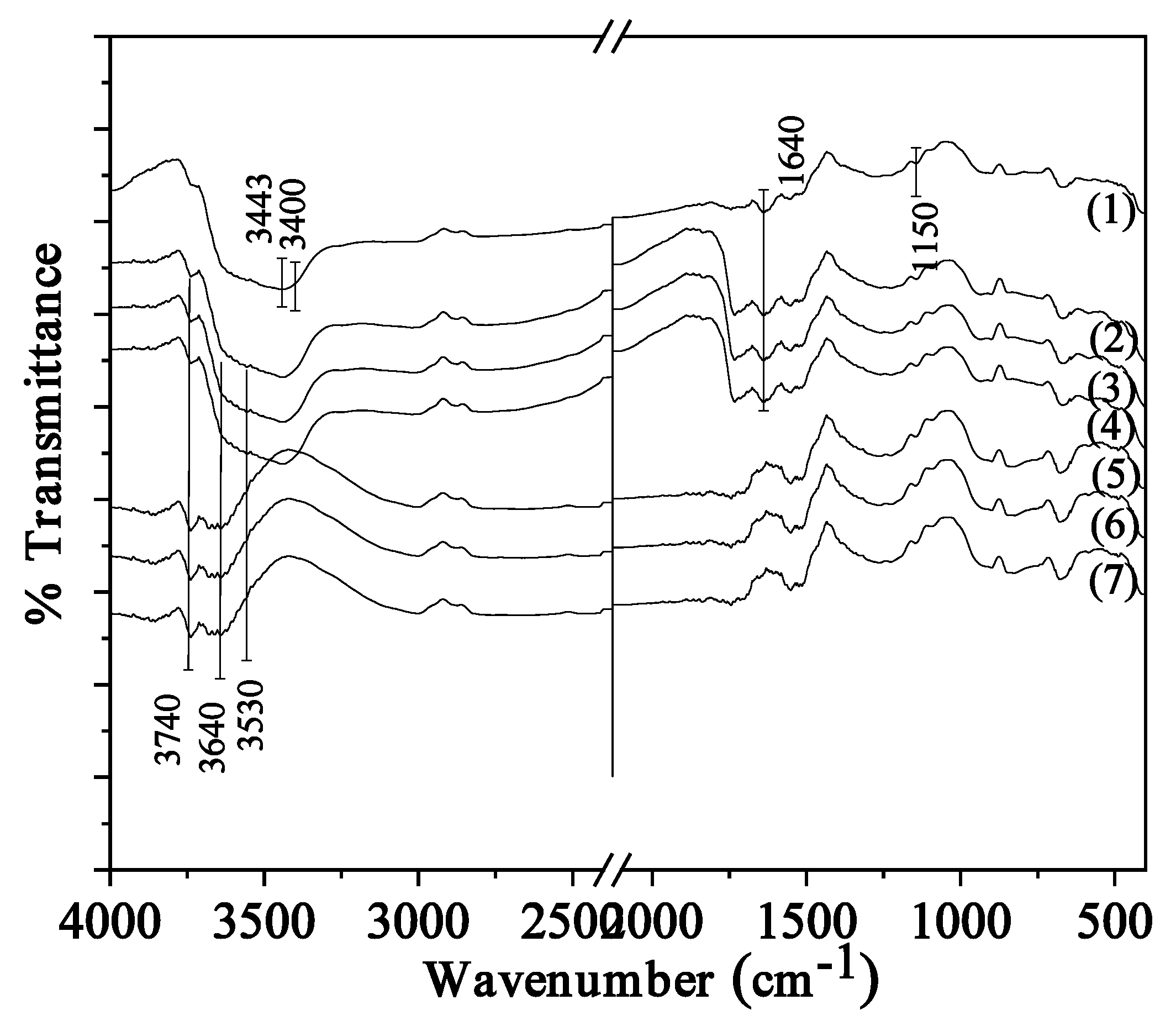
2.2. FTIR spectroscopy of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
2.3. Tensile properties of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
2.4. Water/oxygen barrier properties of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
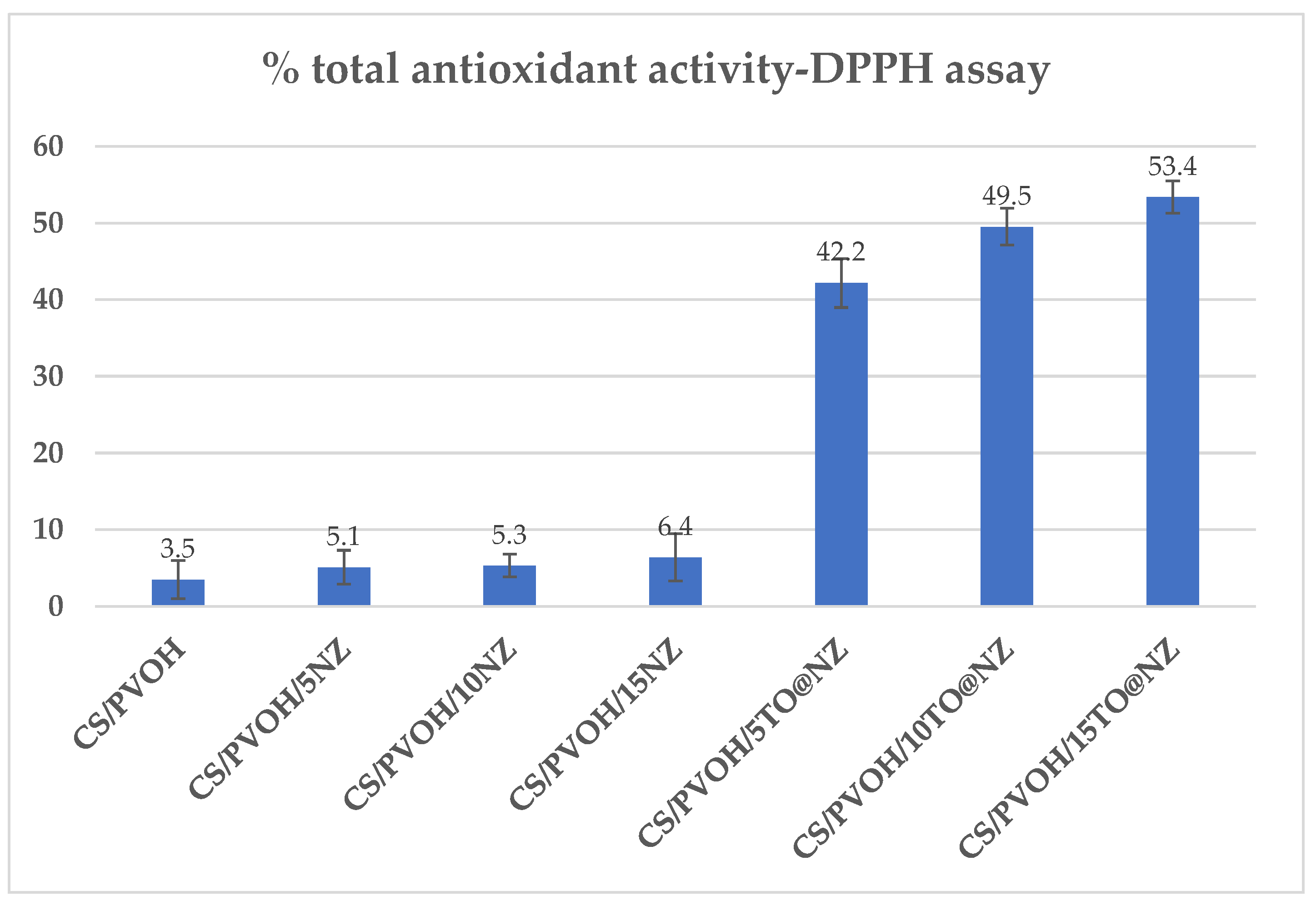
2.5. Total antioxidant activity of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
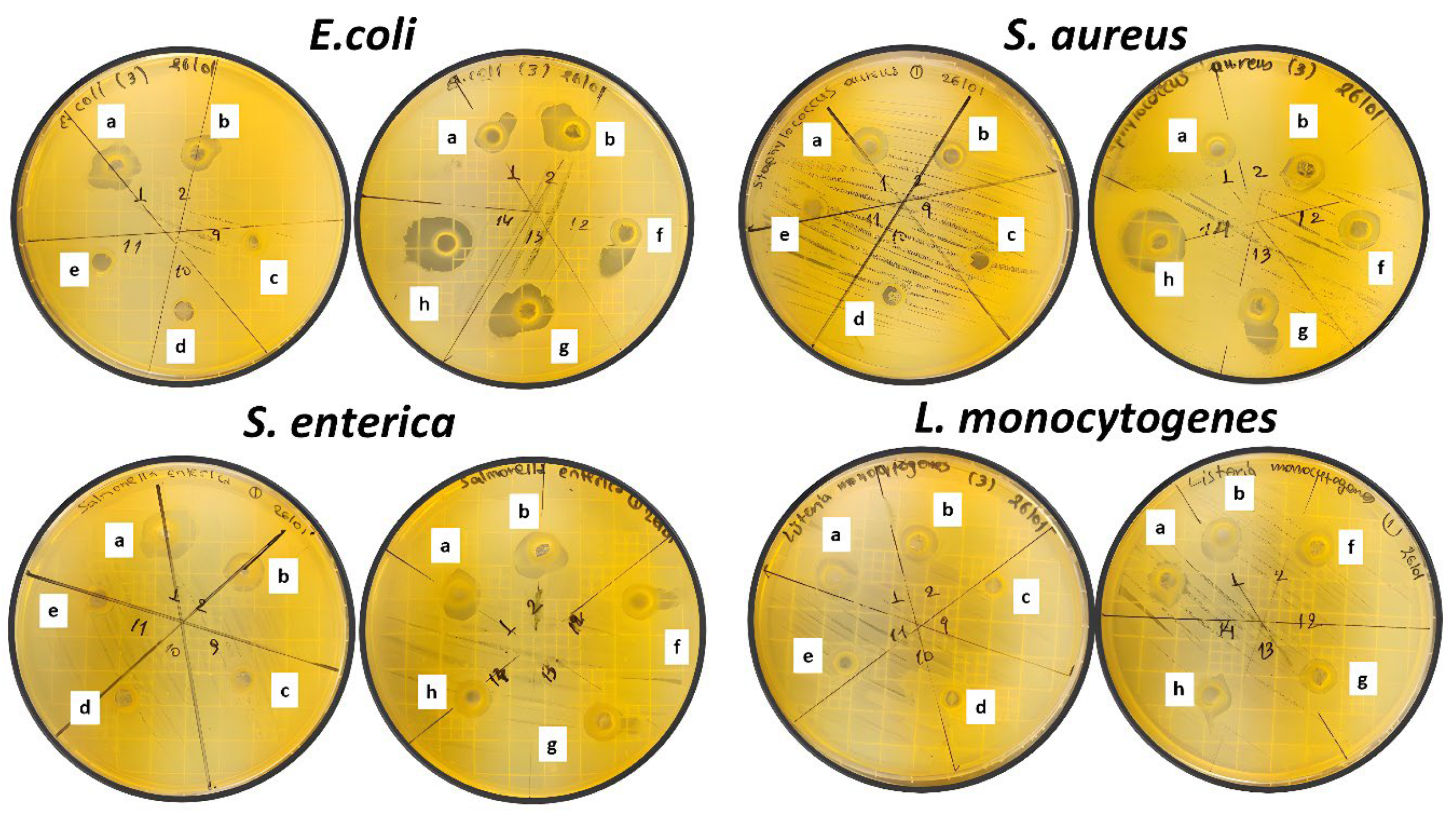
2.6. Antibacterial properties of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
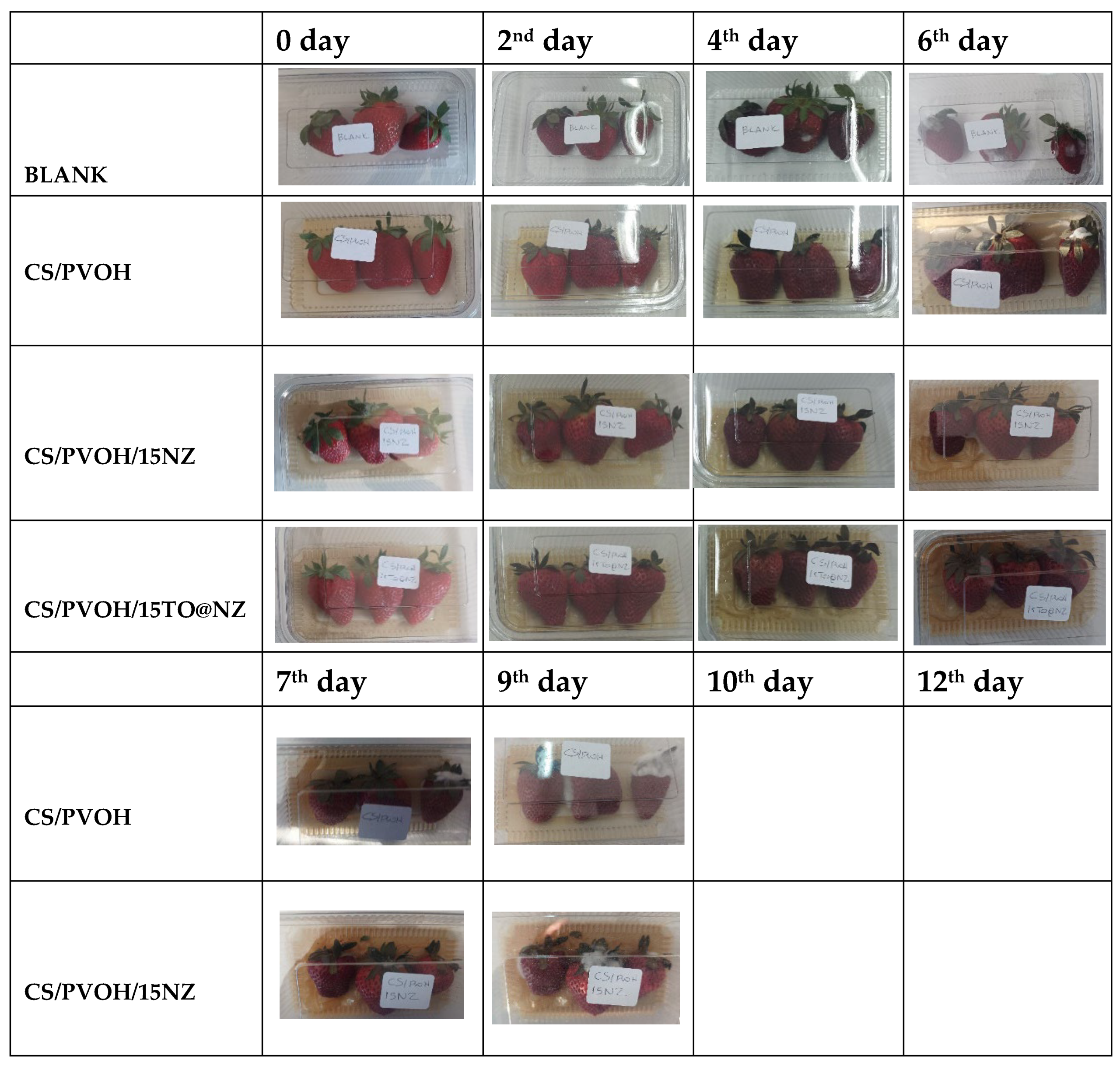
2.7. Preservation of fresh Strawberries-protection against mold of fungi
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Modification of NZ with thymol
4.3. Preparation of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
4.4. XRD analysis of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
4.5. FTIR spectroscopy of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
4.6. Tensile measurements of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
4.7. Water Vapor Transmission Rate Measurements and Water Diffusion Coefficient Calculation
4.8. Oxygen Transmission Rate Measurements and Oxygen Permeability Calculation
4.9. Total antioxidant activity of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
4.10. Antibacterial Activity Tests of CS/PVOH/xNZ and CS/PVOH/xTO@NZ films
4.11. Packaging Test of CS/PVOH/HNT and CS/PVOH/TO@HNT based active pads in Strawberries protection against the fungi of mold
4.12. Statistical analysis
Author Contributions
Data Availability Statement
Conflicts of Interest
References
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and Vegetable Waste Management and the Challenge of Fresh-Cut Salad. Trends in Food Science & Technology 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Scarano, P.; Sciarrillo, R.; Tartaglia, M.; Zuzolo, D.; Guarino, C. Circular Economy and Secondary Raw Materials from Fruits as Sustainable Source for Recovery and Reuse. A Review. Trends in Food Science & Technology 2022, 122, 157–170. [Google Scholar] [CrossRef]
- Yousuf, B.; Qadri, O.S. 11 - Preservation of Fresh-Cut Fruits and Vegetables by Edible Coatings. In Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press, 2020; pp. 225–242 ISBN 978-0-12-816184-5.
- Barrett, D.M.; Lloyd, B. Advanced Preservation Methods and Nutrient Retention in Fruits and Vegetables. Journal of the Science of Food and Agriculture 2012, 92, 7–22. [Google Scholar] [CrossRef]
- Fang, Y.; Wakisaka, M. A Review on the Modified Atmosphere Preservation of Fruits and Vegetables with Cutting-Edge Technologies. Agriculture 2021, 11, 992. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Fan, K. Recent Advances in Polysaccharide-Based Edible Coatings for Preservation of Fruits and Vegetables: A Review. Crit Rev Food Sci Nutr 2022, 1–16. [Google Scholar] [CrossRef]
- Salehi, F. Edible Coating of Fruits and Vegetables Using Natural Gums: A Review. International Journal of Fruit Science 2020, 20, S570–S589. [Google Scholar] [CrossRef]
- Park, H.J. Development of Advanced Edible Coatings for Fruits. Trends in Food Science & Technology 1999, 10, 254–260. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. Reactive and Functional Polymers 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Applications of Chitosan as Food Packaging Materials. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Sustainable Agriculture Reviews; Springer International Publishing: Cham, 2019; pp. 81–123. ISBN 978-3-030-16581-9. [Google Scholar]
- Elsabee, M.Z.; Abdou, E.S. Chitosan Based Edible Films and Coatings: A Review. Materials Science and Engineering: C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Trajkovska Petkoska, A.; Khojah, E.; Sami, R.; Al-Mushhin, A.A.M. Chitosan Edible Films Enhanced with Pomegranate Peel Extract: Study on Physical, Biological, Thermal, and Barrier Properties. Materials (Basel) 2021, 14, 3305. [CrossRef]
- GRAS Notices Available online:. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=443&sort=GRN_No&order=DESC&startrow=1&type=basic&search=chitosan (accessed on 23 February 2023).
- Oun, A.A.; Shin, G.H.; Rhim, J.-W.; Kim, J.T. Recent Advances in Polyvinyl Alcohol-Based Composite Films and Their Applications in Food Packaging. Food Packaging and Shelf Life 2022, 34, 100991. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications. Nanomaterials 2022, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- Constantinos E. Salmas; Aris E. Giannakas; Dimitrios Moschovas; Eleni Kollia; Stsvros Georgopoulos; Christina Gioti; Areti Leontiou; Apostolos Avgeropoulos; Anna Kopsacheili; Learda Avdulai; et al. Kiwi Fruits Preservation Using Novel Edible Active Coatings Based on Rich in Thymol Halloysite Nanostructures and Chitosan/Polyvinyl Alcohol Gels. Gels Bioactive Gel Films and Coatings Applied in Active Food Packaging.
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chemistry 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.; Tsagkalias, I.; Achilias, D.S.; Ladavos, A. A Novel Method for the Preparation of Inorganic and Organo-Modified Montmorillonite Essential Oil Hybrids. Applied Clay Science 2017, 146, 362–370. [Google Scholar] [CrossRef]
- Cheikh, D.; Majdoub, H.; Darder, M. An Overview of Clay-Polymer Nanocomposites Containing Bioactive Compounds for Food Packaging Applications. Applied Clay Science 2022, 216, 106335. [Google Scholar] [CrossRef]
- Li, Q.; Ren, T.; Perkins, P.; Hu, X.; Wang, X. Applications of Halloysite Nanotubes in Food Packaging for Improving Film Performance and Food Preservation. Food Control 2021, 124, 107876. [Google Scholar] [CrossRef]
- Giannakas, A.; Stathopoulou, P.; Tsiamis, G.; Salmas, C. The Effect of Different Preparation Methods on the Development of Chitosan/Thyme Oil/Montmorillonite Nanocomposite Active Packaging Films. Journal of Food Processing and Preservation 2019. [Google Scholar] [CrossRef]
- Villa, C.C.; Valencia, G.A.; López Córdoba, A.; Ortega-Toro, R.; Ahmed, S.; Gutiérrez, T.J. Zeolites for Food Applications: A Review. Food Bioscience 2022, 46, 101577. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Preservation of Strawberry Fruit Quality via the Use of Active Packaging with Encapsulated Thyme Essential Oil in Zein Nanofiber Film. International Journal of Food Science & Technology 2021, 56, 4239–4247. [Google Scholar] [CrossRef]
- Dhital, R.; Mora, N.B.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of Limonene Nano Coatings on Post-Harvest Shelf Life of Strawberries. LWT 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Nikolova, R.; Petkova, N.; Ivanov, I.; Lante, A. Biopreservation of Fresh Strawberries by Carboxymethyl Edible Coatings Enriched with a Bacteriocin from Bacillus Methylotrophicus BM47. Food Technol Biotechnol 2019, 57, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Popescu, P.-A.; Palade, L.M.; Nicolae, I.-C.; Popa, E.E.; Miteluț, A.C.; Drăghici, M.C.; Matei, F.; Popa, M.E. Chitosan-Based Edible Coatings Containing Essential Oils to Preserve the Shelf Life and Postharvest Quality Parameters of Organic Strawberries and Apples during Cold Storage. Foods 2022, 11, 3317. [Google Scholar] [CrossRef] [PubMed]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of Chitosan–Lemon Essential Oil Coatings on Storage-Keeping Quality of Strawberry. Postharvest Biology and Technology 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Pizato, S.; Vega-Herrera, S.S.; Chevalier, R.C.; Pinedo, R.A.; Cortez-Vega, W.R. Impact of Chitosan Coatings Enriched with Clove Essential Oil on Quality of Minimally Processed Strawberries. Braz. arch. biol. technol. 2022, 65. [Google Scholar] [CrossRef]
- Martínez, K.; Ortiz, M.; Albis, A.; Gilma Gutiérrez Castañeda, C.; Valencia, M.E.; Grande Tovar, C.D. The Effect of Edible Chitosan Coatings Incorporated with Thymus Capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria x Ananassa) during Cold Storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of Edible Coating Enriched with Natural Antioxidant Extract and Bergamot Essential Oil on the Shelf Life of Strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H.; Shokrgozar, M.A.; Farokhi, M. Nanoclay-Reinforced Electrospun Chitosan/PVA Nanocomposite Nanofibers for Biomedical Applications. RSC Adv. 2015, 5, 10479–10487. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Aspanut, Z.; Majid, S.R.; Arof, A.K. FTIR Studies of Plasticized Poly(Vinyl Alcohol)–Chitosan Blend Doped with NH4NO3 Polymer Electrolyte Membrane. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2011, 78, 1068–1074. [Google Scholar] [CrossRef]
- TVARUZKOVA, Z.; V, B. CHARACTERIZATION OF HYDROXYL GROUPS OF Y ZEOLITES BY INFRARED SPECTRA. CHARACTERIZATION OF HYDROXYL GROUPS OF Y ZEOLITES BY INFRARED SPECTRA 1975. [Google Scholar]
- Ward, J.W. The Nature of Active Sites on Zeolites: III. The Alkali and Alkaline Earth Ion-Exchanged Forms. Journal of Catalysis 1968, 10, 34–46. [Google Scholar] [CrossRef]
- Giannakas, A.; Salmas, C.; Leontiou, A.; Tsimogiannis, D.; Oreopoulou, A.; Braouhli, J. Novel LDPE/Chitosan Rosemary and Melissa Extract Nanostructured Active Packaging Films. Nanomaterials 2019, 9, 1105. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Castillo-Ortega, M.M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Extruded Films of Blended Chitosan, Low Density Polyethylene and Ethylene Acrylic Acid. Carbohydrate Polymers 2013, 91, 666–674. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food Applications of Chitin and Chitosans. Trends in Food Science & Technology 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Proestos, C. Effect of Copper and Titanium-Exchanged Montmorillonite Nanostructures on the Packaging Performance of Chitosan/Poly-Vinyl-Alcohol-Based Active Packaging Nanocomposite Films. Foods 2021, 10, 3038. [Google Scholar] [CrossRef]
- Milenkovic, J.; Hrenovic, J.; Matijasevic, D.; Niksic, M.; Rajic, N. Bactericidal Activity of Cu-, Zn-, and Ag-Containing Zeolites toward Escherichia Coli Isolates. Environ Sci Pollut Res 2017, 24, 20273–20281. [Google Scholar] [CrossRef]
- Pajnik, J.; Dikić, J.; Milovanovic, S.; Milosevic, M.; Jevtic, S.; Lukić, I. Zeolite/Chitosan/Gelatin Films: Preparation, Supercritical CO2 Processing, Characterization, and Bioactivity. Macromolecular Materials and Engineering 2022, 307, 2200009. [Google Scholar] [CrossRef]
- Król, M.; Syguła-Cholewińska, J.; Sawoszczuk, T. Zeolite-Supported Aggregate as Potential Antimicrobial Agents in Gypsum Composites. Materials 2022, 15, 3305. [Google Scholar] [CrossRef]
- Pajnik, J.; Lukić, I.; Dikić, J.; Asanin, J.; Gordic, M.; Misic, D.; Zizović, I.; Korzeniowska, M. Application of Supercritical Solvent Impregnation for Production of Zeolite Modified Starch-Chitosan Polymers with Antibacterial Properties. Molecules 2020, 25, 4717. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals (Basel) 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Kalaycı, S.; Demirci, S.; Sahin, F. Determination of Antimicrobial Properties of Picaridin and DEET against a Broad Range of Microorganisms. World J Microbiol Biotechnol 2014, 30, 407–411. [Google Scholar] [CrossRef]
- Kasperkowiak, M.; Strzemiecka, B.; Voelkel, A. Characteristics of Natural and Synthetic Molecular Sieves and Study of Their Interactions with Fragrance Compounds. Physicochemical Problems of Mineral Processing; ISSN 2084-4735 2016, 467 kB. [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. [Google Scholar] [CrossRef]
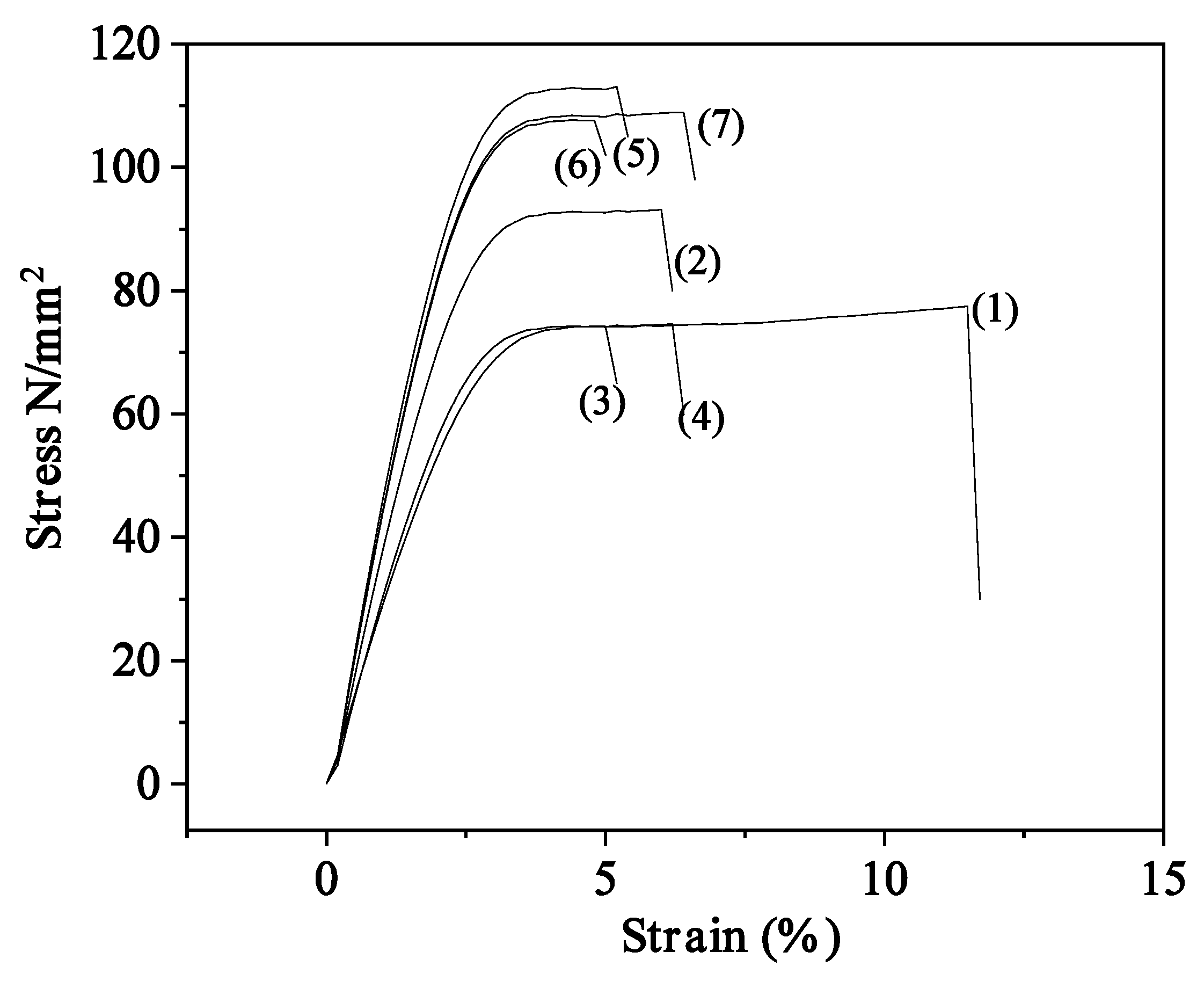
| E | σ uts | ε% | |
|---|---|---|---|
| CS/PVOH | 2249.3(200.3) | 71.2(1.8) | 11.8(0.9) |
| CS/PVOH/5NZ | 3064.3(26.3) | 89.0(4.6) | 6.9(1.1) |
| CS/PVOH/10NZ | 2736.0(351.3) | 71.0(16.7) | 6.7(2.8) |
| CS/PVOH/15NZ | 2803.4(345.3) | 73.3(4.5) | 7.0(2.1) |
| CS/PVOH/5TO@NZ | 3304.0(279.5) | 109.3(17.2) | 6.8(1.2) |
| CS/PVOH/10TO@NZ | 3186.5(125.2) | 103.7(1.4) | 6.7(2.3) |
| CS/PVOH/15TO@NZ | 3010.0(481.3) | 104.7(5.5) | 7.1(2.2) |
| Film thickness (mm) | Water Vapor Transmition Rate (10-6 g/cm2.day) | Dw - Water Diffusion Coefficient (10-4 cm2/s) | Oxygen Transmition Rate (ml/m2.day) | PeO2 (10-7cm2/s) | |
| CS/PVOH | 0.17 | 1.06(0.12) | 3.65(0.11) | 38.2(0.2) | 6.5(0.3) |
| CS/PVOH/5NZ | 0.11 | 1.17(0.10) | 3.21(0.09) | 33.7(0.2) | 3.7(0.1) |
| CS/PVOH/10NZ | 0.12 | 1.01(0.13) | 2.67(0.12) | 26.1(0.2) | 2.6(0.1) |
| CS/PVOH/15NZ | 0.10 | 0.92(0.09) | 2.33(0.08) | 27.5(0.3) | 2.2(0.2) |
| CS/PVOH/5TO@NZ | 0.11 | 0.92(0.07) | 2.03(0.06) | 15.5(0.2) | 1.8(0.2) |
| CS/PVOH/10TO@NZ | 0.08 | 0.81(0.07) | 1.6(0.05) | 34.4(0.2) | 3.4(0.1) |
| CS/PVOH/15TO@NZ | 0.10 | 0.79(0.05) | 1.8(0.04) | 15.0(0.2) | 1.7(0.2) |
| Film material | E. coli | S. aureus | S. enterica | L. monocytogenes |
| Inhibition1 (diameter of clear zone) |
Inhibition1 (diameter of clear zone) |
Inhibition1 (diameter of clear zone) |
Inhibition1 (diameter of clear zone) |
|
| CSPVOH | 3.57 ± 0.55 | 4.23 ± 0.48 | 3.26 ± 0.17 | 3.40 ± 0.70 |
| CSPVOH5NZ | 0.00 | 0.00 | 0.00 | 0.00 |
| CSPVOH10NZ | 0.00 | 0.00 | 0.00 | 0.00 |
| CSPVOH15NZ | 0.00 | 0.00 | 0.00 | 0.00 |
| CSPVOH5%TO@NZ | 3.93 ± 0.53 | 4.73 ± 0.15 | 3.37 ± 0.16 | 3.70 ± 0.14 |
| CSPVOH10TO@NZ | 5.35 ± 0.30 | 5.32 ± 0.19 | 3.53 ± 0.18 | 4.05 ± 0.18 |
| CSPVOH15TO@NZ | 8.35 ± 0.45 | 7.93 ± 0.54 | 3.55 ± 0.07 | 4.48 ± 0.08 |

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





