Submitted:
08 June 2023
Posted:
09 June 2023
You are already at the latest version
Abstract
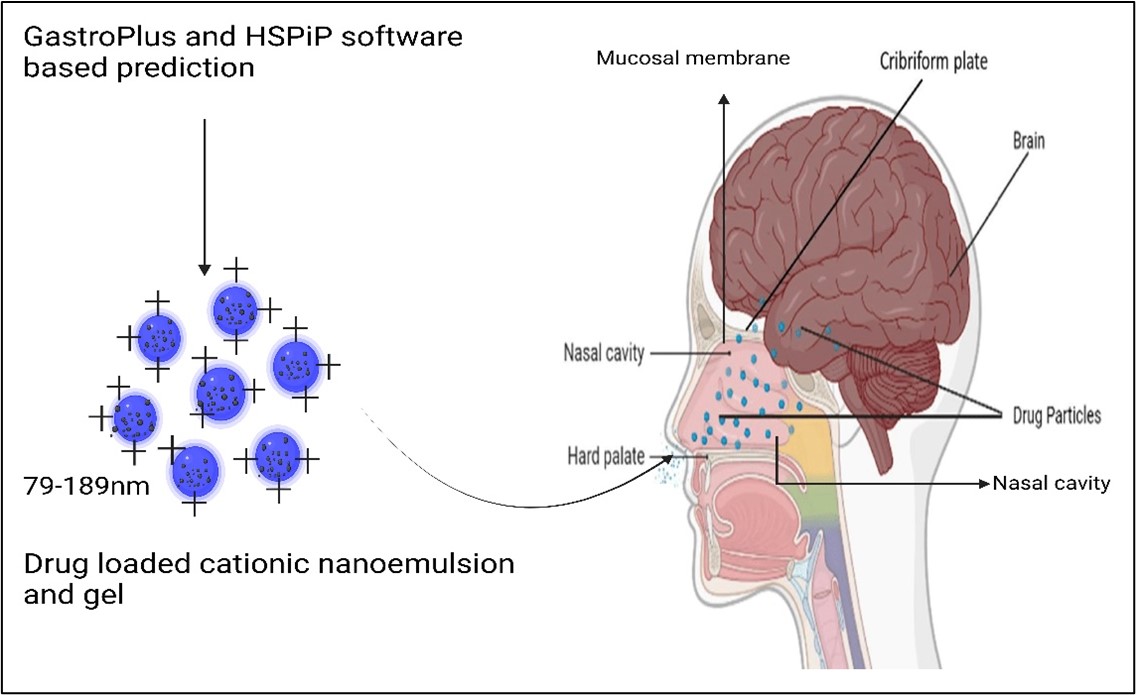
Keywords:
1. Introduction
2. Materials and methods
2.1. Materials
2.2. Methods
2.2.1. Prediction and simulation study using GastroPlus for oral tablet
Hansen solubility parameters for VA and excipients
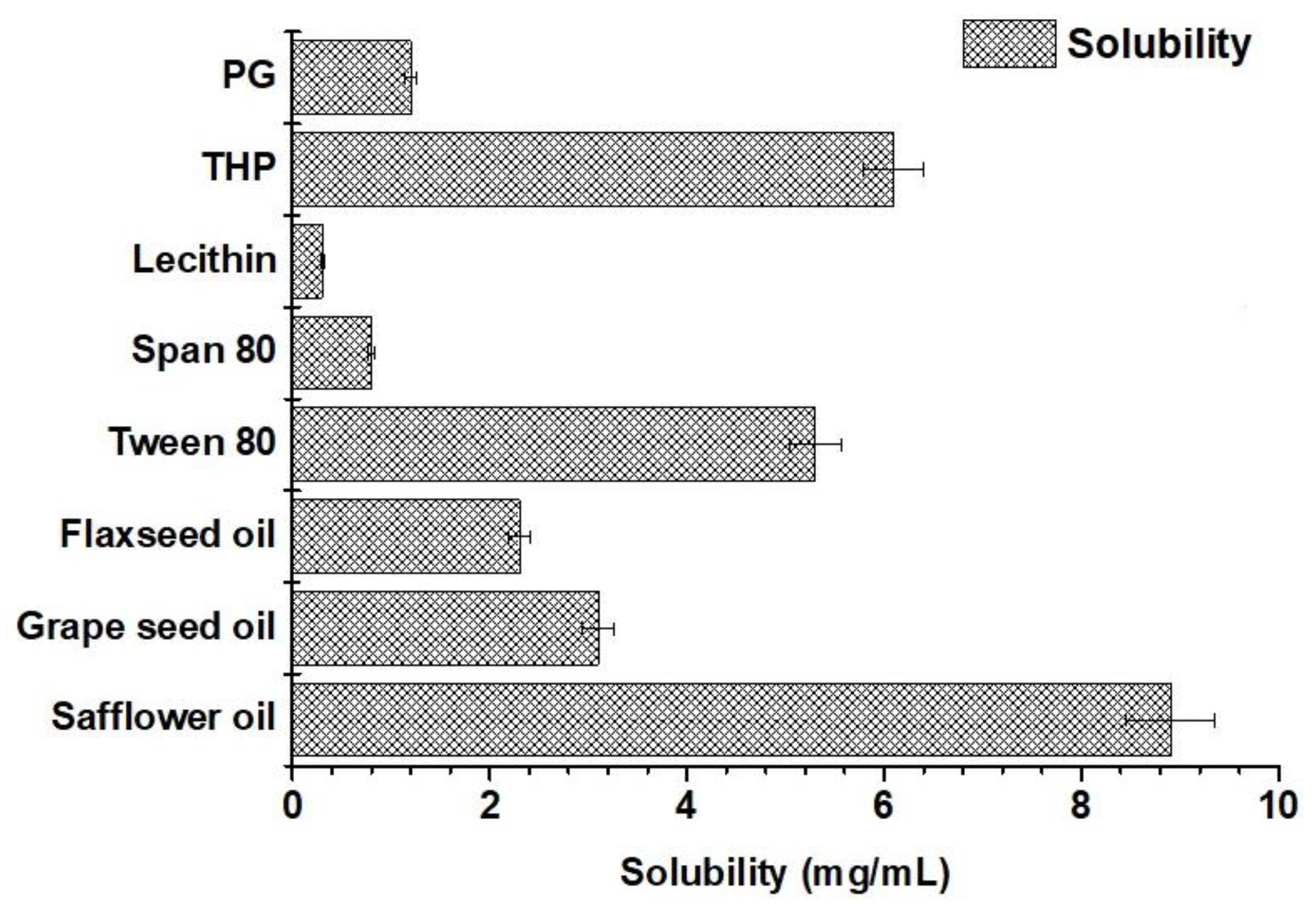
Solubility of valproate sodium in various excipients
Pseudo ternary phase diagram, cationic nanoemulsions, and nanoemulsion gel
Thermodynamic stability of cationic nanoemulsion: Freeze–thaw cycle and ultracentrifugation
2.2.2. Evaluation of cationic nanoemulsions and gels
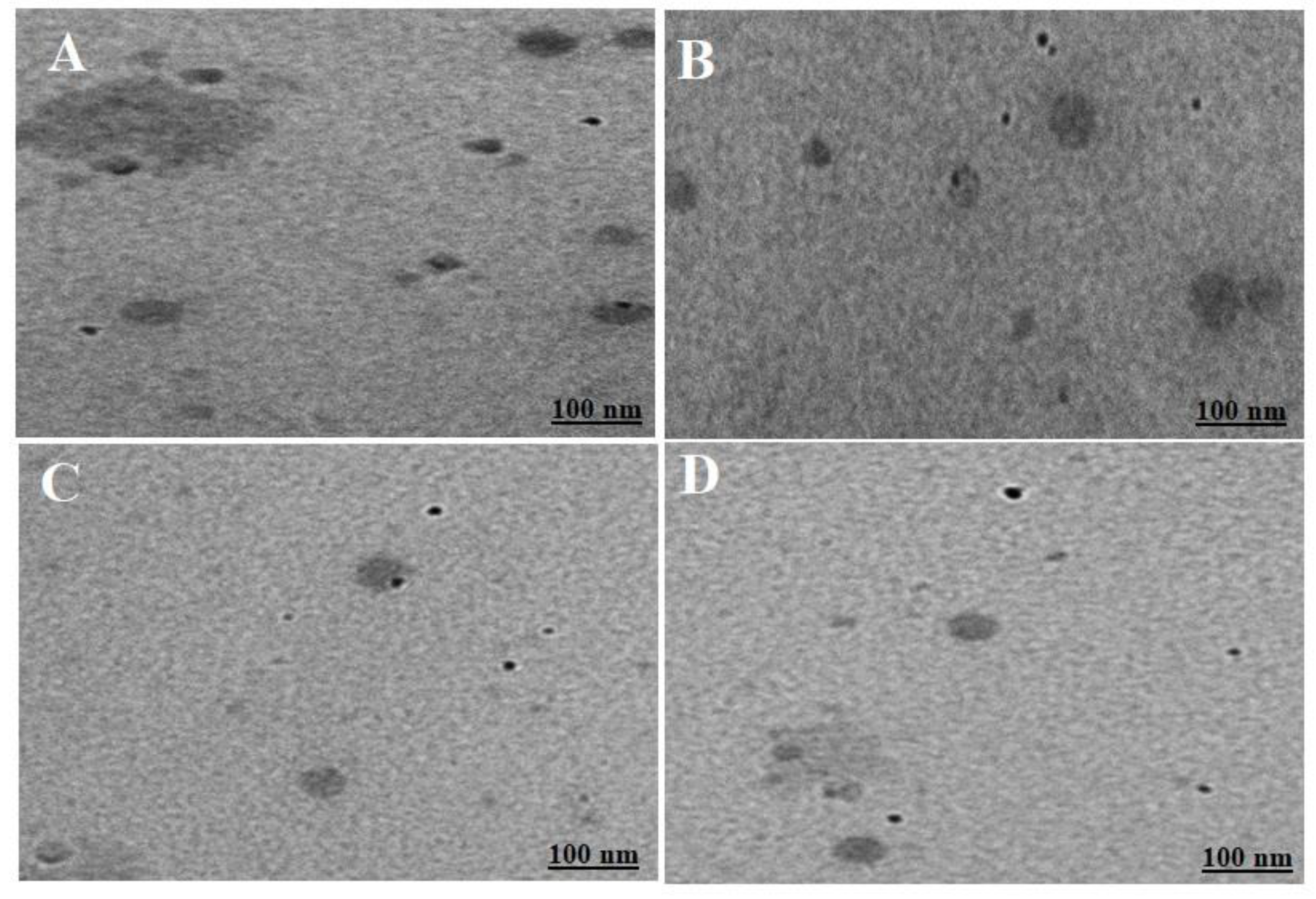
Morphological evaluation of the optimized cationic nanoemulsion and respective gel
Drug content estimation
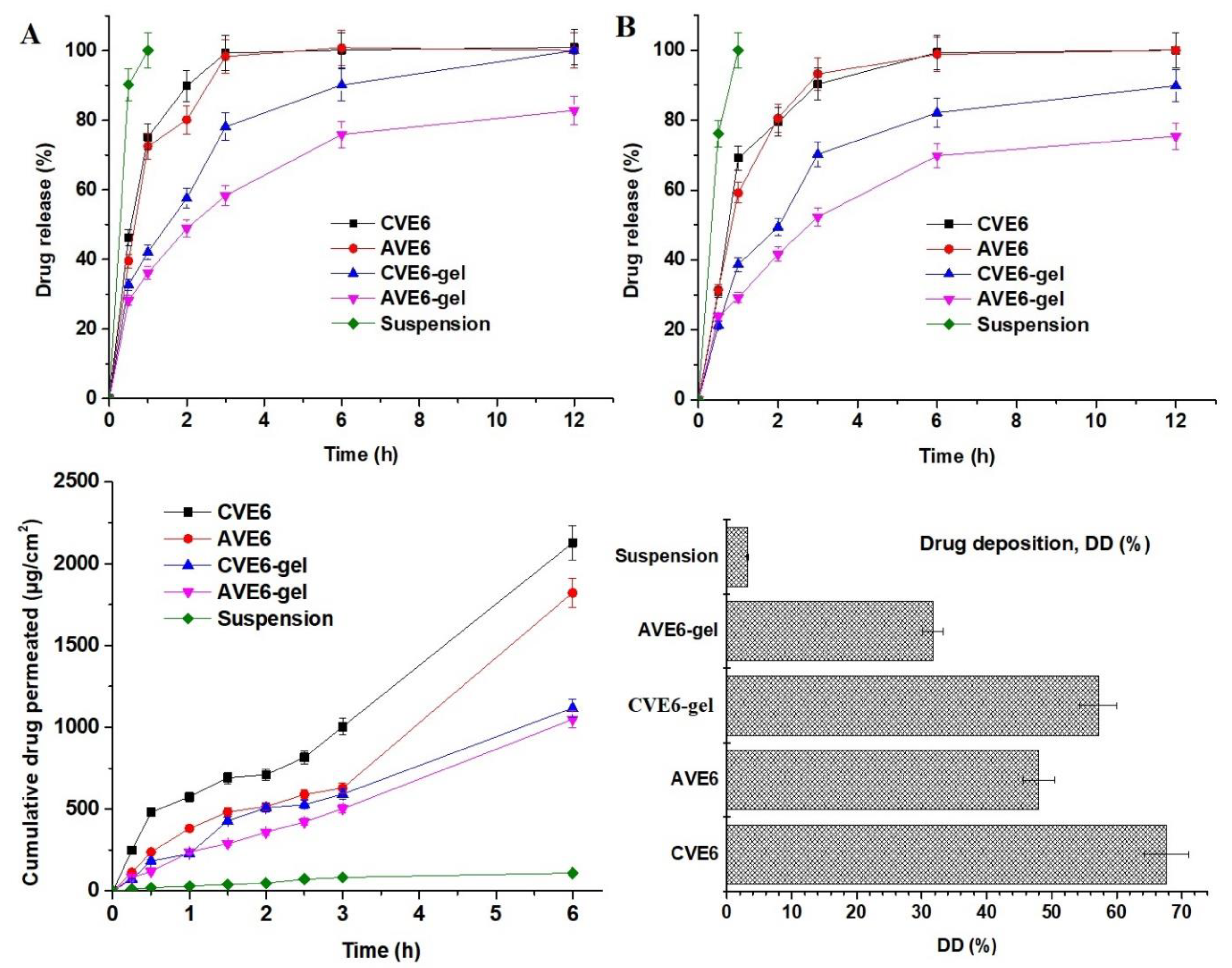
2.2.3. In vitro drug release profile
2.2.4. Ex vivo drug permeation and drug deposition using goat nasal mucosal tissue
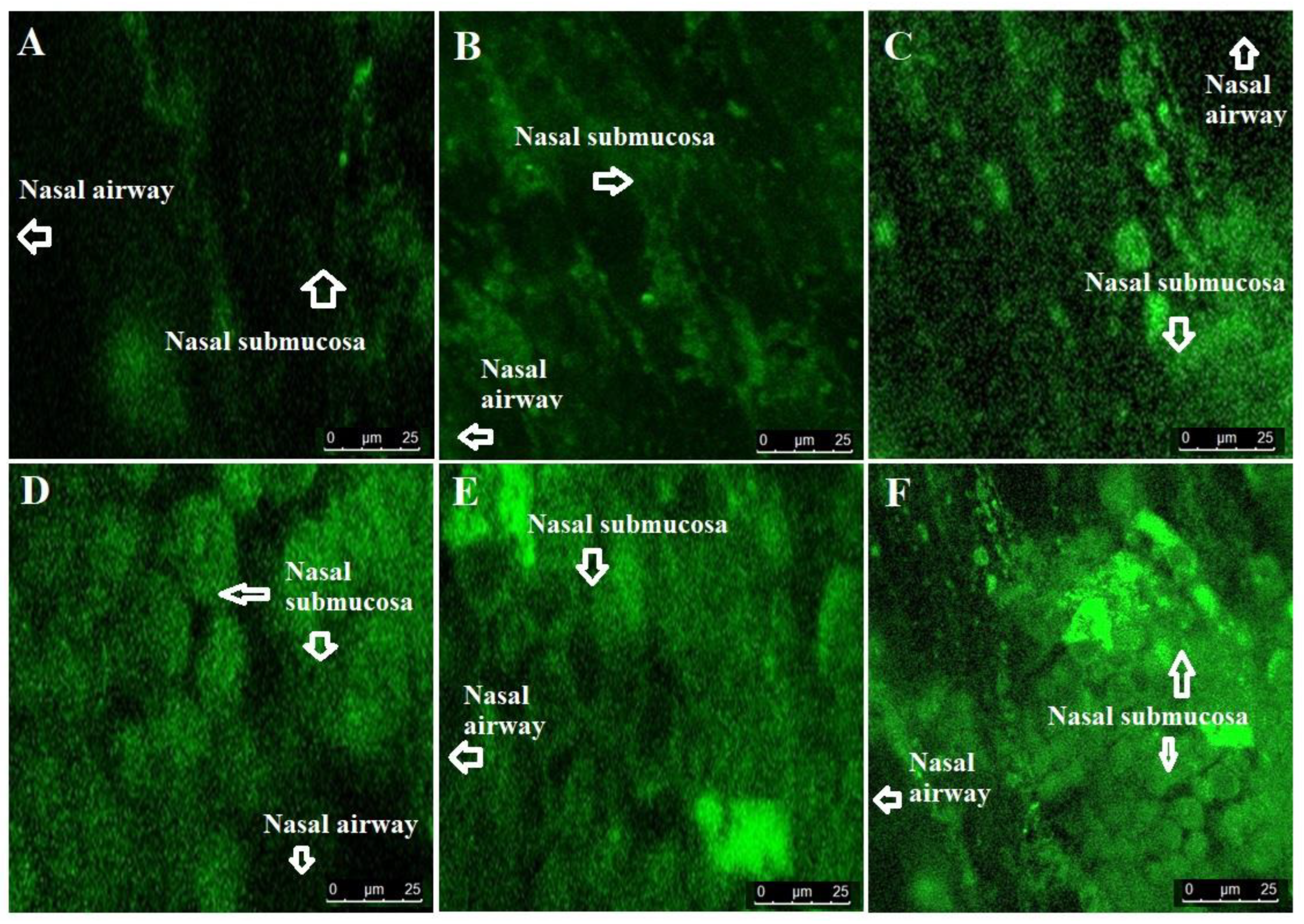
2.2.5. Confocal laser scanning microscopy (CLSM)
3. Results and discussion
3.1. Prediction and simulation study using GastroPlus for oral tablets
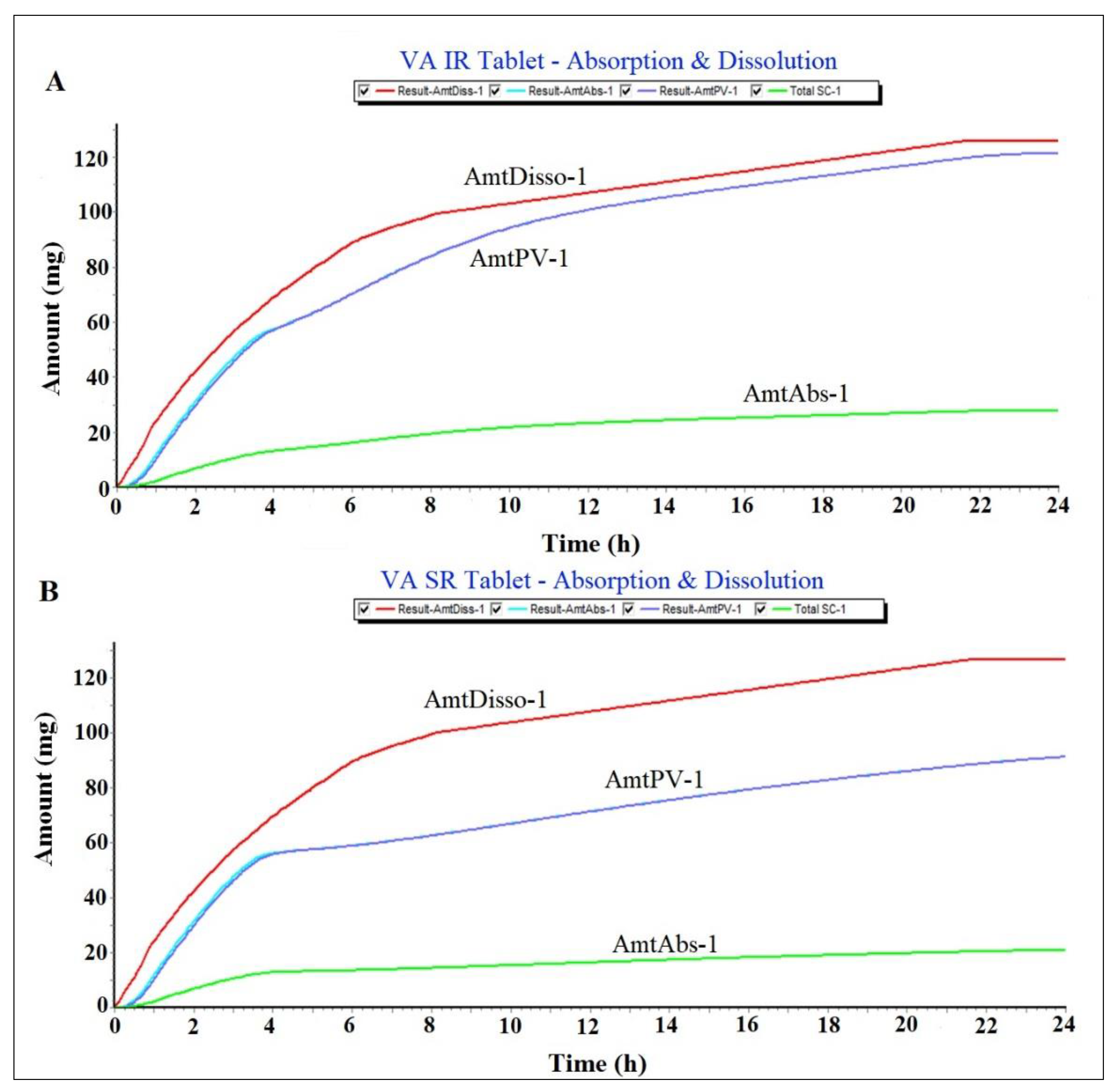
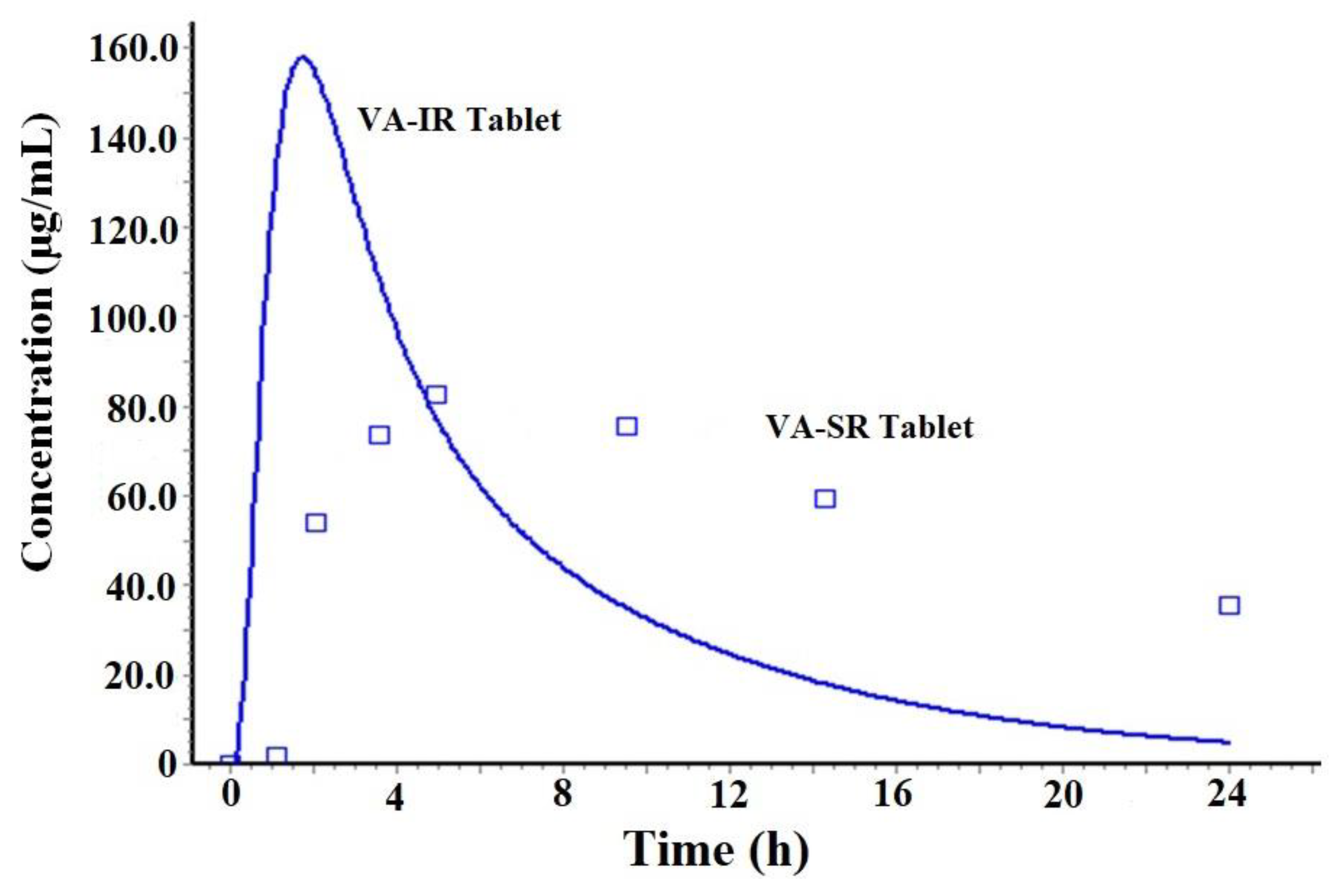
Prediction of plasma drug concentration time profile
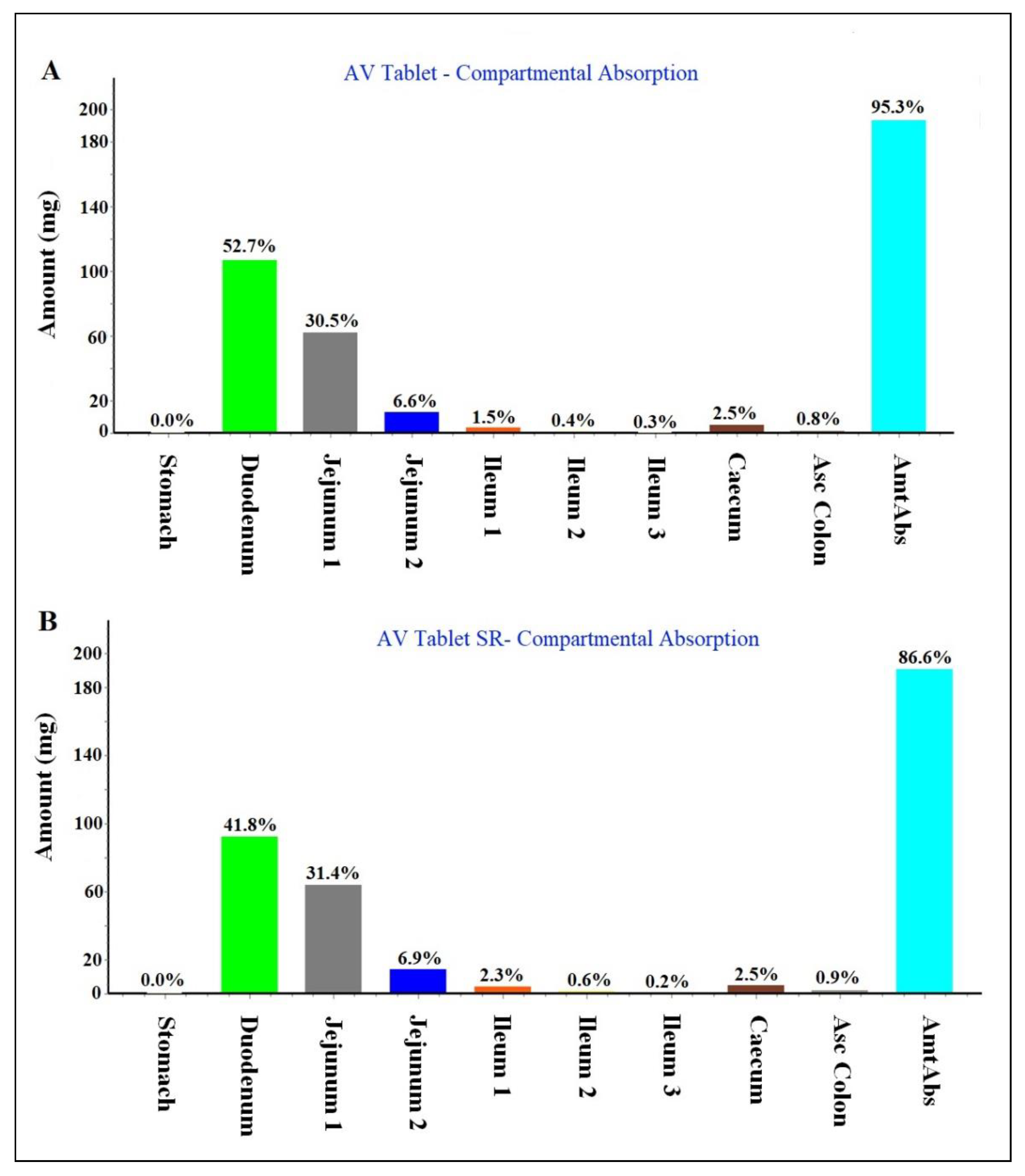
Regional compartmental absorption of both tablets
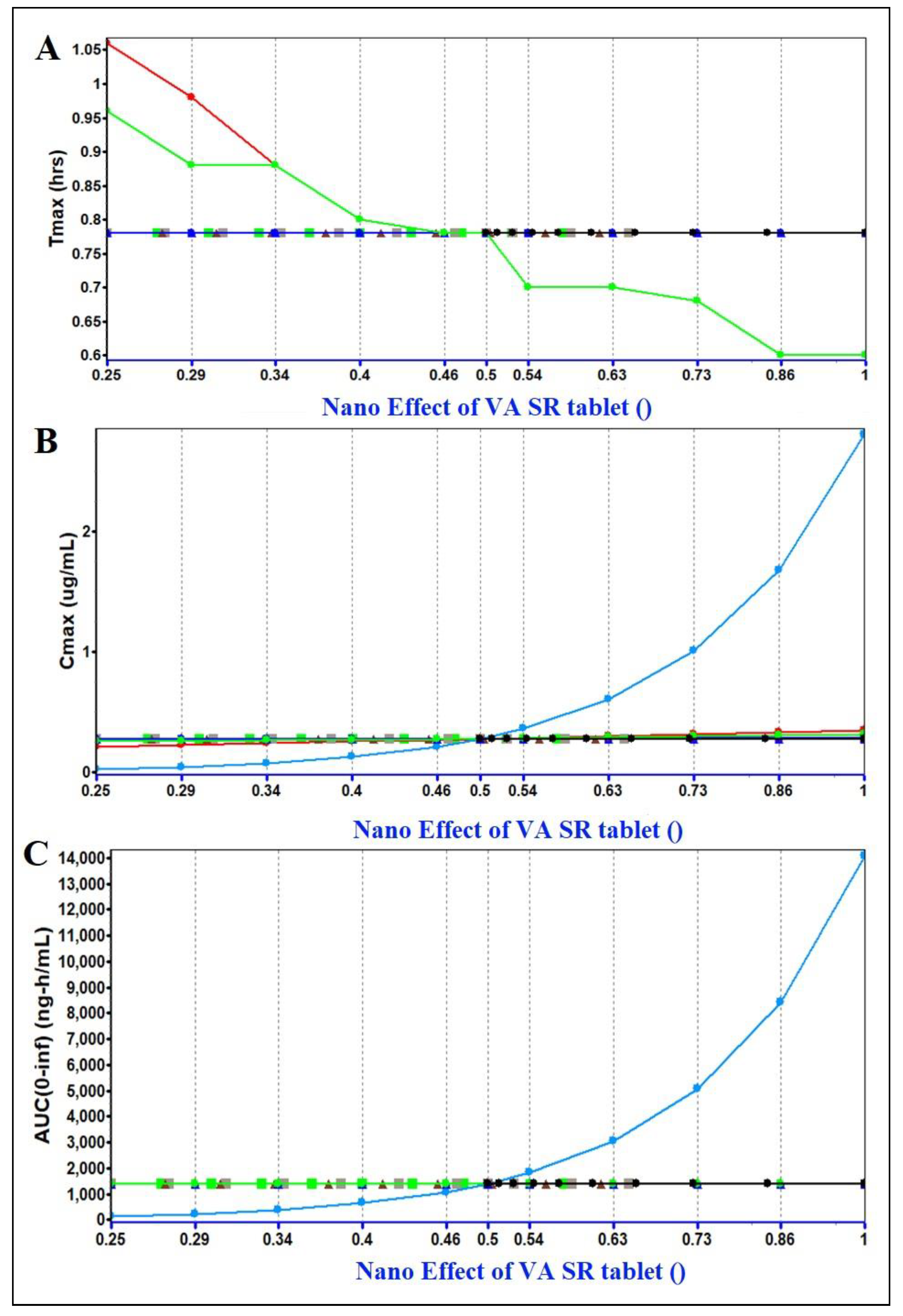
PSA assessment (parameter sensitivity analysis)
| Parameter | Values |
|---|---|
| Molecular formula** | C8H15NaO2 |
| Molecular weight (g/mol)** | 166.19 |
| Melting point (°C) | 300 |
| Aqueous solubility (mg/ml) at 25 °C | ˂ 1 |
| Density (g/mL) | 0.9 |
| Pka | 5.14 |
| Log p | 3.08 |
| ***Apparent permeability coefficient (cm/h) across hCMEC/D3 and CC-2565 of in vitro blood brain barrier | 0.625 |
| Dose (mg) | 200 |
| Body weight (Kg) | 70 |
| Dosing volume (mL) | 1 |
| Mean precipitation time (s) | 30 |
| AUC (µg. h /mL)ψ | 10 – 160 |
| Cmax (mg/L)## | ~ 120 |
| Tmax (h) (mean) | 5 |
| Elimination half-life (h)* | 8-15 |
| Clearance (L/h)# | 0.206 – 1.154 |
| Plasma protein binding (%)# | 90 - 95 |
| Vd (L)# | 8.4-23.3 |
| pH for reference solubility** | 7.0 |
| Simulation time (h)** | 24 |
Hansen solubility parameters for VA and excipients
| Drug and excipient | δd (MPa1/2) | δp (MPa1/2) | δh (MPa1/2) |
|---|---|---|---|
| AV | 16.1 | 4.3 | 9.0 |
| Safflower seed oil (87%)* | 14.5 | 2.7 | 5.3 |
| Grape seed oil (70%)* | 11.69 | 2.17 | 4.27 |
| Flaxseed oil (60%)* | 10.02 | 1.86 | 3.66 |
| Tween 80 | 16.6 | 5.3 | 7.5 |
| Span 80 | 16.7 | 6.1 | 12.4 |
| Lecithin (PC as 20%)* | 3.2 | 0.54 | 0.64 |
| Linoleic acid* | 16.7 | 3.1 | 6.1 |
| Transcutol HP | 16.0 | 2.8 | 6.2 |
| PGϕ | 16.8 | 10.4 | 21.3 |
| PC* | 16 | 2.7 | 3.2 |
Solubility of valproate (VA) in various excipients
VA loaded cationic nanoemulsions prepared
| Code | SO (%) | Smix (%) | Water (%) | Smix ratio | ST (%) | Size (nm) | PDI | ZP (mV) | %T | Product strength (%w/w) |
|---|---|---|---|---|---|---|---|---|---|---|
| CVE1 | 16.46 | 30.21 | 48.59 | 1:2 | 0.04 | 162 | 0.27 | + 24.7 | 98.5 | 0.4 |
| CVE2 | 20.75 | 23.5 | 50.22 | 1:3 | 0.05 | 189 | 0.32 | + 26.8 | 96.8 | 0.5 |
| CVE3 | 14.72 | 21.67 | 57.12 | 1:2 | 0.06 | 185 | 0.31 | + 31.6 | 97.2 | 0.6 |
| CVE4 | 19.88 | 43.51 | 32.16 | 1:2 | 0.04 | 148 | 0.18 | + 23.9 | 96.9 | 0.4 |
| CVE5 | 9.8 | 21.84 | 63.04 | 2:1 | 0.05 | 79 | 0.11 | + 27.1 | 95.3 | 0.5 |
| CVE6 | 14.46 | 17.15 | 60.99 | 3:1 | 0.07 | 113 | 0.26 | + 34.7 | 97.8 | 0.7 |
| AVE6 | 14.46 | 17.15 | 60.92 | 3:1 | 0.0 | 126 | 0.29 | – 22.8 | 95.6 | 0.7 |
| Nanoemulsion gel (0.5%w/w) composition (VA strength) | Evaluated parameters | |||||||||
| 0.5% VE gel | NE (g) | Gel-blank (g) | Triethanolamine (g) | CVE6:gel ratio | Size (nm) | PDI | ZP (mV) | Viscosity (cP) | pH | |
| CVE6 gel (0.35%) | 1 | 0.95 | 0.05 g | 1:1 | 129 | 0.24 | + 21.9 | 1837.3 | 6.8 | |
| AVE6 gel (0.35%) | 1 | 0.95 | 0.05 g | 1:1 | 142 | 0.31 | – 26.5 | 1907.1 | 7.1 | |
Freeze –thaw cycle and ultracentrifugation of nanoemulsions
| Formulations | Freezing (- 21 °C) | Room temperature | Thaw (40 °C) | Centrifugation | Inference* |
|---|---|---|---|---|---|
| CVE1 | ✓ | ✓ | ✓ | ✓ | Stable |
| CVE2 | ✓ | ✓ | ✓ | ✓ | Stable |
| CVE3 | ✓ | ✓ | ✓ | ✓ | Stable |
| CVE4 | ✓ | ✓ | ✓ | ✓ | Stable |
| CVE5 | ✓ | ✓ | ✓ | ✓ | Stable |
| CVE6 | ✓ | ✓ | ✓ | ✓ | Stable |
| AVE6 | ✓ | ✓ | ✓ | ✓ | Stable |
3.2.2. Evaluation of cationic and anionic nanoemulsions gels
Morphological evaluation of the optimized cationic nanoemulsion and respective gel
Drug content estimation
3.2.3. In vitro drug release profile
3.2.4. Ex vivo drug permeation and drug deposition using goat nasal mucosal tissue
3.2.6. Confocal laser scanning microscopy (CLSM)
4. Conclusion
Author Contributions
Ethical statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 20 December 2021).
- Available online: https://www.ninds.nih.gov/current-research/focus-disorders/focus-epilepsy-research/curing-epilepsies-promise-research (accessed on 20 December 2021).
- Al Rajeh S, Awada A, Bademosi O, Gunniyi A. The prevalence of epilepsy and other seizure disorders in an Arab population: a community-based study. Seizure. 2001, 10, 410–414. [CrossRef]
- N. Ishizue, S. Niwano, M. Saito, H. Fukaya, H. Nakamura, T. Igarashi, Fujiishi T., Yoshizawa, T., Oikawa, J., Satoh, A., Kishihara, J., Murakami, M., Niwano, H., Miyaoka, H., Ako, J. Polytherapy with Sodium Channel-Blocking Antiepileptic Drugs Is Associated with Arrhythmogenic ST-T Abnormality in Patients with Epilepsy. Seizure, 2016, 40, 81–87.
- Hammond, E.J., Perchalski, R.J., Villarreal, H.J., Wilder, B. J. (1982). In vivo uptake of valproic acid into brain. Brain Research, 240, 195–198.
- US FDA (US Food and Drug Administration). chrome-extension. Available online: https://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018081s065_018082s048lbl.pdf.
- S. Eskandari, J. Varshosaz, M. Minaiyan, M. Tabbakhian, Brain delivery of valproic acid via intranasal administration of nanostructured lipid carriers: in vivo pharmacodynamic studies using rat electroshock model, Int. J. Nanomed. 2011, 6, 363–371.
- H. Wang, Q. Huang, H. Chang, J. Xiao, Y. Cheng, Stimuli-responsive dendrimers in drug delivery, Biomater. Sci. 4 (3) (2016) 375–390.
- Lopez, T. Biocompatible Titania Microtubes Formed by Nanoparticles and its Application in the Drug Delivery of Valproic Acid. Optical Materials, 2006, 29, 70–74. [Google Scholar] [CrossRef]
- an, B.P. Kirby, J. Stanslas, H Bin Basri, Characterization, in-vitro and in-vivo evaluation of valproic acid-loaded nanoemulsion for improved brain bioavailability, J. Pharm. Pharmacol. 2017, 69, 1447–1457.
- Mori, N., Ohta, S. Comparison of anticonvulsant effects of valproic acid entrapped in positively and negatively charged liposomes in amygdaloid-kindled rats, Brain Res. 593 (2), 1992, 329–331.
- Tan, S. F., Masoumi, H. R. F., Karjiban, R. A., Stanslas, J., Kirby, B. P., Basri, M., & Basri, H. B. Ultrasonic emulsification of parenteral valproic acid-loaded nanoemulsion with response surface methodology and evaluation of its stability. Ultrasonics Sonochemistry, 2016, 29, 299–308.
- Yadav, S. , Gandham, S. K., Panicucci, R., Amiji, M. M. Intranasal brain delivery of cationic nanoemulsion-encapsulated TNFα siRNA in prevention of experimental neuroinflammation. Nanomedicine: Nanotechnology, Biology and Medicine, 2016, 12, 987–1002. [Google Scholar]
- Azambuja, J. H. , Schuh, R. S., Michels, L. R., Gelsleichter, N. E., Beckenkamp, L. R., Iser, I. C., Lenz, G.S., de Oliveira, F.H., Venturin, G., Greggio, S., daCosta, J.C., Wink, M.R., Sevigny, J., Stefani, M.A., Battastini, A.M.O., Teixieria H.F., Braganhol, E. Nasal Administration of Cationic Nanoemulsions as CD73-siRNA Delivery System for Glioblastoma Treatment: a New Therapeutical Approach. Molecular Neurobiology. 2020, 57, 635–649. [Google Scholar] [PubMed]
- Hussain, A.; Alshehri, S.; Ramzan, M.; Afzal, O.; Altamimi, A.S.A.; Alossaimi, M.A. Biocompatible solvent selection based on thermodynamic and computational solubility models, in-silico GastroPlus prediction, and cellular studies of ketoconazole for subcutaneous delivery. Journal of Drug Delivery Science and Technology 2021, 65, 102699. [Google Scholar] [CrossRef]
- Alsarra, I. A. , Al-Omar, M., Belal, F. Valproic Acid and Sodium Valproate: Comprehensive Profile. Profiles of Drug Substances, Excipients and Related Methodology, 2005, 32, 209–240. [Google Scholar] [CrossRef]
- Ezati, N.; Roberts, M.S.; Zhang, Q.; Moghimi, H.R. Measurement of Hansen Solubility Parameters of Human Stratum Corneum. Iran J. Pharm. Res. 2020, 19, 572–578. [Google Scholar]
- Hansen, C.M.; Andersen, B. The affinities of organic solvents in biological systems. Am. Ind. Hyg. Assoc. 1988, 49, 301–308. [Google Scholar] [CrossRef]
- De La Peña-Gil, A. , Toro-Vazquez, J. F., Rogers, M.A. Simplifying Hansen Solubility Parameters for Complex Edible Fats and Oils. Food Biophysics, 2016, 11, 283–291. [Google Scholar] [CrossRef]
- Jones, M. N. , Song, Y. -H., Kaszuba, M., Reboiras, M.D. The Interaction of Phospholipid Liposomes with Bacteria and Their Use in the Delivery of Bactericides. Journal of Drug Targeting, 1997, 5, 25–34. [Google Scholar] [CrossRef]
- Hussain, A. , Singh, S.K. Evidences for anti-mycobacterium activities of lipids and surfactants. World Journal of Microbiology and Biotechnology, 2015, 32. [Google Scholar] [CrossRef]
- Christensen, J. M.; Chuong, M. C.; Le, H.; Pham, L.; Bendas, E. Hydrocortisone diffusion through synthetic membrane, mouse skin, and Epiderm™ cultured skin. Archives of Drug Information, 2011, 4, 10–21. [Google Scholar] [CrossRef]
- Hansen, C. M. The significance of the surface condition in solutions to the diffusion equation: explaining “anomalous” sigmoidal, Case II, and Super Case II absorption behavior. European Polymer Journal, 2010, 46, 651–662. [Google Scholar] [CrossRef]
- Yuwanda, A. , Surini, S.; Harahap, Y.; Jufri. M. Study of valproic acid liposomes for delivery into the brain through an intranasal route. Heliyon, 2022, 8, e09030). [Google Scholar] [CrossRef]
- Basu, S. , Maity, S.Preparation and Characterisation of Mucoadhesive Nasal Gel of Venlafaxine Hydrochloride for Treatment of Anxiety Disorders. Indian J. Pharm. Sci., 2012, 74, 428–433. [Google Scholar] [CrossRef]
- Trenkel, M.; Scherlie, R. Nasal Powder Formulations: In-Vitro Characterisation of the Impact of Powders on Nasal Residence Time and Sensory Effects. Pharmaceutics, 2021, 13, 385. [Google Scholar] [CrossRef]
- Khuroo, T. , Khuroo, A., Hussain, A., Mirza, M.A., Panda, A.K., Iqbal, Z. Qbd based and Box-Behnken design assisted oral delivery of stable lactone (active) form of topotecan as polymeric nanoformulation: Cytotoxicity, pharmacokinetic, in vitro, and ex vivo gut permeation studies. Journal of Drug Delivery Science and Technology Available online 6 October 2022, 103850, In press. [CrossRef]
- G。 Nava, E. Pin˜on, L. Mendoza, N. Mendoza, D. Quintanar, A. Ganem, Formulation and in vitro, ex vivo and in vivo evaluation of elastic liposomes for transdermal delivery of ketorolac tromethamine, Pharm. Times 2011, 3, 954–970.
- Hussain, A. , Samad, A.; Singh, S.K.; Ahsan, M.N.; Haque, M.W.; Faruk, A.; Ahmed, F.J. Drug Deliv. 2016, 23, 652–667; [Google Scholar]
- Chen, H.; Chang, X.; Du, D.; Liu, W.; Liu, J.; Weng, T.; Yang, Y.; Xu, H.; Yang, X. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. Journal of Controlled Release, 2006, 110, 296–306. [Google Scholar] [CrossRef]
- Teixeira-da-Silva, P.; Pérez-Blanco, J.S.; Santos-Buelga, D.; Otero, M.J.; García, M.J. Population pharmacokinetics of valproic acid in pediatric and adult caucasian patients. Pharmaceutics 2022, 14, 811. [Google Scholar] [CrossRef]
- Zaccara, G.; Messori, A.; Moroni, F. Clinical Pharmacokinetics of Valproic Acid - 1988. Clinical Pharmacokinetics, 1988, 15, 367–389. [Google Scholar] [CrossRef]
- Levy, R.H. CSF and plasma pharmacokinetics: relationship to mechanisms of action as exemplified by valproic acid in monkey. In J. S. Lockard and A. A. Ward (Eds.), Epilepsy: A Window to Brain Mechanisms, Raven Press, New York, 1980, 191-200.
- Johannessen, C.U.; Johannessen, S.I. Valproate: past, present, and future. CNS Drug Rev. 2003, 9, 199–216. [Google Scholar] [CrossRef]
- Winter, M.E. Basic Clinical Pharmacokinetics, 5th edn. Philadelphia, PA: Lippincott Williams & Wilkins Health, 2010.
- Loscher, W. Serum protein binding and pharmacokinetics of valproate in man, dog, rat and mouse. J. Pharmacol. Exp. Ther. 1978, 204, 255–261. [Google Scholar] [PubMed]
- Dickinson, R.G.; Harland, R.C.; Ilias, A.M.; Rodgers, R.M.; Kaufman, S.N.; Lynn, R.K.; Gerber, N. Disposition of valproic acid in the rat: dose dependent metabolism, distribution, enterohepatic recirculation and choleretic effect. J. Pharmacol. Exp. Ther. 1979, 211, 583–595. [Google Scholar]
- Hansen, S.; Lehr, C.-M.; Schaefer, U.F. Improved input parameters for diffusion models of skin absorption. Advanced Drug Delivery Reviews, 2013, 65, 251–264. [Google Scholar] [CrossRef]
- Methaneethorn, J. A systematic review of population pharmacokinetics of valproic acid. Br. J. Clin. Pharmacol. 2018, 84, 816–834. [Google Scholar] [CrossRef] [PubMed]
- Gugler, R.; von Unruh, G.E. Clinical Pharmacokinetics of Valproic Acid1. Clinical Pharmacokinetics, 1980, 5, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Vay, K.; Scheler, S.; Frie, W. Application of Hansen solubility parameters for understanding and prediction of drug distribution in microspheres. International Journal of Pharmaceutics, 2011, 416, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.; Brunaldi, K.A. model for fatty acid transport into the brain. J. Mol. Neurosci. 2007, 33, 12–17. [Google Scholar] [CrossRef]
- Hussain, A.; Altamimi, M.A.; Afzal, O.; Altamimi, A.S.A.; Ali, A.; Ali, A.; Martinez, F.; Siddique, M.U.M.; Acree Jr., W. E.; Jouyban, A. Preferential solvation study of the synthesized aldose reductase inhibitor (SE415) in the {PEG400 (1) + Water (2)} cosolvent mixture and GastroPlus-based prediction. ACS Omega, 2022, 7, 1197–1210. [Google Scholar] [CrossRef]
- Han, X.; Cheng, L.; Zhang, R.; Bi, J. Extraction of safflower seed oil by supercritical CO2. Journal of Food Engineering, 2009, 92, 370–376. [Google Scholar] [CrossRef]
- Afzal, O.; Alshammari, H.A.; Altamimi, M.A.; Hussain, A.; Almohaywi, B. ; Altamimia, A.S. A. Hansen solubility parameters and green nanocarrier based removal of trimethoprim from contaminated aqueous solution. Journal of Molecular Liquids, 2022, 361, 119657 doiorg/101016/jmolliq2022119657. [Google Scholar] [CrossRef]
- Abdullah, G.Z.; Abdulkarim, M.F.; Mallikarjun, C.; Mahdi, E.S.; Basri, M.; Sattar, M.A.; Noor, A.M. Carbopol 934, 940 and Ultrez 10 as viscosity modifiers of palm olein esters based nano-scaled emulsion containing ibuprofen. Pak. J. Pharm. Sci., 2013, 26, 75–83. [Google Scholar] [PubMed]
- Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S.; Alnemer, U.A. Development and evaluations of transdermally delivered luteolin loaded cationic nanoemulsion: In vitro and ex vivo evaluations. Pharmaceutics, 2021, 13, 1218. [Google Scholar] [CrossRef]
- Edmond, J. Essential Polyunsaturated Fatty Acids and the Barrier to the Brain: The Components of a Model for Transport. J. Mol. Neurosci. 2001, 16, 181–194. [Google Scholar] [CrossRef]
- Trenkel, M.; Scherlie, R. Nasal Powder Formulations: In-Vitro Characterisation of the Impact of Powders on Nasal Residence Time and Sensory Effects. Pharmaceutics 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, P.; Vibhavari, C.; Chatur, M. Development of Valproic Acid Niosomal in situ Nasal Gel Formulation for Epilepsy. Indian Journal of Pharmaceutical Education and Research, 2013, 47, 31–41. [Google Scholar]
- Ehrick, J.D. , Shah, S.A., Shaw, C., Kulkarni, V.S., Coowanitwong, I., De, S., Suman, J.D. Considerations for the Development of Nasal Dosage Forms. AAPS Advances in the Pharmaceutical Sciences Series, 2013, 6, 99–144. [Google Scholar]
- Vidgren, M.T.; Kublik, H. Nasal delivery systems and their effect on deposition and absorption. Adv. Drug Deliv. Rev. 1998, 29, 157–177. [Google Scholar] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Kumar, S.; Wong, H.; Yeung, S.A.; Riggs, K.W.; Abbott, F.S.; Rurak, D.W. Disposition of valproic acid in maternal, fetal, and newborn sheep II: metabolism and renal elimination. Drug Metab. Dispos. 2000, 28, 845–856. [Google Scholar]
- Tang, W.; Borel, A.G.; Fujimia, T.; Abbott, F.S. Fluorinated analogs as mechanistic probes in valproic acid hepatotoxicity: hepatic microvesicular steatosis and glutathione status. Chem. Res. Toxicol. 1995, 8, 671–682. [Google Scholar] [CrossRef]
- Rossi, MA. Targeting anti-epileptic drug therapy without collateral damage: nanocarrier-based drug delivery. Epilepsy Curr. 2012, 12, 199–200. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E. ; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Andersen I, Proctor DF. Measurement of nasal mucociliary clearance. Eur. J. Respir. Dis. Suppl. 1983, 127, 37–40.
- Patel, R. B. , Patel, M.R., Bhatt, K. K., Patel, B. G. Formulation consideration and characterization of microemulsion drug delivery system for transnasal administration of carbamazepine. Bulletin of Faculty of Pharmacy, Cairo University, 2013, 51, 243–253. [Google Scholar] [CrossRef]
- Yang, Y. , Jing, Y., Yang, J., Yang, Q. Effects of intranasal administration with Bacillus subtilis on immune cells in the nasal mucosa and tonsils of piglets. Experimental and Therapeutic Medicine. 2018, 15, 5189–5198. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





