Submitted:
08 June 2023
Posted:
09 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Microbial cultures
2.2. In vitro screening for probiotic properties
2.2.1. Resistance to low pH, pepsin, pancreatin and tolerance to bile salts
2.2.2. Antibiotic susceptibility
2.3. Production of freeze-dried immobilized P. acidilactici ORE5 on pistachio nuts
2.4. Functional Katiki Domokou type cheese production
2.5. Resistance to spoilage assessment
2.6. Physicochemical Analysis
2.7. Microbiological Analyses
2.7.1. Monitoring Pediococcus acidilactici ORE5 cell viability
2.7.2. Determination of cheese microbiota
2.8. Minor component analysis by HS-SPME GC/MS
2.9. DNA Extraction, PCR Amplification and 16S rRNA Sequencing
2.10. Preliminary sensory evaluation
2.11. Statistical Analysis
3. Results
3.1. Molecular identification
3.2. In vitro screening for probiotic properties
3.3. Safety profile - antibiotic susceptibility
3.4. Physicochemical Characteristics of functional Katiki Domokou type cheese
3.5. Microbiological Analysis of Functional Katiki Domokou type cheese
3.6. Resistance of functional Katiki Domokou type cheese to microbial contamination
3.7. Minor volatiles in functional Katiki Domokou type cheese
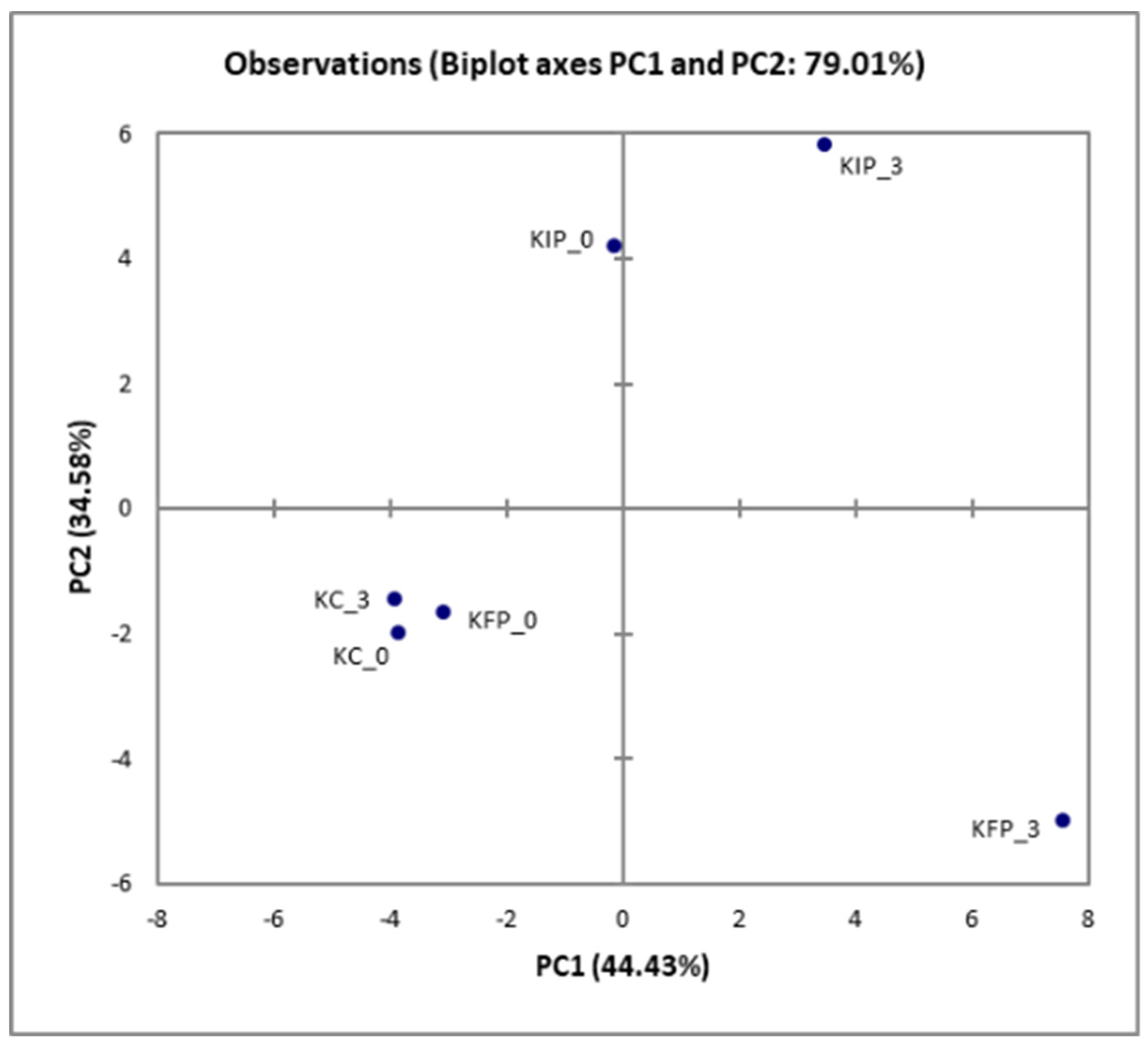
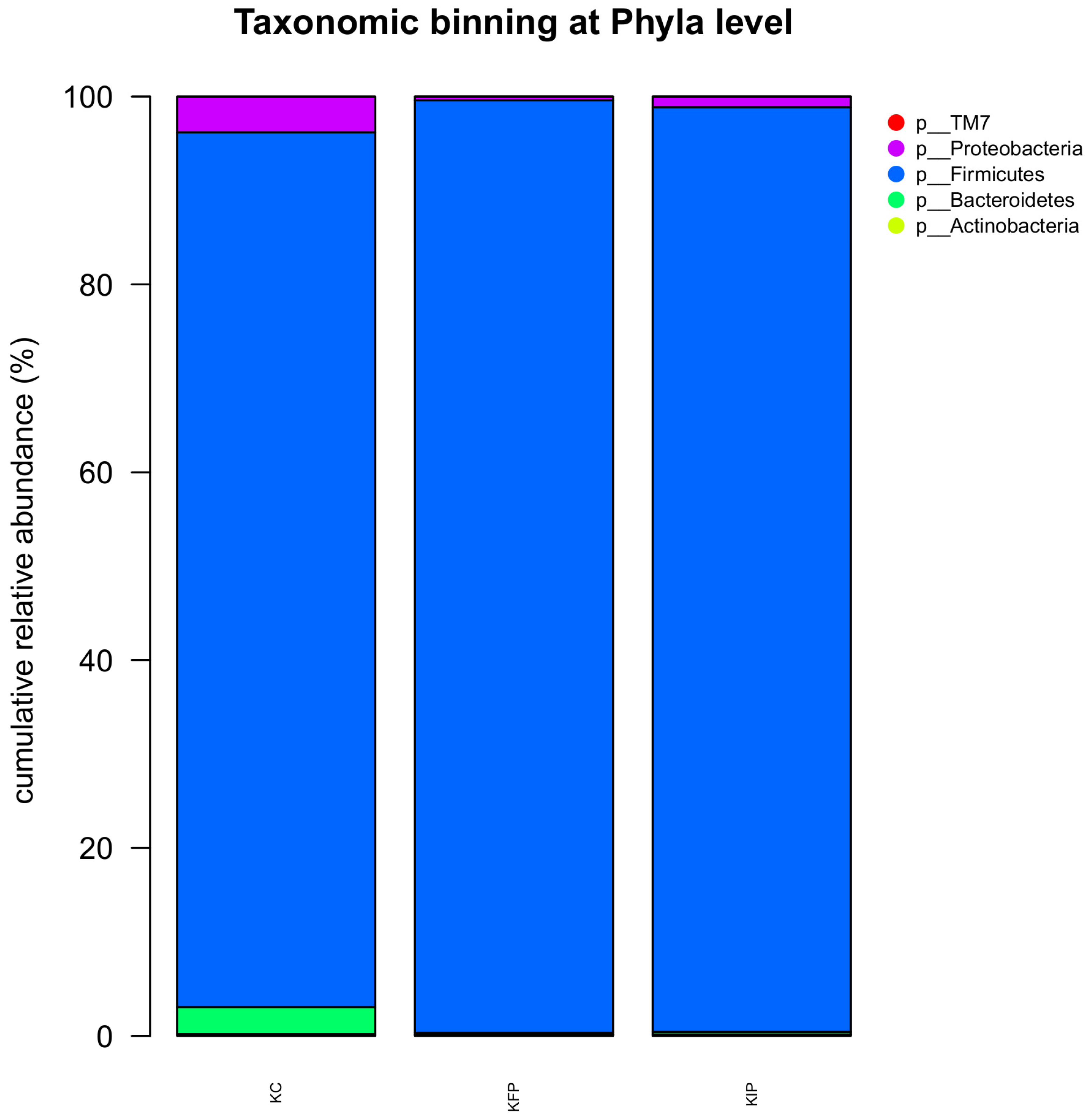
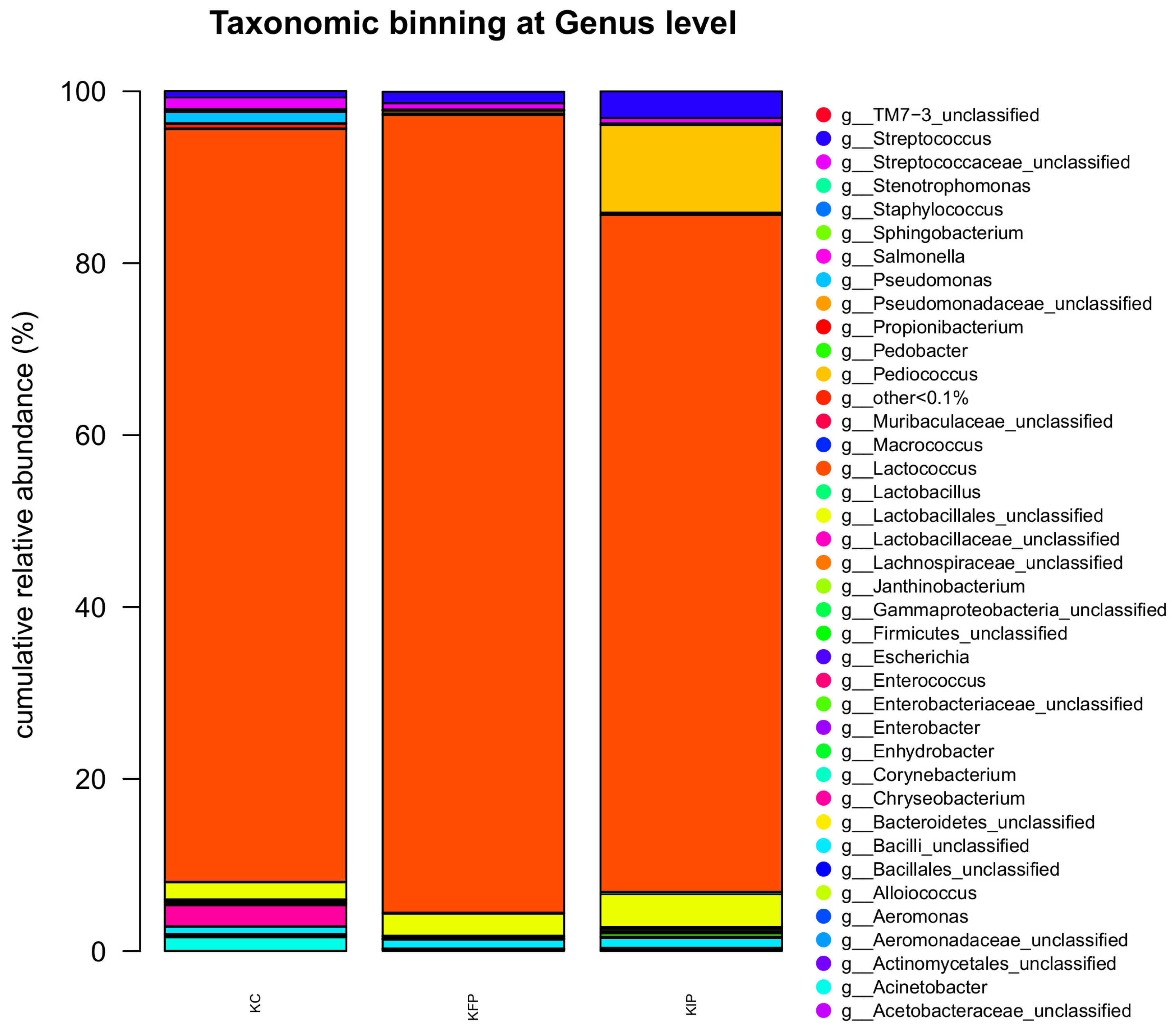
3.8. Effect of Immobilized Pediococcus acidilactici ORE5 culture on bacteria microbiome of Katiki Domokou type cheese
3.9. Preliminary Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agricultural Organization of the United Nations; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. FAO Food Nutr. Paper 2006, 85. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 29 January 2023).
- Kvakova, M.; Bertkova, I.; Stofilova, J.; Savidge, T.C. Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation. Foods 2021, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Prapa, I.; Nikolaou, A.; Panas, P.; Tassou, C.; Kourkoutas, Y. Developing Stable Freeze-Dried Functional Ingredients Containing Wild-Type Presumptive Probiotic Strains for Food Systems. Appl. Sci. 2023, 13, 630. [Google Scholar] [CrossRef]
- Nikolaou, A.; Mitropoulou, G.; Nelios, G.; Kourkoutas, Y. Novel Functional Grape Juices Fortified with Free or Immobilized Lacticaseibacillus rhamnosus OLXAL-1. Microorganisms 2023, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Deepika, G.; Charalampopoulos, D. Surface and Adhesion Properties of Lactobacilli. Adv. Appl. Microbiol. 2010, 70, 127–152. [Google Scholar] [CrossRef]
- Yanni AE, Mitropoulou G, Prapa I, Agrogiannis G, Kostomitsopoulos N, Bezirtzoglou E, Kourkoutas Y, Karathanos VT. Functional modulation of gut microbiota in diabetic rats following dietary intervention with pistachio nuts (Pistacia vera L.). Metabol Open 2020, 7, 100040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prapa, I.; Yanni, A.E.; Nikolaou, A.; Kostomitsopoulos, N.; Kalogeropoulos, N.; Bezirtzoglou, E.; Karathanos, V.T.; Kourkoutas, Y. Dietary Pistachio (Pistacia vera L.) Beneficially Alters Fatty Acid Profiles in Streptozotocin-Induced Diabetic Rat. Appl. Sci. 2022, 12, 4606. [Google Scholar] [CrossRef]
- Karimi R., Mortazavian A. M., da Cruz A. G. Viability of probiotic microorganisms in cheese during production and storage: a review. Dairy Science and Technology 2011, 91, 283–308. [Google Scholar] [CrossRef]
- Kagkli DM, Iliopoulos V, Stergiou V, Lazaridou A, Nychas GJ. Differential Listeria monocytogenes strain survival and growth in Katiki, a traditional Greek soft cheese, at different storage temperatures. Appl Environ Microbiol. 2009, 75, 3621–6. [Google Scholar] [CrossRef] [PubMed]
- Pappa, E.C.; Kondyli, E. Descriptive Characteristics and Cheesemaking Technology of Greek Cheeses Not Listed in the EU Geographical Indications Registers. Dairy 2023, 4, 43–67. [Google Scholar] [CrossRef]
- Blaya J, Barzideh Z, LaPointe G. Symposium review: Interaction of starter cultures and nonstarter lactic acid bacteria in the cheese environment. J Dairy Sci. 2018, 101, 3611–3629. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou D, Kourkoutas Y, Koutinas AA, Kanellaki M. Thermally- dried immobilized kefir on casein as starter culture in dried whey cheese production. Food Microbiol. 2009, 26, 809–20. [Google Scholar] [CrossRef] [PubMed]
- Araújo, V.S.; Pagliares, V.A.; Queiroz, M.L.P. and Freitas-Almeida, A.C. Occurrence Staphylococcus and enteropathogens in soft cheese commercialized in the city of Rio de Janeiro, Brazil. Journal of Applied Microbiology 2002, 92, 1172–1177. [Google Scholar] [CrossRef]
- Arena, M. P., Silvain, A., Normanno, G., Grieco, F., Drider, D., Spano, G. and Fiocco, D. Use of Lactobacillus plantarum strains as a bio-control strategy against food-borne pathogenic microorganisms. Frontiers in Microbiology 2016, 7, 464. [Google Scholar] [CrossRef]
- Sherwani, K. F., Bukhari, D., A., A. Probiotics in processed dairy products and their role in gut microbiota health. 2022, 2022. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Chen, S.; Wang, X.; Deng, X.; Liu, G.; Chang, W.; Beckers, Y.; Cai, H. Screening and Characterization of Pediococcus acidilactici LC-9-1 toward Selection as a Potential Probiotic for Poultry with Antibacterial and Antioxidative Properties. Antioxidants 2023, 12, 215. [Google Scholar] [CrossRef]
- ISO 5534:2004; Cheese and Processed Cheese. Determination of the Total Solids Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- Dan T, Wang D, Jin RL, Zhang HP, Zhou TT, Sun TS. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. J Dairy Sci. 2017, 100, 2488–2500. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; Sahl, J.W.; Stres, B.; Thallinger, G.G.; Van Horn, D.J.; Weber, C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009, 75, 7537–41. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kourkoutas, Y. High-Temperature Semi-Dry and Sweet Low Alcohol Wine-Making Using Immobilized Kefir Culture. Fermentation 2021, 7, 45. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP). Guidance on the Assessment of Bacterial Susceptibility to Antimicrobials of Human and Veterinary Importance. EFSA J. 2012, 10, 2740. [Google Scholar] [CrossRef]
- Litopoulou-Tzanetaki E, Tzanetakis N. The Microfloras of Traditional Greek Cheeses. Microbiol Spectr. 2014, 2, CM-0009-2012. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Iraporda, C.; Garrote, G.L.; Abraham, A.G. Kefir micro-organisms: their role in grain assembly and health properties of fermented milk. J. Appl. Microbiol. 2019, 126, 686–700. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.A.; Tsakalidou, E.; Nychas, G.J.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef]
- Danielsen, M.; Wind, A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int. J. Food Microbiol. 2003, 82, 1–11. [Google Scholar] [CrossRef]
- Kastner, S.; Perreten, V.; Bleuler, H.; Hugenschmidt, G.; Lacroix, C.; Meile, L. Antibiotic susceptibility patterns and resistance genes of starter cultures and probiotic bacteria used in food. Syst. Appl. Microbiol. 2006, 29, 145–155. [Google Scholar] [CrossRef]
- Rojo-Bezares, Beatriz, et al. Assessment of antibiotic susceptibility within lactic acid bacteria strains isolated from wine. International journal of food microbiology 2006, 111, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, Dimitra, et al. Free and immobilized Lactobacillus casei ATCC 393 on whey protein as starter cultures for probiotic Feta-type cheese production. Journal of Dairy Science 2014, 97, 4675–4685. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, Dimitra, Panagiotis Kandylis, and Yiannis Kourkoutas. Assessment of freeze-dried immobilized Lactobacillus casei as probiotic adjunct culture in yogurts. Foods 2019, 8, 374. [Google Scholar] [CrossRef]
- Shehata, Mohamed G., et al. Lacticaseibacillus paracasei KC39 immobilized on prebiotic wheat bran to manufacture functional soft white cheese. Fermentation 2022, 8, 496. [Google Scholar] [CrossRef]
- Terpou, Antonia, et al. Growth capacity of a novel potential probiotic Lactobacillus paracasei K5 strain incorporated in industrial white brined cheese as an adjunct culture. Journal of food science 2018, 83, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D., Kandylis, P., Kourkoutas, Y., & Kanellaki, M. Novel probiotic whey cheese with immobilized lactobacilli on casein. LWT 2017, 86, 627–634. [Google Scholar] [CrossRef]
- Choi J, Lee SI, Rackerby B, Goddik L, Frojen R, Ha SD, et al. Microbial Communities of a Variety of Cheeses and Comparison between Core and Rnd Region of Cheeses. Journal of Dairy Science 2020, 103, 4026–4042. [Google Scholar] [CrossRef] [PubMed]
- Shi, C., & Maktabdar, M. Lactic acid bacteria as biopreservation against spoilage molds in dairy products–A review. Frontiers in microbiology 2022, 12, 4283. [Google Scholar] [CrossRef]
- Gonzales-Barron U, Campagnollo FB, Schaffner DW, Sant'Ana AS, Cadavez VAP. Behavior of Listeria monocytogenes in the presence or not of intentionally- added lactic acid bacteria during ripening of artisanal Minas semi-hard cheese. Food Microbiol. 2020, 91, 103545. [Google Scholar] [CrossRef] [PubMed]
- Afzali, S., Edalatian Dovom, M. R., Habibi Najafi, M. B., & Mazaheri Tehrani, M. Determination of the anti-yeast activity of Lactobacillus spp. isolated from traditional Iranian cheeses in vitro and in yogurt drink (Doogh). cientific reports 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Belessi, C. I. A., Papanikolaou, S., Drosinos, E. H., & Skandamis, P. N. Survival and acid resistance of Listeria innocua in feta cheese and yogurt, in the presence or absence of fungi. Journal of food protection 2008, 71, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q., Wang, G., Zhang, Q., Tian, F., Xiao-Ming, L., Zhao, J., ... & Chen, W. Enhancement of ester formation in Camembert cheese by addition of ethanol. International Journal of Dairy Technology 2017, 70, 220–227. [Google Scholar] [CrossRef]
- Mileriene, J.; Serniene, L.; Kondrotiene, K.; Santarmaki, V.; Kourkoutas, Y.; Vasiliauskaite, A.; Lauciene, L.; Malakauskas, M. Indigenous Lactococcus lactis with Probiotic Properties: Evaluation of Wet, Thermally- and Freeze-Dried Raisins as Supports for Cell Immobilization, Viability and Aromatic Profile in Fresh Curd Cheese. Foods 2022, 11, 1311. [Google Scholar] [CrossRef]
- Pastorino, A.J.; Hansen, C.L.; McMahon, D.J. Effect of pH on the Chemical Composition and Structure-Function Relationships of Cheddar Cheese. J. Dairy Sci. 2003, 86, 2751–2760. [Google Scholar] [CrossRef]
- Hayaloglu, A.A. Comparisons of different single-strain starter cultures for their effects on ripening and grading of Beyaz cheese. International Journal of Food Science & Technology, 2007, 42, 930–938. [Google Scholar] [CrossRef]
- Curioni PM, G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. International Dairy Journal, 2002, 12, 959–984. [Google Scholar] [CrossRef]
- Bezerra, T.K.A.; de Oliveira Arcanjo, N.M.; de Araújo, A.R.R.; de Queiroz, A.L.M.; de Oliveira, M.E.G.; Gomes, A.M.P.; Madruga, M.S. Volatile profile in goat coalho cheese supplemented with probiotic lactic acid bacteria. LWT-Food Science and Technology, 2017, 76, 209–215. [Google Scholar] [CrossRef]
- Quigley L, O’Sullivan O, Beresford TP, Ros, RP, Fitzgerald GF, Cotter PD. High-throughput sequencing for detection of subpopulations of bacteria not previously associated with artisanal cheeses. [CrossRef]
- Mitropoulou, G., Prapa, I., Nikolaou, A., Tegopoulos, K., Tsirka, T., Chorianopoulos, N., ... & Kourkoutas, Y. Effect of Free or Immobilized Lactiplantibacillus plantarum T571 on Feta-Type Cheese Microbiome. Frontiers in Bioscience-Elite 2022, 14, 31. [Google Scholar] [CrossRef]
- Itoi S, Yuasa K, Washio S, Abe T, Ikuno E, Sugita H. Phenotypic variation in Lactococcus lactis subsp. lactis isolates derived from intestinal tracts of marine and freshwater fish. J Appl Microbiol. 2009, 107, 867–74. [Google Scholar] [CrossRef] [PubMed]
- Papadakis P, Konteles S, Batrinou A, Ouzounis S, Tsironi T, Halvatsiotis P, Tsakali E, Van Impe JFM, Vougiouklaki D, Strati IF, Houhoula D. Characterization of Bacterial Microbiota of P.D.O. Feta Cheese by 16S Metagenomic Analysis. Microorganisms 2021, 9, 2377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, Z., Zhang, L., Xin, L., Lin, K., Yi, H., & Han, X. Technological characterization of Lactobacillus in semihard artisanal goat cheeses from different Mediterranean areas for potential use as nonstarter lactic acid bacteria. Journal of Dairy Science 2018, 101, 2887–2896. [Google Scholar] [CrossRef]
- Kamilari, E., Anagnostopoulos, D. A., Papademas, P., Kamilaris, A., & Tsaltas, D. Characterizing Halloumi cheese's bacterial communities through metagenomic analysis. Lwt 2020, 126, 109298. [Google Scholar] [CrossRef]
- Kourkoutas, Y., Bosnea, L., Taboukos, S., Baras, C., Lambrou, D., & Kanellaki, M. Probiotic cheese production using Lactobacillus casei cells immobilized on fruit pieces. Journal of dairy science 2006, 89, 1439–1451. [Google Scholar] [CrossRef]
- Crippa, C., Pasquali, F., Lucchi, A., Gambi, L., & De Cesare, A. Investigation on the microbiological hazards in an artisanal soft cheese produced in northern Italy and its production environment in different seasonal periods. Italian Journal of Food Safety 2022, 11. [Google Scholar] [CrossRef]
- Spyrelli, E.D.; Stamatiou, A.; Tassou, C.C.; Nychas, G.J.E.; Doulgeraki, A.I. Microbiological and metagenomic analysis to assess the effect of container material on the microbiota of Feta cheese during ripening. Fermentation. 2020, 6, 12. [Google Scholar] [CrossRef]
| Final counts (log cfu/mL) | |||
|---|---|---|---|
| Time (h) |
P. acidilactici ORΕ 5 | L. plantarum ATCC 14971 | |
| Resistance to low pH | 0 | 8.4± 0.21 | 8.3± 0.13 |
| pH=2 | 2 | 6.4± 0.11 | 7.2± 0.07 |
| pH=3 | 2 | 6.9± 0.13 | 7.7± 0.15 |
| pH=4 | 2 | 8.1± 0.07 | 80± 0.12 |
| Pepsin | 0 | 7.6± 0.09 | 7.4± 0.17 |
| 3 | 6.5± 0.19 | 6.8± 0.21 | |
| Pancreatin | 0 | 8.0± 0.37 | 8.1± 0.14 |
| 4 | 7.4± 0.13 | 7.4± 0.21 | |
| Bile salts | 0 | 8.4± 0.22 | 8.5± 0.13 |
| 4 | 8.0± 0.19 | 8.1± 0.08 | |
| Agent | P. acidilactici ORΕ 5 | Cut-Offa |
|---|---|---|
| (MIC μg/mL) | ||
| Amoxycillin | 2.41 ± 0.95 | n.r.b |
| Amoxycillin + Clavulanic acid | 0.25 ± 0.19 | n.r.b |
| Ampicillin | 1.28 ± 0.58 | 4 |
| Clindamycin | 1.91 ± 0.27 | 1 |
| Erythromycin | 1.77 ± 0.19 | 1 |
| Gentamycin | 7.23 ± 2.08 | 16 |
| Metronidazole | 159.2 ± 27.8 | n.r.b |
| Tetracycline | 10.97 ± 1.48 | 8 |
| Tigecycline | 0.49 ± 0.08 | n.r.b |
| Vancomycin | >256 | n.r.b |
| Cheese1 | Days of storage | Water Activity (aw) |
pH | Acidity (g lactic acid/100g cheese) | % Moisture content | Overall sensory evaluation2 | |
|---|---|---|---|---|---|---|---|
| KC | 0 | 0.889 ± 0.01 | 4.28 ± 0.05 | 0.085 ± 0.02 | 72.71 ± 0.01 | 3.41 ± 0.70 | |
| 1 | 0.892 ± 0.02 | 4.27 ± 0.01 | 0.088 ± 0.04 | 73.32 ± 0.01 | |||
| 2 | 0.889 ± 0.01 | 4.33 ± 0.02 | 0.091 ± 0.05 | 74.20 ± 0.00 | |||
| 3 | 0.899 ± 0.05 | 4.29 ± 0.01 | 0.095 ± 0.03 | 74.71 ± 0.01 | |||
| 7 | 0.906 ± 0.01 | 4.31 ± 0.02 | 0.085 ± 0.01 | 75.51 ± 0.05 | |||
| 10 | 0.908 ± 0.02 | 4.45 ± 0.08 | 0.079 ± 0.02 | 77.82 ± 0.01 | |||
| 14 | 0.888 ± 0.03 | 4.72 ± 0.01 | 0.071 ± 0.01 | 78.90 ± 0.00 | |||
| KFP | 0 | 0.900 ± 0.01 | 4.08 ± 0.01 | 0.11± 0.02 | 73.94 ± 0.00 | 3.11 ± 0.74 | |
| 1 | 0.918 ± 0.02 | 4.11 ± 0.03 | 0.12± 0.07 | 74.11 ± 0.00 | |||
| 2 | 0.922 ± 0.03 | 4.14 ± 0.01 | 0.20± 0.05 | 74.21 ± 0.01 | |||
| 3 | 0.925 ± 0.05 | 4.12 ± 0.01 | 0.29± 0.03 | 74.42 ± 0.05 | |||
| 7 | 0.904 ± 0.07 | 4.35 ± 0.01 | 0.22 ± 0.02 | 74.81 ± 0.01 | |||
| 10 | 0.894 ± 0.06 | 4.38 ± 0.02 | 0.17 ± 0.01 | 75.50 ± 0.02 | |||
| 14 | 0.889 ± 0.01 | 4.45 ± 0.01 | 0.09 ± 0.01 | 78.35 ± 0.04 | |||
| KIP | 0 | 0.910 ± 0.01 | 4.15 ± 0.01 | 0.15 ± 0.05 | 63.91 ± 0.01 | 4.00 ± 0.54 | |
| 1 | 0.912 ± 0.02 | 4.18 ± 0.02 | 0.18 ± 0.05 | 65.72 ± 0.02 | |||
| 2 | 0.915 ± 0.02 | 4.23 ± 0.01 | 0.24 ± 0.01 | 71.51 ± 0.01 | |||
| 3 | 0.919 ± 0.03 | 4.22 ± 0.01 | 0.31 ± 0.02 | 72.11 ± 0.00 | |||
| 7 | 0.915 ± 0.05 | 4.66 ± 0.02 | 0.25 ± 0.03 | 72.91 ± 0.01 | |||
| 10 | 0.929 ± 0.01 | 4.68 ± 0.05 | 0.18 ± 0.01 | 73.51 ± 0.02 | |||
| 14 | 0.908 ± 0.05 | 4.70 ± 0.05 | 0.10 ± 0.01 | 74.13 ± 0.05 | |||
| Cheese1 | Days of storage | Total Aerobic Count (TAC) (logcfu/g) |
Psychrophilic bacteria (logcfu/g) |
Lactococci (logcfu/g) |
Lactobacilli (logcfu/g) |
Yeasts (logcfu/g) |
Staphylococci (logcfu/g) |
|---|---|---|---|---|---|---|---|
| KC | 0 | 6.50 ± 0.11 | 4.58 ± 0.01 | 4.80 ± 0.05 | 6.95 ± 0.01 | 6.39 ± 0.03 | 3.92 ± 0.03 |
| 1 | 6.44 ± 0.01 | 5.21 ± 0.03 | 5.00 ± 0.02 | 7.27 ± 0.01 | 6.70 ± 0.07 | 4.19 ± 0.06 | |
| 2 | 6.62 ± 0.02 | 5.38 ± 0.01 | 5,06 ± 0.03 | 7.42 ± 0.03 | 6.89 ± 0.03 | 4.33 ± 0.07 | |
| 3 | 6.74 ± 0.02 | 5.60 ± 0.08 | 5.43 ± 0.10 | 7.52 ± 0.02 | 7.33 ± 0.02 | 4.65 ± 0.01 | |
| 7 | 6.88 ± 0.01 | 5.85 ± 0.14 | 4.46 ± 0.09 | 7.08 ± 0.02 | 7.60 ± 0.08 | 5.35 ± 0.04 | |
| 10 | 6.74 ± 0.05 | 5.62 ± 0.01 | 4.25 ± 0.01 | 6.99 ± 0.01 | 7.99 ± 0.02 | 5.15 ± 0.01 | |
| 14 | 6.53 ± 0.01 | 5.52 ± 0.03 | 4.13 ± 0.07 | 6.78 ± 0.06 | 8.27 ± 0.01 | 5.07 ± 0.01 | |
| KFP | 0 | 8.30 ± 0.13 | 6.92 ± 0.04 | 4.81 ± 0.01 | 8.81 ± 0.01 | 6.38 ± 0.01 | 3.80 ± 0.01 |
| 1 | 8.63 ± 0.07 | 7.42 ± 0.02 | 5.10 ± 0.01 | 8.88 ± 0.07 | 6.50 ± 0.04 | 3.94 ± 0.05 | |
| 2 | 8.85 ± 0.03 | 7.38 ± 0.01 | 5.33 ± 0.02 | 8.91 ± 0.09 | 6.73 ± 0.02 | 4.09 ± 0.05 | |
| 3 | 8.96 ± 0.02 | 7.60 ± 0.08 | 5.76 ± 0.06 | 8.99 ± 0.09 | 7.03 ± 0.01 | 4.30 ± 0.08 | |
| 7 | 8.78 ± 0.05 | 7.85 ± 0.14 | 4.65 ± 0.01 | 8.57 ± 0.02 | 7.21 ± 0.05 | 4.70 ± 0.06 | |
| 10 | 8.63 ± 0.01 | 7.02 ± 0.01 | 4.42 ± 0.01 | 8.22 ± 0.01 | 7.52 ± 0.02 | 4.65 ± 0.02 | |
| 14 | 8.55 ± 0.01 | 6.52 ± 0.03 | 4.37 ± 0.04 | 8.09 ± 0.03 | 7.82 ± 0.11 | 4.57 ± 0.03 | |
| KIP | 0 | 8.88 ± 0.03 | 6.92 ± 0.04 | 4.82 ± 0.01 | 8.89 ± 0.02 | 6.35 ± 0.01 | 3.63 ± 0.02 |
| 1 | 8.95 ± 0.01 | 7.42 ± 0.02 | 5.13 ± 0.01 | 8.99 ± 0.05 | 6.43 ± 0.07 | 3.89 ± 0.00 | |
| 2 | 9.01 ± 0.04 | 7.38 ± 0.01 | 5.44 ± 0.01 | 9.09 ± 0.01 | 6.52 ± 0.03 | 4.02 ± 0.01 | |
| 3 | 9.07 ± 0.03 | 7.60 ± 0.08 | 5.97 ± 0.01 | 9.12 ± 0.02 | 6.97 ± 0.02 | 4.22 ± 0.05 | |
| 7 | 9.12 ± 0.02 | 7.85 ± 0.14 | 4.92 ± 0.08 | 8.96 ± 0.04 | 7.12 ± 0.01 | 4.54 ± 0.00 | |
| 10 | 8.99 ± 0.01 | 7.12 ± 0.02 | 4.72 ± 0.01 | 8.88 ± 0.01 | 7.45 ± 0.03 | 4.42 ± 0.01 | |
| 14 | 8.95 ± 0.02 | 6.52 ± 0.03 | 4.53 ± 0.03 | 8.85 ± 0.03 | 7.67 ± 0.02 | 4.38 ± 0.03 |
| Cheese1 | Days of storage | Total Aerobic Count (TAC) (logcfu/g) |
Psychrophilic bacteria (logcfu/g) |
Lactococci (logcfu/g) |
Lactobacilli (logcfu/g) |
Yeasts (logcfu/g) |
Staphylococci (logcfu/g) |
L. monocytogenes (logcfu/g) |
|
|---|---|---|---|---|---|---|---|---|---|
| KC | 0 | 7.52 ± 0.04 | 4.69 ± 0.01 | 4.77 ± 0.03 | 6.89 ± 0.00 | 6.42 ± 0.03 | 3.99 ± 0.01 | 5.11 ± 0.01 | |
| 1 | 7.64 ± 0.06 | 5.42 ± 0.02 | 4.56 ± 0.02 | 6.93 ± 0.02 | 6.65 ± 0.01 | 4.11 ± 0.04 | 5.12 ± 0.01 | ||
| 2 | 8.23 ± 0.01 | 5.53 ± 0.01 | 4.44 ± 0.01 | 7.21 ± 0.05 | 6.95 ± 0.01 | 4.42 ± 0.01 | 5.15 ± 0.02 | ||
| 3 | 8.52 ± 0.03 | 6.28 ± 0.06 | 4.33 ± 0.04 | 7.36 ± 0.02 | 7.48 ± 0.02 | 4.65 ± 0.01 | 6.34 ± 0.06 | ||
| 7 | 8.96 ± 0.02 | 6.01 ± 0.04 | 4.11 ± 0.02 | 6.74 ± 0.02 | 7.68 ± 0.03 | 5.25 ± 0.04 | 6.28 ± 0.02 | ||
| 10 | 8.81 ± 0.01 | 5.89 ± 0.01 | 3.98 ± 0.01 | 6.65 ± 0.01 | 8.07 ± 0.05 | 4.79 ± 0.01 | 6.25 ± 0.05 | ||
| 14 | 8.77 ± 0.04 | 5.7 ± 0.06 | 3.66 ± 0.05 | 6.56 ± 0.02 | 8.25 ± 0.06 | 4.72 ± 0.03 | 6.22 ± 0.04 | ||
| KFP | 0 | 8.13 ± 0.06 | 6.69 ± 0.01 | 4.89 ± 0.05 | 8.32 ± 0.01 | 6.43 ± 0.01 | 3.78 ± 0.01 | 5.09 ± 0.06 | |
| 1 | 8.54 ± 0.01 | 7.21 ± 0.03 | 5.10 ± 0.01 | 8.38 ± 0.04 | 6.54 ± 0.07 | 3.89 ± 0.08 | 5.12 ± 0.01 | ||
| 2 | 8.57 ± 0.01 | 7.14 ± 0.02 | 5.33 ± 0.02 | 8.42 ± 0.07 | 6.85 ± 0.01 | 4.02 ± 0.02 | 5.15 ± 0.02 | ||
| 3 | 8.62 ± 0.04 | 7.54 ± 0.01 | 5.46 ± 0.06 | 8.45 ± 0.02 | 7.11 ± 0.04 | 4.28 ± 0.01 | 5.27 ± 0.02 | ||
| 7 | 8.56 ± 0.01 | 7.43 ± 0.04 | 4.65 ± 0.01 | 7.97 ± 0.03 | 7.32 ± 0.01 | 4.22 ± 0.01 | 5.43 ± 0.01 | ||
| 10 | 8.28 ± 0.02 | 6.89 ± 0.01 | 4.45 ± 0.01 | 7.88 ± 0.04 | 7.68 ± 0.05 | 4.15 ± 0.02 | 5.69 ± 0.05 | ||
| 14 | 8.18 ± 0.01 | 6.11 ± 0.07 | 4.37 ± 0.04 | 7.78 ± 0.04 | 7.96 ± 0.01 | 4.02 ± 0.01 | 5.49 ± 0.02 | ||
| KIP | 0 | 8.71 ± 0.02 | 6.77 ± 0.01 | 4.89 ± 0.01 | 8.42 ± 0.02 | 6.37 ± 0.01 | 3.70 ± 0.01 | 5.08 ± 0.04 | |
| 1 | 8.65 ± 0.05 | 7.24 ± 0.03 | 5.09 ± 0.01 | 8.49 ± 0.01 | 6.43 ± 0.07 | 3.83 ± 0.04 | 5.11 ± 0.04 | ||
| 2 | 8.67 ± 0.03 | 7.17 ± 0.02 | 5.31 ± 0.03 | 8.51 ± 0.01 | 6.59 ± 0.03 | 3.97 ± 0.03 | 5.14 ± 0.01 | ||
| 3 | 8.97 ± 0.01 | 7.57 ± 0.00 | 5.79 ± 0.05 | 8.54 ± 0.01 | 7.05 ± 0.02 | 4.27 ± 0.01 | 5.23 ± 0.01 | ||
| 7 | 8.76 ± 0.01 | 7.46 ± 0.02 | 4.69 ± 0.05 | 8.28 ± 0.01 | 7.20 ± 0.01 | 4.15 ± 0.01 | 5.55 ± 0.01 | ||
| 10 | 8.61 ± 0.02 | 6.94 ± 0.01 | 4.52 ± 0.01 | 8.11 ± 0.02 | 7.35 ± 0.01 | 4.12 ± 0.02 | 5.62 ± 0.04 | ||
| 14 | 8.54 ± 0.04 | 6.14 ± 0.07 | 4.39 ± 0.04 | 8.06 ± 0.04 | 7.72 ± 0.02 | 4.10 ± 0.01 | 5.45 ± 0.02 | ||
| Compound | Identification Method | KC | KFP | KIP | |||
|---|---|---|---|---|---|---|---|
| d0 | d3 | d0 | d3 | d0 | d3 | ||
| Esters | |||||||
| Ethyl acetate | KI | N. D. | 0.1 | 0.6 | 13.8 | 0.5 | 6.4 |
| Ethyl propanoate | KI | N. D. | N. D. | N. D. | 0.1 | 0.0 | 0.1 |
| Ethyl butyrate | KI | N. D. | N. D. | 0.1 | 0.2 | 0.2 | 0.1 |
| 3- methylbutyl acetate | KI | N. D. | N. D. | N. D. | 0.2 | N. D. | 0.2 |
| Ethyl hexanoate | KI | N. D. | 0.2 | 0.8 | 1.8 | 0.6 | 0.6 |
| 2- phenylethyl acetate | KI | N. D. | 0.2 | 1.6 | 8.0 | 1.0 | 3.0 |
| Ethyl decanoate | KI | N. D. | N. D. | 0.1 | 1.8 | 0.2 | 0.2 |
| Organic acids | |||||||
| Hexanoic acid | KI | 0.1 | N. D. | N. D. | 0.8 | 0.2 | 0.4 |
| Sorbic acid | KI | 0.5 | 0.5 | 1.0 | 1.5 | 0.4 | 0.2 |
| Octanoic acid | KI | 0.1 | 0.4 | 0.1 | 3.5 | 0.1 | 0.1 |
| Benzoic acid | KI | 0.3 | 0.6 | 0.1 | 1.0 | 0.1 | 0.1 |
| Decanoic acid | KI | N. D. | 0.1 | 0.1 | 1.9 | 0.3 | 0.1 |
| Alcohols | KI | ||||||
| 2- methyl-1 propanol | KI | N. D. | N. D. | 0.1 | 0.2 | 0.3 | 0.5 |
| 3- methyl- 1 butanol | KI | 0.2 | 1.0 | 1.4 | 3.1 | 2.4 | 9.4 |
| 2- methyl- 1 butanol | KI | N. D. | N. D. | 0.3 | 0.4 | 0.4 | 1.4 |
| 1-hexanol | KI | N. D. | N. D. | N. D. | N. D. | 0.1 | 0.1 |
| 2- methyl- phenol | KI | N. D. | N. D. | N. D. | N. D. | 0.1 | 0.1 |
| 2- phenylethanol | KI | 0.2 | 1.3 | 0.9 | 1.3 | 1.1 | 3.1 |
| Carbonyl compounds | |||||||
| 2- methyl- propanal | KI | N. D, | N. D, | N. D. | N. D. | N.D. | 0.1 |
| Butanal | KI | N. D, | N. D. | N. D. | N. D. | N. D. | N. D. |
| 3- methyl- butanal | KI | N. D, | N. D. | N. D. | 0.4 | 0.1 | 0.2 |
| 2- methyl- butanal | KI | N. D, | N. D. | N. D. | N. D. | 0.1 | 0.1 |
| 2- pentanone | KI | N. D, | 0.1 | N. D. | N. D. | N. D. | N. D. |
| 3- hydroxy- butanone | KI | N. D, | N. D. | N. D. | N. D. | 0.3 | 0.4 |
| Hexanal | KI | N. D, | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 |
| 2- heptanone | KI | N. D, | 0.1 | N. D. | N. D. | 0.1 | N. D. |
| Heptanal | KI | N. D, | N. D. | 0.1 | N. D. | N. D. | N. D. |
| Benzeneacetaldehyde | KI | N. D, | N. D. | N. D. | 0.1 | 0.1 | 0.1 |
| 2- nonanone | KI | N. D, | N. D. | N. D. | 0.1 | N. D. | N. D. |
| Miscellaneous Compounds | |||||||
| 2- methyl- 1,3- pentadiene | KI | N. D. | N. D. | N. D. | 0.1 | 0.1 | 0.2 |
| Octane | KI | N. D. | N. D. | 0.1 | 0.1 | 0.1 | 0.1 |
| 2,4- dimethyl- 1- heptane | KI | N. D. | N. D. | N. D. | N. D. | 0.3 | 0.4 |
| Styrene | KI | N. D. | N. D. | N. D. | 0.1 | N. D. | 0.1 |
| 2,6- dimethyl- pyrazine | KI | N. D. | N. D. | N. D. | N. D. | 0.2 | 0.1 |
| a- pinene | KI | N. D. | 0.1 | N. D. | N. D. | 0.2 | 0.1 |
| Decane | KI | N. D. | N. D. | 0.1 | 1.5 | 0.7 | 0.9 |
| Limonene | KI | N. D. | 0.1 | N. D. | N. D. | 0.3 | 0.2 |
| 3-ethyl-2,5-dimethyl-pyrazine | KI | N. D. | N. D. | N. D. | N. D. | 0.2 | 0.4 |
| Dodecane | KI | N. D. | N. D. | N. D. | 8.0 | 1.2 | 1.6 |
| 1,3-bis(1,1-dimethylethyl)-benzene | KI | N. D. | N. D. | 0.1 | 1.2 | 0.3 | 0.4 |
| Tetradecane | KI | N. D. | N. D. | N. D. | 1.0 | 0.1 | 0.1 |
| 16S rRNA OTUs | KC | KFP | KIP |
|---|---|---|---|
| Shannon’s Index | 0.71 ± 0.05b,c | 0.50 ± 0.03a,c | 0.89 ± 0.01 a,b |
| Simpson’s Index | 0.23 ± 0.02b, c | 0.14 ± 0.05a | 0.17 ± 0.02a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





