Submitted:
07 June 2023
Posted:
12 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Objects
2.3. Methods
3. Results
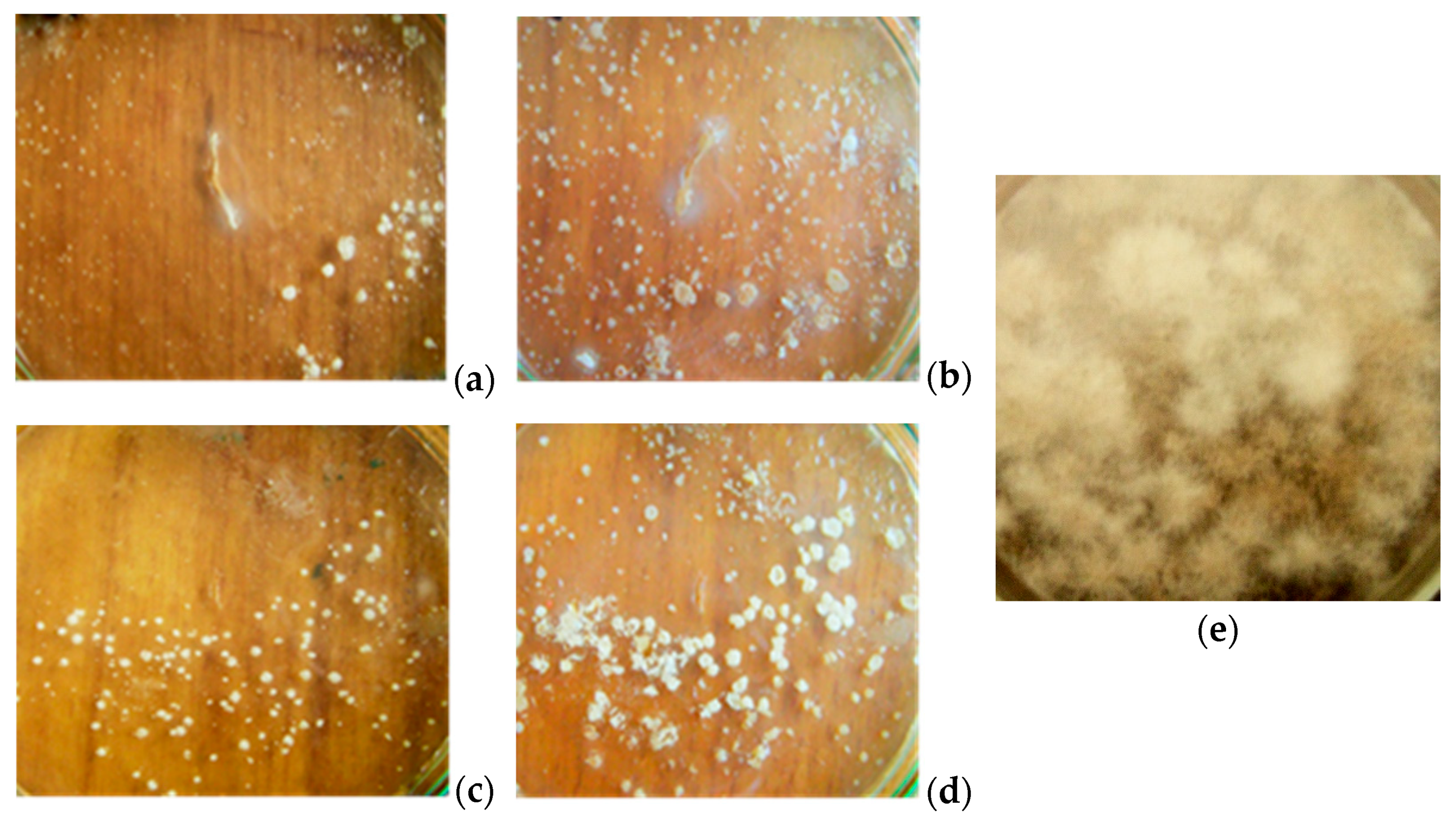
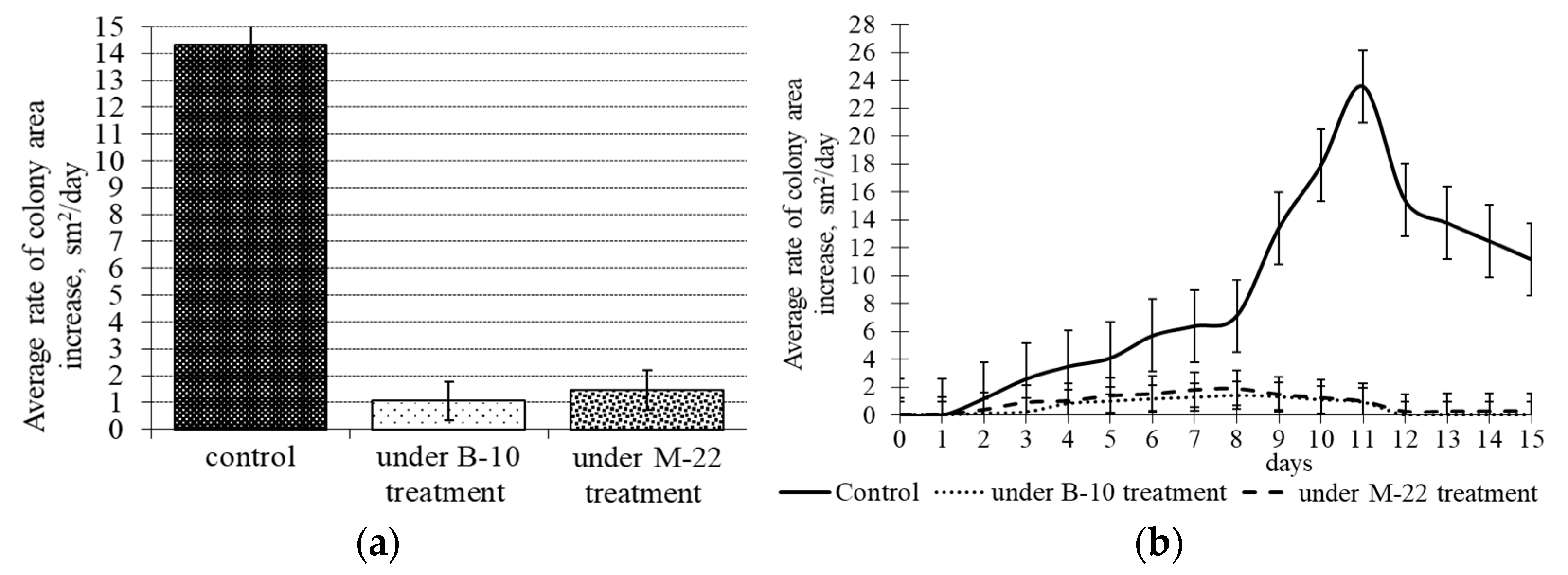
3.1. Antifungal activity of B. subtilis strains against 1-day-old M. perniciosa mycelium
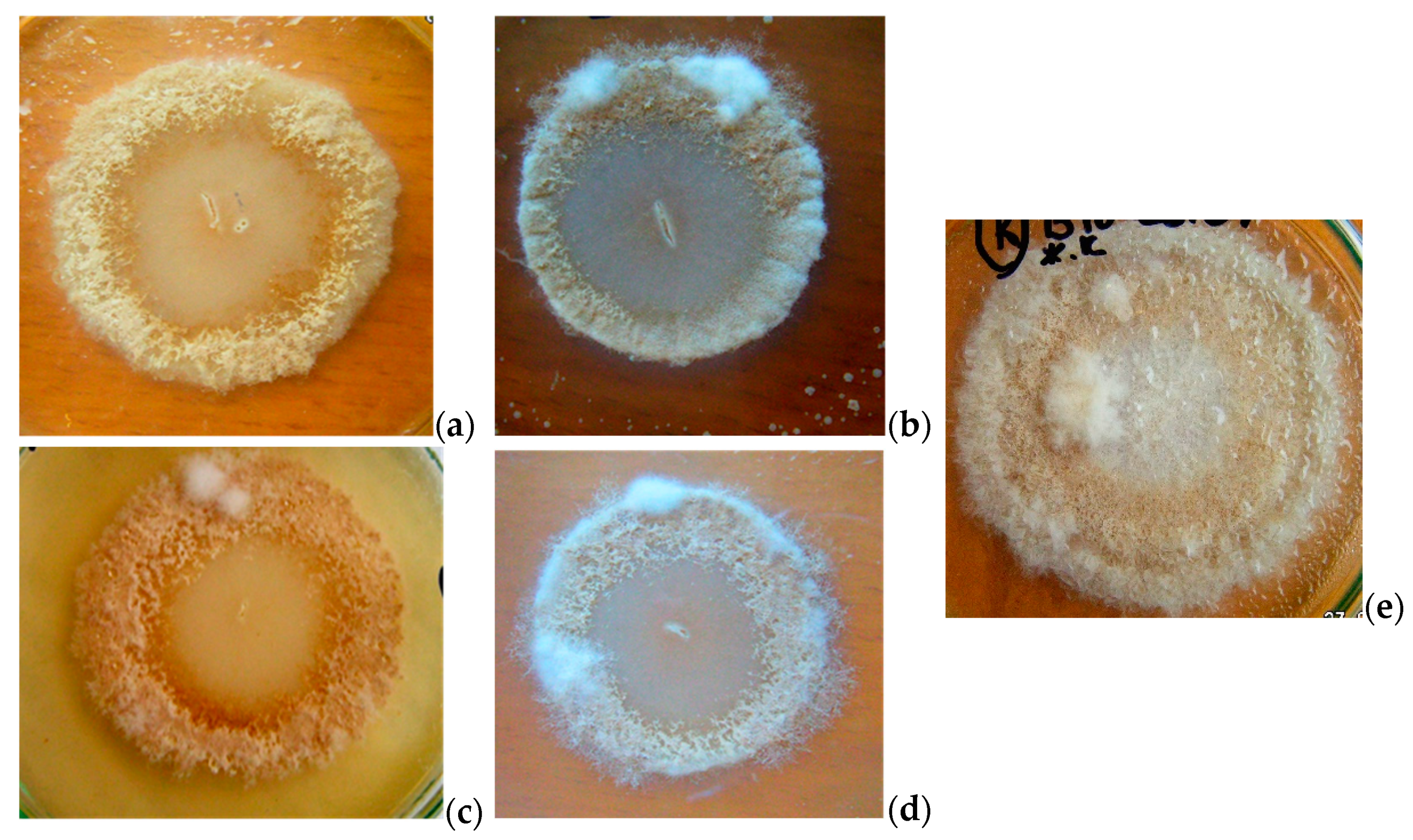
3.2. Antifungal activity of B. subtilis strains against 7-day-old M. perniciosa mycelium
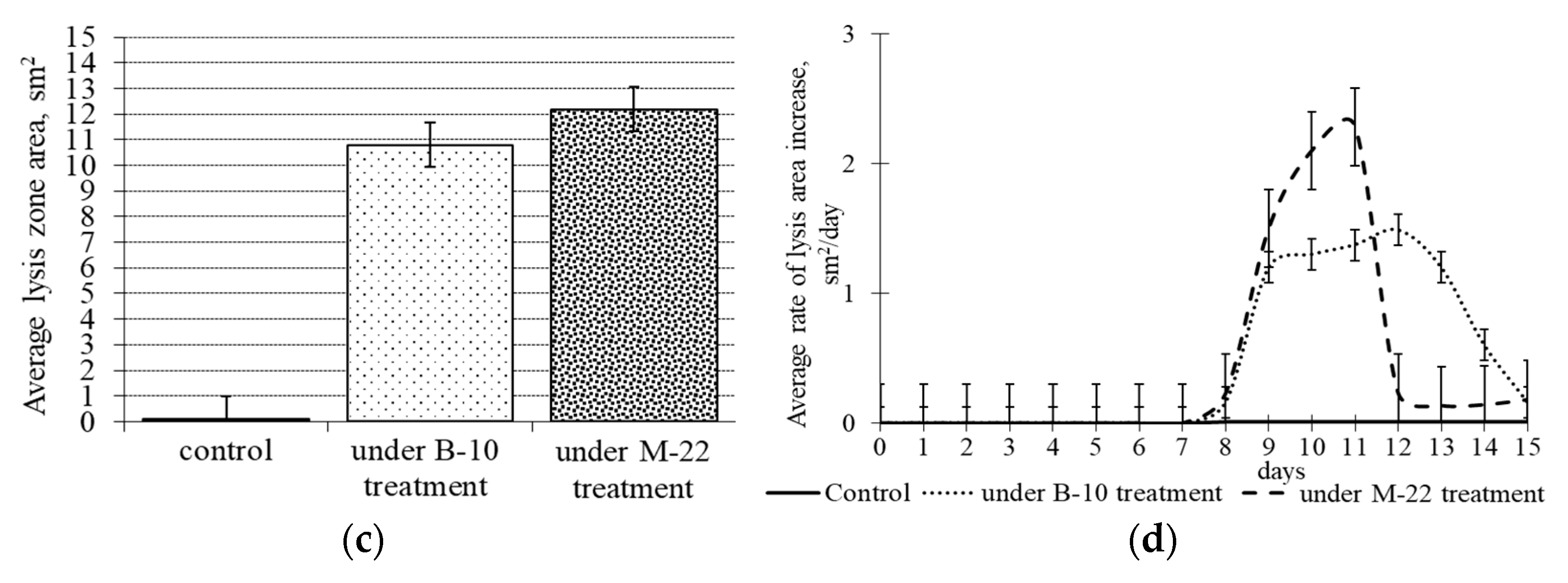
3.3. Antifungal activity of B. subtilis strains secondary metabolites against M. perniciosa
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarka, A.; Archana, T.S. Production of Button Mushroom (Agaricus bisporus), Pharmacological Applications and Pest Management. In Recent Advances in Mushroom Cultivation Technology and Its Application; Kulshreshtha, S., Prince Onyedinma, U., Siddhant, Eds.; Bright Sky Publications: New Delhi, India, 2022; Volume 2, pp. 111–134. [Google Scholar] [CrossRef]
- Lu, H.; Lou, H.; Hu, J.; Liu, Z.; Chen, Q. Macrofungi: A review of cultivation strategies, bioactivity, and application of mushrooms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2333–2356. [Google Scholar] [CrossRef] [PubMed]
- Ab Rhaman, S.M.S.; Naher, L.; Siddiquee, S. Mushroom Quality Related with Various Substrates’ Bioaccumulation and Translocationof Heavy Metals. J. Fungi 2022, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Amin, Z.; Wani, F.F.; Gulzar, H.; Dar, W.A.; Sheikh, P.A. Diseases of White Button Mushroom (Agaricus bisporus)–A Potential Threat to Mushroom Industry. Int. J. Curr. Microbiol. App. Sci, 2021, 10, 2076–2085. [Google Scholar] [CrossRef]
- Aminuzzaman, F.M.; Shahi, S.; Thapa, S.; Das, K. Mushroom Diseases and Their Management: A Review. In Recent Advances in Mushroom Cultivation Technology and Its Application; Kulshreshtha, S., Prince Onyedinma, U., Siddhant, Eds.; Bright Sky Publications: New Delhi, India, 2022; Volume 2, pp. 1–27. [Google Scholar] [CrossRef]
- Munshi, N.A.; Dar, G.H.; Ghani, M.Y.; Kauser, S. , Mughal, N. Button Mushroom Cultivation; Communication and Publication Centre SKUAST-K.1: New Delhi, India, 2010; pp. 1–33. [Google Scholar]
- Gupta, S.; Summuna, B.; Singh, R.; Gupta, M.; Gupta, A. Mushroom Diseases: A potential threat to mushroom cultivation. In Transformation of Indian Agriculture through Innovative technologies; Astral International Pvt. Ltd.: 2018; 5: 57–70.
- Archana, T.S.; Vaja, S. Archana, T.S.; Vaja, S. A Review on Diseases of Button Mushroom (Agaricus spp.): A Potential Hazard to Mushroom Cultivation. In Recent Advances in Mushroom Cultivation Technology and Its Application; Kulshreshtha, S., Prince Onyedinma, U., Siddhant, Eds.; Bright Sky Publications: New Delhi, India, 2022; Volume 2, pp. 135–149. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, X.; Wen, D.; Huang, H.; Chen, Y.; Mukhtar, I.; Yue, L.; Wang, L.; Wen, Z. Intraspecific mitochondrial DNA comparison of mycopathogen Mycogone perniciosa provides insight into mitochondrial transfer RNA introns. Phytopathology 2021, 111, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Shi, N.; Ruan, H.; Chen, F. Three Mycogone species, including a new species, cause wet bubble disease of Agaricus bisporus in China. Plant Dis. 2021, 105, 105. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Kumar, S.; Sharma, V.P. Diseases and Competitor Moulds of Mushrooms and their Management; National Research Centre for Mushroom, Chambaghat, Solan-173 213 (HP): New Delhi, India, 2007; pp. 1–86. [Google Scholar]
- Shi, N.; Ruan, H.; Jie, Y.; Chen, F.; Du, Y. Sensitivity and efficacy of fungicides against wet bubble disease of Agaricus bisporus caused by Mycogone perniciosa. Eur J Plant Pathol 2020, 157, 873–885. [Google Scholar] [CrossRef]
- Altaf, S.; Jan, S.K.; Ahanger, S.A.; Basu, U.; Rather, R.A.; Wani, O.A.; Rasool, F.; Mushtaq, M.; Yassin, M.T.; Mostafa, A.A.-F.; Elgorban, A.M.; El-Haroun, E.; El-Sabrout, A.M.; Casini, R.; Elansary, H.O. Management of Green Mold Disease in White Button Mushroom (Agaricus bisporus) and Its Yield Improvement. J. Fungi 2022, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Titova, J.A. Mycobiota associated with the various stages of Agaricus bisporus morphogenesis. Mycol. and Phytopathol. 2000, 34, 32–39. (in Russian). [Google Scholar]
- Titova, J.A. Mycosinusia on the different Agaricus bisporus sorts. Mycol. and Phytopathol. 2000, 34, 25–32. (in Russian). [Google Scholar]
- Titova, J.A. The influence of temperature on the interactions between cultivated mushroom Agaricus bisporus (Lange) Imbach and its pathogens. In Proceedings of the International Conference “Mycology and cryptogamic botany in Russia: traditions and modern state”, devoted to 100th Anniversary of investigations on mycology and cryptogamic botany in V.L. Komarov Botanical Institute RAS, St.-Petersburg, Russia, 24–28 April 2000; pp. 260–262. (in Russian). [Google Scholar]
- Titova, J.A. Biological bases for the white button mushroom fungal diseases control. PhD Thesis, All-Union Research Institute of Plant Protection, St-Petersburg, Russia, 2000. Available online: https://www.dissercat.com/content/biologicheskie-osnovy-borby-s-gribnymi-boleznyami-shampinonov (accessed on 31 May 2023). (in Russian).
- Miller, F.C.; Spair, M. Very large-scale commercial trial of a biological control for mushroom blotch disease. In Science and Cultivation of Edible Fungi; Elliott, T.J., Balkema, A.A., Eds.; Publisher: Rotterdam, NL, 1995; pp. 635–642. [Google Scholar]
- Altaf, S.; Jan, S.K.; Basu, U.; Ahanger, S.A.; Dave, A.; Kakraliya, S.S.; Baazeem, A.; Mishra, A.K.; Kumar, A.; Shah, I.A.; Mushtaq, M. Sustainable Management of Green Mold Disease of White Button Mushroom Using Botanicals and Biocontrol Agents under Temperate Conditions. Horticulturae 2022, 8, 768. [Google Scholar] [CrossRef]
- Pavlyusin, V.A.; Novikova, I.I.; Boikova, I.V. Microbiological control in phytosanitary optimization technologies for agroecosystems: research and practice (review). Agric. Biol. 2020. [Google Scholar] [CrossRef]
- State catalog of pesticides and agrochemicals permitted for use in Russian Federation territory; Publisher: Moscow, Russia, 2023; Volume 3, pp. 905–939. (in Russian).
- Titova, J.A.; Lobanova, N.V.; Novikova, I.I. Wet bubble disease microbiocontrol of white button mushroom Agaricus bisporus (Lange) Imbach. In Proceedings of the Scientific-practical Conference “Mushroom growing and tied biotechnologies. Innovations to invest”, Moscow, Russia; 2005; pp. 10–11. (in Russian). [Google Scholar]
- Titova, J.A. Spent Mushroom Substrates Valorization via Brand New Multirecycled Polyfunctional Biologics Producing on Them. Waste Biomass Valor 2021, 13, 1089–1100. [Google Scholar] [CrossRef]
- Titova, J.A. Multirecycled biotechnology for liquid-phase polyfunctional biopesticides obtaining. In Proceedings of the 5th International Mycological Forum “Modern Mycology in Russia”, Moscow: National Academy of Mycology, Russia, 2022, 9, 410–412. (in Russian). (in Russian)
- Experimental Mycology Methods: A Handbook; Publisher: Nauk. dumka, Kiev, Ukraine, 1982; (in Russian).
- Peacock, J.L.; Peacock, P.J. Oxford Handbook of Medical Statistics; Oxford University Press: GB, 2011. [Google Scholar] [CrossRef]
- Petrie, A.; Sabin, C. Medical statistics at a glance; Publisher: Wiley Blackwell, USA, 2019. [Google Scholar]
- Ivanova, T.V.; Boyko, O.A.; Melnychuk, M.D. Monitoring of mushrooms Agaricus bisporus (J. Lge) Imbach: diagnostics and prevention of diseases. Living and biocosmic systems 2014, 8. URL: http://www.jbks.ru/archive/issue-8/article-2. (in Russian). [CrossRef]
- Stanojević, O.; Berić, T.; Potočnik, I.; Rekanović, E.; Stanković, S.; Milijašević-Marčić, S. Biological control of green mould and dry bubble diseases of cultivated mushroom (Agaricus bisporus L.) by Bacillus spp. Crop Prot. 2019, 126. [Google Scholar] [CrossRef]
- Pandin, C.; Le Coq, D.; Deschamps, J.; Védie, R.; Rousseau, T.; Aymerich, S.; Briandet, R. Complete genome sequence of Bacillus velezensis QST713: A biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J Biotechnol. 2018, 278, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Pandin, C.; Védie, R.; Rousseau, T.; Le Coq, D.; Aymerich, S.; Briandet, R. Dynamics of compost microbiota during the cultivation of Agaricus bisporus in the presence of Bacillus velezensis QST713 as biocontrol agent against Trichoderma aggressivum. Biol. Contr. 2018, 127, 39–54. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Drakes, G.D.; Talent, C.J.W. The control of wet bubble disease of mushrooms caused by Mycogone perniciosa. Ann. Appl. Biol. 2008, 79, 35–41. [Google Scholar] [CrossRef]
- Szumigaj-Tarnowska, J.; Uliński, Z.; Ślusarski, C. Efficacy assessment of selected fungicides in the control of wet bubble (Mycogone perniciosa) in white button mushroom (Agaricus bisporus). Prog. Plant Prot. 2015, 55, 107–113. [Google Scholar] [CrossRef]
- Shi, N.; Ruan, H.; Jie, Y.; Chen, F.; Du, Y. Sensitivity and efficacy of fungicides against wet bubble disease of Agaricus bisporus caused by Mycogone perniciosa. Eur J Plant Pathol 2020, 157, 873–885. [Google Scholar] [CrossRef]
- Sharma, V.P.; Kumar, S.; Kamal, S. Management of wet bubble disease (Mycogone perniciosa) in Agaricus bisporus. Mushroom Res. 2018, 26. Retrieved from https://epubs.icar.org.in/index.php/MR/article/view/76214. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




