Submitted:
10 June 2023
Posted:
12 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentation
2.3. Green Synthesis of Gold Nanozymes
2.4. Dye Degradation Protocol
3. Results and Discussion
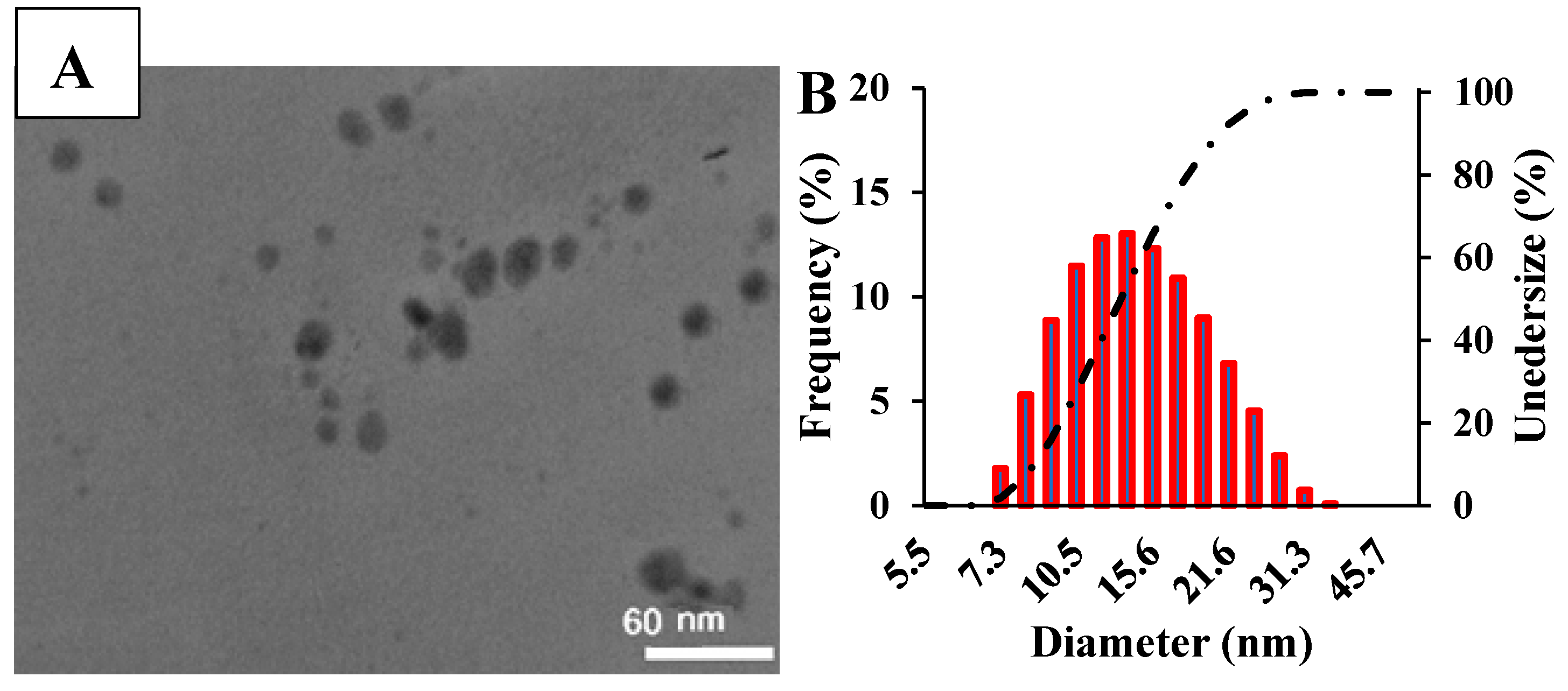
3.1. Characterization of as-Prepared Nanozymes
3.2. Evaluation of Nanozymatic Properties
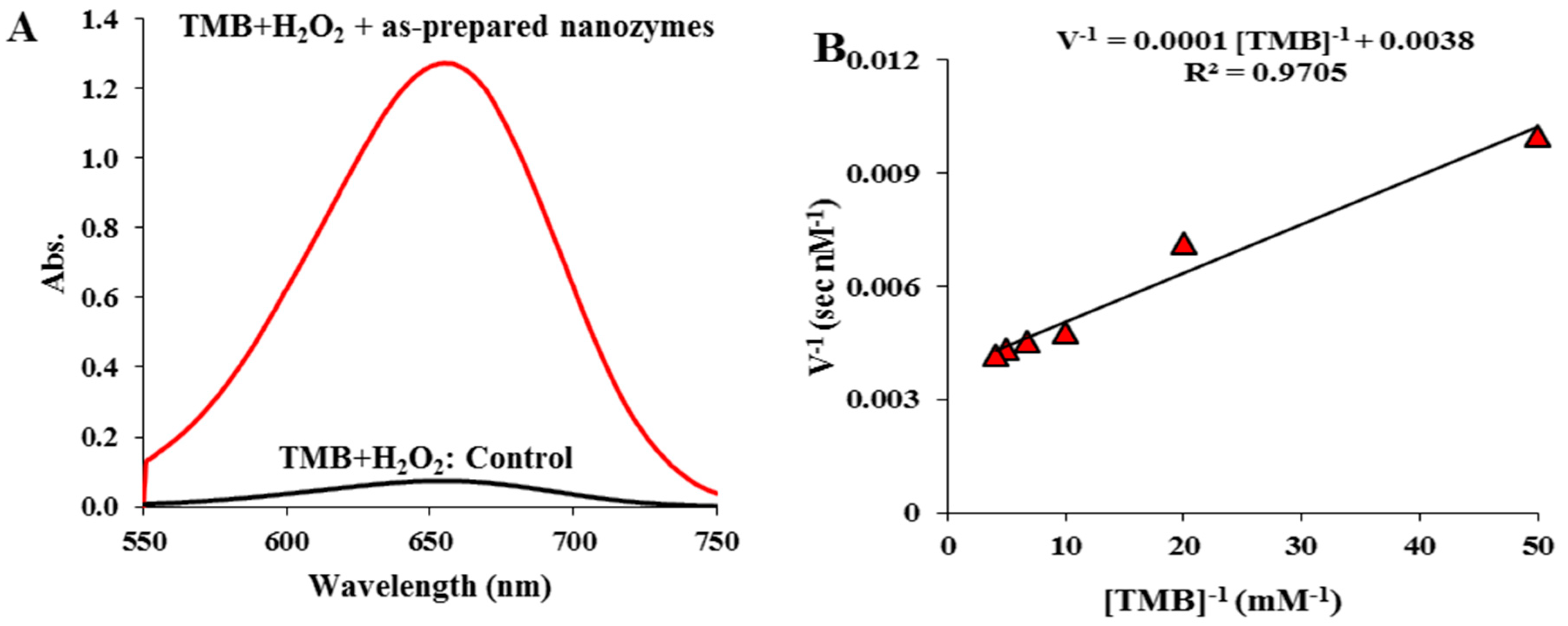
3.2.1. Peroxidase-like Activity the As-Prepared Nanozymes
3.2.2. Kinetics Behavior of the as-Prepared Nanozymes
3.3. Nanozyme-Mediated Organic Dye Degradation
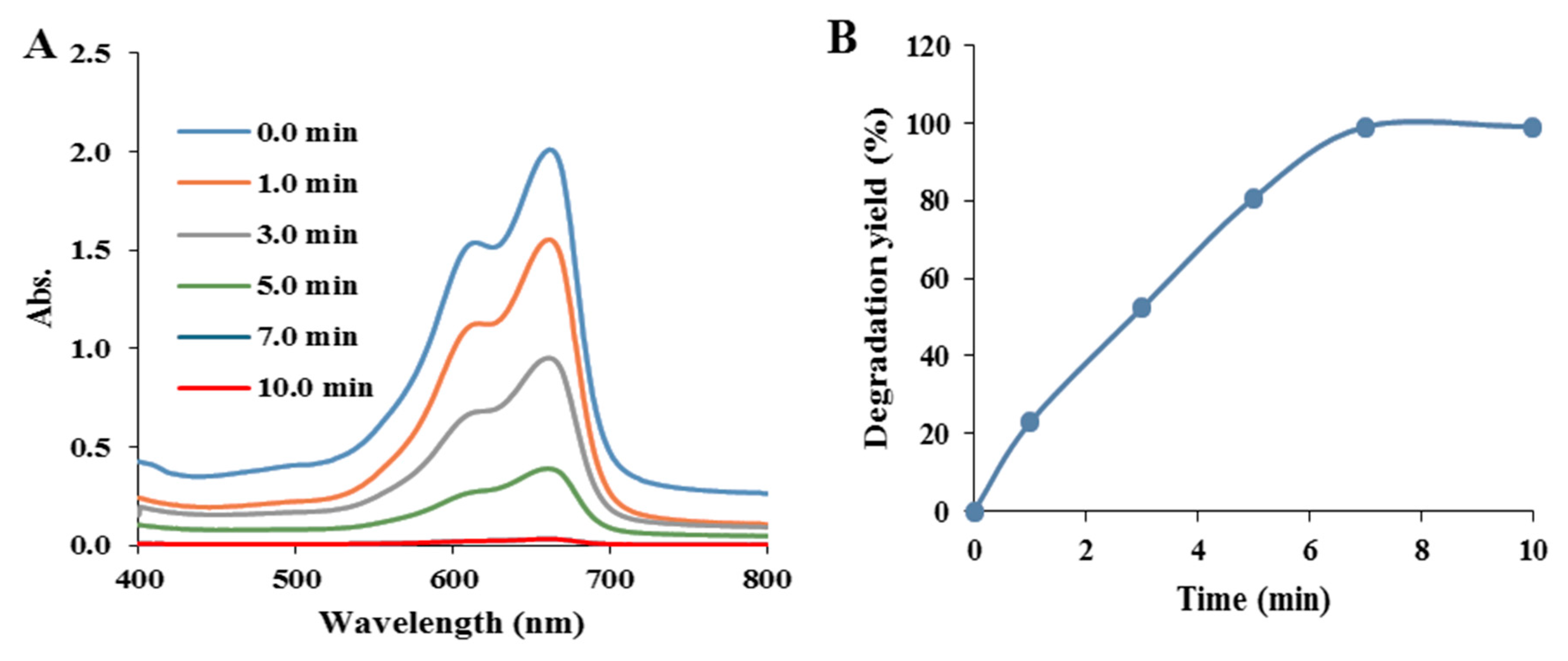
3.3.2. Effect of Degradation Time
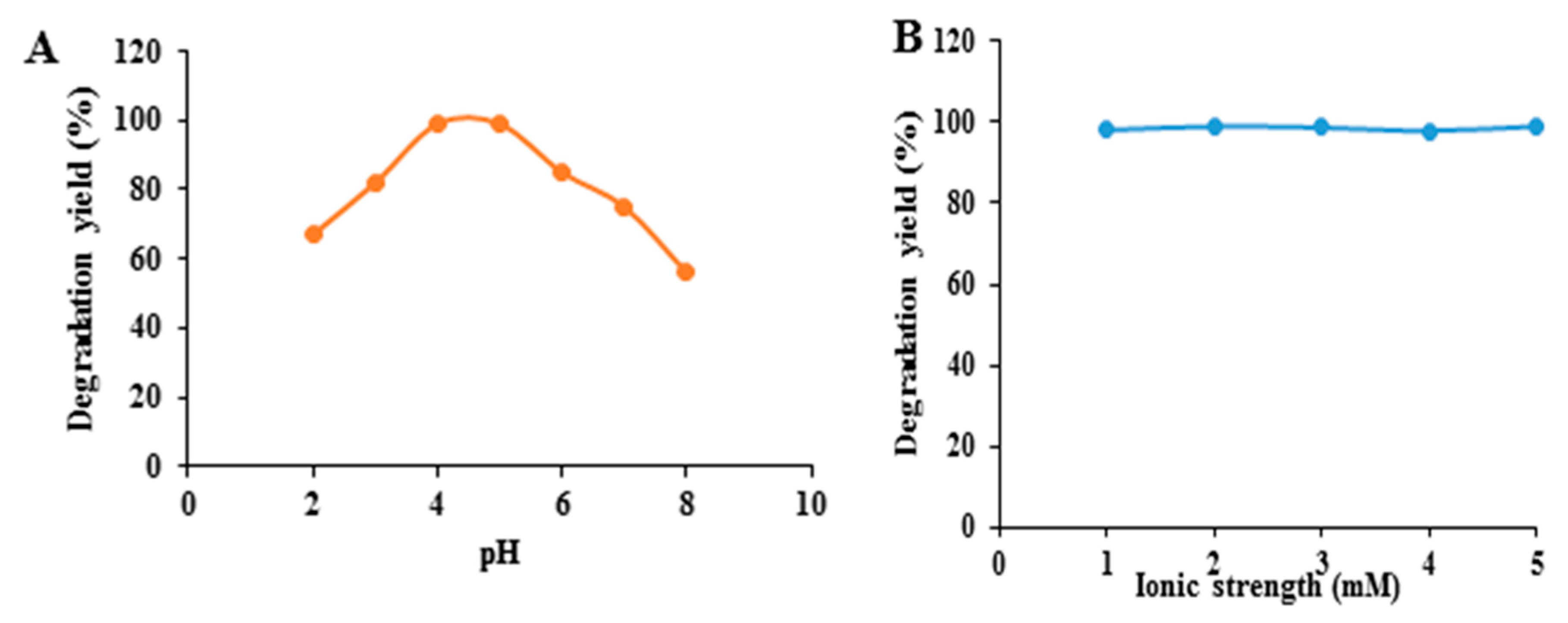
3.3.1. Effect of pH and Ionic Strength
3.3.3. Effect of Hydrogen Peroxide and Nanozyme Volume
3.5. Practical Application
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicology letters. 2002 May 10;131(1-2):5-17.
- Ahmadi-Leilakouhi B, Hormozi Jangi SR, Khorshidi A. Introducing a novel photo-induced nanozymatic method for high throughput reusable biodegradation of organic dyes. Chemical Papers. 2023 Feb;77(2):1033-46.
- Nitin KA, Kishor KS, Chandrakant LP. Integrated chemical and biological processes for the degradation of synthetic dyes. InCurrent Developments in Bioengineering and Biotechnology 2023 Jan 1 (pp. 567-600). Elsevier.
- Chopra L. Photo-Degradation of dyes and drugs using aloe vera synthesized zinc oxide nanoparticles–A review. Materials Today: Proceedings. 2023 Jan 1;72:1613-7.
- Yuan Y, Yin W, Huang Y, Feng A, Chen T, Qiao L, Cheng H, Liu W, Li Z, Ding C, Chen F. Intermittent electric field stimulated reduction-oxidation coupled process for enhanced azo dye biodegradation. Chemical Engineering Journal. 2023 Jan 1;451:138732.
- Hormozi Jangi SR, Davoudli HK, Delshad Y, Hormozi Jangi MR, Hormozi Jangi AR. A novel and reusable multinanozyme system for sensitive and selective quantification of hydrogen peroxide and highly efficient degradation of organic dye. Surfaces and Interfaces. 2020 Dec 1;21:100771.
- Nanjani DS, Keharia H, Paul D. Deciphering the Shift in Soil Functional and Microbial Diversity Upon Perturbation with Reactive Blue 28 Dye. Available at SSRN 4360527.
- Hormozi Jangi AR, Hormozi Jangi MR, Hormozi Jangi SR. Detection mechanism and classification of design principles of peroxidase mimic based colorimetric sensors: A brief overview. Chinese Journal of Chemical Engineering. 2020 Jun 1;28(6):1492-503.
- Jangi SR, Akhond M. Ultrasensitive label-free enantioselective quantification of d-/l-leucine enantiomers with a novel detection mechanism using an ultra-small high-quantum yield N-doped CDs prepared by a novel highly fast solvent-free method. Sensors and Actuators B: Chemical. 2021 Jul 15;339:129901.
- Jangi SR, Akhond M. Synthesis and characterization of a novel metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) and its application for constructing a reusable nanozyme-based sensor for selective and sensitive glutathione quantification. Microchemical Journal. 2020 Nov 1;158:105328.
- Hormozi Jangi SR. Synthesis and characterization of magnesium-based metal-organic frameworks and investigating the effect of coordination solvent on their biocompatibility. Chemical Research and Nanomaterials. 2023;1(4):1-9.
- Hormozi Jangi SR. Low-temperature destructive hydrodechlorination of long-chain chlorinated paraffins to diesel and gasoline range hydrocarbons over a novel low-cost reusable ZSM-5@ Al-MCM nanocatalyst: a new approach toward reuse instead of common mineralization. Chemical Papers. 2023 Apr 21:1-5.
- Hormozi Jangi SR, Akhond M. High throughput green reduction of tris (p-nitrophenyl) amine at ambient temperature over homogenous AgNPs as H-transfer catalyst. Journal of Chemical Sciences. 2020 Dec;132:1-8.
- Hormozi Jangi, SR, Dehghani Z. Spectrophotometric quantification of hydrogen peroxide utilizing silver nanozyme. Chemical Research and Nanomaterials. https://crn.shiraz.iau.ir/article_701960.html?lang=en. 2023.
- Hormozi Jangi SR. Effect of daylight and air oxygen on nanozymatic activity of unmodified silver nanoparticles: Shelf-stability. Qeios 2023. [CrossRef]
- Hormozi Jangi SR. A comparative study on kinetics performances of BSA-gold nanozymes for nanozyme-mediated oxidation of 3,3’,5,5’-tetramethylbenzidine and 3,3’-diaminobenzidine. Preprints.org 2023, 2023060387. [CrossRef]
- Hormozi Jangi SR. Evaluating the Effect of Shelf-Storage, Daylight, and Air Oxygen on the Peroxidase-like Activity of Unmodified Silver Nanoparticles. Preprints.org 2023, 2023052106. [CrossRef]
- Hormozi Jangi SR. Determining kinetics parameters of bovine serum albumin-protected gold nanozymes toward different substrates. Qeios. 2023. [CrossRef]
- Chen H, Ma X, Zhang X, Hu G, Deng Y, Li S, Chen Z, He N, Wu Y, Jiang Z. Novel aerosol detection platform for SARS-CoV-2: Based on specific magnetic nanoparticles adsorption sampling and digital droplet PCR detection. Chinese Chemical Letters. 2023 Jan 1;34(1):107701.
- Zeng X, Ruan Y, Chen Q, Yan S, Huang W. Biocatalytic cascade in tumor microenvironment with a Fe2O3/Au hybrid nanozyme for synergistic treatment of triple negative breast cancer. Chemical Engineering Journal. 2023 Jan 15;452:138422.
- Chen X, Wang Y, Feng M, Deng D, Xie X, Deng C, Khattak KN, Yang X. Dual-active-site Fe/Cu single-atom nanozymes with multifunctional specific peroxidase-like properties for S2− detection and dye degradation. Chinese Chemical Letters. 2023 Jun 1;34(6):107969.
- Hormozi Jangi SR, Akhond M, Absalan G. A field-applicable colorimetric assay for notorious explosive triacetone triperoxide through nanozyme-catalyzed irreversible oxidation of 3, 3′-diaminobenzidine. Microchimica Acta. 2020 Aug;187:1-0.
- Akhond M, Hormozi Jangi SR, Barzegar S, Absalan G. Introducing a nanozyme-based sensor for selective and sensitive detection of mercury (II) using its inhibiting effect on production of an indamine polymer through a stable n-electron irreversible system. Chemical Papers. 2020 Apr;74:1321-30.
- Hormozi Jangi SR, Akhond M, Absalan G. A novel selective and sensitive multinanozyme colorimetric method for glutathione detection by using an indamine polymer. Analytica Chimica Acta. 2020 Aug 29;1127:1-8.
- Hormozi Jangi SR, Akhond M. Introducing a covalent thiol-based protected immobilized acetylcholinesterase with enhanced enzymatic performances for biosynthesis of esters. Process Biochemistry. 2022 Sep 1;120:138-55.
- Hormozi Jangi SR, Akhond M, Dehghani Z. High throughput covalent immobilization process for improvement of shelf-life, operational cycles, relative activity in organic media and enzymatic kinetics of urease and its application for urea removal from water samples. Process Biochemistry. 2020 Mar 1;90:102-12.
- Hormozi Jangi SR, Akhond M. High throughput urease immobilization onto a new metal-organic framework called nanosized electroactive quasi-coral-340 (NEQC-340) for water treatment and safe blood cleaning. Process Biochemistry. 2021 Jun 1;105:79-90.

| Real sample | Spiked MB conc. (mg L-1) | Degradation yield (%) | RSD (%, n=3) |
| Tap water | 10.0 20.0 |
99.0 98.4 |
2.7 3.1 |
| Pool water | 10.0 20.0 |
97.5 96.4 |
3.5 3.8 |
| Mineral water | 10.0 20.0 |
99.0 98.5 |
3.1 3.5 |
| River water | 10.0 20.0 |
96.1 94.3 |
4.3 4.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





