Submitted:
12 June 2023
Posted:
12 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
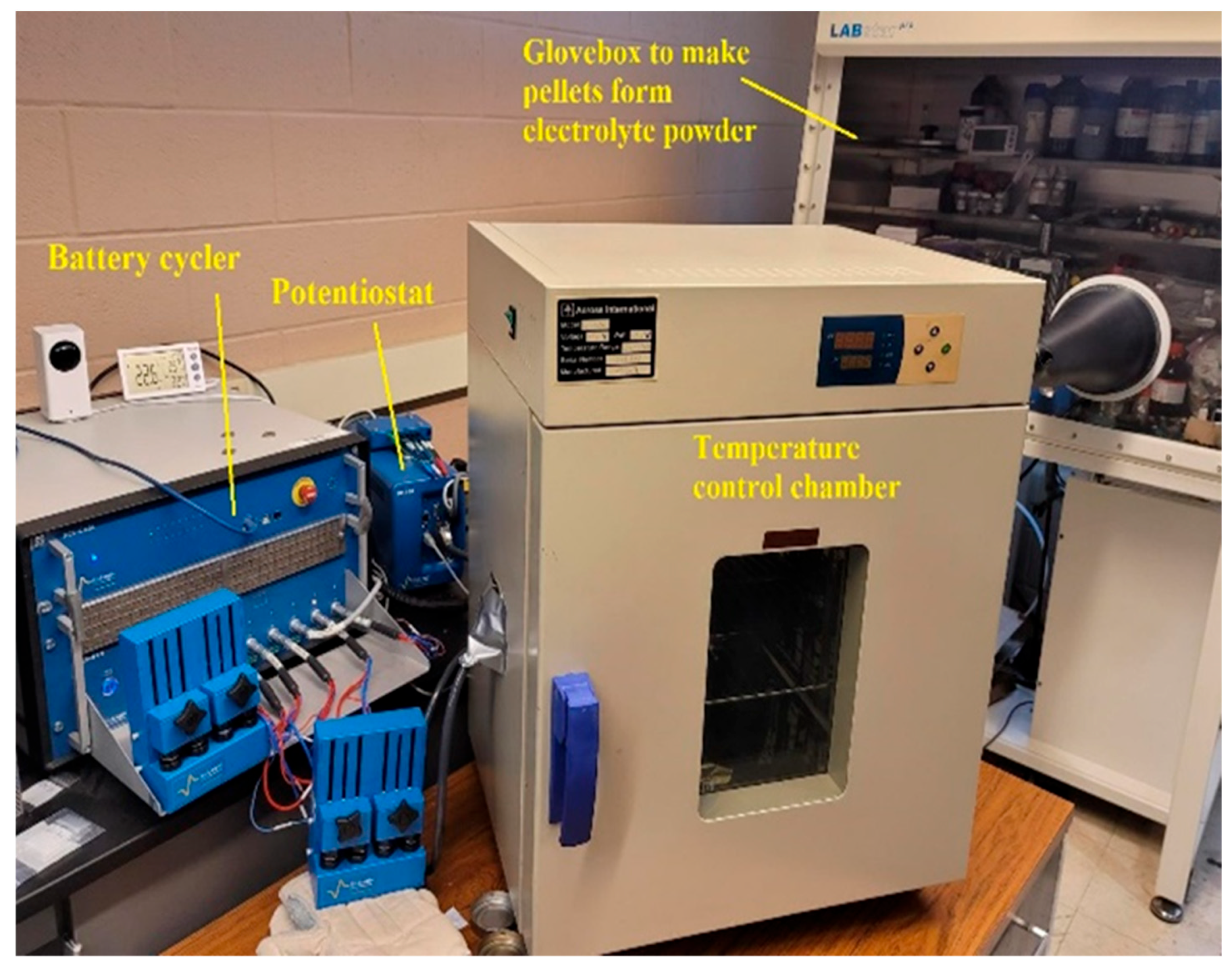
2. Materials and Methods
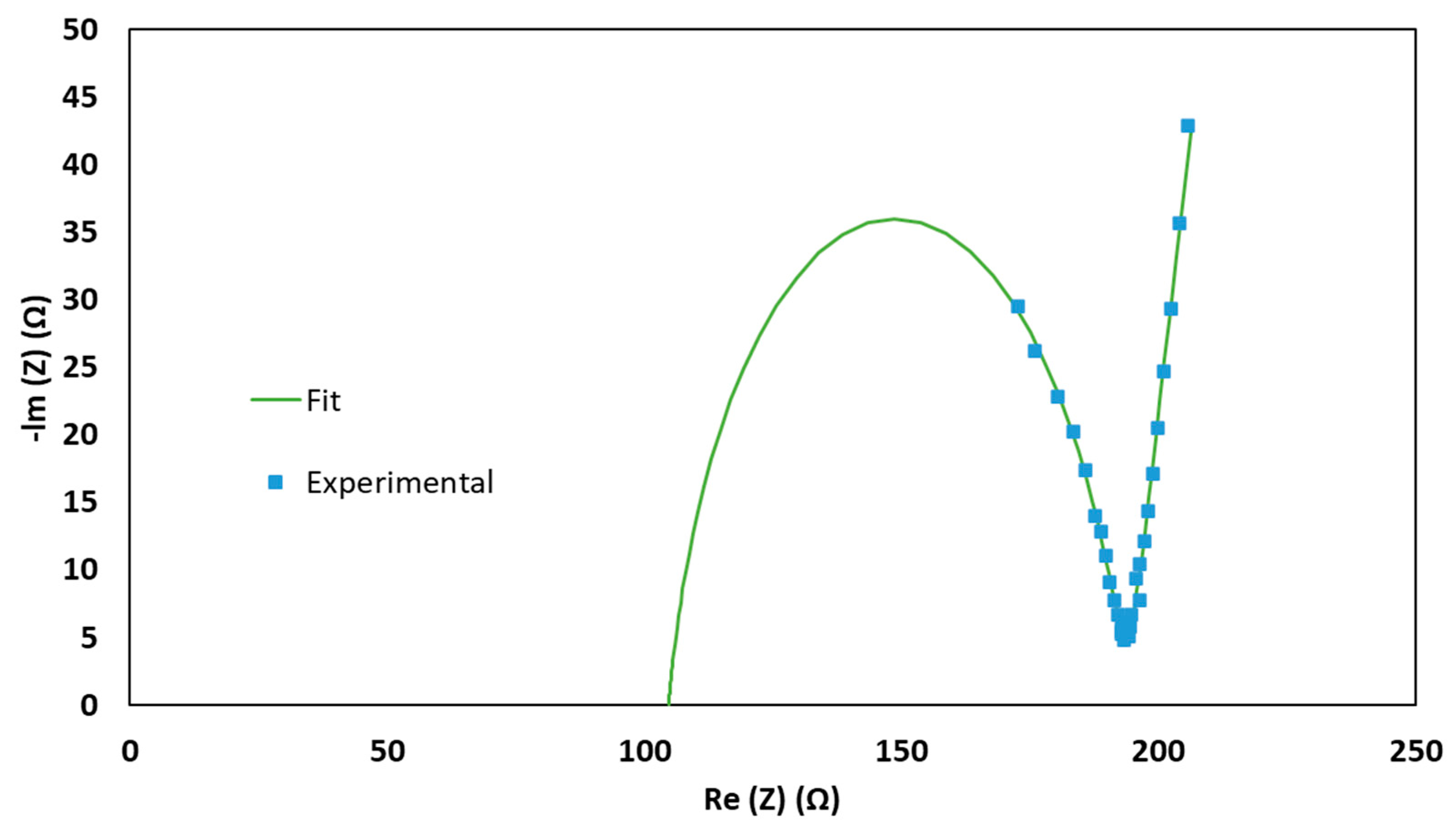
3. Results and Discussion
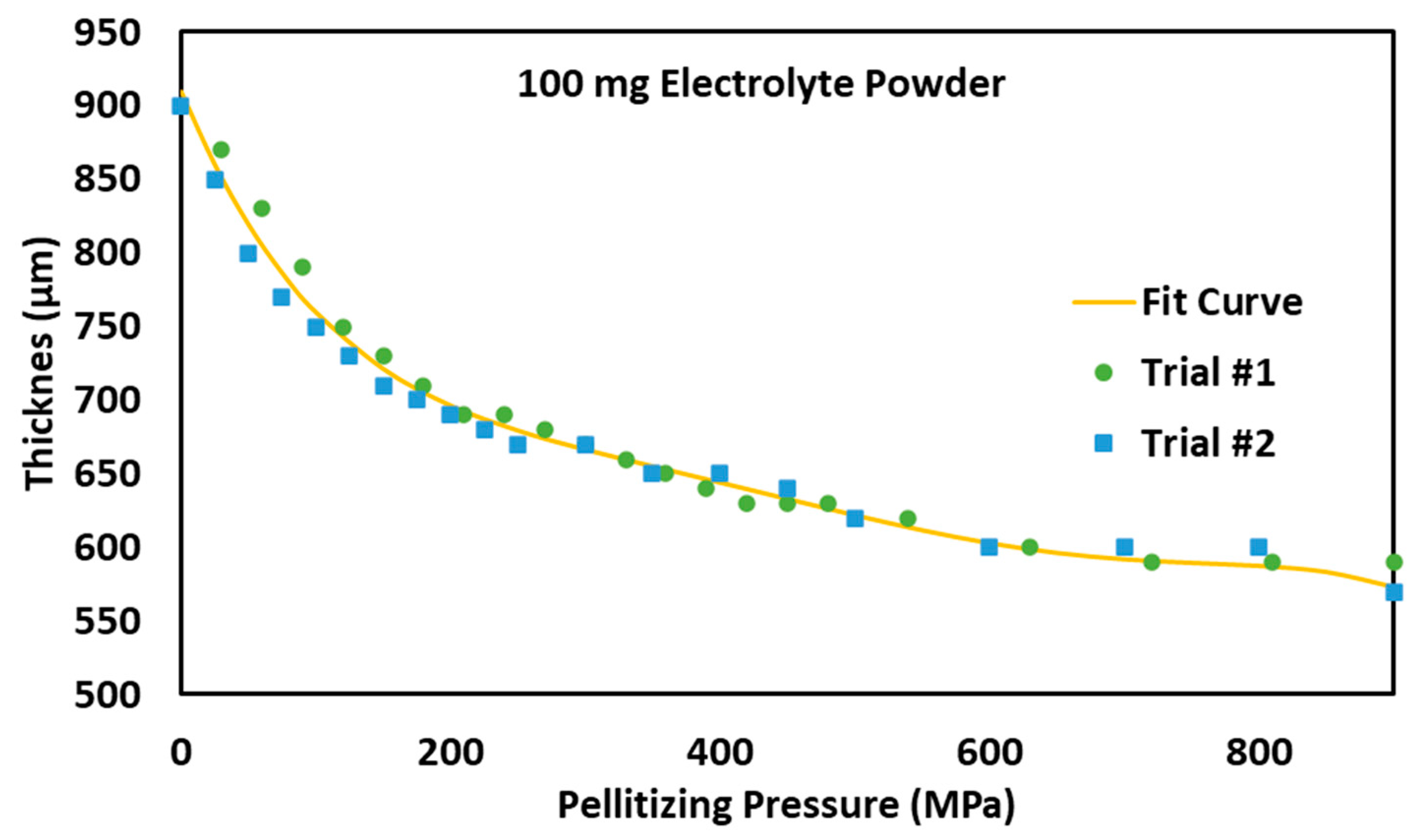
3.1. Effect of Pelletizing Pressure on Electrolyte Pellet Thickness
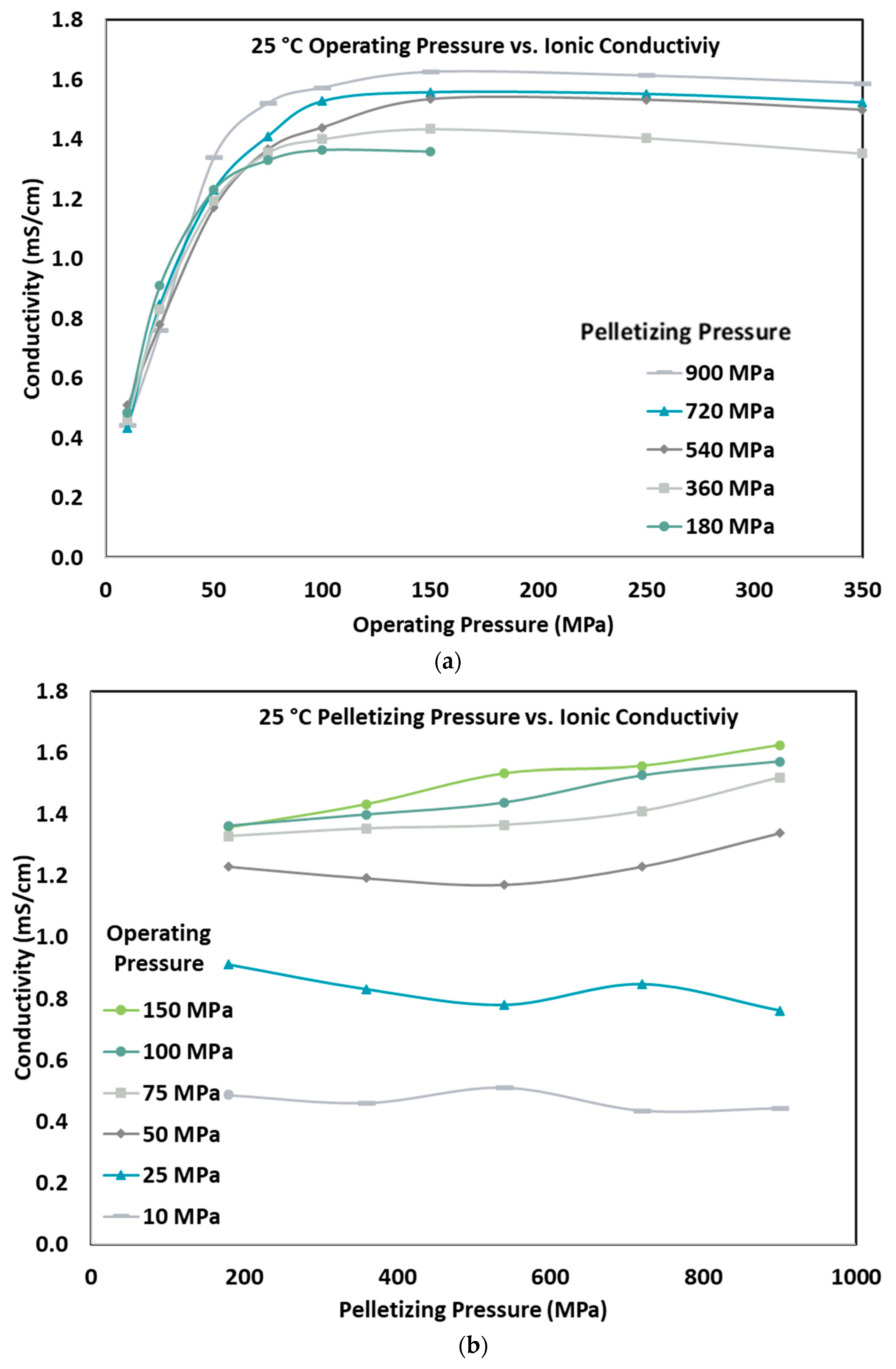
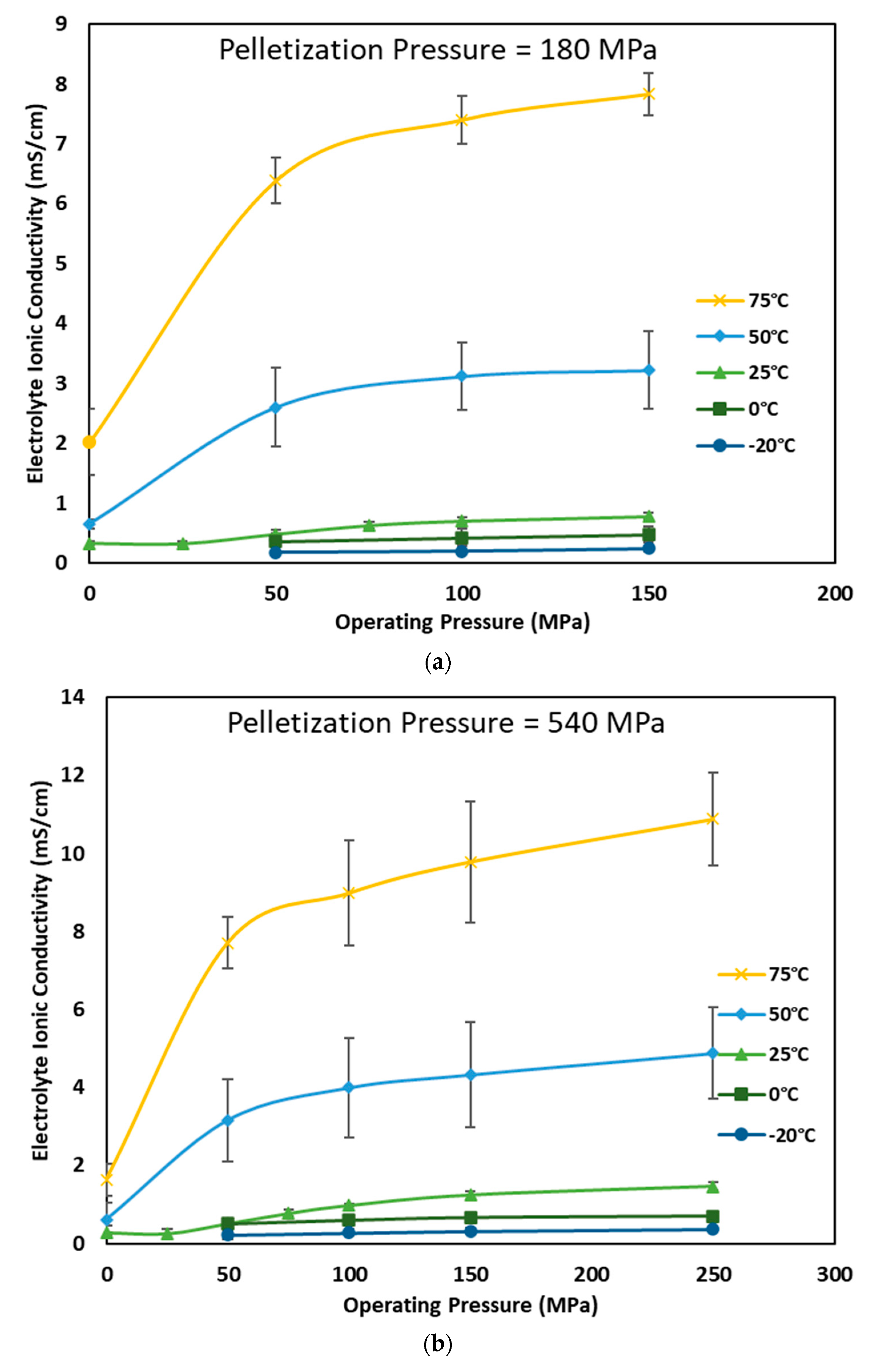
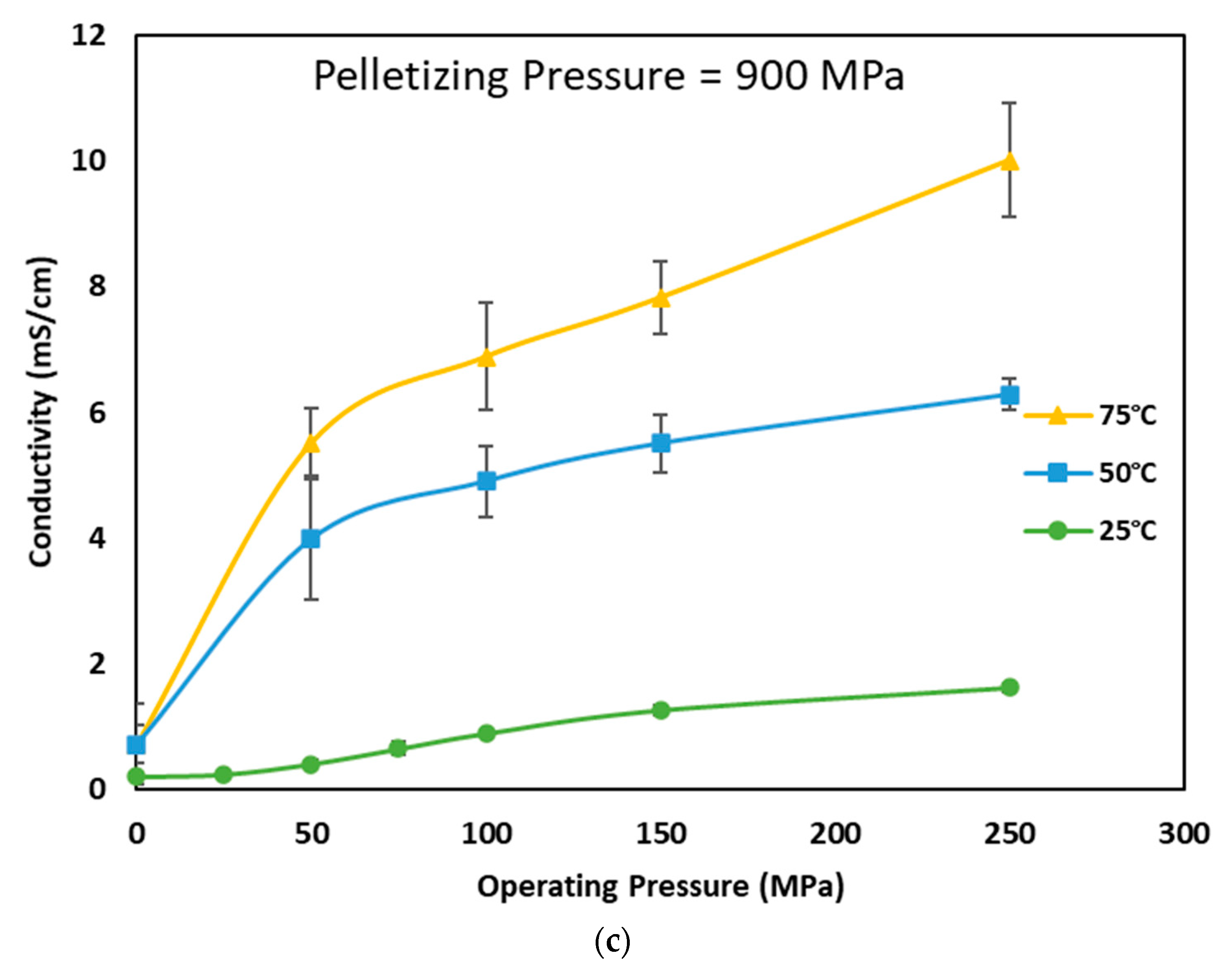
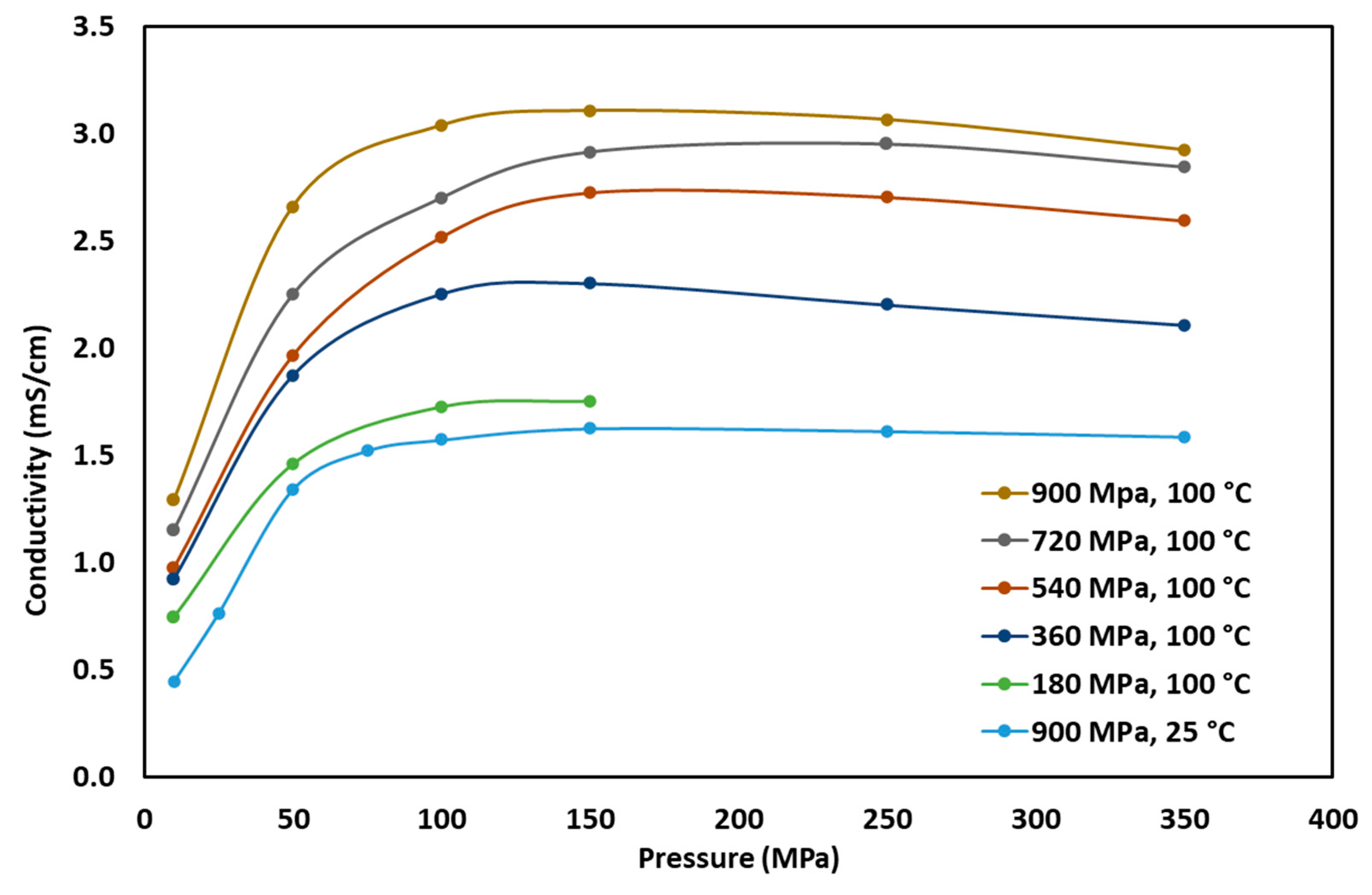
3.2. Effect of Pelletizing and Operating Pressure on Ionic Conductivity
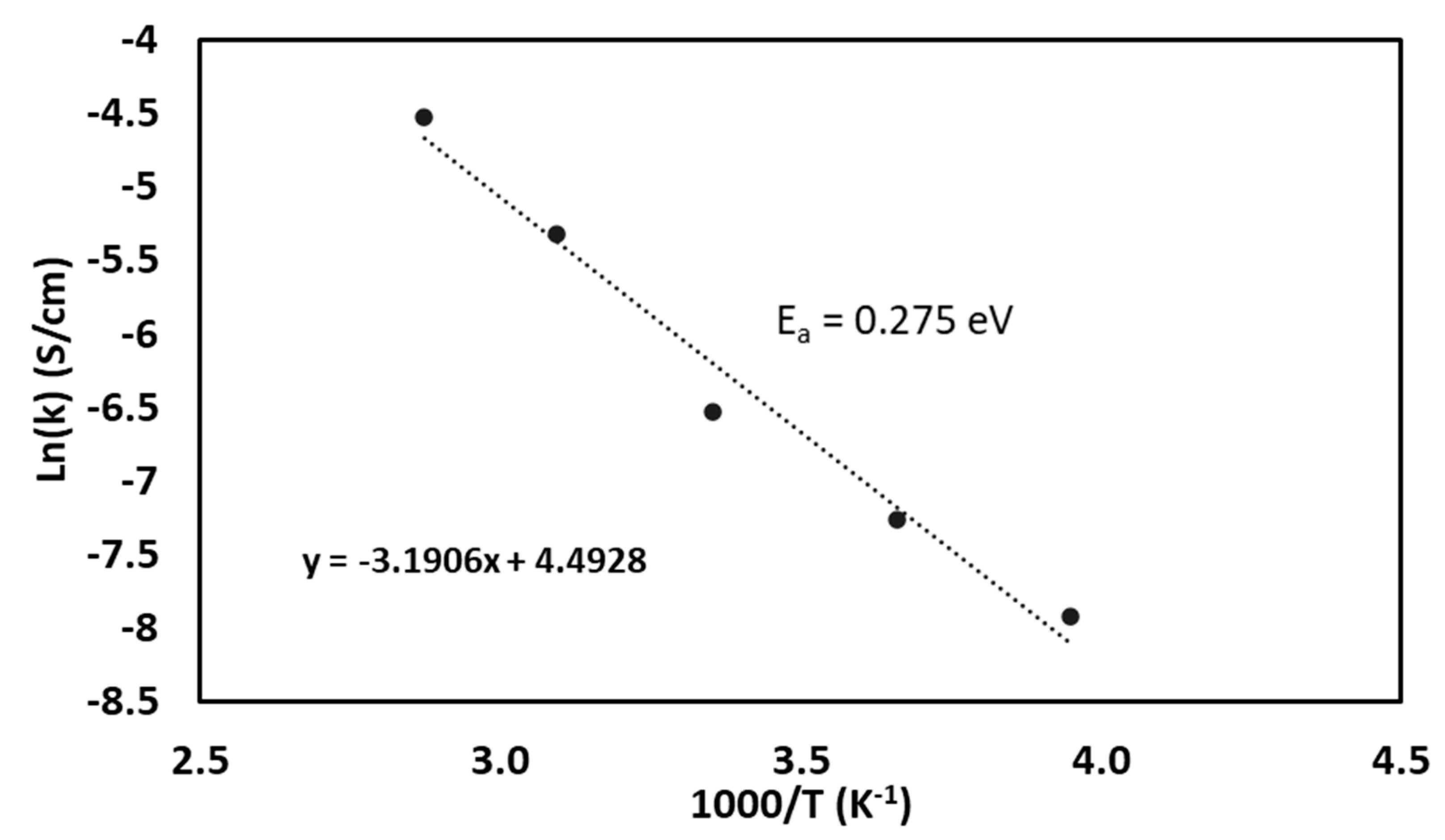
3.3. Effect of Operating Temperature on Ionic Conductivity
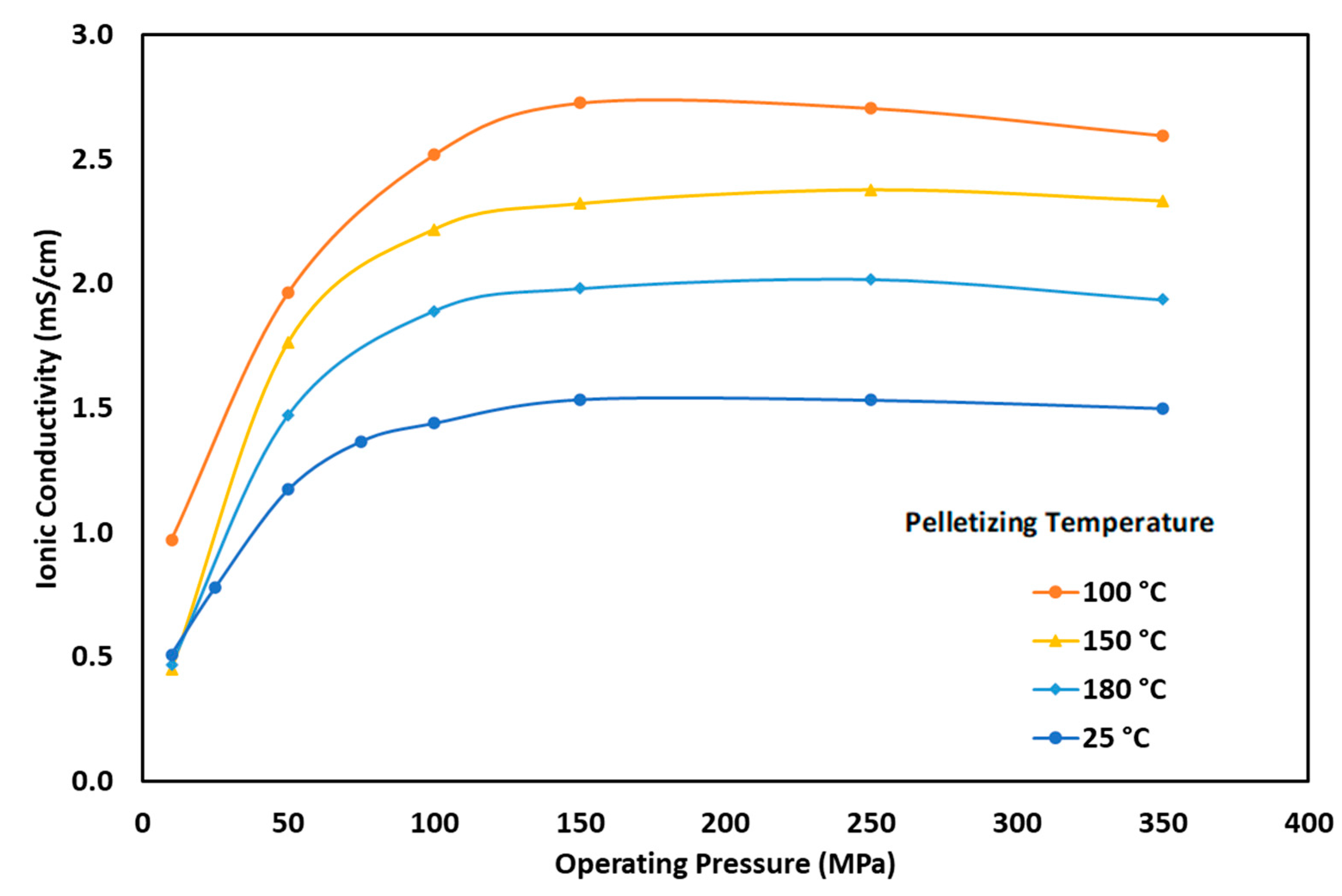
3.4. Effect of Pelletizing Temperature on Ionic Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgement
Conflicts of Interest
References
- Etacheri, V., Marom, R., Elazari, R., Salitra, G., & Aurbach, D. (2011). Challenges in the development of advanced Li-ion batteries: A review. Energy & Environmental Science, 4(9), 3243–3262. [CrossRef]
- Xu, C., Dai, Q., Gaines, L., Hu, M., Tukker, A., & Steubing, B. (2020). Future material demand for automotive lithium-based batteries. Communications Materials, 1(1), Article 1. [CrossRef]
- Goodenough, J. B., & Kim, Y. (2010). Challenges for Rechargeable Li Batteries. Chemistry of Materials, 22(3), 587–603. [CrossRef]
- Famprikis, T., Canepa, P., Dawson, J. A., Islam, M. S., & Masquelier, C. (2019). Fundamentals of inorganic solid-state electrolytes for batteries. Nature Materials, 18(12), Article 12. [CrossRef]
- Hao, F., Han, F., Liang, Y., Wang, C., & Yao, Y. (2018). Architectural design and fabrication approaches for solid-state batteries. MRS Bulletin, 43(10), 775–781. [CrossRef]
- Zhu, X., Wang, K., Xu, Y., Zhang, G., Li, S., Li, C., Zhang, X., Sun, X., Ge, X., & Ma, Y. (2021). Strategies to Boost Ionic Conductivity and Interface Compatibility of Inorganic—Organic Solid Composite Electrolytes. Energy Storage Materials, 36, 291–308. [CrossRef]
- Zhang, B., Tan, R., Yang, L., Zheng, J., Zhang, K., Mo, S., Lin, Z., & Pan, F. (2018). Mechanisms and properties of ion-transport in inorganic solid electrolytes. Energy Storage Materials, 10, 139–159. [CrossRef]
- Qiu, J., Yang, L., Sun, G., Yu, X., Li, H., & Chen, L. (2020). A stabilized PEO-based solid electrolyte via a facile interfacial engineering method for a high voltage solid-state lithium metal battery. Chemical Communications, 56(42), 5633–5636. [CrossRef]
- Senevirathne, K., Day, C. S., Gross, M. D., Lachgar, A., & Holzwarth, N. A. W. (2013). A new crystalline LiPON electrolyte: Synthesis, properties, and electronic structure. Solid State Ionics, 233, 95–101. [CrossRef]
- Zhou, L., Park, K.-H., Sun, X., Lalère, F., Adermann, T., Hartmann, P., & Nazar, L. F. (2019). Solvent-Engineered Design of Argyrodite Li6PS5X (X = Cl, Br, I) Solid Electrolytes with High Ionic Conductivity. ACS Energy Letters, 4(1), 265–270. [CrossRef]
- Wang, H., Yu, C., Ganapathy, S., van Eck, E. R. H., van Eijck, L., & Wagemaker, M. (2019). A lithium argyrodite Li6PS5Cl0.5Br0.5 electrolyte with improved bulk and interfacial conductivity. Journal of Power Sources, 412, 29–36. [CrossRef]
- Zhang, X., Wang, Q. J., Harrison, K. L., Roberts, S. A., & Harris, S. J. (2020). Pressure-Driven Interface Evolution in Solid-State Lithium Metal Batteries. Cell Reports Physical Science, 1(2), 100012. [CrossRef]
- Doux, J.-M., Nguyen, H., Tan, D. H. S., Banerjee, A., Wang, X., Wu, E. A., Jo, C., Yang, H., & Meng, Y. S. (2020). Stack Pressure Considerations for Room-Temperature All-Solid-State Lithium Metal Batteries. Advanced Energy Materials, 10(1), 1903253. [CrossRef]
- Doux, J.-M., Yang, Y., S. Tan, D. H., Nguyen, H., A. Wu, E., Wang, X., Banerjee, A., & Shirley Meng, Y. (2020). Pressure effects on sulfide electrolytes for all solid-state batteries. Journal of Materials Chemistry A, 8(10), 5049–5055. [CrossRef]
- Zarrin, H., Farhad, S., Hamdullahpur, F., Chabot, V., Yu, A., Fowler, M., & Chen, Z. (2014). Effects of Diffusive Charge Transfer and Salt Concentration Gradient in Electrolyte on Li-ion Battery Energy and Power Densities. Electrochimica Acta, 125, 117–123. [CrossRef]
- Mastali, M., Samadani, E., Farhad, S., Fraser, R., & Fowler, M. (2016). Three-dimensional Multi-Particle Electrochemical Model of LiFePO4 Cells based on a Resistor Network Methodology. Electrochimica Acta, 190, 574–587. [CrossRef]
- Kashkooli, A. G., Amirfazli, A., Farhad, S., Lee, D. U., Felicelli, S., Park, H. W., Feng, K., De Andrade, V., & Chen, Z. (2017). Representative volume element model of lithium-ion battery electrodes based on X-ray nano-tomography. Journal of Applied Electrochemistry, 47(3), 281–293. [CrossRef]
- Farhad, S., & Nazari, A. (2019). Introducing the energy efficiency map of lithium-ion batteries. International Journal of Energy Research, 43(2), 931–944. [CrossRef]
- Mohammed, A. H., Esmaeeli, R., Aliniagerdroudbari, H., Alhadri, M., Hashemi, S. R., Nadkarni, G., & Farhad, S. (2019). Dual-purpose cooling plate for thermal management of prismatic lithium-ion batteries during normal operation and thermal runaway. Applied Thermal Engineering, 160, 114106. [CrossRef]
- Yu, C., Ganapathy, S., Hageman, J., van Eijck, L., van Eck, E. R. H., Zhang, L., Schwietert, T., Basak, S., Kelder, E. M., & Wagemaker, M. (2018). Facile Synthesis toward the Optimal Structure-Conductivity Characteristics of the Argyrodite Li6PS5Cl Solid-State Electrolyte. ACS Applied Materials & Interfaces, 10(39), 33296–33306. [CrossRef]
- Boulineau, S., Courty, M., Tarascon, J.-M., & Viallet, V. (2012). Mechanochemical synthesis of Li-argyrodite Li6PS5X (X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ionics, 221, 1–5. [CrossRef]
- Yubuchi, S., Teragawa, S., Aso, K., Tadanaga, K., Hayashi, A., & Tatsumisago, M. (2015). Preparation of high lithium-ion conducting Li6PS5Cl solid electrolyte from ethanol solution for all-solid-state lithium batteries. Journal of Power Sources, 293, 941–945. [CrossRef]
- Wang, S., Zhang, Y., Zhang, X., Liu, T., Lin, Y.-H., Shen, Y., Li, L., & Nan, C.-W. (2018). High-Conductivity Argyrodite Li6PS5Cl Solid Electrolytes Prepared via Optimized Sintering Processes for All-Solid-State Lithium–Sulfur Batteries. ACS Applied Materials & Interfaces, 10(49), 42279–42285. [CrossRef]
- Sakuda, A., Hayashi, A., & Tatsumisago, M. (2013). Sulfide Solid Electrolyte with Favorable Mechanical Property for All-Solid-State Lithium Battery. Scientific Reports, 3(1), Article 1. [CrossRef]
- MIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150001, China, & Hwang, A. (2017). Fabrication and Electrochemical Properties of Li4Ti5O12@Li6PS5Cl for All-solid-state Lithium Batteries using Simple Mechanical Method. International Journal of Electrochemical Science, 7795–7806. [CrossRef]
- Choi, S., Ann, J., Do, J., Lim, S., Park, C., & Shin, D. (2018). Application of Rod-Like Li6PS5Cl Directly Synthesized by a Liquid Phase Process to Sheet-Type Electrodes for All-Solid-State Lithium Batteries. Journal of The Electrochemical Society, 166(3), A5193. [CrossRef]
- Zhang, J., Zhong, H., Zheng, C., Xia, Y., Liang, C., Huang, H., Gan, Y., Tao, X., & Zhang, W. (2018). All-solid-state batteries with slurry coated LiNi0.8Co0.1Mn0.1O2 composite cathode and Li6PS5Cl electrolyte: Effect of binder content. Journal of Power Sources, 391, 73–79. [CrossRef]
- Ohno, S., Bernges, T., Buchheim, J., Duchardt, M., Hatz, A.-K., Kraft, M. A., Kwak, H., Santhosha, A. L., Liu, Z., Minafra, N., Tsuji, F., Sakuda, A., Schlem, R., Xiong, S., Zhang, Z., Adelhelm, P., Chen, H., Hayashi, A., Jung, Y. S., … Zeier, W. G. (2020). How Certain Are the Reported Ionic Conductivities of Thiophosphate-Based Solid Electrolytes? An Interlaboratory Study. ACS Energy Letters, 5(3), 910–915. [CrossRef]
- Zhang, Z., Zhang, L., Liu, Y., Yu, C., Yan, X., Xu, B., & Wang, L. (2018). Synthesis and characterization of argyrodite solid electrolytes for all-solid-state Li-ion batteries. Journal of Alloys and Compounds, 747, 227–235. [CrossRef]
- Cronau, M., Szabo, M., König, C., Wassermann, T. B., & Roling, B. (2021). How to Measure a Reliable Ionic Conductivity? The Stack Pressure Dilemma of Microcrystalline Sulfide-Based Solid Electrolytes. ACS Energy Letters, 6(9), 3072–3077. [CrossRef]
- Zhang, W., Weber, D. A., Weigand, H., Arlt, T., Manke, I., Schröder, D., Koerver, R., Leichtweiss, T., Hartmann, P., Zeier, W. G., & Janek, J. (2017). Interfacial Processes and Influence of Composite Cathode Microstructure Controlling the Performance of All-Solid-State Lithium Batteries. ACS Applied Materials & Interfaces, 9(21), 17835–17845. [CrossRef]
- Zhou, L., Minafra, N., Zeier, W. G., & Nazar, L. F. (2021). Innovative Approaches to Li-Argyrodite Solid Electrolytes for All-Solid-State Lithium Batteries. Accounts of Chemical Research, 54(12), 2717–2728. [CrossRef]
- Dixit, M., Beamer, C., Amin, R., Shipley, J., Eklund, R., Muralidharan, N., Lindqvist, L., Fritz, A., Essehli, R., Balasubramanian, M., & Belharouak, I. (2022). The Role of Isostatic Pressing in Large-Scale Production of Solid-State Batteries. ACS Energy Letters, 7(11), 3936–3946. [CrossRef]
| Conductivity | Pelletizing Pressure | Operating Pressure | Reference | Notes |
|---|---|---|---|---|
| (mS/cm) | (MPa) | (MPa) | ||
| 0.22-3.02 | 50-370 | 5-70 | Doux [14] | Li6PS5Cl |
| 4.96 | 1000 | - | Yu [20] | Li6PS5Cl |
| 1.33 | 333 | - | Boulineau [21] | Li6PS5Cl |
| 1.40 | 360 | - | Yubuchi [22] | Li6PS5Cl |
| 3.15 | 150 | - | Wang [23] | Li6PS5Cl |
| 1.10 | 750 | - | Sakuda [24] | Li6PS5Cl |
| 1.60 | 330 | - | Hwang [25] | Li6PS5Cl |
| 1.40 | 140 | - | Choi [26] | Li6PS5Cl |
| 1.29 | 350 | - | Zhang [27] | Li6PS5Cl |
| 1.79 | 380 | 50 | Ohno [28] | Li6PS5Cl |
| 1.53 | 375 | - | Ohno [28] | Li6PS5Cl |
| 2.6 | 370 | 75 | Ohno [28] | Li6PS5Cl |
| 1.9 | 720 | 0 | Ohno [28] | Li6PS5Cl |
| 2.14 | 325 | 0 | Ohno [28] | Li6PS5Cl, isostatic pelletizing |
| 0.443 | 275 | - | Ohno [28] | Li6PS5Cl |
| 2.98 | 441 | 315 | Ohno [28] | Li6PS5Cl |
| 0.79 | - | - | Wang [11] | Li6PS5Cl |
| 0.63 | 1000 | 10 | Ohno [28] | Li6PS5Cl |
| 1.00 | 300 | - | Zhang [29] | Li6PS5Cl without modified synthesizing process |
| 1.80 | 300 | - | Zhang [29] | Li6PS5Cl with modified synthesizing process |
| 0.50 | 300 | - | Zhang [29] | Li6PS5Br without modified synthesizing process |
| 1.30 | 300 | - | Zhang [29] | Li6PS5Br with modified synthesizing process |
| 0.36 | - | - | Wang [11] | Li6PS5Br |
| 0.30-2.45 | 100-500 | 5-500 | Cronau [30] | Li6PS5Br, with and without annealing at 550 °C (similar results reported whether using high operating pressure with no annealing or low operating pressure with annealing) |
| 3.63 | - | - | Wang [11] | Li6PS5Cl0.5Br0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





