1. Introduction
Cough reflex deficits can occur in those individuals who have experienced a neurological event that may alter the afferent, central, or efferent components of this reflex temporarily (such as after general anesthesia) or permanently (such as after a stroke, cervical cord damage, Parkinson’s disease, or amyotrophic lateral sclerosis) [
1,
2,
3]. This can occur due to damage to the areas of the brain responsible for controlling the cough reflex, including the medulla oblongata. In these cases, the patient may have difficulty initiating a cough and may be at risk for aspiration and pulmonary infections. Treatment options may include the use of assisted coughing techniques, such as the use of an incentive spirometer or vibration therapy, and/or the use of medications to help stimulate the cough reflex [
4,
5].
In clinical practice, to assess the integrity of the cough response to airway invasion and the possibility of silent aspiration, the cough reflex test can be employed as a screening tool [
7]. It includes a nebulizer to inhale a cough-evoking aerosol at a particular concentration in order to elicit coughing. In addition to self-reported ratings of the perceived severity of airway irritation, clinicians can note the presence, absence, and quantity of coughs that are evoked. It offers information that would not otherwise be available on the consistency of upper airway sensation. One factor raising patients’ risk of silent aspiration is impaired upper airway feeling. A patient with impaired upper airway feeling may not aspirate if their swallowing biomechanics are not altered. Therefore, it is necessary to interpret the cough reflex test results considering the full clinical swallowing evaluation. On instrumental examination, citric acid has first been proven to be a reliable compound for silent aspiration [
7]. This test exposes the patient to a small amount of citric acid measuring the response, including the time to onset of coughing and the number of coughs produced. Other commonly reported cough-evoking techniques are the nebulized 2.5% hypertonic saline, epinephrine, or mannitol [
8].
The capsaicin inhalation test is another method used to evaluate the cough reflex in neurological patients [
7,
8]. This is currently the most widely utilized non-acid tussive substance in cough inhalation tests. Several guidelines, including those from the Chinese Thoracic Society (CTS), European Respiratory Society (ERS), and American College of Chest Physician (ACCP), recommend the use of capsaicin inhalation challenges [
9,
10]. Capsaicin is a flavorful compound with the chemical formula C
18H
27NO
3 found in chili peppers, which can cause reflex coughing in humans. Since the 1980s, the test is performed by having the patient inhale a small amount of capsaicin through a nebulizerè [
11,
12]. The patient’s coughing response is then measured and recorded, including the time to onset of coughing and the number of coughs produced. The test can be used to evaluate the patient’s ability to produce an effective cough and to detect any underlying problems with the cough reflex, with a documented excellent safety record in healthy volunteers as well as in patients with asthma, chronic obstructive pulmonary disease, pathologic cough, and other respiratory conditions [
13,
14]. This test has been applied either for diagnosis of cough reflex deficits or for therapeutic purposes in patients with neurogenic dysphagia [
15], progressive supranuclear palsy [
16], Parkinson’s disease [
17], and in patients with hemorrhagic stroke [
18].
The wide range of techniques described in the literature presents one of the biggest obstacles to integrating cough reflex text into clinical practice. In particular, in patients with acquired brain injury (ABI), such as stroke, traumatic or anoxic injury, there is no defined standard procedure (i.e., nebulizer flow rates, duration and concentration of substances exposure) to evaluate cough reflex. Therefore, in a clinical neurological setting, a feasible, less-invasive, less time-consuming/labor-consuming, and simple-to-comprehend technique is advantageous.
For this reason, in this preliminary study, we sought to evaluate the effectiveness of a different kind of simple and timesaving cough reflex induction method based on a commercial capsaicin spray stimulation applied directly on the tongue of patients with ABI.
2. Methods
2.1. Participants
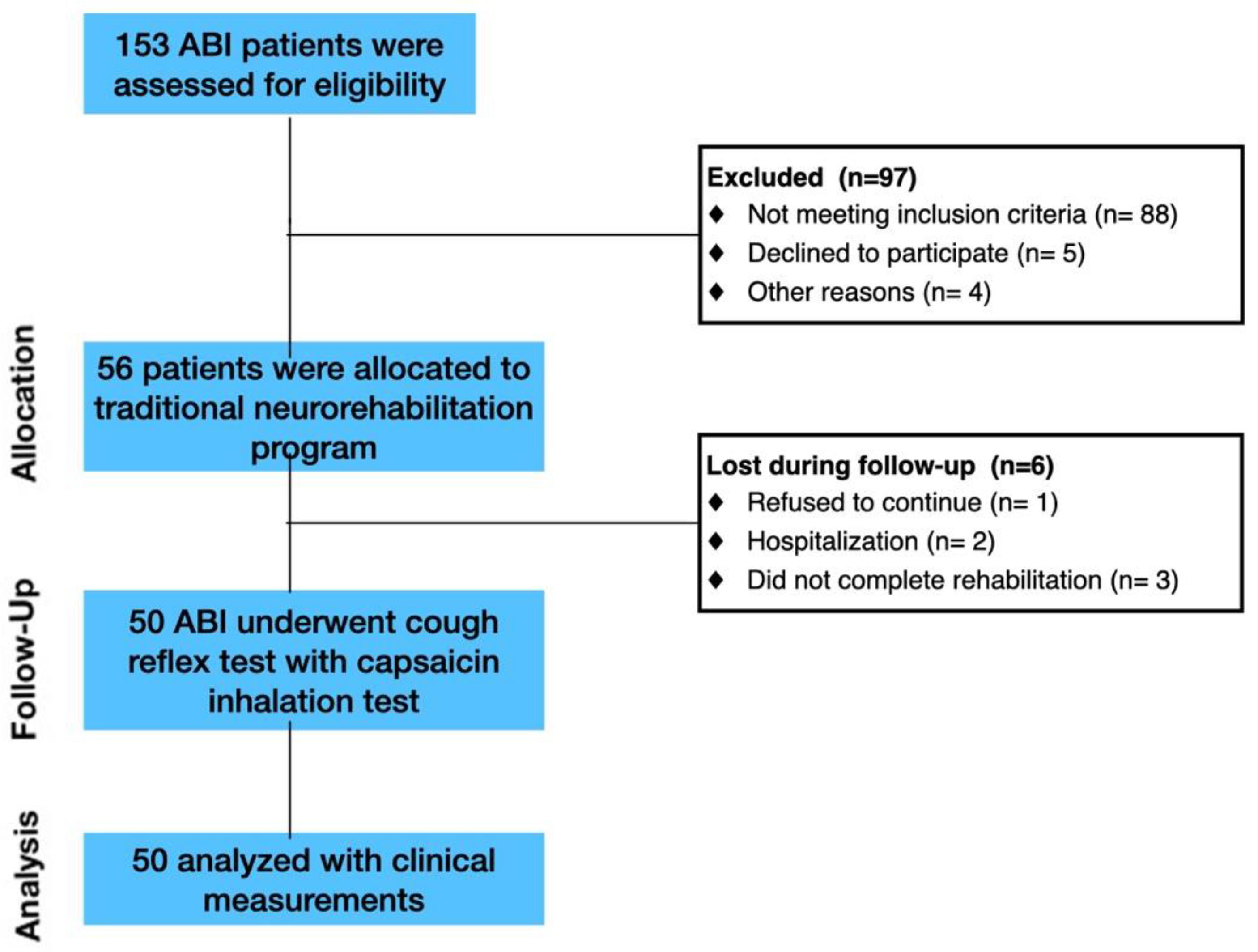
The study involved patients affected by ABI. Inpatients consecutively admitted to the Intensive Rehabilitation Unit (IRU) of the Institute S. Anna (Crotone, Italy) between January 2020 and December 2022 were screened for possible inclusion. From an initial cohort of 153 ABI patients, we enrolled only ABI patients who fulfilled the following inclusion criteria: (1) age ≥ 18 years; (2) first admission to the neurorehabilitation unit; (3) Level of Cognitive Functioning Scale (LCF) ≥ 4; and (4) ability to perform three trials of capsaicin stimulation. The exclusion criteria included: (1) history of asthma or other respiratory conditions; (2) patients with active respiratory infection; (3) hypersensitivity to low levels of capsaicin. Following inclusion and exclusion criteria, 103 ABI patients were excluded (
Figure 1).
Healthy controls (HCs) were recruited from universities, community recreational centers and hospital personnel through local advertisements. Inclusion criteria were: (1) no evidence of neuro logical and psychiatric symptoms according to DSM-V criteria; (2) no use of antidepressant, anxiolytic or antipsychotic drugs and (3) absence of chronic medical conditions (i.e., history of asthma or other respiratory conditions) (4) no smokers. Following inclusion and exclusion criteria, 100 HCs ABI patients were enrolled.
All patients and/or their caregivers gave written informed consent, and the study was approved by the Ethical Committee of the Central Area Regione Calabria of Catanzaro (CZ-Prot 24-2019), according to the Helsinki Declaration.
2.2. Design and Procedure
All the included normal controls and patients underwent preliminary baseline reflex and voluntary cough clinical evaluations. The Gugging Swallowing Screen (GUSS) scale was used at admission to determine the dysphagia severity [
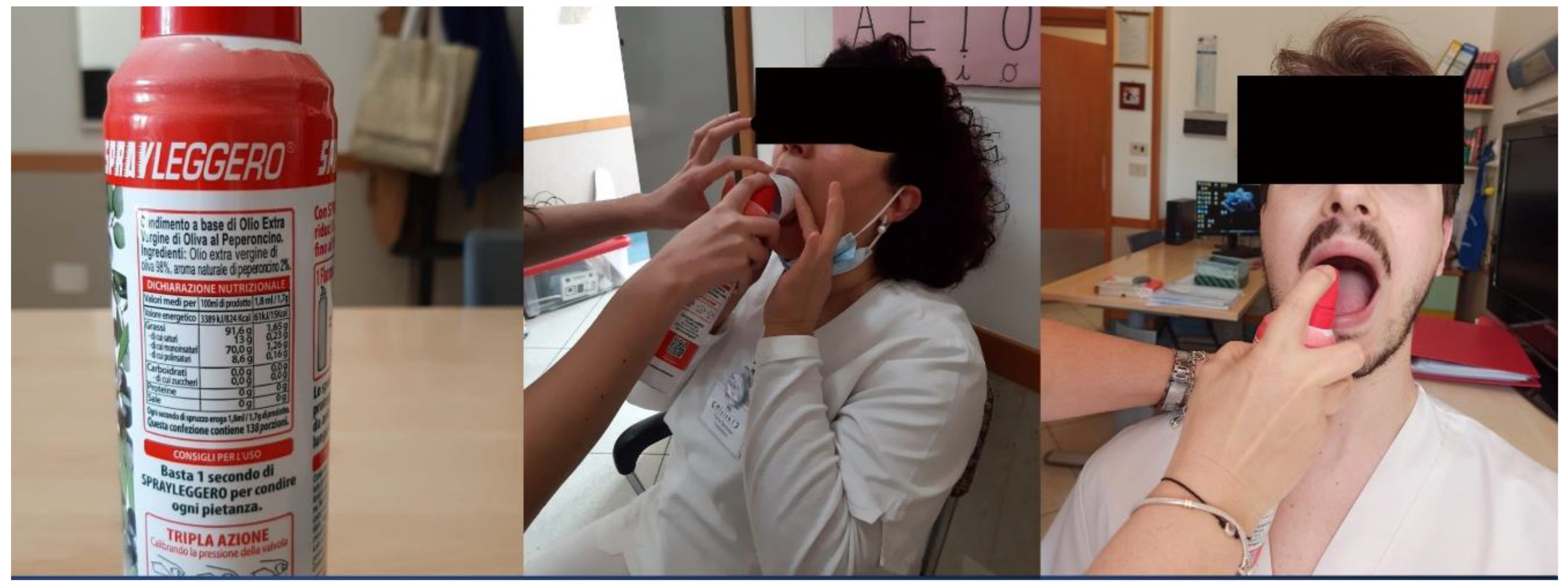
19] in ABI patients. After admission to the IRU, the enrolled patients were tested with our cough reflex test (called, Chili Pepper Test), as the first component of a standard bedside swallow examination. All patients were examined only after the decannulation. A spray oil that contains capsaicin was used for the test. During the stimulation, the subject’s nose was pinched closed. Open-mouthed, on the posterior third of the tongue, a spray of oil containing 1.7 g of 2% capsaicin equivalent dose was applied (
Figure 2). We waited from 0 to 60 seconds after stimulation to note the presence or absence and the duration of the cough response’s latency. After the first administration (0.034 g), a second stimulation with a double concentration (0.068 g) was made only in the absence of a first response. If no reactivity was noticed too, a third and final dose with a tripled concentration (1.02 g) was then given. The expected result of a normal cough reflex test was an immediate series of coughs, which are primarily expiratory “airway clearing” in character. If the subject had a normal cough reflex after the first administration, additional stimulations were not performed. Each single spray dose corresponds to the basic capsaicin concentration (1.8 mL/1.7 g).
The cough reflex test was performed by one clinically certified speech-language pathologist, with more than 10 years of experience rating swallows and cough reflexes in ABI patients (L.S.).
Figure 2.
Chili Pepper administration. A) Spray; B) administration in the case of patients with troubles in opening his mouth. They can receive treatment while having his mouth held open by a tube. C) traditional administration directly on the patient’s tongue.
Figure 2.
Chili Pepper administration. A) Spray; B) administration in the case of patients with troubles in opening his mouth. They can receive treatment while having his mouth held open by a tube. C) traditional administration directly on the patient’s tongue.
2.3. Outcome Measures
An additional ad-hoc questionnaire was included in the protocol to assess the intensity of cough reflex after the three capsaicin administrations. The questionnaire was structured as a Likert scale and included 4 items: 0–3 multiple answers, scored from lowest to the highest intensity level of cough reflex (0 “absent”—no cough reflex; 1 “feeble”—cough reflex present but ineffective; 2 “wheezing”—Cough reflex present and bothersome; 3 “persistent/irritating”—persistent cough reflex with duration > 5 seconds).
2.4. Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Science software (SPSS, v20.0, Chicago, IL, USA) for Macintosh. Assumptions of normality were tested for all the continuous variables. Normality was tested using the Kolmogorov–Smirnov test. X2, unpaired t-test and One-Way Anova were used to evaluate the differences in demographic and clinical factors between groups, and to evaluate significant changes detected during the cough reflex test. For all the tests, a p-value < 0.05 was statistically significant.
3. Results
3.1. Clinical Data
There was no difference between patients and controls concerning age (49.6 ± 13.7 years HCs; 55.5 ± 18.1; t-test = 2-1; p-level = 0.11), and gender distribution (male: 45% HCs; male: 65% ABI; X2 = 3; p-level = 0.08). At admission, 24 ABI patients had dysphagia (58% characterized by a severe condition according to GUSS). For this reason, we decided to group patients according to this critical symptom.
Table 1 showed the demographical characteristics of ABI patients with or without severe dysphagia.
3.2. Cough Reflex Test
No adverse events or parasympathetic responses were detected in all controls and patients. Descriptive statistics for cough reflex response and parameters are included in
Table 2.
As concerns the presence of cough reflex, there was a significant difference among the groups. Indeed, 24% of HCs did not show any significant cough reflex after three administrations of capsaicin spray, whereas in ABI groups the absence of a response was minimal but similar (ABI without dysphagia 8%; ABI with dysphagia 13%). In the remaining individuals, 63% showed significant cough reflex after the first administration.
ABI patients without dysphagia showed the most sensitive response to tongue stimulation (77% with relevant cough reflex at 1° administration), with respect to controls group (63%) and ABI patients with dysphagia (50%). Both controls and ABI patients without dysphagia showed the fastest response (11.8 s and 12.1 s, respectively) with respect to ABI patients with dysphagia (50%). Finally, the cough intensity was feeble and similar among the three groups.
Table 2.
Cough Reflex Response in healthy controls and ABI patients.
Table 2.
Cough Reflex Response in healthy controls and ABI patients.
| |
Healthy Controls (n°100) |
ABI without dysphagia (n°26) |
ABI with dysphagia (n°24) |
p-level |
| % response at 1° Administration |
63% |
77% |
50% |
X2 = 15.7; p < 0.001 |
| % response at 2° Administration |
10% |
15% |
21% |
X2 = 5.6; n.s. |
| % response at 3° Administration |
3% |
0% |
16% |
X2 = 23.3; p < 0.001 |
| Absence of response |
24% |
8% |
13% |
X2 = 10.5; p = 0.005 |
| Cough Reflex Trigger time (s) |
11.8 ± 14.2 |
12.1 ± 7.5 |
23.8 ± 20.1 |
F = 3.38; p = 0.043 |
| Cough Intensity |
1.3 ± 0.6
1 [0–3] |
1.3 ± 0.6
1 [0–3] |
1.5± 0.6
1 [0–3] |
n.s. |
4. Discussion
This study aimed to evaluate the effectiveness of a new kind of capsaicin stimulation test to evaluate the cough reflex in patients with ABI. Considering patients without dysphagia, capsaicin spray stimulation was able to induce a robust, fast and immediate cough response similar to those detected in healthy controls (all post-hoc t-test > 0.05). Whereas a significantly slower and delayed response was detected in ABI patients with dysphagia with respect to controls (t-test = 0.003) and ABI without dysphagia (t-test = 0.02). According to the current pilot investigation, our capsaicin stimulation test consistently causes coughing in healthy volunteers as well as in ABI cohorts with or without dysphagia.
It is crucial to emphasize that distinct cough reflex reactions are caused by different nebulizers, speed, and tussigenic substances [
13]. The approaches used for the cough reflex test have varied among researches, which may be the potential cause of the significant differences in the methods and prevent fair comparison [
20]. Basically, the two most frequently employed tussigenic substances reported in cough-evoking aerosols literature are capsaicin and citric acid. Citric acid, in contrast to capsaicin, has been shown to activate both chemoreceptors and mechanoreceptors [
10]. Laryngeal coughing, instead, is reported to be induced by citric acid [
13]. The choice of aerosol should be carefully considered, as it has implications for the underlying neurophysiology of the induced cough. Citric acid preferentially stimulates neural pathways and rapidly adapts laryngeal receptors that play a role in coughing to aspiration [
21]. Capsaicin preferentially stimulates slowly adapting sensory receptors that mediate coughing to prolonged airway irritation. With respect to previously reported cough-evoking aerosol methods we decided to use a commercial spray solution in order to directly stimulate the oropharynx tract. Indeed, it is generally known that vagal afferents that innervate the larynx, trachea, and airways mediate the cough reflex. The glossopharyngeal nerve in the nasopharynx supplies sensory innervation to the pharynx, although the oropharynx and laryngopharynx get dual innervation from the glossopharyngeal and vagus nerves. Therefore, it is conceivable that oropharynx-related treatments may affect cough by affecting the local vagal afferent fibers. Furthermore, there is evidence that the cough reflex can also be mechanically elicited from the pharynx, even though mechanical stimulation of the oropharynx can cause adverse effects [
22]. The larynx and esophagus’ vagal afferent fibers are additional potential sites of action. Therefore, we believe that the effect of capsaicin spray on the oropharynx in our study suggests that the activation of pharyngeal sensory fibers plays a different role in contributing to cough reflex with respect to previously reported cough-evoking aerosols methods.
Two important findings of our study needed to be highlighted. First, the Cough Reflex Trigger time is longer in patients with dysphagia, as well as the response to the first stimulation is reduced in patients with dysphagia than in ABI patients without dysphagia and healthy controls. This is because, as evidenced by the Barthel Index at admission, patients with dysphagia are more severe than those without (see
Table 1). This is an important observation that gives clinicians the opportunity to understand that the cough reflex in patients with dysphagia can be evoked over the 3 administrations and is of valid intensity but has a delayed trigger. This should advise therapists and phoniatrics of the risk of pre-swallowing falls with the risk of inhalation and the consequent probability of aspiration pneumonia. Next, during our experimental procedures no adverse events were detected. This is coherent with the current literature that described only a skin facial irritation self-limited without the use of drugs [
12,
23,
24]. Moreover, according to a previous study no alteration of the heart rate, respiratory rate, and oxygen saturation were observed during capsaicin administration [
25].
4.2. Limitations
We acknowledge that the main issue of this study is the absence of an instrumental assessment, as well as the lack of intra/inter-rater evaluation of cough response. Indeed, using spirometry we might have measured the intensity and duration of cough reflex. Although our primary goal was to realize a proof-of-concept study to evaluate the effectiveness of our test in inducing cough reflex response in HC and ABI patients, further RCT studies are needed for assessing the reliability of our method with respect to others [
26,
27]. Moreover, a direct comparison between our cough test (spray stimulation on the tongue) with respect to another well-validated approach (nebulizer) is mandatory. However, it should be considered that the rationale behind the choice of using this kind of cough stimulation test is twofold: a) to prevent irritation of the nasal and oral mucous membranes using a natural compound, and b) to apply the selected amount of capsaicin with greater accuracy. Finally, it should be noted that in almost a quarter of healthy controls, there is an absent cough response to the capsaicin test. Briefly, we hypothesize that first, the concentration of capsaicin to provoke cough in pathological populations is more than in healthy subjects [
28]; and it is possible that in some healthy the concentration used was not sufficient. Second the absence of cough response after capsaicin stimulation in a quarter of HC could be dependent upon the fact that chili pepper is widely used in Italian culinary food. Thus, a diet based on capsaicin (permanent exposure to chili peppers) could reduce the perceptive threshold thus influencing the cough response test.
5. Conclusions
In this study, we provide preliminary evidence on the effectiveness of a new kind of a simple and timesaving cough reflex induction method using a capsaicin commercial spray stimulation. In a heterogeneous population of ABI patients with or without dysphagia this proof-of-concept study demonstrates a robust and consistent elicitation of cough reflex after a single session of capsicin. These results are encouraging because they demonstrate that individuals with ABI respond to this oil solution. This finding may lead to the development of a novel form of therapy since reflexive cough modulation may be an effective strategy for enhancing cough strength in the presence of blunted reflex. A possible hypothesis is that capsaicin should aid the clearance of the laryngopharynx and lower airways. In addition, a repeated trigger of the cough reflex might induce a central recovery of the cough reflex contributing to dysphagia rehabilitation as demonstrated in a recent case report in subjects affected by stroke [
29] and in tracheostomized subjects affected by hemorrhagic stroke [
30]. Another study conducted by Cui et al. demonstrated an improvement in the swallowing function by combining ice and capsaicin stimulation in subjects with dysphagia after stroke [
31]. According to this preliminary but promising evidence, spray capsaicin can be used in clinical practice not only to diagnose cough reflex alterations but also for rehabilitation purposes. This could eventually help in the complex management of patients with severe ABI.
Author Contributions
Conceptualization, L.S.; methodology, D.C., M.Q., L.P. and F.L.L.; investigation, L.S.; resources, P.T.; data curation, A.C. and L.S.; writing—original draft preparation, G.M., R.S.C. and A.C.; writing—review and editing, G.M., R.S.C. and A.C.; supervision, P.T.; funding acquisition, G.P.T. All authors have read and agreed to the published version of the manuscript.
Funding
Nothing to declare.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fontana, G.A.; Pantaleo, T.; Lavorini, F.; Benvenuti, F.; Gangemi, S. Defective motor control of coughing in Parkinson’s disease. Am J Respir Crit Care Med 1998, 158, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Hadjikoutis, S.; Wiles, C.M.; Eccles, R. Cough in motor neuron disease: A review of mechanisms. Qjm 1999, 92, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Hoshino, M.; Okayama, K.; Sekizawa, K.; Sasaki, H. Swallowing and cough reflexes after onset of stroke. Chest 1994, 105, 1623. [Google Scholar] [CrossRef]
- Belal, E.S.; Selim, S.; Aboul fotouh, A.M.; et al. Detection of airway protective level of the cough reflex in acute stroke patients. Egypt J Neurol Psychiatry Neurosurg 2020, 56, 21. [Google Scholar] [CrossRef]
- Ward, K.; Seymour, J.; Steier, J.; Jolley, C.J.; Polkey, M.I.; Kalra, L.; et al. Acute ischaemic hemispheric stroke is associated with impairment of reflex in addition to voluntary cough. Eur Respir J 2010, 36, 138–390. [Google Scholar] [CrossRef]
- Watts, S.A.; Tabor, L.; Plowman, E.K. To cough or not to cough? Examining the potential utility of cough testing in the clinical evaluation of swallowing. Curr Phys Med Rehabil Rep 2016, 4, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.; Guiu Hernandez, E.; Ang, A.; Hiew, S.; Macrae, P. A systematic review of methods of citric acid cough reflex testing. Pulm Pharmacol Ther 2019, 58, 101827. [Google Scholar] [CrossRef]
- Mai, Y.; Fang, L.; Zhong, S.; de Silva, S.D.S.H.; Chen, R.; Lai, K. Methods for assessing cough sensitivity. J Thorac Dis 2020, 12, 5224–5237. [Google Scholar] [CrossRef]
- Boulet, L.P.; Coeytaux, R.R.; McCrory, D.C.; et al. Tools for assessing outcomes in studies of chronic cough: CHEST guideline and expert panel report. Chest 2015, 147, 804–814. [Google Scholar] [CrossRef]
- Lai, K.; Shen, H.; Zhou, X.; et al. Clinical Practice Guidelines for Diagnosis and Management of Cough-Chinese Thoracic Society (CTS) Asthma Consortium. J Thorac Dis 2018, 10, 6314–6351. [Google Scholar] [CrossRef]
- Mazzone, S.B. An Overview of the Sensory Receptors Regulating Cough. Cough 2005, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Morice, A.H. Inhalation Cough Challenge in the Investigation of the Cough Reflex and Antitussives. Pulm. Pharmacol. 1996, 9, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Morice, A.H.; Fontana, G.A.; Belvisi, M.G.; Birring, S.S.; Chung, K.F.; Dicpinigaitis, P.V.; Kastelik, J.A.; McGarvey, L.P.; Smith, J.A.; Tatar, M.; et al. ERS Guidelines on the Assessment of Cough. Eur. Respir. J. 2007, 29, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, P.V. Review: Effect of drugs on human cough reflex sensitivity to inhaled capsaicin. Cough 2012, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Lüthi-Müller, E.; Kool, J.; Mylius, V.; Diesener, P. A New Therapeutic Approach for Dystussia and Atussia in Neurogenic Dysphagia: Effect of Aerosolized Capsaicin on Peak Cough Flow. Dysphagia 2022, 37, 1814–1821. [Google Scholar] [CrossRef]
- Borders, J.C.; Curtis, J.A.; Sevitz, J.S.; et al. Immediate Effects of Sensorimotor Training in Airway Protection (smTAP) on Cough Outcomes in Progressive Supranuclear Palsy: A Feasibility Study. Dysphagia 2022, 37, 74–83. [Google Scholar] [CrossRef]
- Hegland, K.W.; Troche, M.S.; Brandimore, A.; Okun, M.S.; Davenport, P.W. Comparison of Two Methods for Inducing Reflex Cough in Patients With Parkinson’s Disease, With and Without Dysphagia. Dysphagia 2016, 31, 66–73. [Google Scholar] [CrossRef]
- Chao, W.; You-Qin, M.; Hong, C.; Hai-Ying, Z.; Yang-Li Su-Xue, J.; Lan, X.; Zhong, W. Effect of Capsaicin Atomization on Cough and Swallowing Function in Patients with Hemorrhagic Stroke: A Randomized Controlled Trial. J Speech Lang Hear Res 2023, 66, 503–512. [Google Scholar] [CrossRef]
- Trapl, M.; Enderle, P.; Nowotny, M.; Teuschl, Y.; Matz, K.; Dachenhausen, A.; Brainin, M. Dysphagia bedside screening for acute-stroke patients: The Gugging Swallowing Screen. Stroke 2007, 38, 2948–2952. [Google Scholar] [CrossRef]
- Enrichi, C.; Zanetti, C.; Gregorio, C.; Koch, I.; Vio, A.; Palmer, K.; Meneghello, F.; Piccione, F.; Battel, I. The assessment of the peak of reflex cough in subjects with acquired brain injury and tracheostomy and healthy controls. Respir Physiol Neurobiol 2020, 274, 103356. [Google Scholar] [CrossRef]
- Mazzone, S.B.; Undem, B.J. Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 2016, 96, 975–1024. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T. Physiological and pathophysiological implications of upper airway reflexes in humans. Jpn J Physiol 2000, 50, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Dicpinigaitis, P.V.; Alva, R.V. Safety of capsaicin cough challenge testing. Chest 2005, 128, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Imoto, Y.; Kojima, A.; Osawa, Y.; Sunaga, H.; Fujieda, S. Cough reflex induced by capsaicin inhalation in patients with dysphagia. Acta Otolaryngol 2011, 131, 96–100. [Google Scholar] [CrossRef]
- Chao, W.; You-Qin, M.; Hong, C.; Hai-Ying, Z.; Yang-Li Su-Xue, J.; Lan, X.; Zhong, W. Effect of Capsaicin Atomization on Cough and Swallowing Function in Patients With Hemorrhagic Stroke: A Randomized Controlled Trial. Journal of speech, language, and hearing research: JSLHR 2023, 66, 503–512. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Kurokawa, R.; Akamatsu, T.; Fukumitsu, K.; Fukuda, S.; Ito, Y.; Takeda, N.; Nishiyama, H.; Ito, K.; Tajiri, T.; et al. Decreased capsaicin cough reflex sensitivity predicts hospitalisation due to COPD. BMJ Open Respir Res 2023, 10, e001283. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Kurokawa, R.; Akamatsu, T.; et al. Decreased capsaicin cough reflex sensitivity predicts hospitalisation due to COPD. BMJ Open Resp Res 2023, 10, e001283. [Google Scholar] [CrossRef]
- Pullerits, T.; Ternesten-Hasséus, E.; Johansson, E.L.; Millqvist, E. Capsaicin cough threshold test in diagnostics. Respir Med 2014, 108, 1371–1376. [Google Scholar] [CrossRef]
- Pekacka-Egli, A.M.; Herrmann, J.; Spielmanns, M.; Goerg, A.; Schulz, K.; Zenker, E.; Windisch, W.; Kulnik, S.T. Inhalation Therapy with Nebulized Capsaicin in a Patient with Oropharyngeal Dysphagia Post Stroke: A Clinical Case Report. Geriatrics 2022, 7, 27. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Yang, L.; Shen, F.; Ma, C.; Shen, M. Effect of Capsaicin Atomization-Induced Cough on Sputum Excretion in Tracheotomized Patients After Hemorrhagic Stroke: A Randomized Controlled Trial. J. Speech Lang. Hear. Res. 2021, 64, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Yin, Q.; Wu, C.; Shen, M.; Zhang, Y.; Ma, C.; Zhang, H.; Shen, F. Capsaicin combined with ice stimulationimproves swallowing function in patients with dysphagia afterstroke: A randomised controlled trial. Journal of Oral Rehabilitation 2020, 47, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).