Submitted:
12 June 2023
Posted:
12 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. B cells
2.2. T cells
3. Discussion
4. Materials and Methods
4.1. Patient samples
4.2. Cell isolation, storage and counting
4.3. Flow cytometry
| marker | fluorophore | clone | |
|---|---|---|---|
| CD127 | Alexa-Fluor 647 | HIL-7R-M21 | |
| CD183 | BV480 | CXCR3 | |
| CD19 | APC-R700 | HIB19 | |
| CD194 (CCR4) | PE | 1G1 | |
| CD196 (CCR6) | APC-R700 | CCR6 | |
| CD20 | BB700 | 2H7 | |
| CD21 | BV421 | B-Ly4 | |
| CD25 | BV421 | M-A251 | |
| CD27 | APC | L128 | |
| CD294 (CRTH2) | BV650 | BM16 | |
| CD3 | BV786 | SK7 | |
| CD31 | BV421 | L133.1 | |
| CD38 | PE | HB-7 | |
| CD4 | PE-CF594 | SK3 | |
| CD43 | BV605 | 1G10 | |
| CD45RO | BV605 | UCHL1 | |
| CD5 | BV650 | L17F12 | |
| CD62L | BB700 | SK11 | |
| CD69 | BV480 | FN50 | |
| CD8 | APC-R700 | SK1 | |
| IgD | PE-CF594 | IA6-2 | |
| IgM | BV480 | G20-127 | |
| TCRαβ | FITC | WT31 | |
| TCRγδ | PE | 11F2 |



| Lymphocyte subsets | Definition | |
|---|---|---|
| B lymphocytes | B cells | CD19+ |
| Innate B cells | CD19+CD27-IgD-IgM- | |
| Naive B cells | CD19+CD27-IgD+IgM+ | |
| Memory B1 cells | CD19+CD27+ | |
| Marginal zone memory B cells | CD19+CD27+IgD+IgM+ | |
| Class switched memory B cells | CD19+CD27+IgD-IgM- | |
| Late memory B cells | CD19+CD27+CD38+IgM+ | |
| Plasmablasts | CD19+CD27+CD38++IgM- | |
| Transitional B cells | CD19+CD20+CD27-CD38+ | |
| B1 cells | CD20+CD27+CD43+ | |
| B2 cells | CD20+CD27+CD43- | |
| T lymphocytes | T cells | CD3+ |
| T helper cells | CD3+CD4+ | |
| Cytotoxic T cells | CD3+CD8+ | |
| T helper cells with αβ-TCR | TCRαβ+CD4+ | |
| Cytotoxic T helper cells with αβ-TCR | TCRαβ+CD8+ | |
| Activated T helper cells αβ-TCR | TCRαβ+CD4+CD69+ | |
| Activated cytotoxic T helper cells with αβ-TCR | TZR αβ+ CD8+CD69+ | |
| Memory effector T helper cells | CD3+CD4+CD62L-CD45RO+ | |
| Naive central T helper cells | CD3+CD4+CD62L+CD45RO+ | |
| Naive effector T helper cells | CD3+CD4+CD62L-CD45RO- | |
| Naive central T helper cells | CD3+CD4+CD62L+CD45RO- | |
| Memory effector cytotoxic T cells | CD3+CD8+CD62L-CD45RO+ | |
| Memory central cytotoxic T cells | CD3+CD8+CD62L+CD45RO+ | |
| Naive effector cytotoxic T cells | CD3+CD8+CD62L-CD45RO- | |
| Naive central cytotoxic T cells | CD3+CD8+CD62L+CD45RO- | |
| Naive thymus negative T helper cells | CD3+CD4+CD31-CD45RO- | |
| Naive thymus positive T helper cells | CD3+CD4+CD31+CD45RO- | |
| T helper cells 1 | CD3+CD4+CD183+CCR6+ | |
| Naive T helper cells 1 | CD3+CD4+CD183+CD45RO- | |
| Memory T helper cells 1 | CD3+CD4 CD183+CD45RO+ | |
| T helper cells 2 | CD3+CD4+CCR4+CRTH2+ | |
| Naive T helper cells 2 | CD3+CD4+CD45RO-CRTH2+ | |
| Memory T helper cells 2 | CD3+CD4+CD45RO+CRTH2+ | |
| Regulatory T cells | CD3+CD4+CD25+CD127- | |
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013, 13, 118–132. [Google Scholar] [CrossRef]
- Kumar SKM, Bhat BV. Distinct mechanisms of the newborn innate immunity. Immunology Letters. 2016, 173, 42–54. [Google Scholar] [CrossRef]
- Basha S, Surendran N, Pichichero M. Immune Responses in Neonates. Expert Rev Clin Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef]
- Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70-. J Exp Med. 2011, 208, 67–80. [CrossRef]
- Prabhu SB, Rathore DK, Nair D, Chaudhary A, Raza S, Kanodia P, et al. Comparison of Human Neonatal and Adult Blood Leukocyte Subset Composition Phenotypes. PLOS ONE. 2016, 11, e0162242. [Google Scholar] [CrossRef]
- Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunological Reviews. 2004, 197, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Murphy K, Weaver C. Die Entwicklung der B- und T-Lymphocyten. Janeway Immunologie. 2018; 377–440. [CrossRef]
- Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping Precursor Movement through the Postnatal Thymus Reveals Specific Microenvironments Supporting Defined Stages of Early Lymphoid Development. Journal of Experimental Medicine. 2001, 194, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Arrieta M-C, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science Translational Medicine. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmunity Reviews. 2003, 2, 119–125. [Google Scholar] [CrossRef]
- Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997, 84, 223–243. [Google Scholar] [CrossRef]
- Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Bereshchenko O, Bruscoli S, Riccardi C. Glucocorticoids, Sex Hormones, and Immunity. Front Immunol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Lai J-J, Lai K-P, Zeng W, Chuang K-H, Altuwaijri S, Chang C. Androgen Receptor Influences on Body Defense System via Modulation of Innate and Adaptive Immune Systems: Lessons from Conditional AR Knockout Mice. The American Journal of Pathology. 2012, 181, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005, 434, 400–404. [Google Scholar] [CrossRef]
- Lyn-Cook BD, Xie C, Oates J, Treadwell E, Word B, Hammons G, et al. Increased expression of Toll-like receptors (TLRs) 7 and 9 and other cytokines in systemic lupus erythematosus (SLE) patients: ethnic differences and potential new targets for therapeutic drugs. Mol Immunol. 2014, 61, 38–43. [Google Scholar] [CrossRef]
- Spitzer, JA. Gender differences in some host defense mechanisms. Lupus. 1999, 8, 380–383. [Google Scholar] [CrossRef]
- Lott N, Gebhard CE, Bengs S, Haider A, Kuster GM, Regitz-Zagrosek V, et al. Sex hormones in SARS-CoV-2 susceptibility: key players or confounders? Nat Rev Endocrinol. 2023, 19, 217–231. [CrossRef]
- Abdullah M, Chai P-S, Chong M-Y, Tohit ERM, Ramasamy R, Pei CP, et al. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cellular Immunology. 2012, 272, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Forest MG, Cathiard AM, Bertrand JA. Evidence of testicular activity in early infancy. J Clin Endocrinol Metab. 1973, 37, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Chamekh M, Deny M, Romano M, Lefèvre N, Corazza F, Duchateau J, et al. Differential Susceptibility to Infectious Respiratory Diseases between Males and Females Linked to Sex-Specific Innate Immune Inflammatory Response. Front Immunol. 2017, 8, 1806. [Google Scholar] [CrossRef] [PubMed]
- Lamason R, Zhao P, Rawat R, Davis A, Hall JC, Chae JJ, et al. Sexual dimorphism in immune response genes as a function of puberty. BMC Immunology. 2006, 7, 2. [Google Scholar] [CrossRef]
- Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011, 118, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Kondo Y, Miyazato A, Okamoto K, Tanaka H. Impact of Sex Differences on Mortality in Patients With Sepsis After Trauma: A Nationwide Cohort Study. Front Immunol. 2021, 12, 678156. [Google Scholar] [CrossRef]
- Laffont S, Rouquié N, Azar P, Seillet C, Plumas J, Aspord C, et al. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-α production of plasmacytoid dendritic cells from women. J Immunol. 2014, 193, 5444–5452. [Google Scholar] [CrossRef]
- Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008, 451, 1125–1129. [Google Scholar] [CrossRef]
- Haneklaus M, Gerlic M, O’Neill LAJ, Masters SL. miR-223: infection, inflammation and cancer. J Intern Med. 2013, 274, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Comans-Bitter WM, de Groot R, van den Beemd R, Neijens HJ, Hop WC, Groeneveld K, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1997, 130, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Paavonen T, Andersson LC, Adlercreutz H. Sex hormone regulation of in vitro immune response. Estradiol enhances human B cell maturation via inhibition of suppressor T cells in pokeweed mitogen-stimulated cultures. J Exp Med. 1981, 154, 1935–1945. [Google Scholar] [CrossRef]
- Baumgarth, N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011, 11, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H. Human B-1 cells take the stage. Annals of the New York Academy of Sciences. 2013, 1285, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Kawikova I, Paliwal V, Szczepanik M, Itakura A, Fukui M, Campos RA, et al. Airway hyper-reactivity mediated by B-1 cell immunoglobulin M antibody generating complement C5a at 1 day post-immunization in a murine hapten model of non-atopic asthma. Immunology. 2004, 113, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Deng J, Wang X, Chen Q, Sun X, Xiao F, Ko K-H, et al. B1a cells play a pathogenic role in the development of autoimmune arthritis. Oncotarget. 2016, 7, 19299–19311. [Google Scholar] [CrossRef]
- Fournier, C. Where Do T Cells Stand in Rheumatoid Arthritis? Joint Bone Spine. 2005, 72, 527–532. [Google Scholar] [CrossRef]


| sex | Mean ± standard deviation [%] | p-value | |
|---|---|---|---|
| Innate B cells | male | 6.6 ± 4.0 | 0.8 |
| (percent of CD19+ cells ) | female | 6.2 ± 3.7 | |
| Naive B cells | male | 72.0 ± 11.7 | 0.53 |
| (percent of CD19+ cells) | female | 75.3 ± 8.3 | |
| Marginal zone memory B cells | male | 1.7 ± 2.1 | 0.01 |
| (percent of CD19+ cells) | female | 0.8 ± 1.2 | |
| Class switched memory B cells | male | 5.7 ± 4.1 | 0.84 |
| (percent of CD19 + cells) | female | 5.3 ± 2.6 | |
| Late memory B cells | male | 3.2 ± 2.7 | 0.073 |
| (percent of CD19+ cells) | female | 1.7 ± 1.4 | |
| Plasmablasts | male | 5.5 ± 4.0 | 0.84 |
| (percent of CD19+ cells) | female | 5.2 ± 2.6 | |
| Transitonal B cells | male | 82.7 ± 11.0 | 0.89 |
| (percent of CD19+ cells) | female | 85.4 ± 6.7 | |
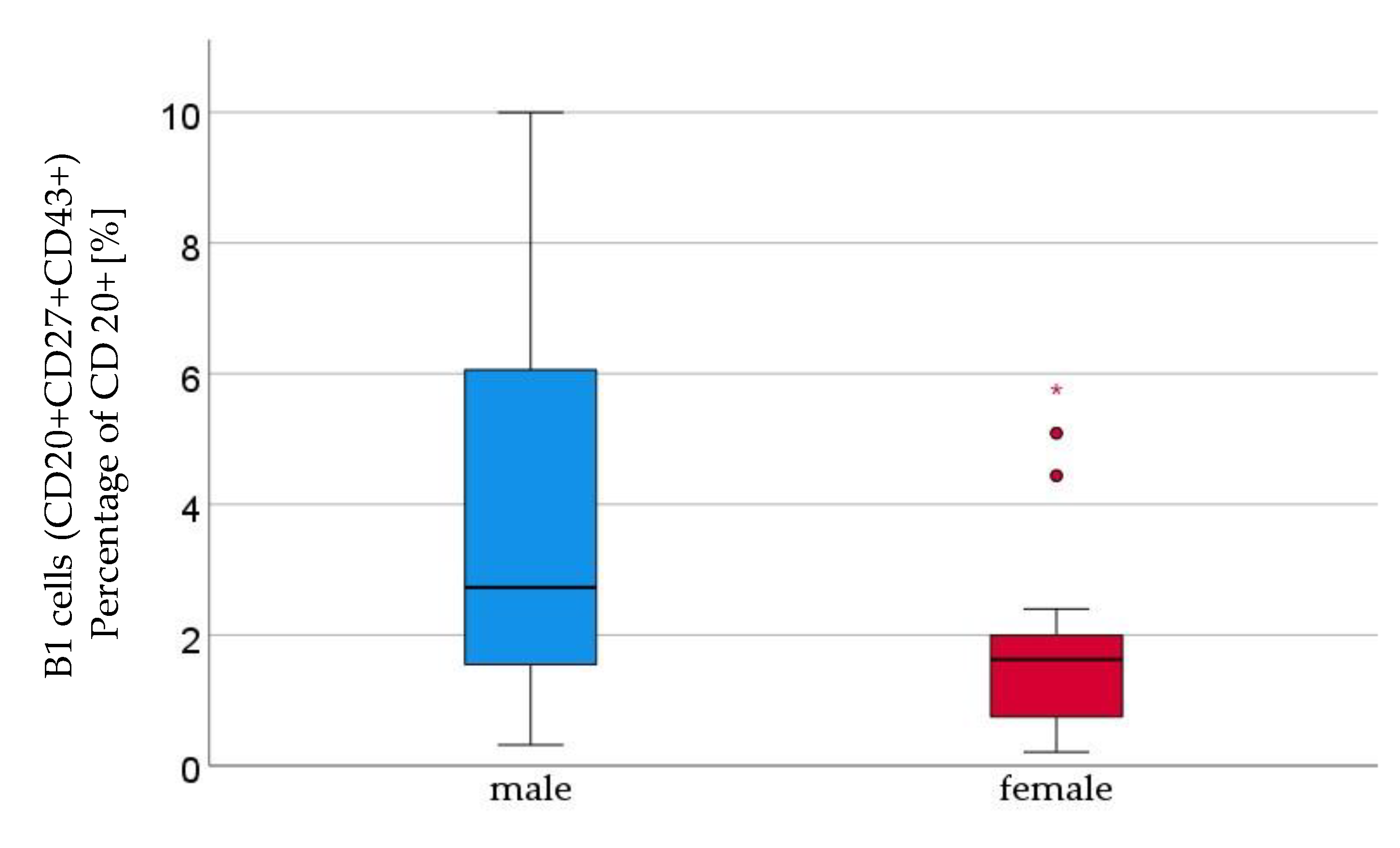
| B1 cells | male | 3.7 ± 2.9 | 0.023 |
| (percent of CD20+ cells) | female | 1.8 ± 1.5 | |
| B2 cells | male | 0.3 ± 0.3 | 0.13 |
| (percent of CD20+ cells) | female | 0.2 ± 0.3 |
| n | sex | Mean ± standard deviation [%] | p-value | |
|---|---|---|---|---|
| Th cells | 16 | male | 74.4 ± 6.6 | 0.22 |
| (percent of CD3+ cells) | 21 | female | 76.8 ± 7.2 | |
| Cytotoxic T cells | 16 | male | 27.9 ± 7.6 | 0.12 |
| (percent of CD3+ cells) | 21 | female | 24.2 ± 7.7 | |
| Th cells with αβ-TCR CD4+CD6+ | 16 | male | 76.0 ± 6.4 | 0.5 |
| (percent of TCRab+ cells) | 21 | female | 77.3 ± 7.1 | |
| Activated cytotoxic Th-cells with αβ-TCR | 16 | male | 0.6 ± 0.6 | 0.27 |
| (percent of TCRab+ cells) | 21 | female | 0.9 ± 0.8 | |
| Memory effector Th cells | 16 | male | 49.5 ± 13.6 | 0.48 |
| (percent of CD3+CD4+ cells) | 21 | female | 52.1 ± 15.8 | |
| Memory central Th cells | 16 | male | 5.7 ± 2.8 | 0.58 |
| (percent of CD3+CD4+ cells) | 21 | female | 6.7 ± 4.1 | |
| Naive effector Th cells | 16 | male | 40.9 ± 15.2 | 0.17 |
| (percent of CD3+CD4+ cells) | 21 | female | 34.9 ± 11.8 | |
| Naive central Th cells | 16 | male | 3.9 ± 1.4 | 0.62 |
| (percent of CD3+CD4+ cells) | 21 | female | 6.4 ± 9.4 | |
| Memory effector cytotoxic T cells | 16 | male | 50.8 ± 13.8 | 0.4 |
| (percent of CD3+CD8+ cells) | 21 | female | 53,.7 ± 14.1 | |
|
Memory central cytotoxic T cells
(percent of CD3+CD8+ cells) |
16 | male | 6.2 ± 4.3 | 0.66 |
| 21 | female | 7.8 ± 6.4 | ||
| Naive effector cytotoxic T cells | 16 | male | 39.4 ± 14.6 | 0.24 |
| (percent of CD3+CD8+ cells) | 21 | female | 34.1 ±1 0.6 | |
| Naive central cytotoxic T cells | 16 | male | 3.6 ± 1.6 | 0.73 |
| (percent of CD3+CD8+ cells) | 21 | female | 4.5 ± 4.1 | |
| Naïve thymus negative Th cells | 16 | male | 24.4 ± 9.8 | 0.005 |
| (percent of CD3+CD4+ cells) | 21 | female | 15.9 ± 5.7 | |
|
Naïve thymus positive Th cells CD31+
(percent of CD3+CD4+ cells) |
16 | male | 65.7 ± 9.4 | 0.6 |
| 21 | female | 70.8 ± 9.6 | ||
|
Th1 cells
(percent of CD3+ cells) |
11 | male | 2.8 ± 1.6 | 0.61 |
| 16 | female | 2.8 ± 1.9 | ||
|
Naive Th1 cells
(percent of CD3+ CD4+ CD183+ cells) |
11 | male | 87.2 ± 7.0 | 0.54 |
| 16 | female | 88.1 ± 6.8 | ||
|
Memory Th1 cells
(percent of CD3+ CD4+ CD183+ cells) |
11 | male | 12.8 ± 7.0 | 0.51 |
| 16 | female | 11.9 ± 6.8 | ||
|
Th2 cells
(percent of number of CD3+ cells) |
11 | male | 0.7 ± 0.5 | 0.54 |
| 16 | female | 0.5 ± 0.3 | ||
|
Naive Th2 cells
(percent of CD3+ CD4+ CRTH2+ cells) |
11 | male | 66.8 ± 8.6 | 0.9 |
| 16 | female | 67.6 ± 11.2 | ||
|
Memory Th2 cells
(percent of CD3+ CD4+ CRTH2+ cells) |
11 | male | 33.2 ± 8.6 | 0.9 |
| 16 | female | 32.4 ± 11.2 | ||
|
Regulatory T cells
(percent of CD3+ cells) |
11 | male | 2.3 ± 0.6 | 0.8 |
| 16 | female | 2.4 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





