Submitted:
12 June 2023
Posted:
12 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Materials
2.2. Preparation of BMF Microparticle
2.3. Preparation of Physically Crosslinked PVA/GC/BMF Hydrogel
2.4. Characteristics
2.4.1. Mechanical performance testing Compression and low cycle fatigue testing
2.4.2. Scanning electron microscopy and infrared spectroscopy testing
2.5. Results and Discussion
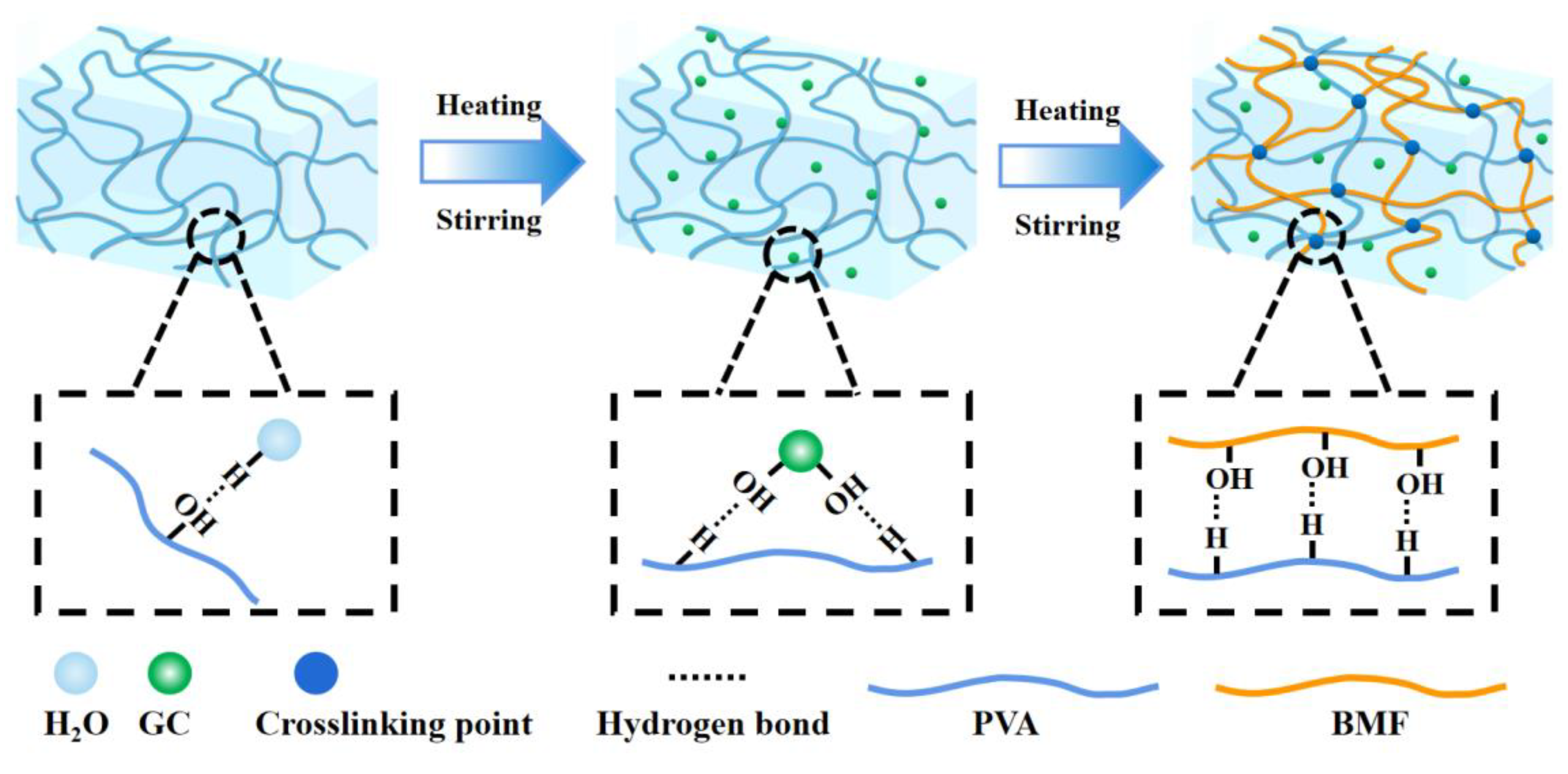
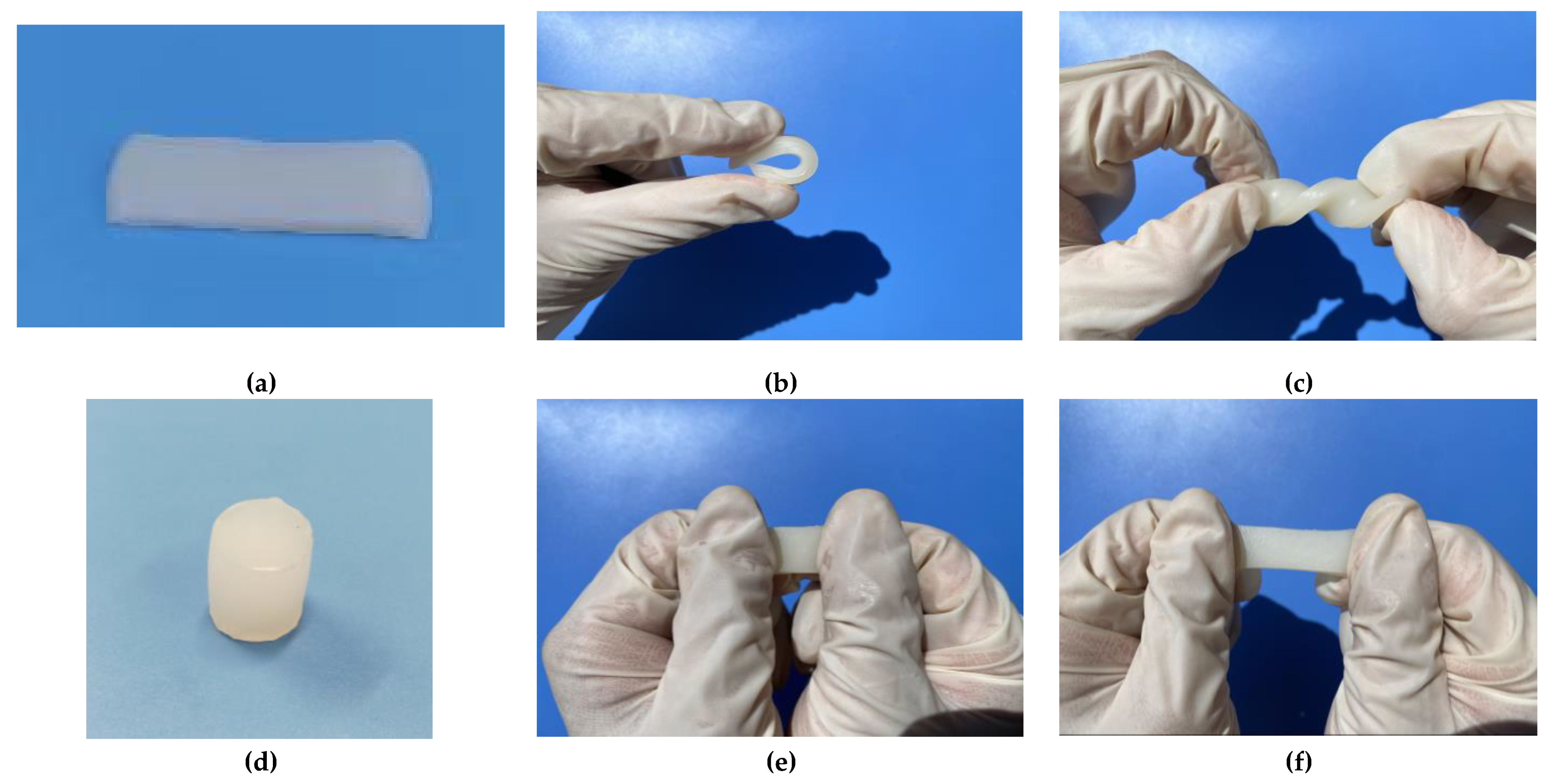
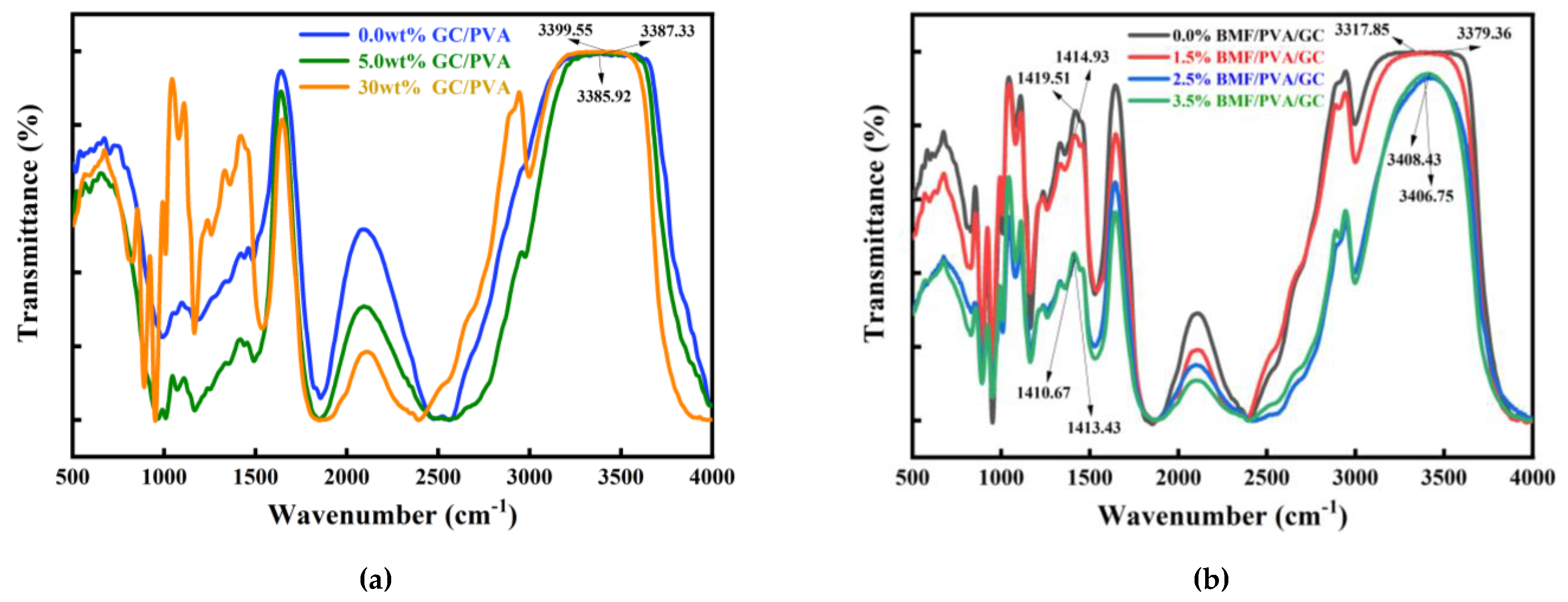
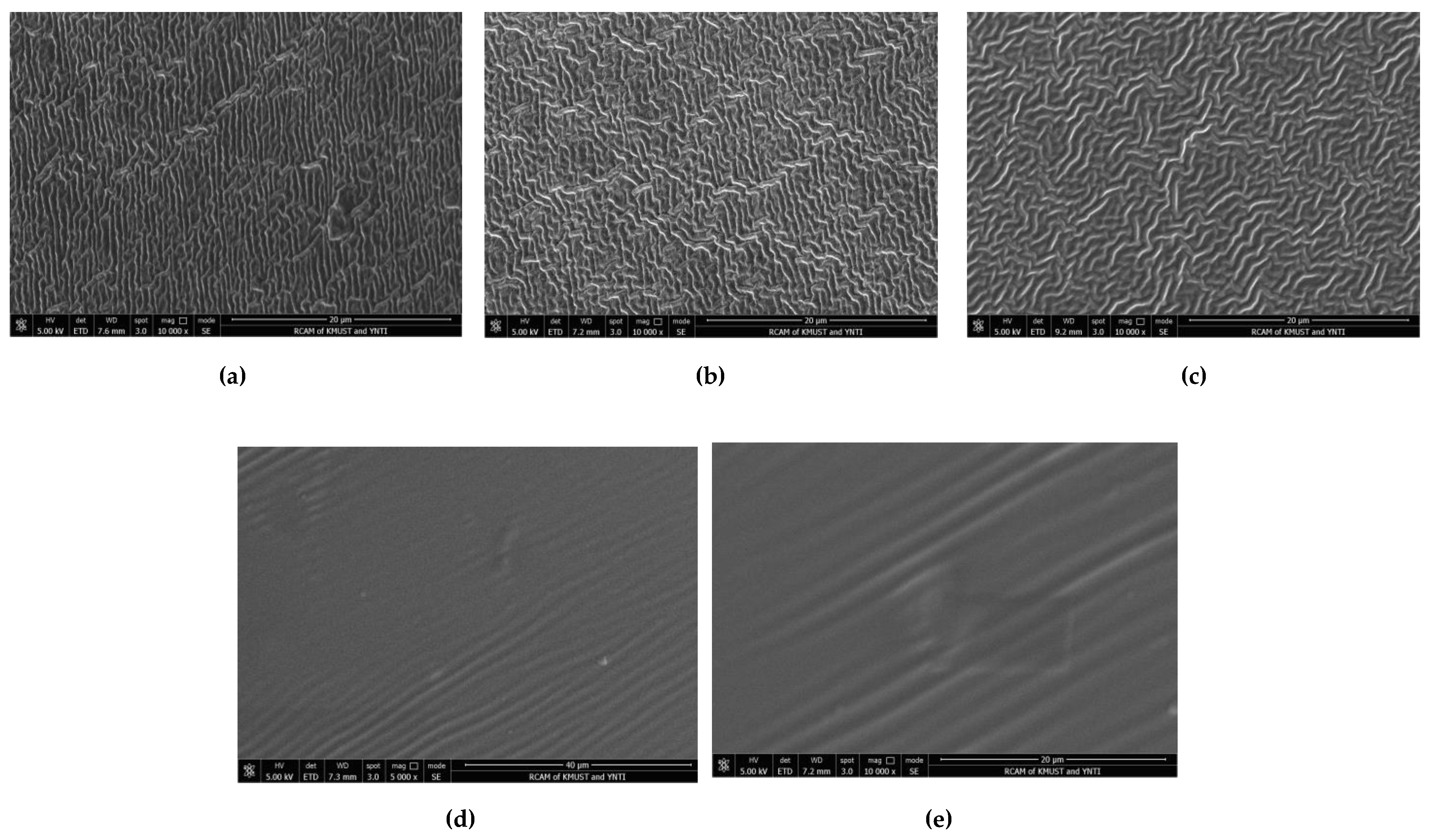
2.5.1. Design strategy and structural characteristics of hydrogel
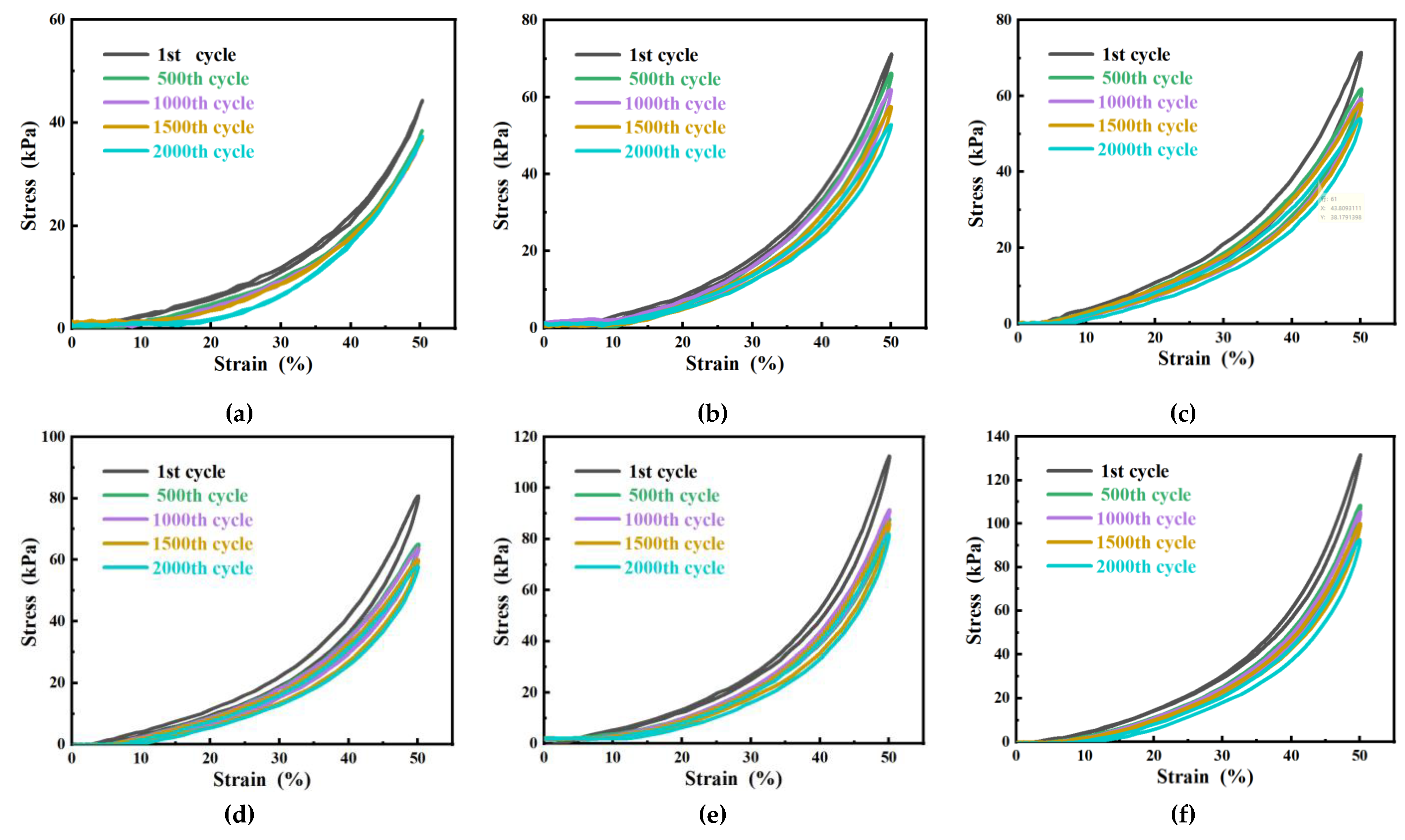
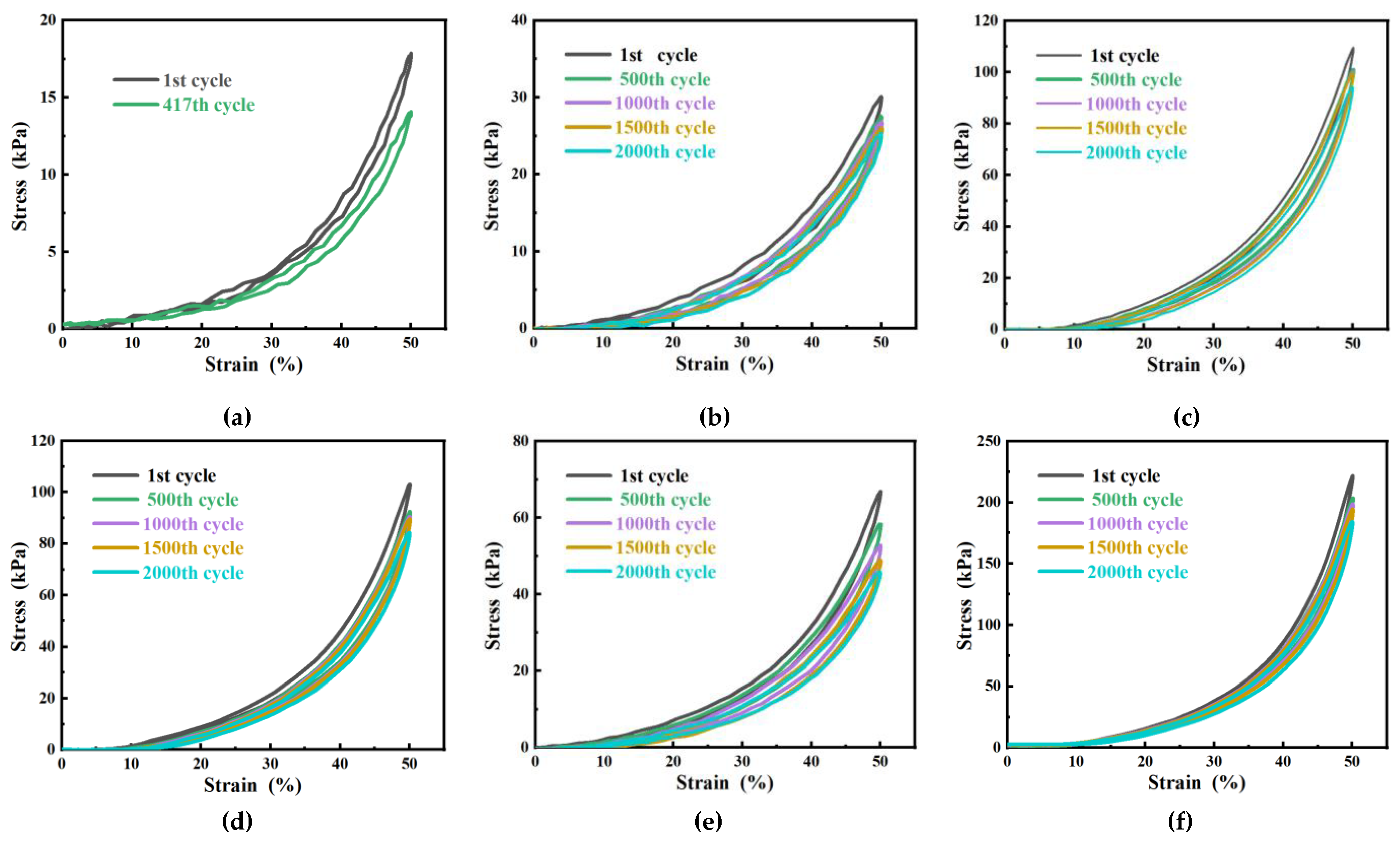
2.5.2. Mechanical property test of hydrogel
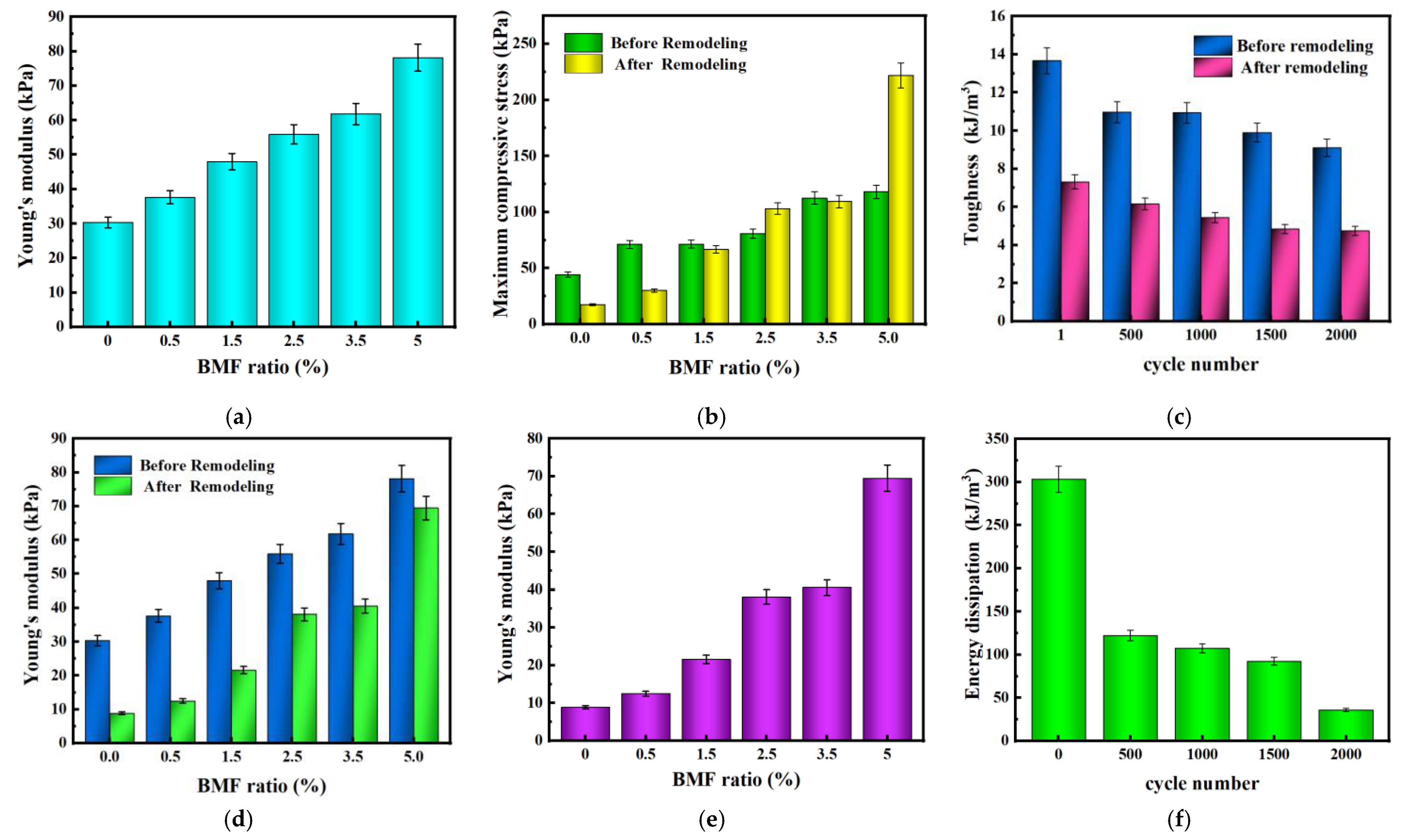
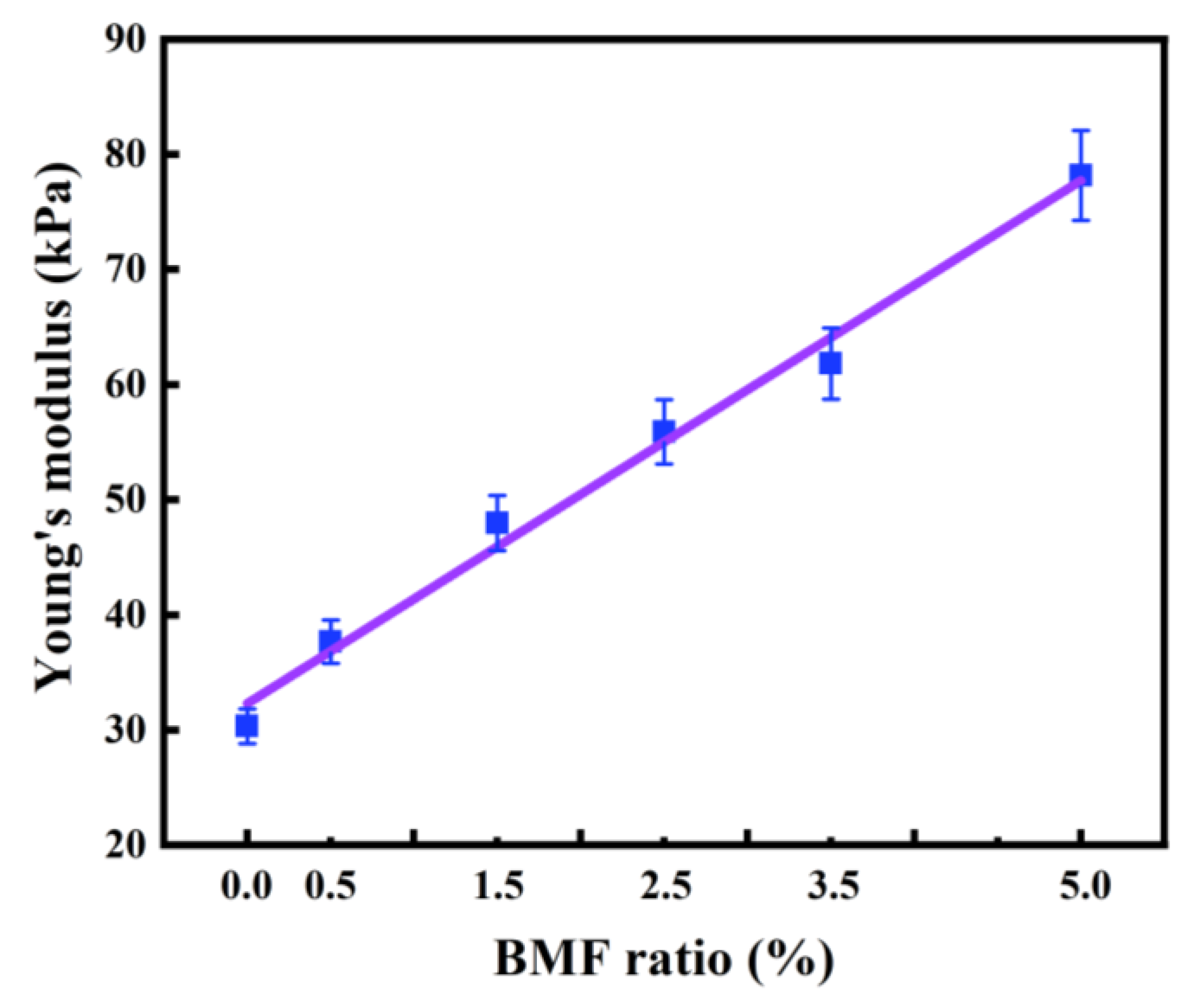
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Wu, Q.; Xiao, H.; Han, J. Self-Recovery, Fatigue-Resistant, and Multifunctional Sensor Assembled by a Nanocellulose/Carbon Nanotube Nanocomplex-Mediated Hydrogel. ACS. Appl Mater Inter. 2021, 42, 13. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, J.; Koshut, W.J.; Watt, J.; Wiley, B.J. A Synthetic Hydrogel Composite with the Mechanical Behavior and Durability of Cartilage. Adv. Funct Mater. 2020, 2003451. [Google Scholar] [CrossRef]
- Liu, T.; Peng, X.; Chen, Y.N.; Bai, Q.W.; Shang, C.; Zhang, L.; Wang, H. Hydrogen-Bonded Polymer–Small Molecule Complex es with Tunable Mechanical Properties. Macromol. Rapid. Comm. 2018, 180, 0050. [Google Scholar]
- Wen, N.; Jiang, B.; Wang, X.; Wang, X.J.; Shang, Z.F.; Jiang, D.W.; Zhang, L.; Sun, C.Y.; Wu, Z.J.; Yan, H.; Liu, C.T.; Guo, Z.H. Overview of Polyvinyl Alcohol Nanocomposite Hydrogels for Electro-Skin, Actuator, Supercapacitor and Fuel Cell. Chem. Rec. 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Kerativitayanan, P.; Carrow, J.K.; Gaharwar, A.K. Nanomaterials for Engineering Stem Cell Responses. Adv. Healthc. Mater. 2015, 4, 1600–1627. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.J.; Peng, X.; Liu, T.Q.; Chen, Y.N.; He, C.C.; Wang, H.L. Facile preparation of hydrogen-bonded supramolecular polyvinyl alcohol-glycerol gels with excellent thermoplasticity and mechanical properties. Polym. Int. 2017. [Google Scholar] [CrossRef]
- Yingkun, S.; Baohu, W.; Shengtong, S.; Peiyi, W. Aqueous spinning of robust, self-healable, and crack-resistant hydrogel microfibers enabled by hydrogen bond nanoconfinement. Nat. Commun. 2023, 14, 1370. [Google Scholar]
- Meng, X.H.; Qiao, Y.; Do, C.; Bras, W.; He, C.Y.; Ke, Y.B.; Russell, T.P.; Qiu, D. Hysteresis-Free Nanoparticle-Reinforced Hydrogels. Adv. Mater. 2021, e2108243. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.P.; Xu, H.X.; Yang, Y; Wang, X.L.; An, W.L.; Hu, Y.; Xu, S.M.A. nonswellable gradient hydrogel with tunable mechanical properties. J Mater Chem. B 2020, 8, 13. [Google Scholar] [CrossRef]
- Dutta, A.; Das, R.K. Dual Cross-Linked Hydrogels with High Strength, Toughness, and Rapid Self-Recovery Using Dynamic Metal–Ligand Interactions. Macromol Mater Eng 2019, 8, 304. [Google Scholar] [CrossRef]
- Mu, Q.F.; Cui, K.P.; Wang, Z.J.; Matsuda, T.; Cui, W.; Kato, H.; Namiki, S.; Yamazaki, T.; Frauenlob, M.; Nonoyama, T.; Tsuda, M.; Tanaka, S.; Nakajima, T.; Gong, J.P. Force-triggered rapid microstructure growth on hydrogel surface for on-demand functions. Nat. Commun. 2022, 13, 6213. [Google Scholar] [CrossRef]
- Xia, S.; Song, S.X.; Gao, G.H. Robust and flexible strain sensors based on dual physically cross-linked double network hydrogels for monitoring human-motion. Chem Eng J 2018, 354. [Google Scholar]
- Wang, X.Q.; Chan, K.H.; Lu, W.H.; Ding, T.P.; Ng, S.W.L.; Cheng, Y.; Li, T.T.; Hong, M.H.; Tee, B.C.K.; Ho, G.W. Macromolecule conformational shaping for extreme mechanical programming of polymorphic hydrogel fibers. Nat. Commun. 2022, 13, 3369. [Google Scholar] [CrossRef]
- Wang, T.T.; Wang, J.Q.; Li, Z.P.; Yue, M.Q.; Qing, X.L.; Zhang, P.X.; Liao, X.Z.; Fan, Z.J.; Yang, S.R. PVA/SA/MXene dual-network conductive hydrogel for wearable sensor to monitor human motions. J Appl Polym Sci 2021. [Google Scholar] [CrossRef]
- Xiao, G.; XinYu, D.; GuiJin, Z.; Huajian, G. Strong and tough fibrous hydrogels reinforc ed by multiscale hierarchical structures with multimechanisms. Sci.Adv. 2023, 9, 11. [Google Scholar]
- Hua, M.; Wu, S.; Ma, Y.; Zhao, Y.; Chen, Z.; Frenkel, I.; Strzalka, J.; Zhou, H.; Zhu, X.; He, X. Strong tough hydrogels via the synergy of freeze-casting and salting out. Nature 2021, 590, 594–599. [Google Scholar] [CrossRef]
- Liang, X.; Chen, G.; Lin, S.; Zhang, J.; Wang, L.; Zhang, P.; Wang, Z.; Wang, Z.; Lan, Y.; Ge, Q.; Liu, J. Anisotropically fatigue-resistant hydrogels. Adv. Mater. 2021, 33, 210. [Google Scholar] [CrossRef] [PubMed]
- Conley, G.M.; Zhang, C.; Aebischer, P.; Harden, J.L.; Scheffold, F. Relationship between rheology and structure of interpenetrating, deforming and compressing microgels. Nat. Commun. 2019, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, Y.; Chen, G.; Chu, Z.; Zhu, Z. Self-healable poly(vinyl alcohol) photonic crystal hydrogel. ACS Applied Polymer Materials, 2020, 2, 5, 2086–2092. [Google Scholar]
- Truong, N.F.; Kurt, E.; Tahmizyan, N.; Lesher-Pérez, S.C.; Chen, M.; Darling, N. J.; Xi, W.; Segura, T. Microporous annealed particle hydrogel stiffness, void space size, and adhesion properties impact cell proliferation, cell spreading, and gene transfer. Acta Biomater. 2019, 94, 160–172. [Google Scholar] [PubMed]
- Morikawa, Y.; Yamagiwa, S.; Sawahata, H.; Numano, R.; Koida, K.; Ishida, M.; Kawano, T. Ultrastretchable kirigami bioprobes. Adv. Healthc. Mater. 2018, 7, 1701100. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T.; Fan, S. Effects of clay content on the properties of nanocomposite hydrogels composed of poly (N-isopropylacrylamide) and clay. Macromolecules 2002, 35, 10162–10171. [Google Scholar] [CrossRef]
- Liang, S.; Wu, Z.L.; Hu, J.; Kurokawa, T.; Yu, Q.M.; Gong, J.P. Direct observation on the surface fracture of ultrathin film double-network hydrogels. Macromolecules 2011, 44, 3016–3020. [Google Scholar] [CrossRef]
- Kim, Y.S.; Liu, M.; Ishida, Y.; Ebina, Y.; Osada, M.; Sasaki, T.; Hikima, T.; Takata, M.; Aida, T. Thermoresponsive actuation enabled by permittivity switching in an electrostatically anisotropic hydrogel. Nat. Mater. 2015, 14, 1002–1007. [Google Scholar] [CrossRef]
- Huang, X.; Ge, G.; She, M.; Ma, Q.; Lu, Y.; Zhao, W.; Shen, Q.; Wang, Q.; Shao, J. Self-healing hydrogel with multiple dynamic interactions for multifunctional epidermal sensor. Appl. Surf. Sci. 2022, 598, 153803. [Google Scholar] [CrossRef]
- Xin, H. Double-Network Tough Hydrogels: A Brief Review on Achievements and Challenges. Gels 2022, 8, 247. [Google Scholar] [CrossRef]
- Nonoyama, T.; Wada, S.; Kiyama, R.; Kitamura, N.; Mredha, M.T.I.; Zhang, X.; Kurokawa, T.; Nakajima, T.; Takagi, Y.; Yasuda, K. Double-network hydrogels strongly bondable to bones by spontaneous osteogenesis penetration. Adv. Mater. 2016, 28, 6740–6745. [Google Scholar] [CrossRef]
- Arakaki, K.; Kitamura, N.; Fujiki, H.; Kurokawa, T.; Iwamoto, M.; Ueno, M.; Kanaya, F.; Osada, Y.; Gong, J.P.; Yasuda, K. Artificial cartilage made from a novel double-network hydrogel: In vivo effects on the normal cartilage and ex vivo evaluation of the friction property. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 93, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Sun, Y.N.; Gao, G.R.; Du, G.L.; Cheng, Y.J.; Fu, J. Super Tough, Ultrastretchable, and Thermoresponsive Hydrogels with Functionalized Triblock Copolymer Micelles as Macro-Cross-Linkers. ACS Macro. Lett. 2014, 3, 496–500. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Mooney, D.J. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015, 6, 6365. [Google Scholar] [CrossRef]
- Cacopardo, L.; Guazzelli, N.; Nossa, R.; Mattei, G.; Ahluwalia, A. Engineering hydrogel viscoelasticity. J. Mech. Behav. Biomed. Mater. 2019, 89, 162–167. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Yuk, H.; Lin, S.; Liu, X.; Parada, G. Soft Materials by Design: Unconventional Polymer Networks Give Extreme Properties. Chem. Rev. 2021, 121, 4309–4372. [Google Scholar] [CrossRef]
- Wang, Z.J.; Jiang, J.; Mu, Q.; Maeda, S.; Nakajima, T.; Gong, J.P. Azo-Crosslinked Double-Network Hydrogels Enabling Highly Efficient Mechanoradical Generation. J. Am. Chem. Soc. 2022, 144, 3154–3161. [Google Scholar] [CrossRef]
- Millereau, P.; Ducrot, E.; Clough, J.M.; Wiseman, M.E.; Brown, H.R.; Sijbesma, R.P.; Creton, C. Mechanics of elastomeric molecular composites. Proc. Natl. Acad. Sci. USA 2018, 115, 9110–9115. [Google Scholar] [CrossRef]
- Gong, J.P. Materials both tough and soft. Science 2014, 344, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 5124. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Choi, S.; Choi, Y.; Kim, J. Anisotropic hybrid hydrogels with superior mechanical properties reminiscent of tendons or ligaments. Adv. Funct. Mater. 2019, 29, 1904342. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D. A Universal Soaking Strategy to Convert Composite Hydrogels into Extremely Tough and Rapidly Recoverable Double-Network Hydrogels. Adv. Mater. 2016, 28, 7178–7184. [Google Scholar] [CrossRef]
- Yu, J.; Xu, K.; Chen, X.; Zhao, X.; Yang, Y.; Chu, D.; Xu, Y.; Zhang, Q.; Zhang, Y.; Cheng, Y. Highly Stretchable, Tough, Resilient, and Antifatigue Hydrogels Based on Multiple Hydrogen Bonding Interactions Formed by Phenylalanine Derivatives. Biomacromolecules 2021, 22, 1297–1304. [Google Scholar] [CrossRef]
- Ahmed, S.; Nakajima, T.; Kurokawa, T.; Haque, M.A.; Gong, J.P. Brittle–ductile transition of double network hydrogels: Mechanical balance of two networks as the key factor. Polymer 2014, 55, 914–923. [Google Scholar] [CrossRef]
- Chen, G.; Tang, W.W.; Wang, X.H.; Zhao, X.L.; Chen, C.; Zhu, Z.G. Applications of Hydrogels with Special Physical Properties in Biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-Inspired Multifunctional Autonomic-Intrinsic Conductive Self-Healing Hydrogels with Pressure Sensitivity, Stretchability, and 3D Printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef]
- Cui, K.; Sun, T.L.; Liang, X.; Nakajima, K.; Ye, Y.N.; Chen, L.; Kurokawa, T.; Gong, J.P. Multiscale energy dissipation mechanism in tough and self-healing hydrogels. Phys. Rev. Lett. 2018, 121, 185501. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, J.; Chen, Y.; Zhang, L.; Zhou, J. Dual Physical Crosslinking Strategy to Construct Moldable Hydrogels with Ultrahigh Strength and Toughness. Adv. Funct. Mater. 2018, 28, 1800739. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, K.; Fang, L.; Zhang, H.; Tang, Y.; Ding, Y.; Weng, L.-T.; Xu, J.; Weng, J. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017, 9, e372. [Google Scholar] [CrossRef]
- Samp, M.A.; Iovanac, N.C.; Nolte, A.J. Sodium Alginate Toughening of Gelatin Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3176–3182. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Jin, Z. Tough, Swelling-Resistant, Self-Healing, and Adhesive Dual-Cross-Linked Hydrogels Based on Polymer–Tannic Acid Multiple Hydrogen Bonds. Macromol. 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- El Salmawi, K.M. Gamma radiation-induced cross-linked PVA/chitosan blends for wound dressing. J. Macromol. Sci. Part A-Pure Appl. Chem. 2007, 44, 541–545. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, X.; Ouchi, T.; Kouznetsova, T.B.; Beech, H.K.; Av-Ron, S.; Matsuda, T.; Bowser, B.H.; Wang, S.; Johnson, J.A.; et al. Toughening hydrogels through force-triggered chemical reactions that lengthen polymer strands. Science 2021, 374, 193–196. [Google Scholar] [CrossRef]
- Fei, C.; Huang, D.; Feng, S. Adsorption behavior of amphoteric double-network hydrogel based on poly(acrylic acid) and silica gel. J. Polym. Res. 2012, 19, 1–7. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically cross-linked with glutaraldehyde. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Lin, S.; Liu, J.; Liu, X.; Zhao, X. Muscle-like fatigue-resistant hydrogels by mechanical training. Proc. Natl. Acad. Sci. USA 2019, 116, 10244–10249. [Google Scholar] [CrossRef]
- Wang, P.; Wu, M.; Li, R.; Cai, Z.; Zhang, H. Fabrication of a Double-Network Hydrogel Based on Carboxymethylated Curdlan/Polyacrylamide with Highly Mechanical Performance for Cartilage Repair. ACS Appl. Polym. Mater. 2021, 3, 5857–5869. [Google Scholar] [CrossRef]
- Ma, M.Y.; Liu, S.H.; Chen, S.F. Strong double network hydrogels reinforced by silk fibroin microfibers. J. Chem. Eng. Chin. Univ. 2021, 35, 148–154. [Google Scholar]
- Li, S.; Wang, X.; Zhu, J.; Wang, Z.; Wang, L. Preparation and characterization of double network hydrogel with high-strength and self-healing. Mater. Today Commun. 2021, 27, 102450. [Google Scholar] [CrossRef]
- Kimura, T.; Urayama, K. Multiaxial stress relaxation of dual-cross-link Poly(vinyl alcohol) hydrogels. ACS Macro Lett. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Majmudar, T.S.; Behringer, R.P. Contact force measurements and stress-induced anisotropy in granular materials. Nat. 2005, 435, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, S.; Weitz, D.A. Microfluidic fabrication of smart microgels from macromolecular p re cursors. Polym. 2010, 51, 5883–5889. [Google Scholar]
- Nasrollahzadeh, N.; Karami, P.; Pioletti, D.P. Control of Dissipation Sources: A Central Aspect for Enhancing the Mechanical and Mechanobiological Performances of Hydrogels. ACS App Mater & Inter 2019. [Google Scholar]
- Lu, Y.; Yue, Y.Y.; Ding, Q.Q.; Mei, C.T.; Xu, X.W.; Wu, Q.L.; Xiao, H.N.; Han, J.Q. Self-Recovery, Fatigue-Resistant, and Multifunctional Sensor Assembled by a Nanocellulose/Carbon Nanotube Nanocomplex-Mediated Hydrogel. ACS App Mater & Inter 2021, 42, 13. [Google Scholar]
- Zhang, F.; Xiong, L.G.; Ai, Y.J.; Liang, Z.; Liang, Q.L. Stretchable Multiresponsive Hydrogel with Actuatable, Shape Memory, and Self-Healing Properties. Adv. Sci 2018, 5, 8. [Google Scholar] [CrossRef]
- Young, D.A.; Ibrahim, D.O.; Hu, D.; Christman, K.L. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2011, 7, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Nurazzi, N.M.; Harussani, M.M.; Aisyah, H.A.; Ilyas, R.A.; Norrrahim, M.N.F.; Khalina, A.; Abdullah, N. Treatments of natural fiber as reinforcement in polymer composites—A short review. Funct. Compos. Struct. 2021, 3, 024002. [Google Scholar] [CrossRef]
- Guo, X.W; Li, J.; Wang, J.X.; Huang, L.Q.; Cheng, G.J.; Zhang, Q.; Zhu, H.; Zhang, M.Y.; Zhu, S.P. Stretchable Hydrogels with Low Hysteresis and High Fracture Toughness for Flexible Electronics. Macromol Rapid Comm 2022, 43, e21007,16. [Google Scholar] [CrossRef]
- Hossain, S.I.; Hasan, M.; Hasan, M.N.; Hassan, A. Effect of chemical treatment on physical, mechanical and thermal properties of ladies finger natural fiber. Adv. Mater. Sci. Eng. 2013, 2013, 824274. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Li, Y.; Zhang, Y.; Geng, L.; Shen, F.; Ren, J. Hydrogelation Landscape Engineering and a Novel Strategy to Design Radically Induced Healable and Stimuli-Responsive Hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 19605–19612. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tian, Y.; Tong, Q.; Zhang, Z.; Jin, Z. Effect of Pullulan on the Water Distribution, Microstructure and Textural Properties of Rice Starch Gels during Cold Storage. Food Chem 2017, 214, 702–709. [Google Scholar] [CrossRef]
- Gong, J. P. Why are Double Network Hydrogels so Tough. Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, F.; Shen, H.; Wu, D. A Universal Soaking Strategy to Convert Composite Hydrogels into Extremely Tough and Rapidly Recoverable Double-Network Hydrogels. Adv. Mater 2016, 28, 7178–7184. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J. T.; Kostiainen, M. A.; Santos, H. A. Properties and Chemical Modifications of Lignin: towards Lignin-based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Salvati, E.; Brandt, L.R; Uzun, F.; Zhang, H.; Papadaki, C.; Korsunsky, A.M. Multiscale analysis of bamboo deformation mechanisms following NaOH treatment using X-ray and correlative microscopy. Acta Biomater 2018. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.L.; Hse, C.Y; De Hoop, C.F.; Hu, T.X.; Qi, J.Q.; Shupe, T.F. Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr. Polym. 2016, 151, 725–734. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, W.; Wen, Y. Improving the production of nanofibrillated cellulose from bamboo pulp by the combined cellulase and refining treatment. J Chemical Tech & Biotech 2019. [Google Scholar]
- Wu, Y.; Wu, X.Y.; Shi, T.L.; Chen, H.; Wang, H.K.; Sun, M.; Zhang, J.L. The Microstructure and Mechanical Properties of Poplar Catkin Fibers Evaluated by Atomic Force Microscope (AFM) and Nanoindentation. Forests 2019, 10, 938. [Google Scholar] [CrossRef]
- Khalil, H.; Bhat, I.; Jawaid, M.; Zaidon, A.; Hermawan, D.; Hadi, Y.S. Bamboo fibre reinforced bio composites: a review. Materi & Des 2012, 42, 353–368. [Google Scholar]
- Rosa, M.F.; Chiou, B.S.; Medeiros, E.S.; Wood, D.F.; Williams, T.G.; Mattoso, L.H.C.; Orts, W.J.; Imam, S.H. Effect of fiber treatments on tensile and thermal properties of starch/ethylene vinyl alcohol copolymers/coir biocomposites. Bioresour. Technol. 2009, 100, 5196–5202. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 11, 37. [Google Scholar] [CrossRef]
- Gratani, L.; Crescente, M.F.; Varone, L.; Fabrini, G.; Digiulio, E. Growth pattern and photosynthetic activity of different bamboo species growing in the Botanical Garden of Rome. Flora - Morphology, Distribution, Funct. Ecolo. Plan. 2008, 203, 77–84. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Arpitha, G.R.; Yogesha, B. Study on Mechanical Properties of Natural - Glass Fibre Reinforced Polymer Hybrid Composites: A Review. Materi. Tod. Procee. 2015, 2, 2959–2967. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Manickam, S.; Ratnam, C.T.; Raghu, M.S.; Parashuram, L.; Prashantha, K.; Jeon, B.H. Surface-treated short sisal fibers and halloysite nanotubes for synergistically enhanced performance of polypropylene hybrid composites. J. Thermoplast. Compos. Mater. 2020, 0892705720946063. [Google Scholar] [CrossRef]
- Yusoff, R.B.; Takagi, H.; Nakagaito, A.N. Tensile and flexural properties of polylactic acid-based hybrid green composites reinforced by kenaf, bamboo and coir fibers. Industl Crop & Pro 2016, 94, 562–573. [Google Scholar]
- Soutis, C. Carbon fiber reinforced plastics in aircraft construction. Mater. Sci. Eng. A. 2005, 412, 171–176. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Zamani, M.H.; Estaji, S.; Tayouri, M.I.; Arjmand, M.; Jafari, S.H.; Nouranian, S.; Khonakdar, H.A. Mechanical properties of bamboo fiber-reinforced polymer composites: a review of recent case studies. J Mater Sci. 2022, 57, 3143–3167. [Google Scholar] [CrossRef]
- Ismail, H.; Edyham, M.R.; Wirjosentono, B. Bamboo fibre filled natural rubber composites: The effects of filler loading and bonding agent. Polym Test 2002, 21, 139–144. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; Ilyas, R.A.; Zainudin, E.S.; Abdan, K. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polym. 2021, 13, 2710. [Google Scholar] [CrossRef] [PubMed]
- Okubo, K.; Fujii, T.; Yamamoto, Y. Development of bamboo-based polymer composites and their mechanical properties. Compos Part A-Appl S 2004, 35, 377–383. [Google Scholar] [CrossRef]
- Thwe, M.M.; Liao, K. Effects of environmental aging on the mechanical properties of bamb oo-glass fiber reinforced polymer matrix hybrid composites. Compos Part A-Appl S 2002, 33, 43–52. [Google Scholar] [CrossRef]
- Shih, Y.F. Mechanical and thermal properties of waste water bamboo husk fiber reinforced epoxy composites. Mater Sci & Eng A 2007, 445, 289–295. [Google Scholar]
- Guimaraes, M.; Botaro, V.R.; Novack, K.M.; Teixeira, F.G.; Tonoli, G.H.D. Starch/PVA-based nanocomposites reinforced with bamboo nanofibrils. Ind Crop Prod 2015, 70, 72–83. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Ishak, M.R.; Sapuan, S.M.; Yidris, N. Influence of Additional Bracing Arms as Reinforcement Members in Wooden Timber Cross-Arms on Their Long-Term Creep Responses and Properties. Appl. Sci. 2021, 11, 2061. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, Z.; Wang, H. The effect of freezing speed and hydrogel concentration on the microstructure and compressive performance of bamboo-based cellulose aerogel. Journal of Wood Science 2015, 61, 595–601. [Google Scholar] [CrossRef]
- Xu, K.; Narayanan, K.; Lee, F.; Bae, K.H.; Gao, S.; Kurisawa, M. Enzyme-mediated hyaluronic acid–tyramine hydrogels for the propagation of human embryonic stem cells in 3D. Acta Biomater. 2015, 24, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Vu, C.M.; Vu, H.T.; Choi, H.J. Micron-size white bamboo fibril-based silane cellulose aerogel: Fabrication and oil absorbent characteristics. Mater 2019, 12, 1407. [Google Scholar] [CrossRef] [PubMed]
- Norul Izani, M.A.; Paridah, M.T.; Anwar, U.M.K.; Mohd Nor, M.Y.; H’Ng, P.S. Effects of fiber treatment on morphology, tensile and thermogravimetric analysis of oil palm empty fruit bunches fibers. Compos. Part B Eng. 2013, 45, 1251–1257. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA Hydrogel Properties for Biomedical Application. J. Mech. Behav. Biomed. Mater 2011, 4, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C. M.; Peppas, N. A. Structure and Morphology of Freeze/Thawed PVA Hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
| Number | Ratio of BMF | PVA | H2O | GC | BMF |
|---|---|---|---|---|---|
| 1 | 0.0wt% | 10g | 60g | 30g | 0.00g |
| 2 | 0.5wt% | 10g | 60g | 30g | 0.50g |
| 3 | 1.5wt% | 10g | 60g | 30g | 1.52g |
| 4 | 2.5wt% | 10g | 60g | 30g | 2.56g |
| 5 | 3.5wt% | 10g | 60g | 30g | 3.63g |
| 6 | 5.0wt% | 10g | 60g | 30g | 5.26g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





