Submitted:
09 June 2023
Posted:
12 June 2023
You are already at the latest version
Abstract
Keywords:
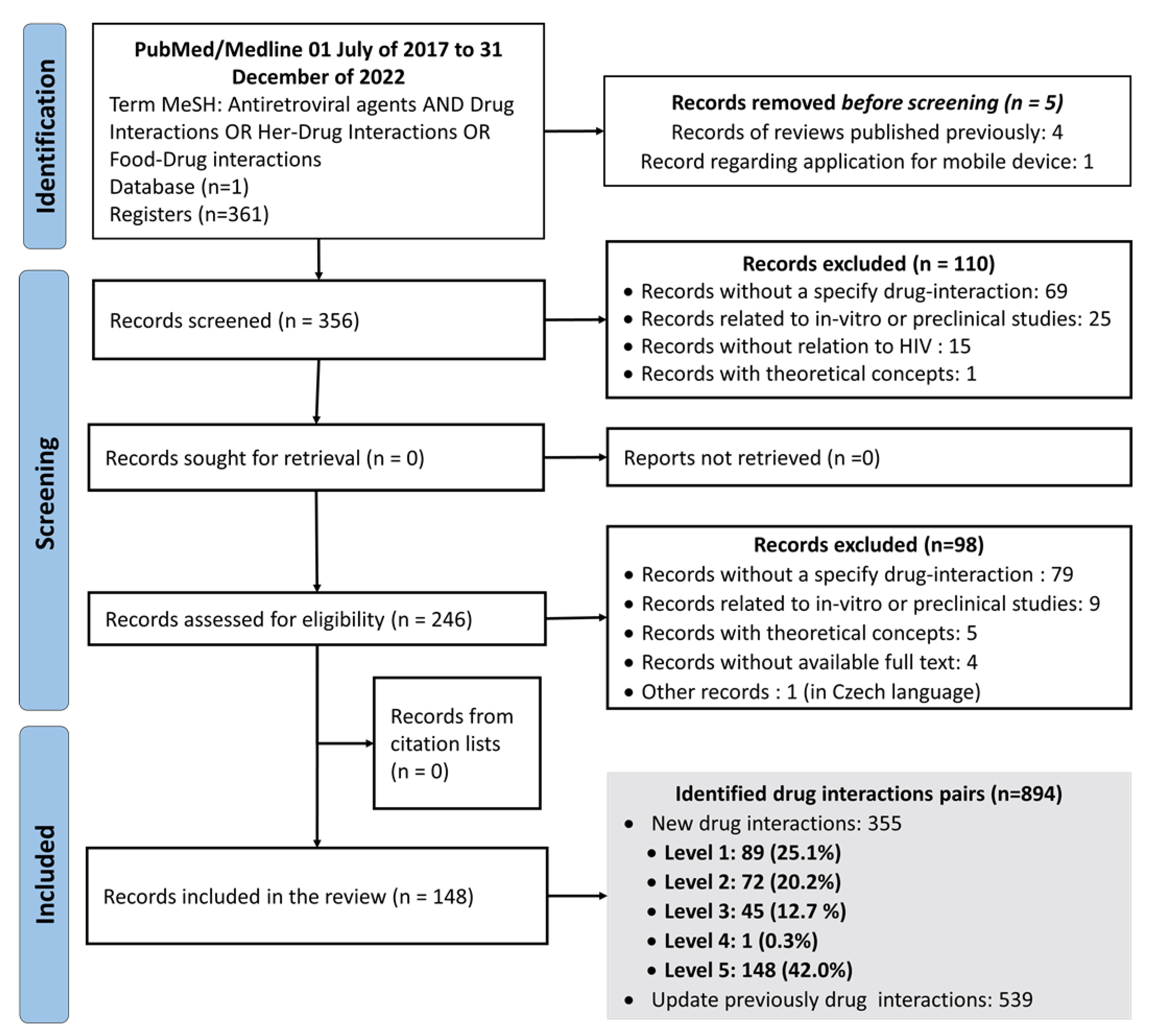
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. HIV/AIDS. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed on 10 February 2022).
- Gandhi RT, Bedimo R, Hoy JF, Landovitz RJ, Smith DM, Eaton EF, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2023, 329, 63–84.
- Amariles P, Giraldo NA, Faus MJ. Interacciones medicamentosas: aproximación para establecer y evaluar su relevancia clínica. Med Clin (Barc). 2007, 129, 27–35.
- Amariles P, Madrigal-Cadavid J, Giraldo NA. Clinical relevance of drug interactions: Proposal to update the classification based on the severity and probability of its occurrence. Rev Chil Infectologia Organo Of Soc Chil Infectologia. 2021, 38, 304–5.
- Amariles, P.; Giraldo, N.; Faus, M. Interacciones medicamentosas en pacientes infectados con el VIH: aproximación para establecer y evaluar su relevancia clínica. Farm. Hosp. 2007, 31, 283–302. [Google Scholar] [CrossRef]
- Giraldo, N.; Amariles, P.; Gutiérrez, F.; Monsalve, M.; Faus, M. Aproximación para establecer y evaluar la relevancia clínica de las interacciones medicamentosas en pacientes infectados con virus de la inmunodeficiencia humana: actualización 2009. Farm. Hosp. 2010, 34, 90–93. [Google Scholar] [CrossRef]
- A Giraldo, N.; Amariles, P.; Marín, D.E.P.; Faus, M.J. Relevancia clínica de las interacciones medicamentosas en pacientes infectados con el virus de la inmunodeficiencia humana: actualización 2009-2014. 2016, 33, 36–53. [CrossRef]
- Osorio, T. L, Rivera C. M, Pino-Marín DE, Giraldo NA, Amariles P, Osorio T. L, et al. Relevancia clínica de las interacciones medicamentosas en pacientes infectados con el virus de la inmunodeficiencia humana: actualización 2015-2017. Rev Chil Infectol. 2019, 36, 475–89. [Google Scholar]
- Giraldo, N.; Amariles, P.; Monsalve, M.; Faus, M. Free software to analyse the clinical relevance of drug interactions with antiretroviral agents (SIMARV®) in patients with HIV/AIDS. Res. Soc. Adm. Pharm. 2017, 13, 831–839. [Google Scholar] [CrossRef]
- Amariles P, Madrigal Cadavid J, Granados J, Giraldo N. Interapp ARV: aplicación móvil para analizar la relevancia clínica de interacciones de antirretrovirales. Vitae. 2019, 26:S33-6.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021, 372:n71.
- Hastain, N.V.; Santana, A.; Schafer, J.J. The Incidence and Severity of Drug Interactions Before and After Antiretroviral Therapy Simplification in Treatment-Experienced Patients With HIV Infection. Ann. Pharmacother. 2019, 54, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Marzolini, C.; Livio, F. Prescribing issues in elderly individuals living with HIV. Expert Rev. Clin. Pharmacol. 2019, 12, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Tyrberg, E.; Edén, A.; Eriksen, J.; Nilsson, S.; Treutiger, C.J.; Thalme, A.; Mellgren. ; Gisslén, M.; Andersson, L.-M. Higher plasma drug levels in elderly people living with HIV treated with darunavir. PLOS ONE 2021, 16, e0246171. [Google Scholar] [CrossRef] [PubMed]
- Zautner, A.E.; Herchenröder, O.; El Moussi, A.; Schwarz, N.G.; Wiemer, D.F.; Groß, U.; Frickmann, H. Pharmaceutical interactions between antiretroviral and antimalarial drugs used in chemoprophylaxis. Acta Trop. 2018, 179, 25–35. [Google Scholar] [CrossRef]
- Haaland, R.E.; Otieno, K.; Martin, A.; Katana, A.; Dinh, C.; Slutsker, L.; Menendez, C.; Gonzalez, R.; Williamson, J.; Heneine, W.; et al. Short Communication: Reduced Nevirapine Concentrations Among HIV-Positive Women Receiving Mefloquine for Intermittent Preventive Treatment for Malaria Control During Pregnancy. AIDS Res. Hum. Retroviruses 2018, 34, 912–915. [Google Scholar] [CrossRef]
- Meintjes, G.; Brust, J.C.M.; Nuttall, J.; Maartens, G. Management of active tuberculosis in adults with HIV. Lancet HIV 2019, 6, e463–e474. [Google Scholar] [CrossRef]
- Chastain DB, Franco-Paredes C, Stover KR. Addressing Antiretroviral Therapy-Associated Drug-Drug Interactions in Patients Requiring Treatment for Opportunistic Infections in Low-Income and Resource-Limited Settings: Journal of Clinical Pharmacology. J Clin Pharmacol. 2017, 57, 1387–99.
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and induction of CYP enzymes in humans: an update. Arch. Toxicol. 2020, 94, 3671–3722. [Google Scholar] [CrossRef]
- Cerrone, M.; Bracchi, M.; Wasserman, S.; Pozniak, A.; Meintjes, G.; Cohen, K.; Wilkinson, R.J. Safety implications of combined antiretroviral and anti-tuberculosis drugs. Expert Opin. Drug Saf. 2019, 19, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.; Moss, C.E.; Marzolini, C.; Khoo, S. Clinical Pharmacodynamics, Pharmacokinetics, and Drug Interaction Profile of Doravirine. Clin. Pharmacokinet. 2019, 58, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Freise, K.J.; Hu, B.; Salem, A.H. Impact of ritonavir dose and schedule on CYP3A inhibition and venetoclax clinical pharmacokinetics. Eur. J. Clin. Pharmacol. 2018, 74, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Mutiti, C.S.; Kapungu, N.N.; Kanji, C.R.; Stadler, N.; Stingl, J.; Nhachi, C.; Hakim, J.; Masimirembwa, C.; Thelingwani, R.S. Clinically relevant enantiomer specific R- and S-praziquantel pharmacokinetic drug-drug interactions with efavirenz and ritonavir. Pharmacol. Res. Perspect. 2021, 9, e00769. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.A.; Tseng, A.; Cooper, R. Managing drug interactions in HIV-infected adults with comorbid illness. Can. Med Assoc. J. 2014, 187, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chary, A.; Nguyen, N.N.; Maiton, K.; Holodniy, M. A review of drug-drug interactions in older HIV-infected patients. Expert Rev. Clin. Pharmacol. 2017, 10, 1329–1352. [Google Scholar] [CrossRef]
- Patel, R.C.; Stalter, R.M.; Thomas, K.K.; Tamraz, B.; Blue, S.W.; Erikson, D.W.; Kim, C.J.; Kelly, E.J.; Nanda, K.; Kourtis, A.P.; et al. A pharmacokinetic and pharmacogenetic evaluation of contraceptive implants and antiretroviral therapy among women in Kenya and Uganda. AIDS 2019, 33, 1995–2004. [Google Scholar] [CrossRef]
- Neary, M.; A Chappell, C.; Scarsi, K.K.; Nakalema, S.; Matovu, J.; Achilles, S.L.; A Chen, B.; Siccardi, M.; Owen, A.; Lamorde, M. Effect of patient genetics on etonogestrel pharmacokinetics when combined with efavirenz or nevirapine ART. J. Antimicrob. Chemother. 2019, 74, 3003–3010. [Google Scholar] [CrossRef]
- Kosloski, M.P.; Oberoi, R.; Wang, S.; Viani, R.M.; Asatryan, A.; Hu, B.; Ding, B.; Qi, X.; Kim, E.J.; Mensa, F.; et al. Drug-Drug Interactions of Glecaprevir and Pibrentasvir Coadministered With Human Immunodeficiency Virus Antiretrovirals. J. Infect. Dis. 2019, 221, 223–231. [Google Scholar] [CrossRef]
- Starbird, L.E.; Hong, H.P.; Sulkowski, M.S.; Farley, J.E.P. Management of the Patient With HIV/Hepatitis C Drug Interactions: A Guide for Nurses and Nurse Practitioners. J. Assoc. Nurses AIDS Care 2020, 31, 241–248. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, O.; Back, D. Drug Interactions of Hepatitis C Direct-Acting Antivirals in the HIV-Infected Person. Curr. HIV/AIDS Rep. 2015, 12, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, A.A.; Patel, T.; Saad, S.; Renner, E.; Mouland, E.; Adie, S.; Ha, N.B. Management of anticoagulation in patients with human immunodeficiency virus/acquired immunodeficiency virus. Thromb. Res. 2021, 200, 102–108. [Google Scholar] [CrossRef]
- Chen, G.-L.; Lin, S.-Y.; Lo, H.-Y.; Wu, H.-C.; Lin, Y.-M.; Chen, T.-C.; Chu, C.-Y.S.; Lee, W.-C.; Chen, Y.-H.; Lu, P.-L. Clinical impact of recreational drug use among people living with HIV in southern Taiwan. J. Microbiol. Immunol. Infect. 2020, 54, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Castro-Granell V, Garin N, Jaén Á, Cenoz S, Galindo MJ, Fuster-RuizdeApodaca MJ. Prevalence, beliefs and impact of drug-drug interactions between antiretroviral therapy and illicit drugs among people living with HIV in Spain. Torti C, editor. PLOS ONE. 2021, 16, e0260334.
- Ryom, L.; Cotter, A.; De Miguel, R.; Béguelin, C.; Podlekareva, D.; Arribas, J.; Marzolini, C.; Mallon, P.; Rauch, A.; Kirk, O.; et al. 2019 update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020, 21, 617–624. [Google Scholar] [CrossRef]
- O’kelly, B.B.M.B.B.M.; Murtagh, R.; Lambert, J.S. Therapeutic Drug Monitoring of HIV Antiretroviral Drugs in Pregnancy: A Narrative Review. Ther. Drug Monit. 2020, 42, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Scarsi, K.K.; Havens, J.P.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V. HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs 2020, 80, 1649–1676. [Google Scholar] [CrossRef]
- Burgess, M.J.; Kasten, M.J.; Zeuli, J.D. Management of HIV/AIDS in older patients–drug/drug interactions and adherence to antiretroviral therapy. HIV/AIDS - Res. Palliat. Care, 7. [CrossRef]
- Nachega, J.B.; Hsu, A.J.; Uthman, O.A.; Spinewine, A.; Pham, P.A. Antiretroviral therapy adherence and drug–drug interactions in the aging HIV population. AIDS 2012, 26, S39–S53. [Google Scholar] [CrossRef] [PubMed]
- Devanathan, A.S.; Anderson, D.J.; Cottrell, M.L.; Burgunder, E.M.; Saunders, A.C.; Kashuba, A.D. Contemporary Drug–Drug Interactions in HIV Treatment. Clin. Pharmacol. Ther. 2019, 105, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo D, Giacomelli A, Pagani G, Filice C, Gervasoni C. Ritonavir/Cobicistat-Induced Cushing Syndrome in HIV Patients Treated With Non-Oral Corticosteroids: A Call for Action? Am J Med Sci. 2021, 361, 137–9.
- Flynn, P.M.; Abrams, E.J. Response to correspondence entitled: Perinatal HIV and response to vaccination. AIDS 2019, 33, 1675–1677. [Google Scholar] [CrossRef] [PubMed]
- Canetti, D.; Spagnuolo, V. An evaluation of cabotegravir for HIV treatment and prevention. Expert Opin. Pharmacother. 2020, 22, 403–414. [Google Scholar] [CrossRef]
- Ford, S.L.; Sutton, K.; Lou, Y.; Zhang, Z.; Tenorio, A.; Trezza, C.; Patel, P.; Spreen, W. Effect of Rifampin on the Single-Dose Pharmacokinetics of Oral Cabotegravir in Healthy Subjects. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Hostench-Junoy N, Ramírez-Montoya M, Arefai-Refai B, Estal-Jiménez J, Santana-Rodríguez ZJ, Costa-Pérez L. Acute Ischemia of Lower Extremities Caused by Ergotamine Toxicity due to Pharmacologic Interaction With Cobicistat in an HIV-Positive Patient. Ann Vasc Surg. 2022, 80:392.e1-392.e6.
- Manzardo, C.; Tuset, M.; Miró, J.M.; Gatell, J.M. Interacciones graves o potencialmente letales entre antirretrovirales y otros medicamentos. Enfermedades Infecc. Y Microbiol. Clin. (english Ed.) 2015, 33, e15–e30. [Google Scholar] [CrossRef]
- Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future: Three decades of antiretroviral therapy. Br J Clin Pharmacol. 2015, 79, 182–94.
- Hiransuthikul, A.; Janamnuaysook, R.; Himmad, K.; Kerr, S.J.; Thammajaruk, N.; Pankam, T.; Phanjaroen, K.; Mills, S.; Vannakit, R.; Phanuphak, P.; et al. Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. J. Int. AIDS Soc. 2019, 22, e25338. [Google Scholar] [CrossRef] [PubMed]
- Ribera E, Podzamczer D. Mecanismo de acción, farmacología e interacciones de dolutegravir. Enfermedades Infecc Microbiol Clínica. 2015, 33:2-8.
- Dooley KE, Sayre P, Borland J, Purdy E, Chen S, Song I, et al. Safety, Tolerability, and Pharmacokinetics of the HIV Integrase Inhibitor Dolutegravir Given Twice Daily With Rifampin or Once Daily With Rifabutin: Results of a Phase 1 Study Among Healthy Subjects. J Acquir Immune Defic Syndr. 2013, 62.
- Speaker Abstracts. J Int AIDS Soc. 2018, 21, e25093. [CrossRef] [PubMed]
- Eke, A.C.; Mirochnick, M. Ritonavir and cobicistat as pharmacokinetic enhancers in pregnant women. Expert Opin. Drug Metab. Toxicol. 2019, 15, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Podany, A.T.; Scarsi, K.K.; Fletcher, C.V. Comparative Clinical Pharmacokinetics and Pharmacodynamics of HIV-1 Integrase Strand Transfer Inhibitors. Clin. Pharmacokinet. 2016, 56, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Chandiwana, N.C.; Chersich, M.; Venter, W.F.; Akpomiemie, G.; Hill, A.; Simmons, B.; Lockman, S.; Serenata, C.M.; Fairlie, L.; Moorhouse, M.A. Unexpected interactions between dolutegravir and folate: randomized trial evidence from South Africa. AIDS 2020, 35, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Bukkems, V.E.; Smolders, E.J.; Jourdain, G.; Burger, D.M.; Colbers, A.P.; Cressey, T.R. ; PANNA Network and iTAP Study Group Effect of Pregnancy and Concomitant Antiretrovirals on the Pharmacokinetics of Tenofovir in Women With HIV Receiving Tenofovir Disoproxil Fumarate-Based Antiretroviral Therapy Versus Women With HBV Receiving Tenofovir Disoproxil Fumarate Monotherapy. J. Clin. Pharmacol. 2020, 61, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.N.; Gufford, B.T.; Lu, J.B.L.; Bushman, L.R.; Anderson, P.L.; Bergstrom, R.F.; Desta, Z.; Gupta, S.K. Inhibitory Effects of Probenecid on Pharmacokinetics of Tenofovir Disoproxil Fumarate and Emtricitabine for On-Demand HIV Preexposure Prophylaxis. Clin. Pharmacol. Ther. 2019, 107, 1200–1208. [Google Scholar] [CrossRef]
- Ewing, A.C.; King, C.C.; Wiener, J.B.; Chasela, C.S.; Hudgens, M.G.; Kamwendo, D.; Tegha, G.; Hosseinipour, M.C.; Jamieson, D.J.; Van der Horst, C.; et al. Effects of concurrent exposure to antiretrovirals and cotrimoxazole prophylaxis among HIV-exposed, uninfected infants. AIDS 2017, 31, 2455–2463. [Google Scholar] [CrossRef]
- Ippolito, M.M.; Jacobson, J.M.; Lederman, M.M.; Winterberg, M.; Tarning, J.; A Shapiro, T.; Flexner, C. Effect of Antiretroviral Therapy on Plasma Concentrations of Chloroquine and Desethyl-chloroquine. Clin. Infect. Dis. 2018, 67, 1617–1620. [Google Scholar] [CrossRef]
- van der Laan, L.E.; Garcia-Prats, A.J.; Schaaf, H.S.; Tikiso, T.; Wiesner, L.; de Kock, M.; Winckler, J.; Norman, J.; McIlleron, H.; Denti, P.; et al. Pharmacokinetics and Drug-Drug Interactions of Lopinavir-Ritonavir Administered with First- and Second-Line Antituberculosis Drugs in HIV-Infected Children Treated for Multidrug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Ramachandran, G.; Kumar, A.H.; Kannan, T.; Sridhar, R.; Guha, S.; Kadam, D.; Gangadevi, N.P.; Rajapandian, T. Pharmacokinetics of rifabutin during atazanavir/ritonavir co-administration in HIV-infected TB patients. Indian J. Tuberc. 2018, 66, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Shieh, E.; A Marzinke, M.; Fuchs, E.J.; Hamlin, A.; Bakshi, R.; Aung, W.; Breakey, J.; Poteat, T.; Brown, T.; Bumpus, N.N.; et al. Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when also taking oestrogen when compared to cisgender men. J. Int. AIDS Soc. 2019, 22, e25405. [Google Scholar] [CrossRef] [PubMed]
- Havens, J.P.; Podany, A.T.; Scarsi, K.K.; Fletcher, C.V. Clinical Pharmacokinetics and Pharmacodynamics of Etravirine: An Updated Review. Clin. Pharmacokinet. 2019, 59, 137–154. [Google Scholar] [CrossRef]
- Serrano López De Las Hazas, JI. Interacciones farmacológicas de los nuevos antirretrovirales. Farm Hosp. 2011, 35, 36–43. [Google Scholar] [CrossRef]
- Thurman, A.R.; Schwartz, J.L.; Brache, V.; Chen, B.A.; Chandra, N.; Kashuba, A.D.; Weiner, D.H.; Mauck, C.; Doncel, G.F. Effect of Hormonal Contraception on Pharmacokinetics of Vaginal Tenofovir in Healthy Women: Increased Tenofovir Diphosphate in Injectable Depot Medroxyprogesterone Acetate Users. Am. J. Ther. 2019, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.; Back, D.J.; Gibbons, S.; Khoo, S.H.; Marzolini, C. Pharmacokinetics and Drug–Drug Interactions of Long-Acting Intramuscular Cabotegravir and Rilpivirine. Clin. Pharmacokinet. 2021, 60, 835–853. [Google Scholar] [CrossRef]
- Trezza C, Ford SL, Gould E, Lou Y, Huang C, Ritter JM, et al. Lack of effect of oral cabotegravir on the pharmacokinetics of a levonorgestrel/ethinyl oestradiol-containing oral contraceptive in healthy adult women: Effect of cabotegravir on combined oral contraceptives. Br J Clin Pharmacol. 2017, 83, 1499–505.
- Wang, S.C.; Kaur, G.; Schulman-Marcus, J.; Purga, S.; Mookherjee, S.; Miller, C.; Sidhu, M.S.; Rosenson, R.S. Implementation of Cholesterol-Lowering Therapy to Reduce Cardiovascular Risk in Persons Living with HIV. Cardiovasc. Drugs Ther. 2020, 36, 173–186. [Google Scholar] [CrossRef]
- Lozano F, Domingo P. [Antiretroviral therapy for HIV infection]. Enferm Infecc Microbiol Clin. 2011, 29, 455–65.
- Burgess S, Partovi N, Yoshida EM, Erb SR, Azalgara VM, Hussaini T. Drug Interactions With Direct-Acting Antivirals for Hepatitis C: Implications for HIV and Transplant Patients. Ann Pharmacother. 2015, 49, 674–87.
- Zhao, A.V.; Crutchley, R.D.; Guduru, R.C.; Ton, K.; Lam, T.; Min, A.C. A clinical review of HIV integrase strand transfer inhibitors (INSTIs) for the prevention and treatment of HIV-1 infection. Retrovirology 2022, 19, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, R.C.; Liedtke, M.D. Antiretroviral Drug Interactions: Overview of Interactions Involving New and Investigational Agents and the Role of Therapeutic Drug Monitoring for Management. Pharmaceutics 2011, 3, 745–781. [Google Scholar] [CrossRef]
- Al Sayed, H.A.H.; Sharif-Askari, N.S.; Rahimi, M.R. Clinically significant drug interactions between antiretroviral and co-prescribed drugs in HIV infected patients: retrospective cohort study. Med. Pharm. Rep. 2022, 95, 260–266. [Google Scholar] [CrossRef]
- Jiménez-Guerrero L, Núñez-Núñez M, Castañeda-Macías I, Sandoval-Fernández del Castillo S, Jiménez-Guerrero L, Núñez-Núñez M, et al. Interacciones potenciales en una cohorte de pacientes VIH positivos de edad avanzada. Farm Hosp. 2018, 42, 163–7.
- Lopera, V.; Rodríguez, A.; Amariles, P. Clinical Relevance of Drug Interactions with Cannabis: A Systematic Review. J. Clin. Med. 2022, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
| Probability | |||
|---|---|---|---|
| Gravity | Defined | Probable | Possible |
| Grave | 1 (very high risk) | 1 (very high risk) | 2 (high risk) |
| Moderate | 2 (high risk) | 2 (high risk) | 3 (medium risk) |
| Minor | 3 (medium risk) | 3 (medium risk) | 4 (low risk) |
| Lack of gravity | 5 (riskless) | N/A | N/A |
| Total of drug interaction pairs, n (%) | 355 (100%) | |||||
|---|---|---|---|---|---|---|
| Pharmacokinetic mechanism | 197 (55.5%) | |||||
|
111 (31.2%) | |||||
|
66 (18.6%) | |||||
|
18 (5.1%) | |||||
|
1 (0.3%) | |||||
|
1 (0.3%) | |||||
| Pharmacodynamic mechanism | 6 (1.7%) | |||||
|
6 (1.7%) | |||||
| Pharmacokinetic/pharmacodynamic mechanism | 4 (1.1%) | |||||
| Enzyme inhibition/synergism | 2 (0.5%) | |||||
| Enzyme induction/synergism | 2 (0.5%) | |||||
| Evidence of no clinically relevant interactions | 148 (42.0%) | |||||
| Level of the clinical relevance of drug interaction | ||||||
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Total, n (%) | |
| 89 (25.1%) | 72 (20.2%) | 45 (12.7%) | 1 (0.3%) | 148 (42.0%) | 355 (100.0%) | |
| Pharmacological group or drugs affected | Antiretroviral agent | Gravity/Probability | Comments/Suggestions |
|---|---|---|---|
| Alpha-1 adrenergic receptor antagonist | |||
| Silodosin [12] | ATV/RTV, DRV/RTV, FPV/RTV, LPV/RTV | (Moderate/Probable): Level 2 | Boosted PIs may increase silodosin plasma levels through the inhibition of CYP3A4 metabolism. To monitor silodosin safety parameters, a dose adjustment may be necessary. |
| Anesthetic/Anxiolytic/Benzodiazepine | |||
| Midazolam [13] | ATV/RTV | (Grave/Defined): Level 1 | Boosted PIs may increase midazolam plasma levels, increasing the probability of respiratory depression, sedation, and muscle weakness. The use of this combination is not recommended. If necessary, parenteral midazolam should be used with close clinical monitoring or use safer alternatives such as lorazepam or propofol. |
| Triazolam [13] | ATV/RTV | (Grave/Defined): Level 1 | Boosted PIs may increase triazolam plasma levels, increasing the probability of respiratory depression, sedation, and muscle weakness. The use of this combination is not recommended, safer alternatives such as oxazepam, lorazepam or temazepam should be used. |
| Antiasthmatic/ Glucocorticoid | |||
| Budesonide [14] | ATV | (Grave/Defined): Level 1 | ATV may increase the budesonide plasma levels, enhancing the side effects produced by steroids. To monitor budesonide safety parameters, a dose adjustment may be necessary. |
| Antimalarial | |||
| Atovaquone [15] | EFV, ETR | (Moderate/Defined): Level 2 | NNRTIs may decrease the AUC and Cmax of atovaquone by inducing glucuronidation. To monitor atovaquone effectiveness parameters, a dose adjustment may be necessary. |
| Mefloquine [16] | NVP | (Moderate/Defined): Level 2 | Mefloquine may decrease NVP plasma levels. Caution is recommended in case of using NVP concomitantly with mefloquine, closely monitor, a dose adjustment may be necessary. |
| Proguanil [15] | EFV, ETR | (Moderate/Defined): Level 2 | NNRTIs may decrease proguanil AUC and Cmax. To monitor proguanil effectiveness parameters, a dose adjustment may be necessary. |
| Antimicrobial/ Antileprotic | |||
| Clofazimine [17] | EFV | (Grave/Defined): Level 1 | The use of EFV and clofazimine may generate QT interval prolongation in the ECG. Heart function should be closely monitored by ECG. |
| Antimicrobial/ Antituberculosis | |||
| Moxifloxacin [17] | EFV | (Grave/Defined): Level 1 | EFV may decrease the AUC of moxifloxacin by 30 %, increasing the development of resistance and the risk of virological failure. Levofloxacin is suggested instead of moxifloxacin when used concomitantly with EFV. |
| Rifabutin [18] | ATV, DRV, FPV, LPV/RTV | (Moderate/Defined): Level 2 | Rifabutin may reduce PIs plasma levels by induction of CYP3A4, causing decreased virological response. It is recommended to adjust the dose of rifabutin to 150 mg daily. |
| Rifabutin [19] | DOR | (Moderate/Defined): Level 2 | Rifabutin may decrease DOR plasma levels by induction of CYP3A4. To monitor rifabutin effectiveness parameters, a dose adjustment may be necessary. |
| Rifabutin [18,20] | EFV, ETR | (Grave/Defined): Level 1 | Rifabutin may induce CYP3A4 and increase EFV and ETR metabolism, causing decrease in their plasma levels and virological response. These combinations are not recommended given the risk of reducing viral susceptibility and developing resistance. |
| Rifampicin (17,18,20] | DRV/RTV | (Grave/Defined): Level 1 | Rifampicin may significantly decrease DRV/RTV plasma levels by 57 %, increasing the development of resistance and the risk of virological failure. Alternatively, it suggests using rifabutin 150 mg daily. |
| Rifampicin [19,20,21] | DOR | (Grave/Defined): Level 1 | Rifampicin may induce CYP3A4, causing decrease DOR plasma levels by 88 %. To monitor rifabutin effectiveness parameters, a dose adjustment may be necessary. |
| Rifampicin [16,20] | RPV | (Grave/Defined): Level 1 | Rifampicin may decrease RPV plasma levels, increasing the risk of virological failure and development of resistance, therefore, this combination is contraindicated. In this sense, rifabutin 300 mg daily and double dose of RPV is recommended. |
| Antineoplastic | |||
| Venetoclax [22] | RTV | (Moderate/Probable): Level 2 | RTV (doses of 50 mg and 100 mg) may inhibit CYP3A4 resulting in an increase of venetoclax Cmax 2.3 to 2.4 times compared with venetoclax alone and AUC 6.1 and 8.1 times, respectively. After completing the gradual increase in dose, venetoclax dose reductions of at least 75 % are recommended when administered concomitantly with potent CYP3A inhibitors to maintain venetoclax exposures within the therapeutic range established for the treatment of lymphocytic leukemia chronic. |
| Antiparasitic/ Anthelmintic | |||
| Praziquantel [23] | EFV | (Moderate/Defined): Level 2 | EFV may decrease praziquantel plasma levels up to 4 times the risk of therapeutic failure. To monitor praziquantel effectiveness parameters, a dose adjustment may be necessary, but not exceed the maximum. |
| Antiplatelet agent | |||
| Ticagrelor [13] | ATV/RTV, DRV/RTV, FPV/RTV, LPV/RTV | (Grave/Defined): Level 1 | Boosted PIs may increase ticagrelor plasma levels, increasing the risk of bleeding. The use of ticagrelor with boosted PIs is not recommended. Other antiplatelet agents are suggested as an alternative. |
| Antiulcer/Anti-H2 | |||
| Famotidine [19] | RPV | (Moderate/Defined): Level 2 | Famotidine may decrease RPV plasma levels, increasing the risk of virological failure and development of resistance, by increasing gastric pH. To monitor RPV effectiveness parameters. This combination may be used as long as doses are temporarily separated, i.e., RPV can be given 4 h before or 12 h after anti-H2. |
| Antiulcer/Proton Pump Inhibitor | |||
| Lansoprazole [24,25] | RPV | (Grave/Defined): Level 1 | Proton pump inhibitors may increase gastrointestinal pH and affect RPV absorption by decreasing its plasma levels. Avoid this combination or use esomeprazole by spacing your intake in 10 hours with RPV. |
| Omeprazole [13, 19] | |||
| Pantoprazole [24,25] | |||
| Rabeprazole [24,25] | |||
| Atypical antipsychotic | |||
| Ziprasidone [25] | EFV | (Moderate/Defined): Level 2 | EFV may decrease ziprasidone plasma levels. To monitor ziprasidone effectiveness parameters, a dose adjustment may be necessary. |
| Contraceptive | |||
| Etonogestrel (Oral contraceptive) [26] | NVP | (Grave/Defined): Level 1 | NVP may reduce etonogestrel plasma levels (Cmin decrease by 39 % and AUC of 0-24 weeks by 37 %), significantly decreasing the contraceptive effect. Etonogestrel implants are not recommended for women receiving long-term treatment with NVP. The patient should be informed of the need to use a barrier method as a complementary method of planning or to seek a safer contraceptive alternative. |
| Levonorgestrel (Implant) [26,27] | EFV | (Grave/Defined): Level 1 | EFV may significantly reduce plasma levonorgestrel concentrations by 61 %, and the contraceptive effect can be decreased. Levonorgestrel implants are not recommended for women receiving long-term treatment with EFV. The patient should be informed of the need to use a barrier method as a complementary method of planning or to seek a safer contraceptive alternative. |
| Direct acting antiviral | |||
| Glecaprevir/Pibrentasvir [28-30] | ATV/RTV, DRV/RTV, FPV/RTV | (Grave/Defined): Level 1 | Boosted PIs increase glecaprevir/pibrentasvir plasma levels. Administration of glecaprevir/pibrentasvir with boosted PIs regimens is completely contraindicated. |
| Glecaprevir/Pibrentasvir [28-30] | EFV | (Grave/Defined): Level 1 | EFV may reduce glecaprevir/pibrentasvir plasma levels. Administration of glecaprevir/pibrentasvir with EFV is completely contraindicated. |
| Grazoprevir/Elbasvir [28-30] | NVP | (Grave/Defined): Level 1 | NVP may decrease grazoprevir/elbasvir plasma levels. Administration of grazoprevir/elbasvir with NVP is completely contraindicated. |
| Sofosbuvir/Velpatasvir/ Voxilaprevir [28-30] | ATV/RTV | (Grave/Defined): Level 1 | ATV/RTV may increase voxilaprevir plasma levels up to 4 times. Concomitant administration between ATV/RTV and voxilaprevir-containing regimens is contraindicated. |
| Sofosbuvir/Velpatasvir/ Voxilaprevir [28-30] | EFV | (Grave/Defined): Level 1 | EFV may decrease voxilaprevir plasma levels. Concomitant administration between EFV and voxilaprevir-containing regimens is contraindicated. |
| Direct acting oral anticoagulant | |||
| Edoxaban [13,31] | ATV | (Moderate/Defined): Level 2 | ATV may increase edoxaban plasma levels by inhibition of Pg-P. The use of these drugs is not recommended, therefore other oral anticoagulants should be considered as an alternative, or if it combination is necessary, a dose adjustment should be made. |
| Edoxaban [13,31] | ATV/RTV, DRV/RTV, FPV/RTV, LPV/RTV | (Moderate/Defined): Level 2 | Boosted PIs may increase edoxaban plasma levels. To monitor edoxaban safety parameters. It is recommended to reduce the dose of edoxaban from 60 mg to 30 mg daily. |
| Edoxaban [13,31] | ETR | (Grave/Defined): Level 1 | ETR may increase edoxaban plasma levels, increasing the risk of bleeding. The use of this combination is not recommended, other anticoagulants are suggested. |
| Edoxaban [13,31] | FPV | (Grave/Defined): Level 1 | FPV may increase/decrease edoxaban plasma levels, increasing the risk of bleeding and thrombus formation, respectively. The use of this combination is not recommended. |
| Drugs of abuse | |||
| Amphetamine [32] | LPV/RTV | (Grave/Possible): Level 2 | LPV/RTV may inhibit amphetamine metabolism, leading to increased plasma levels and toxicity. Warn patients about the risks of this combination. |
| Cocaine [33] | RPV | (Grave/Defined): Level 1 | RPV may inhibit cocaine metabolism, leading to increased plasma levels and toxicity. Warn patients about the risks of this combination. Cocaine overdose is life-threatening due to rhabdomyolysis, arrhythmia, and cardiovascular collapse. |
| Heroin [32] | ETR | (Grave/Possible): Level 2 | Heroin may decrease ETR plasma levels, resulting in a no suppressed viral load. Warn patients of the risk of using this combination. |
| Ketamine [32] | ETR | (Grave/Possible): Level 2 | Ketamine may decrease ETR plasma levels, resulting in a no suppressed viral load. Warn patients of the risk of using this combination. |
| Tetrahydrocannabinol (THC) [32] | EFV | (Grave/Possible): Level 2 | EFV may increase THC plasma levels, prolong its clinical effects and increase toxicity. Warn patients about the risks of this combination. |
| Tetrahydrocannabinol (THC) [32] | ETR | (Grave/Possible): Level 2 | THC may decrease ETR plasma levels, increasing the risk of faster HIV progression, neurocognitive impairment and immune dysfunction in people with HIV. Warn patients of the risk of using this combination. |
| Mineralocorticoid | |||
| Eplerenone [13] | ATV/RTV, DRV/RTV, FPV/RTV, LPV/RTV | (Grave/Defined): Level 1 | Boosted PIs may increase eplerenone plasma levels, increasing the risk of hyperkalemia. The use of this combination is not recommended. |
| Oral anticoagulant/Anti vitamin K | |||
| Warfarin [31] | DRV | (Moderate/Defined): Level 2 | PIs may increase warfarin plasma levels, increasing the risk of bleeding. To monitor warfarin safety parameters, a dose adjustment may be necessary. |
| Special condition | |||
| Pregnancy [34,35] | ATV, DRV | (Grave/Defined): Level 1 | Pregnancy may decrease PIs exposure. Using ritonavir-enhanced IPs in women who become pregnant while on ART treatment. |
| Pregnancy [34] | DOR | (Grave/Defined): Level 1 | Pregnancy may decrease DOR exposure. Currently, its use is not recommended in women who become pregnant while on ART treatment. |
| Pharmacological group or drugs affected | Antiretroviral agent | Gravity/Probability | Comments/Suggestions |
|---|---|---|---|
| Alpha-1 adrenergic receptor antagonist | |||
| Silodosin [12] | EVG/COBI | (Moderate/Probable): Level 2 | EVG/COBI may increase silodosin plasma levels by inhibition of CYP3A4. To monitor silodosin safety parameters, a dose adjustment may be necessary. |
| Anesthetic/Anxiolytic/ Benzodiazepine | |||
| Clonazepam [13] | EVG/COBI | (Moderate/Defined): Level 2 | EVG/COBI may increase benzodiazepines plasma levels. To monitor benzodiazepines safety parameters. It is recommended to use the lowest dose and for a short period of time. |
| Diazepam [13] | |||
| Midazolam [13] | (Grave/Defined): Level 1 | EVG/COBI may increase midazolam plasma levels, increasing the probability of respiratory depression, sedation, and muscle weakness. The use of this combination is not recommended. If necessary, parenteral midazolam should be used with close clinical monitoring or use safer alternatives such as lorazepam or propofol. | |
| Triazolam [13] | (Grave/Defined): Level 1 | EVG/COBI may increase triazolam plasma levels, increasing the probability of respiratory depression, sedation, and muscle weakness. The use of this combination is not recommended, safer alternatives such as oxazepam, lorazepam or temazepam should be used. | |
| Antacids | |||
| Aluminum hydroxide [13,36] | BIC, CAB, RAL | (Moderate/Defined): Level 2 | Antacids may produce chelation and affect InSTIs absorption, mainly antacids containing Ca2+, Al3+, Mg2+ and Fe2+. InSTIs may be given together with antacids containing calcium when taken along with food, but not on an empty stomach because food increases InSTIs exposure. Moreover, any antacid containing aluminum or magnesium should be administered at least 2 h after or 6 h before administration of InSTIs. |
| Calcium carbonate [13,36] | |||
| Ferrous fumarate [13,36] | |||
| Magnesium hydroxide [13,36] | |||
| Aluminum hydroxide [13,24,25,37,38] | DTG | (Moderate/Defined): Level 2 | Antacids may produce chelation and affect DTG absorption, mainly antacids containing polyvalent cations such as Mg2+, Al3+, Fe2+, Ca2+ and Zn2+. To administer DTG 2 hours before or 6 h after antacids. |
| Calcium carbonate [13,24,25,37,38] | |||
| Ferrous fumarate [13,24,25,37,38] | |||
| Magnesium hydroxide [13,24,25,37,38] | |||
| Zinc Gluconate [13,24,25,37,38] | |||
| Aluminum hydroxide [13] | EVG/COBI | (Moderate/Defined): Level 2 | Antacids may produce chelation and affect EVG absorption, mainly antacids containing polyvalent cations such as Mg2+, Al3+, Fe2+, Ca2+ and Zn2+. To separate EVG/COBI for 4 h from antacids. |
| Calcium carbonate [13] | |||
| Ferrous fumarate [13] | |||
| Magnesium hydroxide [13] | |||
| Zinc Gluconate [13] | |||
| Antiarrhythmic | |||
| Dofetilide [39] | DTG | (Grave/Defined): Level 1 | DTG administration causes a 70-90 % increase in dofeltilde plasma levels. Concomitant use is not recommended. |
| Antiasthmatic/Glucocorticoid | |||
| Budesonide [12] | EVG/COBI | (Moderate/Probable): Level 2 | EVG/COBI may increase budesonide and fluticasone plasma levels by inhibition of CYP3A4. To monitor budesonide and fluticasone safety parameters, a dose adjustment may be necessary. |
| Fluticasone [12,13] | |||
| Mometasone [12,13] | EVG/COBI | (Grave/Defined): Level 1 | EVG/COBI may increase mometasone plasma levels, and even lead to the appearance of Cushing's Syndrome. To monitor mometasone safety parameters and cortisol levels. To suspend treatment if necessary and use beclomethasone as an alternative. |
| Triamcinolone (injectable) [12,13,40] | EVG/COBI | (Grave/Probable): Level 1 | EVG/COBI may increase triamcinolone plasma levels by generating adrenal suppression. Do not co-administer unless the benefit outweighs the risk of systemic effect. Safer alternatives should be considered. |
| Anticoagulant/Anti vitamin K | |||
| Warfarin [31] | BIC, DTG, EVG/COBI | (Grave/Defined): Level 1 | InSTIs may increase warfarin plasma levels. To monitor warfarin safety parameters, especially INR, a dose adjustment may be necessary. |
| Anticonvulsant | |||
| Valproic acid [41] | DTG | (Grave/Possible): Level 2 | Valproic acid may decrease DTG plasma levels by inducing the metabolism of the enzymes UGT1A1 and CYP3A4, P-gp protein and BCRP transporter. Closely monitor DTG effectiveness parameters, because it may lead to increased resistance and increased viral load. In addition, other anticonvulsant alternatives such as levetiracetam are suggested. |
| Antidiabetic/Biguanide | |||
| Metformin [20] | BIC | (Moderate/Probable): Level 2 | BIC may increase metformin plasma levels by 39 % dose-dependent, through inhibition of MATE 1 and OCT2. Additionally, there have been cases of lactic acidosis and loss of glycemic control when the metformin dose was reduced empirically in combination with BIC. Efficacy and safety monitoring is recommended and dose adjustment may be required. Preferably start with low doses and increase according to the patient’s response. |
| Antidiabetic/Sulphonylurea | |||
| Chlorpropamide [39] | 3TC | (Moderate/Defined): Level 2 | 3TC administration may cause a significant reduction in exposure to chlorpropamide. It is advisable to strictly control blood glucose levels in diabetic patients treated concomitantly with these drugs. |
| Antimalarial | |||
| Artemether/Lumefantrine [39] | DTG | (Moderate/Defined): Level 2 | Artemether/Lumefantrine may decrease DTG exposure by 40 %. To monitor DTG effectiveness parameters, a dose adjustment may be necessary. |
| Artesunate/Amodiaquine [39] | DTG | (Moderate/Defined): Level 2 | Artesunate/Amodiaquine may decrease DTG exposure by 25-45 %. To monitor DTG effectiveness parameters, a dose adjustment may be necessary. |
| Atovaquone [15] | AZT | (Moderate/Defined): Level 2 | Atovaquone may increase the AUC of AZT. To monitor AZT effectiveness parameters, a dose adjustment may be necessary. |
| Antimicrobial/Oxazolidinone | |||
| Linezolid [17] | AZT | (Grave/Defined): Level 1 | The concomitant use of AZT and linezolid can produce hematologic toxicity, since it may generate suppression of the bone marrow. Avoid the concomitant use of AZT and linezolid due to the risk of hematological toxicity. |
| Antimicrobial/Sulfonamide | |||
| Trimethoprim/Sulfamethoxazole [39] | 3TC | (Moderate/Defined): Level 2 | Trimethoprim administration causes a 40% increase in 3TC plasma levels. There is no need to adjust the dose of 3TC in patients with normal renal function; the dose may be reduced in patients with renal failure. |
| Antimicrobial/Antituberculosis | |||
| Bedaquiline [17] | COBI | (Grave/Defined): Level 1 | COBI may significantly increase bedaquiline exposure. Closely monitor cardiac function using the ECG. |
| Rifabutin [17,20] | ABC, COBI | (Grave/Defined): Level 1 | Rifabutin may induce CYP3A4 and increase ABC and COBI metabolism, causing a decrease in plasma levels and virological response. It is not recommended given the risk of reducing viral susceptibility and the development of resistance associated with therapeutic ARV drug levels. |
| Rifampicin [19,20] | ATV/COBI | (Grave/Defined): Level 1 | Rifampicin may induce CYP3A4 and increase ATV/COBI metabolism, causing decrease in plasma levels and virological response. Administration of rifampicin with ATV/COBI is completely contraindicated. |
| Rifampicin [17,20,36] | BIC | (Grave/Defined): Level 1 | Rifampicin may induce BIC metabolism, causing a significant decrease in plasma levels (46-61 %), resulting in a loss of therapeutic effect. Dose recommendations for co-administration of BIC and rifampicin have not been established, however, the combination is contraindicated. |
| Rifampicin [36,40,42,43] | CAB | (Grave/Defined): Level 1 | Rifampicin may induce CAB metabolism, causing a 60 % decrease in oral CAB AUC, resulting in loss of therapeutic effect. Additionally, oral rifampicin may decrease exposure to intramuscular CAB by 44 %. Dose recommendations for co-administration of CAB and rifampin have not been established, however, the combination is contraindicated. |
| Rifampicin [17] | EVG/COBI | (Grave/Probable): Level 1 | Rifampicin may decrease EVG/COBI plasma levels, causing risk of reduced viral susceptibility and development of resistance. Simultaneous use between EVG/COBI and rifampicin is not recommended. |
| Antimigraine | |||
| Ergotamine/Dipyrone/Caffeine [44] | TDF/FTC/EVG/COBI | (Grave/Possible): Level 2 | COBI may increase plasma ergotamine concentrations by inhibiting CYP3A4, increasing the probability of ergotism: High blood pressure, nervousness, hallucinations, seizures, gastrointestinal and muscle disorders, as well as the appearance of acute ischemia of the lower extremities. To monitor ergotamine safety parameters. Rapid initiation of treatment is important to reduce the risk of ischemic sequelae. |
| Antineoplastic | |||
| Methotrexate [42] | CAB | (Grave/Defined): Level 1 | CAB can inhibit OAT1 and OAT3 causing increased methotrexate plasma levels. This combination requires strict vigilance, because methotrexate is a drug with a narrow therapeutic index. |
| Vinblastine [25] | AZT | (Grave/Defined): Level 1 | AZT may increase the probability of hematologic toxicity with the use of vinblastine. This combination is not recommended due to the possibility of serious adverse effects. |
| Antiplatelet agents | |||
| Clopidogrel [13] | EVG/COBI | (Grave/Defined): Level 1 | Boosted ARVs may alter clopidogrel antiplatelet effect. Co-administration of clopidogrel with boosted regimens is not possible. Use alternative antiplatelet agents or non-boosted regimens. |
| Ticagrelor [13] | EVG/COBI | (Grave/Defined): Level 1 | EVG/COBI may increase ticagrelor plasma levels, increasing the risk of bleeding. The use of ticagrelor with boosted ARVs is not recommended. Other antiplatelet agents are suggested as an alternative. |
| Antiulcer/Proton Pump Inhibitor | |||
| Omeprazole [12] | EVG/COBI | (Grave/Probable): Level 1 | EVG/COBI may increase omeprazole plasma levels by inhibition of CYP3A4. To monitor omeprazole safety parameters, a dose adjustment may be necessary. |
| Antiviral/Purine Analogue | |||
| Ribavirin [45,46] | AZT | (Grave/Defined): Level 1 | Concomitant use between AZT and ribavirin increases the probability of development of bone medulla suppression, associated with the appearance of severe hematological toxicity and increased risk of anemia. This combination is not recommended due to serious adverse effects. |
| Atypical antipsychotic | |||
| Quetiapine [12] | EVG/COBI | (Moderate/Probable): Level 2 | EVG/COBI may increase quetiapine plasma levels by inhibition of CYP3A4. To monitor quetiapine safety parameters, a dose adjustment may be necessary. |
| Direct-acting antiviral | |||
| Grazoprevir/Elbasvir [29] | COBI | (Grave/Defined): Level 1 | COBI may increase grazoprevir plasma levels. The administration of grazoprevir/elbasvir with COBI is completely contraindicated. |
| Direct oral anticoagulant | |||
| Apixaban [13,31] | EVG/COBI | (Grave/Defined): Level 1 | EVG/COBI may inhibit CYP3A4, causing increased apixaban plasma levels. The use of this combination is not recommended. |
| Dabigatran [13,31] | EVG/COBI | (Grave/Defined): Level 1 | EVG/COBI may increased dabigatran plasma levels by inhibition of Pg-P. The use of this combination is not recommended. Other anticoagulants are suggested as an alternative. |
| Edoxaban [13,31] | ATV/COBI | (Grave/Defined): Level 1 | ATV/COBI may increased edoxaban plasma levels. It is not recommended to use this combination; it is suggested to use other anticoagulants as an alternative. |
| Edoxaban [13,31] | DRV/COBI | (Moderate/Defined): Level 2 | DRV/COBI may increase plasma concentrations of edoxaban by inhibition of P-gp. It is recommended to monitor edoxaban safety parameters, especially in patients with renal damage. |
| Edoxaban [13,31] | EVG/COBI | (Moderate/Defined): Level 2 | EVG/COBI may inhibit CYP3A4, causing increase edoxaban plasma levels. To monitor edoxaban safety parameters. It is recommended to reduce the dose of edoxaban from 60 mg to 30 mg. |
| Rivaroxaban [13,31] | EVG/COBI | (Grave/Defined): Level 1 | EVG/COBI may inhibit CYP3A4, causing increase rivaroxaban plasma levels. The use of this combination is not recommended. |
| Disease | |||
| Severe renal impairment (36) | DTG | (Grave/Defined): Level 1 | Severe kidney failure can decrease DTG plasma levels. Caution is recommended in certain populations, for example, those with resistance associated with the use of InSTIs. |
| Drugs abuse | |||
| Amphetamine [32] | EVG/COBI | (Moderate/Probable): Level 2 | EVG/COBI may increase plasma levels of abused drugs, prolong their clinical effects and increase toxicity. Warn patients about the risks of these combinations. |
| Cannabis [32] | |||
| Ecstasy (MDMA) [32] | |||
| Heroin [32] | |||
| Ketamine [32] | |||
| Methamphetamine [32] | |||
| Cocaine [33] | EVG/COBI | (Grave/Probable): Level 1 | Simultaneous administration of cocaine and EVG/COBI may reduce cocaine metabolism, leading to increased plasma levels and toxicity. Warn patients about the risks of this combination. Cocaine overdose is life-threatening due to rhabdomyolysis, arrhythmia, and cardiovascular collapse. |
| Feminizing Hormone Therapy (FHT) | |||
| Estradiol Valerate/Cyproterone Acetate [47] | TDF/FTC | (Moderate/Probable): Level 2 | TDF exposure was reduced by 12 % in the presence of FHT, suggesting that it may potentially affect the effectiveness of PrEP among transgender women. To monitor TDF effectiveness parameters. |
| Mineralocorticoid | |||
| Eplerenone [13] | EVG/COBI | (Grave/Defined): Level 1 | EVG/COBI may increase eplerenone plasma levels, increasing the risk of hyperkalemia. The use of this combination is not recommended. |
| PI | |||
| Ritonavir [19,25,48-50] | DTG | (Moderate/Defined): Level 2 | Ritonavir may significantly reduce DTG plasma levels. To monitor DTG effectiveness parameters, a dose adjustment may be necessary. |
| Special conditions | |||
| Pregnancy [34] | BIC | (Grave/Defined): Level 1 | Pregnancy may decrease BIC exposure. This drug is not currently recommended in women who become pregnant while on ART treatment. |
| Pregnancy [34,36,51,52] | EVG/COBI | (Grave/Defined): Level 1 | Pregnancy may decrease the AUC of EVG and COBI by 24 % and 44 %, respectively, during the second trimester. Additionally, pregnancy may decrease the AUC of COBI by 59 % during the third trimester. This scheme should not be used during pregnancy. If women become pregnant during treatment with COBI it should be changed to an alternative treatment regimen such as ritonavir. |
| Pregnancy [34] | TDF/FTC/RAL | (Grave/Defined): Level 1 | Pregnancy may decrease TDF/FTC/RAL exposure. This scheme is not currently recommended in women who become pregnant while on ART treatment. |
| Pregnancy [36,51,52] | CAB | (Grave/Possible): Level 2 | Three cases of pregnancy were reported in patients who were using CAB. CAB is not recommended during pregnancy. However, more studies are needed to prove the effectiveness of this drug in pregnancy. |
| Pregnancy [34,36,53] | DTG | (Moderate/Defined): Level 2 | Exposure to DTG in pregnancy may be associated with an increased risk of neural tube defects in fetus. Additionally, a decrease in DTG exposure between 10-50 % has been demonstrated compared to postpartum plasma levels. The use of DTG should be avoided, especially in the first trimester of pregnancy, however, some studies claim that it can be administered without dose adjustment during pregnancy, if it is taken with food to increase absorption. |
| Pregnancy [54] | TDF | (Moderate/Defined): Level 2 | Pregnancy may decrease exposure and TDF plasma levels. Monitor viral load continuously. |
| Statins | |||
| Lovastatin [13] | EVG/COBI | (Grave/Defined): Level 1 | EVG/COBI may increase lovastatin and simvastatin plasma levels, increasing the risk of myopathies, rhabdomyolysis and even death. These combinations are not recommended. Safer alternatives such as pravastatin or fluvastatin should be used. |
| Simvastatin [13] | |||
| Tricyclic antidepressant drugs | |||
| Amitriptyline [13] | EVG/COBI | (Moderate/Defined): Level 2 | EVG/COBI may increase plasma levels of tricyclic antidepressants. It is recommended to start with low doses of antidepressant and adjust the dose according to the patient’s response, monitoring safety parameters. |
| Clomipramine [13] | |||
| Imipramine [13] | |||
| Uricosuric agent | |||
| Probenecid [55] | TDF/FTC | (Grave/Possible): Level 2 | Probenecid increases the AUC of TDF and FTC by 64 % and 62 %, respectively. Administer this combination with caution. |
| Pharmacological group or drugs affected | Antiretroviral agent | Comments/Suggestions |
|---|---|---|
| Anesthetic/Anxiolytic/ Benzodiazepine | ||
| Diazepam [40,45,46] | AZT, TDF | Plasma levels of either drug is not affected. These combinations are safe and do not require dose adjustment. |
| Midazolam [40] | CAB | CAB does not modify the pharmacokinetics of midazolam. It is a safe combination, therefore, does not require dose adjustment. |
| Triazolam [45,46] | TDF | Plasma levels of either drug is not affected. This combination is safe and does not require dose adjustment. |
| Anticoagulant/Anti vitamin K | ||
| Warfarin [31] | 3TC | No significant changes in plasma levels of 3TC and warfarin are generated. This combination is safe and does not require dose adjustment. |
| Warfarin [25,31,38] | MVC | No significant changes in plasma levels of MVC and warfarin are generated. This combination is safe and does not require dose adjustment. |
| Warfarin [31] | TDF/FTC/RPV | No significant changes in plasma levels of RPV and warfarin are generated. This combination is safe and does not require dose adjustment. |
| Antifungal | ||
| Clotrimazole [56] | NVP | No significant changes in plasma levels of NVP and clotrimazole are generated. This combination is safe and does not require dose adjustment. |
| Voriconazole [19,56] | BIC | No significant changes in plasma levels of BIC and voriconazole are generated. This combination is safe and does not require dose adjustment. |
| Antimalarial | ||
| Chloroquine [57] | EFV | EFV does not change chloroquine plasma levels. This combination is safe and does not require dose adjustment. |
| Antimicrobial/Antituberculosis | ||
| Ethambutol [58] | LPV/RTV | Plasma levels of either drug is not affected. This combination is safe and does not require dose adjustment. |
| Ethionamide [58] | LPV/RTV | Plasma levels of either drug is not affected. This combination is safe and does not require dose adjustment. |
| Levofloxacin [17,58] | EFV | No significant changes in plasma levels of EFV and levofloxacin are generated. This combination is safe and requires no dose adjustment. |
| Pyrazinamide [58] | LPV/RTV | Plasma levels of either drug is not affected. This combination is safe and does not require dose adjustment. |
| Rifabutin [17,59] | ATV/RTV | No significant changes in plasma levels of ATV/RTV and rifabutin are generated. This combination is safe and does not require dose adjustment. |
| Rifabutin [17,59] | BIC | Plasma levels of either drug is not affected. This combination is safe and does not require dose adjustment. |
| Terizidone [58] | LPV/RTV | Plasma levels of either drug is not affected. This combination is safe and does not require dose adjustment. |
| Antiplatelet agent | ||
| Acetylsalicylic acid [13] | EVG/COBI | There are no significant changes in plasma levels of EVG/COBI and acetylsalicylic acid. This combination is safe and requires no dose adjustment. |
| Conjugated estrogens | ||
| Estradiol [60] | TDF/FTC | The use of conjugated estrogens for the GAHT reduces from 24-32 % in the AUC of TDF and FTC, but there is no statistically significant reduction in plasma levels of TDF/FTC and estradiol. The combination of TDF and FTC on demand (2+1+1) and GAHT may result in plasma levels too low for reliable prevention of HIV infection. |
| Direct acting oral anticoagulant | ||
| Apixaban [25,31,38] | ABC, ABC/AZT, ABC/3TC, BIC, BIC/TAF/FTC, DTG, DTG/ABC/3TC, EFV, MVC, TFV/FTC, TDF/FTC/RPV, T-20, 3TC | There are no significant changes in the plasma levels of apixaban and the ARVs mentioned. These combinations are safe and do not require dose adjustment. |
| Dabigatran [25,31,38] | ABC, ABC/AZT, ABC/3TC, BIC, BIC/TAF/FTC, DTG, DTG/ABC/3TC, EFV, MVC, TFV/FTC, TDF/FTC/RPV, T-20, 3TC | There are no significant changes in the plasma levels of dabigatran and the ARVs mentioned. These combinations are safe and do not require dose adjustment. |
| Edoxaban [25,31,38] | ABC, ABC/AZT, ABC/3TC, BIC, BIC/TAF/FTC, DTG, DTG/ABC/3TC, EFV, MVC, TFV/FTC, TDF/FTC/RPV, T-20, 3TC | There are no significant changes in the plasma levels of edoxaban and the ARVs mentioned. These combinations are safe and do not require dose adjustment. |
| Rivaroxaban [25,31,38] | ABC, ABC/AZT, ABC/3TC, BIC, BIC/TAF/FTC, DTG, DTG/ABC/3TC, EFV, MVC, TFV/FTC, TDF/FTC/RPV, T-20, 3TC | There are no significant changes in the plasma levels of rivaroxaban and the ARVs mentioned. These combinations are safe and do not require dose adjustment. |
| Disease | ||
| Mild to moderate hepatic impairment [36] | DTG, EVG, RAL | No significant differences have been observed in the exposures of InSTIs in people with mild to moderate liver impairment. Dose adjustment is not required in patients with mild to moderate liver impairment. |
| Drugs of abuse | ||
| Amphetamine [32] | ABC, DTG, NVP, RAL, RPV | There are no changes in the plasma levels of the ARVs mentioned. No dose adjustment is required. |
| Cannabis [32] | ABC, NVP, RAL, RPV | There are no changes in the plasma levels of the ARVs mentioned. No dose adjustment is required. |
| Ecstasy (MDMA) [32] | ||
| Methamphetamine [32] | ||
| Heroin [32] | ABC, RAL, RPV | There are no changes in the plasma levels of the ARVs mentioned. No dose adjustment is required. |
| Ketamine [32] | ||
| Nitrates | ABC, EFV, EVG/COBI, NVP, RAL, RPV | There are no changes in the plasma levels of the ARVs mentioned. No dose adjustment is required. |
| Attachment inhibitor | ||
| Fostemsavir [61] | ETR | Plasma levels of either drug is not affected. This combination is safe and does not require dose adjustment. |
| Injectable contraceptive | ||
| Medroxyprogesterone [62] | AZT | AZT does not reduce plasma levels of medroxyprogesterone (progestin). This combination is safe and does not require dose adjustment. |
| Medroxyprogesterone [63] | TDF (Topical) | Medroxyprogesterone affects the systemic and mucosa pharmacokinetics of TDF (topical) in healthy women. However, TDF administered topically for HIV-1 prevention in women resulted in high and protective mucosal concentrations with or without use of medroxyprogesterone. This combination is safe and requires no dose adjustment. |
| NNRTI | ||
| Etravirine [61] | CAB | Plasma levels of either drug is not affected. This combination is safe and does not require dose adjustment. |
| Rilpivirine [64] | CAB | Intramuscular administration of CAB has a low probability of pharmacokinetic interaction with rilpivirine. This combination is safe and does not require dose adjustment. |
| Oral contraceptive | ||
| Ethinylestradiol/Levonorgestrel [65] | CAB | There are no significant changes in plasma levels of CAB and ethinylestradiol/levonorgestrel. This combination is safe and requires no dose adjustment. |
| Ethinylestradiol [45,46,65] | TDF | There are no significant changes in plasma levels of TDF and ethinylestradiol. This combination is safe and requires no dose adjustment. |
| Ethinylestradiol/Levonorgestrel [63] | TDF (Topical) | Ethinylestradiol/Levonorgestrel affects the systemic and mucosa pharmacokinetics of TDF (topical) in healthy women. However, TDF administered topically for HIV-1 prevention in women resulted in high and protective mucosal concentrations with or without use of oral contraceptives. This combination is safe and requires no dose adjustment. |
| PI | ||
| Atazanavir [19] | BIC | Plasma levels of either drug is not affected. This combination is safe and does not require dose adjustment. |
| Special condition | ||
| Adults < 74 years [36] | BIC | There are no changes in plasma levels of BIC in adults < 74 years. Dose adjustment is not required in these patients. |
| Adults > 65 years [36] | EVG | There are no changes in plasma levels of EVG in adults > 65 years. Dose adjustment is not required in these patients. |
| Dialysis [36] | DTG, EVG, RAL | InSTIs are highly bound to plasma proteins (albumin and alpha-1 acid glycoprotein, 83-99 %) and are not significantly eliminated by dialysis (hemodialysis or peritoneal dialysis). Dose adjustment is not required in patients receiving hemodialysis or peritoneal dialysis. |
| Statin | ||
| Lovastatin [45,66] | DOR, RPV | There are no changes in the plasma levels of NNRTIs and lovastatin. These combinations are safe and do not require dose adjustment. |
| Pitavastatin [45,66] | ATV, DRV, LPV/RTV | There are no changes in the plasma levels of PIs and pitavastatin. These combinations are safe and do not require dose adjustment. |
| Pitavastatin [45,66] | ATV/RTV, DRV/RTV, FPV/RTV | There are no changes in the plasma levels of boosted PIs and pitavastatin. These combinations are safe and do not require dose adjustment. |
| Pitavastatin [45,66] | DOR, EFV, NVP | There are no changes in the plasma levels of NNRTIs and pitavastatin. These combinations are safe and do not require dose adjustment. |
| Pitavastatin [45,66] | EVG/COBI | There are no changes in the plasma levels of EVG/COBI and pitavastatin. These combinations are safe and do not require dose adjustment. |
| Pravastatin [45,66] | DOR, RPV | DOR and RPV do not affect the pharmacokinetics of pravastatin. These combinations are safe and do not require dose adjustment. |
| Rosuvastatin [45,66] | DOR, EFV, NVP, RPV | There are no changes in the plasma levels between NNRTIs and rosuvastatin. These combinations are safe and do not require dose adjustment. |
| Simvastatin [45,66] | DOR, NVP, RPV | There are no changes in the plasma levels between NNRTIs and simvastatin. These combinations are safe and do not require dose adjustment. |
| Thiazide antihypertensive | ||
| Hydrochlorothiazide [13] | ATV/RTV, DRV/RTV, FPV/RTV, LPV/RTV | There are no changes in the plasma levels between boosted PIs and hydrochlorothiazide. These combinations are safe and does not require dose adjustment. |
| Hydrochlorothiazide [13] | EVG/COBI | There are no changes in the plasma levels of EVG/COBI and hydrochlorothiazide. This combination is safe and do not require dose adjustment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





