1. Introduction
Dengue virus infection is endemic in several tropical and subtropical regions of the world where the mosquito vectors,
Aedes aegypti and
Aedes albopictus are found [
3]. The disease is estimated to affect approximately 200 to 400 million cases annually in the world [
1,
2].
Dengue is caused by any of the four dengue virus serotypes (DENV-1, DENV-2, DENV-3 and DENV-4). Dengue fever (DF) is usually a mild self-limiting febrile illness that may develop into more severe diseases such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [
4,
5]. To date, there is no approved specific antiviral therapy for dengue. Hence, an effective treatment against dengue is urgently needed.
Flavonoids are polyphenolic compounds with known potential biological activities including antioxidant, anti-inflammatory, anti-cancer, antimicrobial and antiviral activities [
6]. It is also known that flavonoids are synthesized by plants in response to microbial infections [
7]. In earlier studies, baicalein, a flavonoid and its main metabolite, baicalin were reported to exhibit
in vitro anti-DENV replication activities [
8,
9]. Baicalein and baicalin are flavonoids mainly found in the roots of
Scutellaria baicalensis a Chinese medicinal plant [
8,
10]. Previous pharmacokinetic studies of baicalein showed that the compound is metabolized in animal and human to baicalin [
11,
12].
The antiviral properties of flavonoids relate to their chemical structure. However, to elucidate the anti-DENV activity of selected flavonoids in vivo, it is important to know the bioavailability and the type of metabolites that are present in serum after administration of these flavonoids. Therefore, it is essential to study the bioavailability and metabolism of both baicalein and baicalin.
This study is designed to evaluate the bioavailability of baicalein in Albino Wistar rat and evaluate sera of rats fed with baicalein on anti-DENV activity in vitro.
2. Results
Pharmacokinetic study of baicalein in rat
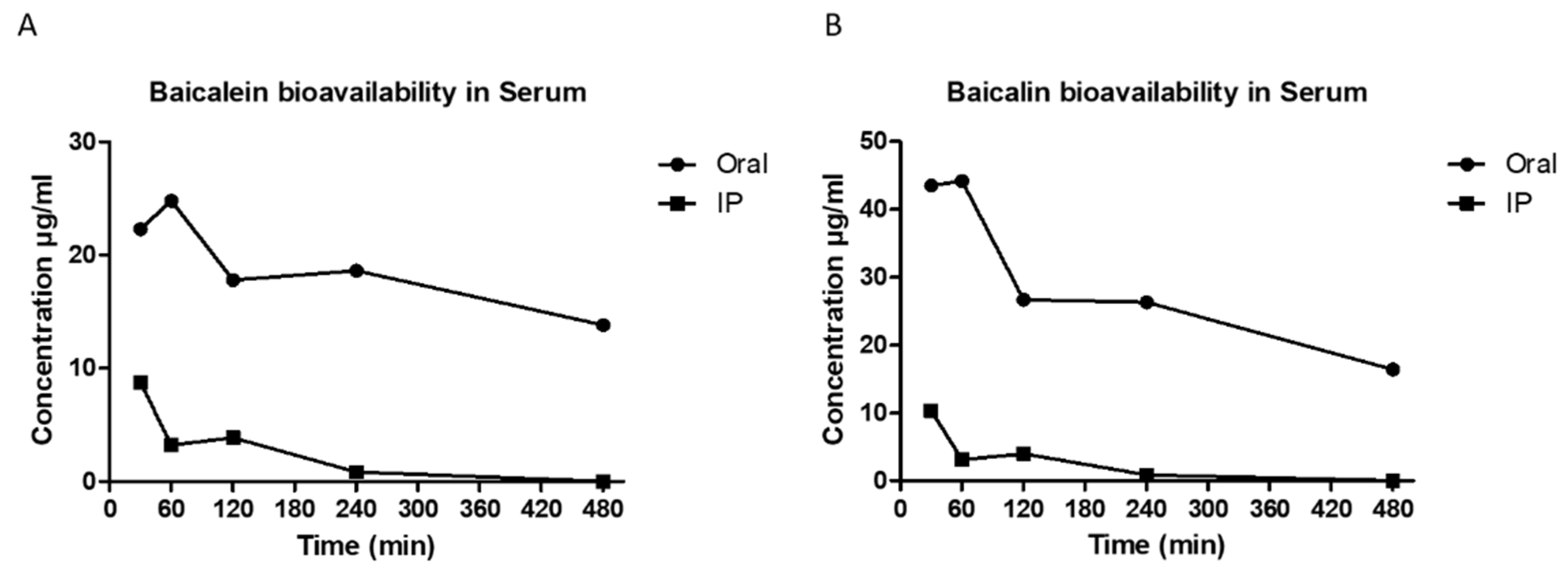
Baicalein was administered orally (500 mg/ml) and intraperitoneal injection (IP) to the Albino Wistar rats. Following thereafter baicalin the metabolite of baicalein, at concentration of 44.20 µg/ml was detected in rat blood serum at 1-hour oral consumption. Baicalin concentration in rat blood serum dropped to 27.70 µg/ml after 2 hours post-oral administration and maintained at the same concentration up to 4 hours. After 8-hours the baicalin concentration was detected 16.40 µg/ml in the rat blood serum. Intraperitoneal injection with baicalein the highest concentration of baicalin detected in IP blood serum was at 10.30 µg/ml at thirty minutes post injection. The concentration dropped to 3.10 µg/ml at one hour and two hours after injection and eventually not detectable in the rat blood serum at 4 hours onwards. (
Figure 1).
Baicalein concentration in the rat serum was measured as well using LC/MS/MS. The results showed that the highest concentration of baicalein was detected at one hour after oral administration (24.80 µg/ml) and it was maintained at 18 µg/ml after two hours and four hours oral administration. Baicalein concentration dropped to 13.80 µg/ml eight hours after oral administration. Similar to baicalin, baicalein bioavailability was detected very low in rat serum when it was administrated intraperitoneally. The highest concentration of baicalein detected was equal to 8.37 µg/ml thirty min after IP injection. The concentration dropped to 3.21 µg/ml and maintained at the same range till 2 hours after injection and eventually became undetectable at 4-8 hours after IP injection.
Cytotoxicity of sera of rats fed baicalein
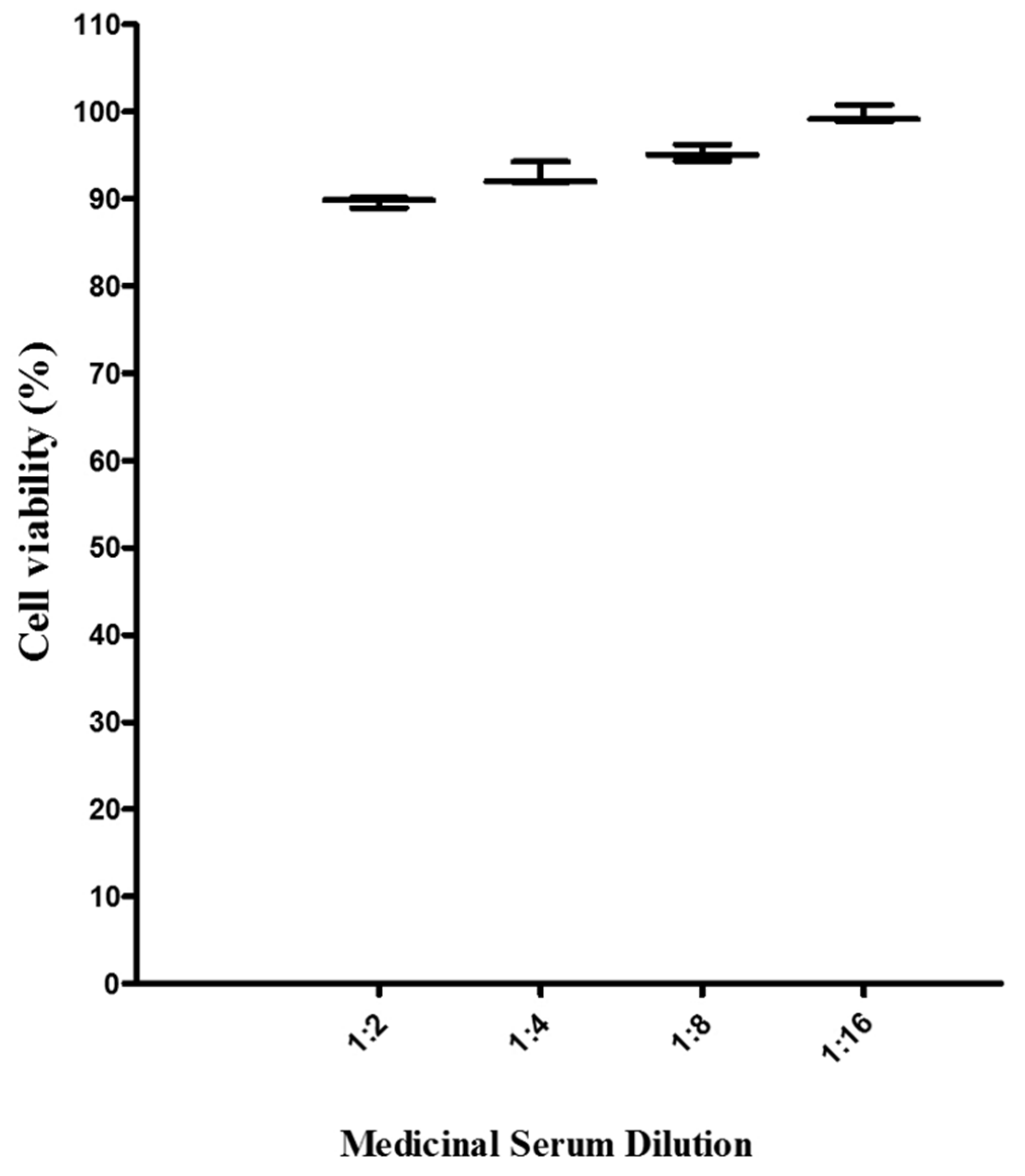
The cytotoxicity of sera of rats fed with baicalein was determined by MTS assay as described above. The cytotoxicity results showed, there was no cytotoxicity from all sera of rats fed baicalein against Vero cells (
Figure 2). In sera with the most concentrated more than 90% of cells were still viable, in comparison to the vehicle control (Sera of rats without compound).
In vitro antiviral activity of sera of rats fed baicalein
The effects of sera of rats fed with baicalein was evaluated against DENV-2 virus
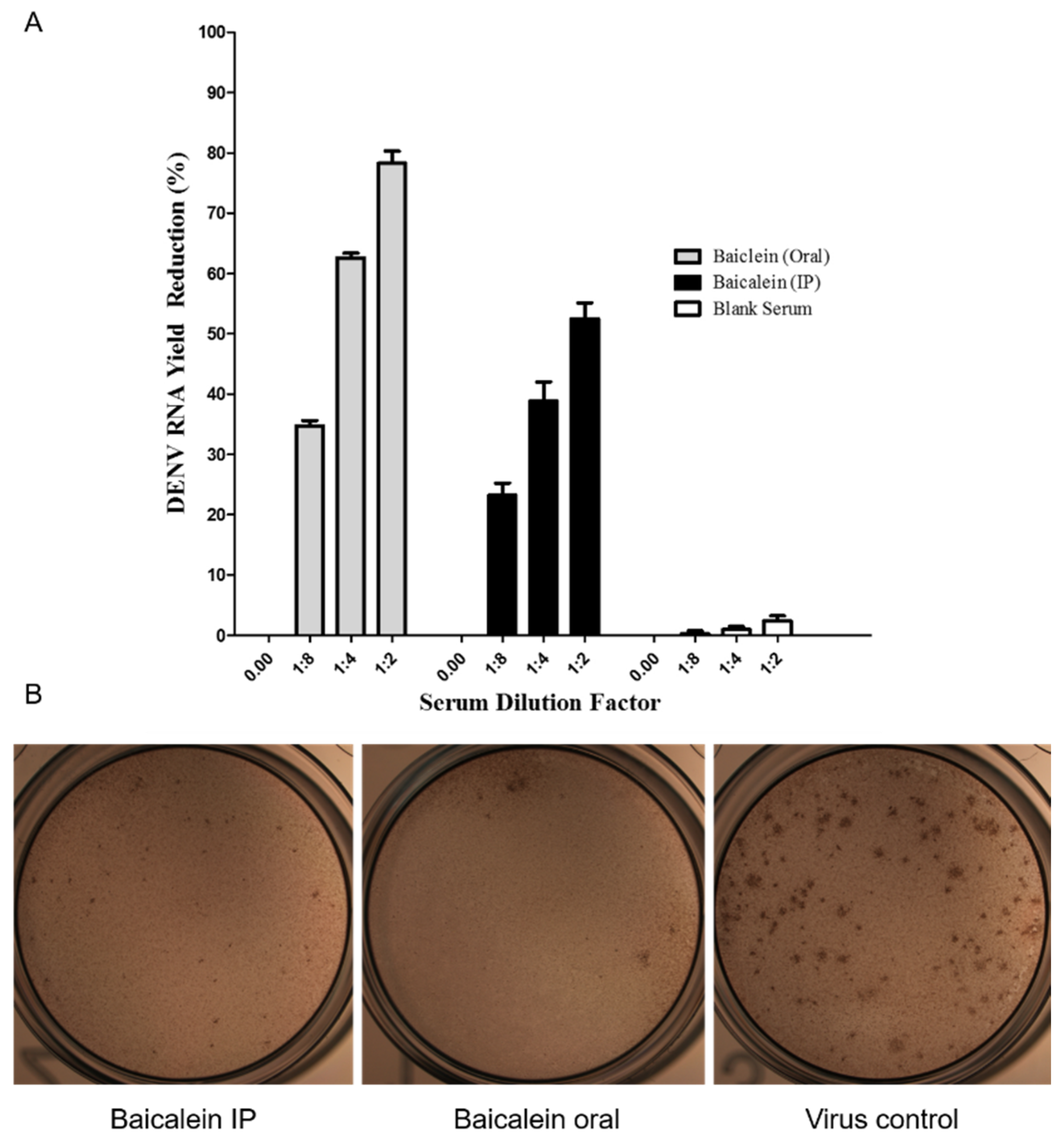
in vitro using the virus yield reduction assay and qT-PCR and the Focus Forming Unit Reduction Assay (FFURA). Results obtained suggested that at, 1:2 diluted serum prepared from the orally administered rat’s blood sample inhibited 78.26% ± 2.04 of DENV-2 replication whereas at the same dilution prepared from the intraperitoneally administered rat’s blood sample, 52.50 % ± 2.04 inhibition against DENV-2 replication was obtained. The same inhibition percentages were observed for other serum dilutions. The FFURA data showed the same reduction in number of dengue virus antigen compared to virus control group (
Figure 3B). As the figure present, number of foci in sera of rat fed with baicalein significantly reduce compared to virus control. The same reduction can be observed in sera of rats injected intraperitoneally with baicalein, however the reduction is not significant.
3. Discussion
Dengue continues to be a major mosquito-borne disease of significant worldwide health concern. To date, there is no specific drug to treat this infection and currently the most applied prevention measures lie in controlling the mosquito populations [
15]. Earlier studies to discover antiviral against dengue showed that, both baicalein and its metabolite baicalin, process good anti-DENV inhibition properties
in vitro [
9,
16]. Baicalein showed direct virucidal activity against DENV-2 with IC
50= 1.55 μg/mL besides its effects against dengue virus adsorption and intracellular replication with IC
50 = 7.14 μg/mL and IC
50= 6.46 μg/mL respectively [
16]. Baicalin on other hand, affected DENV-2 replication at 13.5 µg/ml concentration and showed anti-adsorption effect with IC
50 = 18.07 µg/ml [
9]. Baicalein, a flavonoid isolated mainly from the roots of
S.baicalensis, when administrated to the animals and human is metabolized to baicalin as its main metabolite [
17,
18,
19]. To determine if baicalein possess anti DENV replication property
in vivo, baicalein was fed to adults’ rats to evaluate the bioavailability and
in vitro anti-DENV activity. The sera of rats fed with baicalein was tested for its anti DENV property ex-
vivo since DENV could not productively infect rats.
The sera of rats fed baicalein containing baicalin and baicalein significantly inhibited DENV-2 replication in Vero cells in a dose-dependent manner in consistency with our previous findings [
9,
16].
We found out that the sera prepared from orally administered rats in the first hour after administration contain higher amount of baicalin and baicalein compared to the sera that were taken after 1 h post administration from rats that injected IP. This finding can confirm that the significant bioavailability of orally administered baicalein in Albino Wistar rat is 1 h post administration with significant in vitro anti-DENV activity as well. Since the amount of baicalein detected in serum was neglectable to exert that effect and as baicalin is the predominant metabolite of baicalein, it can be concluded that the baicalin in sera of rats fed baicalein plays an important role in observed anti-DENV-2 activity. We have reported in vitro anti-DENV-2 activity of baicalin in our previous study with various effects against different stages of DENV-2 replication cycle. Baicalin inhibited DENV-2 intracellular replication with IC50= 13.5 μg/mL besides its virucidal activity against DENV-2 extracellular particles with IC 50= 8.74 μg/mL and anti-adsorption effect with IC50 = 18.07 μg/ml. Nevertheless, obtained data in current study showed that sera of rats fed baicalein containing 21.1 μg/mL of baicalin exhibited significant reduction of dengue-2 virus with 78.26% ± 2.04.
Based on previous findings it has been shown that the conversion of baicalein to baicalin occurs during digestion by the removal of a glycoside moiety by ß-glucoronidase with 74% conversion [
11,
12,
20]. ß-glucoronidase enzyme is found in lysosomes mainly available in the intestinal lumen. This enzyme is also produced by some certain intestinal commensal bacteria [
12].
Results from the study suggested that oral administration of baicalein gives significant bioavailability of both baicalein and baicalin to the Albino Wistar rat blood serum compared to intraperitoneal injection. This finding suggests that baicalein can be absorbed through the digestive system in animal model efficiently. The of data this study showed that baicalein is also available as non-metabolized administered compound but with lower concentration compared to baicalin which is consistent with previous studies on pharmacokinetic of baicalein [
10,
12].
4. Materials and Methods
Materials
Baicalein and baicalin were purchased from Sigma Chemical Company (Sigma, St Louis,USA). The compound was dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA), prior to use. All other chemicals and solvents used in LC/MS/MS such as methanol and acetonitrile were of the HPLC grade purchased from Fisher Scientific Company (Fisher Scientific,USA).
Cells and virus
C6/36 mosquito cells and Vero cells (African green monkey kidney) purchased from American Type Culture Collection (ATCC) were cultured and maintained in Eagle’s Minimum Essential Medium (EMEM) (Gibco, NY, USA), supplemented with 10% fetal bovine serum (FBS) (Gibco, NY, USA). Vero and C6/36 cells were incubated in humidified atmosphere at 37°C and 28°C and in the presence of 5% and 3% CO2 respectively.
In this study dengue virus type-2 (DENV-2) New Guinea C strain (NGC) was used. The virus was kindly provided by The Virology laboratory of the Tropical Infectious Disease Research and Education Center (TIDREC), University of Malaya (Kuala Lumpur, Malaysia). DENV-2 was propagated in C6/36 cells and harvested after presentation of cytopathic effects (CPE) on the day seven post-infection (PI). Propagated viral stock was titrated on Vero cells by focus forming assay (FFA) as previously described [
13] and stored at -80°C until needed.
Animal ethical issues
This study received approval from the Animal Experimental Unit (AEU), Faculty of Medicine, University of Malaya, Malaysia (No. MP/17/02/2012/KZ) and in full compliant of the National Academy of Science’s Guide for the Care and Use of Laboratory Animals [
21,
22]
Animal experimental design
Fourteen mature male Albino Wistar rats (weighing approximately 220-250 g) were obtained from the Animal Experimental Unit (AEU) of the University of Malaya. Rats were housed 2 per cage and maintained in a dark room under controlled temperature of 24°C, respectively. Food and water were provided as prescribed by the AEU.
The half maximum lethal dose (LD
50) was not carried out for this study because the maximum non-lethal dose of baicalein has been previously reported [
12] and in compliance to the to Ethical Guidelines
, which recommended not to redo the same test that has been conducted and published.
The rats were fasted for 12 h with free access to water before and throughout the experiment. Rats were randomly assigned to two experimental groups: group 1, normal control (n = 4) and group 2, experimental (n =10). Rats in the experimental group were further divided into two treatment groups with different administration routes, intraperitoneal (IP) (n=5) and oral administration (n=5).
Administration of baicalein
To investigate the bioavailability of baicalein at different time profiles, the compound was dissolved in 0.5% DMSO and administered by an intragastric probe and intraperitoneal injection for baicalein with doses of 500 mg/kg/d (n=5) and 100 mg/kg/d (n=5), respectively. Control rats in all groups were treated with 0.5% DMSO at a final concentration of 1 mL/kg per rat based on previous recommendation as well as different methods of administration. The rats were anesthetized with IP injection of ketamine\ xylazine and blood samples were collected by cardiac puncture separately at 30, 60, 120, 240- and 480-minutes post-dosing. Sera were obtained by centrifugation at 3000 x g for 20 min and stored at -80 C˚.
Cytotoxicity of sera of rats fed baicalein
The cytotoxicity of sera of rats fed baicalein was determined against Vero cells using the MTS assay. Briefly, Vero cells seeded at a concentration of 5×104 cells/well in 96-well cell culture microplate and kept at 37°C for 24 h in a humidified atmosphere with 5% CO2.
When cell monolayer reached 90% confluency, two-fold dilutions of sera of rats fed baicalein were added to the cells in triplicates. After 4 days post treatment, MTS solution (Promega, Madison, WI, USA) was added to each well and the microplate was kept at 37°C for 4 h in a humidified atmosphere with 5% CO2. Then the absorbance values of the wells were measured at 570 nm using a 96-well plate reader (TECAN, Mannendorf, Switzerland). Dose-response curve was plotted using Graph Pad Prism 5 (Graph Pad Software Inc., San Diego, CA, USA, 2005). Results were represented as the means ± standard error of the mean (SEM) from triplicate assay from three independent experiments.
In Vitro Antiviral activity of sera of rats fed baicalein
Antiviral activity of sera of rats fed with baicalein was evaluated by performing the virus yield reduction assay using DENV-2 specific quantitative RT-PCR and Foci Forming Reduction Unit Assay (FFRUA). Briefly, confluent monolayers of Vero cells were prepared in 24-wells cell culture plate and incubated with 100 µl of DENV-2 (NGC) suspension containing 100 FFU of DENV-2 and an equal volume of the diluted two-fold concentrations of sera of rats fed baicalein in FBS-free medium in the presence of blank serum (50%, 25%, 12.5% and 6.25%) which were sterile-filtered using 0.2 µm syringe filter (Sartorius Stedim Biotech, Germany) and added to the respective wells. Virus adsorption was performed with gentle rocking and incubated inside CO2 Incubator for 1 h at 37ºC temperature. Subsequently, cells were washed twice with sterile PBS to remove unabsorbed viruses; then the cells were overlaid with 100 μl medium containing 2% FBS (Sigma-Aldrich, USA) with increasing two-fold dilutions of sera of rats fed baicalein. Treated cells were then incubated for 2 days at 37°C in 5% CO2 atmosphere. After two days post-infection, the supernatants were collected and DENV-2 RNA were extracted using the Viral RNA extraction kit (Qiagen, Hilden, Germany DENV yield was determined using the q-RT-PCR.
DENV quantitative RT-PCR
The quantitative RT-PCR assay for DENV-2 was performed using SensiFAST
TM SYBR
® Hi- ROX One-Step Mix (Bio line, UK) in a total reaction volume of 20 μl which contained of ddH2O (6.4μl), 2× SensiFAST One-Step (10 μl), Reverse transcriptase (0.2 μl), RNase Inhibitor (0.4 μl), 0.5 μl of 400 nM of forward (DNF) and reverse (D2R) primers [
14], and the extracted DENV-2 RNA (2 μl). All samples were performed in triplicates, amplification was performed using the DNA Engine Opticon system (MJ Research/Bio-Rad, Hercules, CA) with the following thermal cycling conditions: reverse transcription at 42°C for 10 min, initial denaturation at 95°C for 10 min, followed by 45 cycles of 95°C for 15 sec, 59°C for 15 sec and 72°C for 30 sec. Melting curve analysis was afterward performed at temperature from 60°C to 95°C to verify the assay specificity. For absolute quantities of viral RNA in the samples, standard curve was established with viral RNA extracted from DENV-2 virus inoculate of known infection virus titer.
5. Conclusions
This The present study was designed to investigate antiviral activities of sera of rats fed baicalein against DENV due to administration of baicalein in Wistar rats. The results showed significant in vitro anti-dengue activity properties for sera of rats fed baicalein. As conclusion, oral administration of baicalein gives higher bioavailability of baicalin compared to the intraperitoneal administration. Based on baicalin effects against different stages of DENV replication, further studies may require revealing the specific antiviral target(s) of the compound. Furthermore, based on the acceptable bioavailability of baicalein and its metabolite baicalin and significant anti-DENV activity of sera of rats fed baicalein, further in vivo study it required using suitable animal models for DENV. It also furthers studies are required to investigate the molecular and intracellular pathways, especially identification of target viral gene(s) and cellular element(s) that could play a vital role in facilitating anti-DENV function of baicalein and baicalin as potential candidates for dengue infection treatment.
Author Contributions
This work was approved by all the co-authors. E.M, P.H, K.Z and SAB, made a significant contribution to the concept study design. Data and logistics for sample acquisition were completed by D.E and Z.C. R.P. E.M and P.H contributed to data analysis and interpretation. E.M P.H and D.E contributed to writing the draft of the article and critically revising it. SAB, K.Z contributed with grant resources. SAB and K.Z took part in project investigation and supervision.
Funding
We acknowledge the funding from the Ministry of Education, Malaysia for niche area research under the Higher Institution Centre of Excellence (HICoE) program (Project MO002-2019).
Conflicts of Interest
The authors declare no conflicts of interest. The work described in this manuscript is original. It has not been published and is not considered for publication elsewhere, partially, or completely. All authors read and approved the final work
References
- Rice, C.M. Flaviviridae: the viruses and their replication. Fields virology. 1996, 3, 931–959. [Google Scholar]
- Organization WH, Research SPf, Diseases TiT, Diseases WHODoCoNT, Epidemic WHO, Alert P: Dengue: guidelines for diagnosis, treatment, prevention and control: World Health Organization; 2009.
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; et al. The global distribution and burden of dengue. Nature. 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C. Flaviviridae: the viruses and their replication. Fields virology. 2001, 1, 991–1041. [Google Scholar]
- Hidari, K.I.; Takahashi, N.; Arihara, M.; Nagaoka, M.; Morita, K.; Suzuki, T. Structure and anti-dengue virus activity of sulfated polysaccharide from a marine alga. Biochem Biophys Res Commun. 2008, 376, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Lim, T.-H.; Rahim, N.-A.; Shu, M.-H.; Teoh, B.-T.; Sam, S.-S.; et al. Extract of Scutellaria baicalensis inhibits dengue virus replication. BMC Complement Altern Med. 2013, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, E.; Teoh, B.-T.; Sam, S.-S.; Lani, R.; Hassandarvish, P.; Chik, Z.; et al. Baicalin, a metabolite of baicalein with antiviral activity against dengue virus. Scientific reports. 2014, 4, 5452. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Y.; Hsiu, S.L.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D.L. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J. Pharm. Pharmacol. 2003, 55, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Xin Wenyu, Tian Shuo, Song Junke, He Guorong, Mu Xin, Qie Xuemei et al. Research Progress on Pharmacological Actions and Mechanism of Baicalein and Baicalin. Curr Opin Complement Alternat Med. 2014, 1, 71–78. [Google Scholar]
- Xu, G.; Dou, J.; Zhang, L.; Guo, Q.; Zhou, C. Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum. Biol Pharm Bull. 2010, 33, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-S.; Abd-Jamil, J.; Abubakar, S. Antibody neutralization and viral virulence in recurring dengue virus type 2 outbreaks. Viral Immunol. 2007, 20, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Seah, C.; Chow, V.; Tan, H.; Chan, Y. Rapid, single-step RT-PCR typing of dengue viruses using five NS3 gene primers. J Virol Methods. 1995, 51, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. Novel antiviral activity of baicalein against dengue virus. BMC Complement Altern Med. 2012, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; He, G.; Song, J.; Wang, S.; Xin, W.; Zhang, D.; et al. Pharmacokinetic study of baicalein after oral administration in monkeys. Fitoterapia. 2012, 83, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Du, L.; Wang, S.; He, G.; Yang, T.; Li, X.; et al. Pharmacokinetic study of baicalein and its major metabolites after iv administration in dogs. CHM. 2011, 3, 196–201. [Google Scholar] [CrossRef]
- Dou, J.; Chen, L.; Xu, G.; Zhang, L.; Zhou, H.; Wang, H.; et al. Effects of baicalein on Sendai virus in vivo are linked to serum baicalin and its inhibition of hemagglutinin-neuraminidase. Arch Virol. 2011, 156, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ruan, Q.; Bedner, E.; Deptala, A.; Wang, X.; Hsieh, T.; Traganos, F.; Darzynkiewicz, Z. Effects of the flavonoid baicalin and its metabolite baicalein on androgen receptor expression, cell cycle progression and apoptosis of prostate cancer cell lines. Cell Prolif. 2001, 34, 293–304. [Google Scholar] [CrossRef] [PubMed]
- CHEMICALS, D (2005) OECD Guidline for testing of chemicals.
- Health, N.I. O (1985) Guide fr the care and use of laboratory animals. National Academies.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).