1. Introduction
Gastric cancer is the fourth leading cause of cancer death worldwide and the fifth most common malignant tumor [
1,
2].
Its occurrence varies markedly across different geographic regions [
3], with Eastern Asian countries, having the highest incidence rates, particularly China (accounting for half of all cases globally), Japan, and Korea [
1].
Radical surgery remains the mainstay of curative treatment for patients with resectable gastric cancer. R0 resection with gastrectomy plus D2 lymphadenectomy is the standard of care (SoC) [
4]. Unfortunately, less than 25% of patients diagnosed can be considered for resection since gastric cancer is most frequently discovered in advanced stages [
2].
Despite optimal surgical treatment, there is a high rate of tumor recurrence with approximately 60% of patients presenting tumor relapse [
2].
In Western countries, the reported five-year survival rate of patients treated with perioperative strategies and surgery is approximately 35 to 45% and between 0 to 10% in patients with metastatic disease, with a median overall survival (OS) of 12 months at most [
2,
4,
5].
Considering this discouraging panorama, new treatment strategies are certainly warranted to further improve clinical outcomes.
Over the last decade, significant progress has been made in better understanding the biology of gastric cancer via the investigation of their molecular characteristics. Molecular characterization is of vital relevance, given that advances in the knowledge of the molecular pathways involved in pathogenesis may allow the detection of new biomarkers capable of selecting patients eligible for targeted therapies.
2. Molecular subtypes in gastric cancer
Gastric cancer is a heterogenous disease [
6]. Traditional morphological classifications by Lauren and the World Health Organization (WHO) have limitations that do not reflect all the molecular complexity.
Several methods have been used to classify gastric cancer into molecular subtypes: next-generation sequencing (NGS) including deoxyribonucleic acid (DNA) sequencing, ribonucleic acid (RNA) sequencing, whole-exome sequencing, copy number variation analysis, and DNA methylation arrays. All these methods afford more detailed information than classic histopathological characteristics.
Molecular classification might increase the complexity and costs of diagnosis, however, it provides valuable information necessary to select targeted treatment that may increase the survival of gastric cancer patients [
7,
8].
2.1. The Cancer Genome Atlas (TCGA) subtypes
The most comprehensive molecular characterization of gastric adenocarcinoma was reported by the TCGA (The Cancer Genome Atlas) Research Network in 2014 [
6].
The study proposed a molecular classification into four molecular subtypes after analyzing 295 resected gastric tumor samples: Epstein-Barr virus (EBV)-positive (representing 9%), microsatellite unstable tumors (MSI) (22%), genomically stable (GS) tumors (20%), and tumors with chromosomal instability (CIN) (50%) [
6,
9,
10].
EBV-positive gastric cancer is due to infection by the Epstein-Barr virus. These tumors are more frequently found in male patients and are mainly located in the gastric fundus or body. This subtype presents high EBV burden, recurrent mutations in AT-rich interactive domain-containing protein 1A (
ARID1A), phosphatidylinositol 3-kinase (
PIK3CA), extreme DNA hypermethylation, B-cell lymphoma 6 corepressor (
BCOR) mutations, amplification of Janus-associated kinase 2 (
JAK2) and Erb-B2 receptor tyrosine kinase 2 (
ERBB2), and programmed death ligand-1/2 (PD-L1/2) overexpression. These findings suggest a potential therapeutic role for PIK3CA and JAK2 inhibitors and immune checkpoint antagonists [
6].
MSI gastric cancers are mostly due to promoter methylation that can lead to transcriptional silencing of the DNA mismatch repair gene
MLH1, resulting in a form of genomic instability. These tumors have a slightly higher prevalence in female and older patients (median age 72 years). The most common location is the gastric antrum. This subtype is characterized by elevated mutation rates, including mutations in
PIK3CA,
ARID1A, epithelial growth factor receptor (
EGFR),
ERBB3, and
TP53. It also presents high levels of PD-L1 expression. Frequent frameshift mutations in repeat DNA tracts cause inactivating mutations of key tumor suppressors genes (TSGs), or frequent missense-activating mutations in oncogenes [
6]. In recent years, immune checkpoint inhibitors have demonstrated relevant antitumor efficacy in this molecular subtype and currently constitute a mainstay of treatment in this population.
GS gastric cancers are more frequently diagnosed at an earlier age (median 59 years). Recurrent mutations in E-cadherin (
CDH1) and Ras homolog family member A (
RHOA) and CLDN18-ARHGAP rearrangements were observed. These genetic alterations may enhance invasiveness and disrupt intercellular cohesion, leading to more diffuse histologies, which confers more aggressiveness to this molecular subtype [
6].
CIN gastric cancers are more frequently located in the gastroesophageal junction/cardia and exhibit intestinal histology. These tumors show marked aneuploidy, focal activation of the receptor tyrosine kinases-Ras (RTK/RAS) pathway, high frequency of
TP53 mutations, amplification of cyclins E1, D1 (
CCNE1,
CCND1), and cell division protein kinase 6 (
CDK6). Amplification of
ERBB2,
KRAS/NRAS,
EGFR,
ERBB3,
FGFR2, and
MET are also observed [
6].
There are no differences in survival outcomes between the four molecular subtypes [
6]. Retrospective studies show that patients with the GS subtype had the least survival benefit with adjuvant chemotherapy and the CIN subtype had the greatest survival benefit [
11].
Of note, samples from the TCGA study were collected from resected gastric tumors. Data regarding the molecular characterization of metastatic disease is scarce. In 2021, an exploratory analysis was performed using samples from three different randomized clinical trials (KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062). The analysis concluded that, in patients with advanced gastric cancer, the EBV and MSI subtypes exhibited a lower prevalence compared with the TCGA dataset [
12].
2.2. Asian Cancer Research Group (ACRG) subtypes
In 2015, the ACRG study proposed a new molecular classification of gastric cancer after analyzing the mRNA expression level of 300 resected tumors [
13].
The study suggested dividing gastric cancer into four subtypes: MSI-high (23%), microsatellite stable/epithelial-mesenchymal transition (MSS/EMT) (15%), microsatellite stable/epithelial/TP53 intact (MSS/TP53+, p53 active) (26%), and microsatellite stable/epithelial/TP53 loss (MSS/TP53-, p53 inactive) (36%). Each of these molecular subtype associates with a different prognosis [
13].
MSI-high cancer locates more often in the antrum and mainly exhibits intestinal histology. It is associated with hypermutation in the
ARID1A gene, the PI3K-PTEN-mTOR pathway,
KRAS, and
ALK. It had the best prognosis and lowest recurrence frequency [
13].
The MSS/EMT subtype is mostly diagnosed in younger patients at advanced stages. These tumors exhibit mainly diffuse histology and include a large set of signet ring cell carcinomas, loss of CDH1 expression, and a number of mutations. It has the worst prognosis and the highest recurrence frequency [
13].
MSS/TP53+ has a higher number of mutations in
KRAS,
SMAD4,
ARID1A,
PIK3CA, and
APC compared with the MSS/TP53- subtype; in addition, EBV infection is more frequently observed. After the MSI subtype, it has the second-best prognosis [
13].
The MSS/TP53- subtype presents recurrent amplifications in
EGFR,
CCNE1,
ROBO2,
MDM2,
CCND1,
GATA6,
MYC, and
ERBB2. It has the highest prevalence of
TP53 mutations [
13].
2.3. Comparison between classifications
When comparing TCGA and ACRG classifications, the TCGA subtypes EBV, MSI, GS and CIN mainly correspond to the ACRG subtypes MSS/TP53+, MSI, MSS/EMT, and MSS/TP53-, respectively. However, there are some differences between the two classifications, with a partial overlap of certain subtype characteristics, probably explained by the different patient populations, tumor sampling, and technological platforms [
10].
Some of the most remarkable differences between classifications include CDH1 mutations (more commonly detected in the GS subtype [37%] compared with the MSS/EMT subtype [2.8%]) and RHOA mutations (characteristic of the GS subtype and observed in the MSS/TP53+ and MSS/TP53- subtypes, yet rarely seen in MSS/EMT). Finally, the CIN and GS subtypes are distributed across all ACRG subtypes.
HER2 gene amplifications are observed in several subtypes including CIN, GS, and EBV, however, they are more commonly associated with the CIN molecular subtype [
6,
9,
13,
14].
In the ACRG classification, recurrent focal amplifications in
HER2 were more commonly detected in the MSS/TP53- subgroup [
9,
13].
Based on these molecular classifications, HER2-positive gastric cancer has been more frequently associated with the CIN and MSS/TP53- molecular subtypes.
3. Human epidermal growth factor receptor 2 (HER2)
Human epidermal growth factor receptor 2 (HER2) is a transmembrane tyrosine kinase receptor involved in the pathogenesis and outcomes of several types of cancer, including advanced gastroesophageal adenocarcinomas [
15].
HER2 expression is most commonly determined by immunohistochemistry (IHC) and/or fluorescence in situ hybridization (ISH), although other methodologies are available. Overexpression of the HER2 protein in gastric cancer was first described in 1986 via IHC [
16].
HER2 protein expression may be classified as 0, 1+, 2+, or 3+ by IHC depending upon the extent and pattern of staining.
HER2 gene amplification is determined by ISH;
HER2 overexpression is currently defined as IHC 3+, or IHC 2+ along with
HER2 gene amplification by ISH (chromosome enumeration probe [CEP] 17 ratio ≥ 2 [ISH positive]) [
17]. However, IHC classification presents limitations, mainly due to remarkable intratumoral heterogeneity, the possibility of incomplete staining of gastric cancer cells, and the inter-pathologist variability associated with the subjective interpretation of the results [
18].
HER2 expression has been associated with gastroesophageal adenocarcinomas, with several studies reporting
HER2 amplification rates varying from 12% to 27% and HER2 overexpression from 9% to 23% [
4,
9,
19,
20,
21,
22]. Its expression is more frequent in the proximal stomach, including the esophageal-gastric junction, than in the distal stomach [
22].
Some studies could not demonstrate the prognostic properties of HER2, however, a larger number of studies indicated that HER2 expression confers a more aggressive biological behavior and higher recurrence frequencies in HER2-positive tumors [
22,
23,
24,
25,
26,
27,
28,
29,
30].
HER2 status is the most studied target in gastroesophageal cancer and has key clinical implications in the management of the disease.
Trastuzumab, the first monoclonal antibody against HER2 receptor, was approved for clinical use, in combination with chemotherapy, as first line treatment in patients with HER2-positive unresectable or metastatic gastric cancer in 2010 [
31], representing a paradigm shift in the management of HER2-positive disease.
Therefore, HER2 testing is strongly recommended for all patients at the time of diagnosis, especially in the metastatic setting, due to its clinical implications [
4].
Advances in the knowledge of gastric cancer, including the role of the HER2 pathway, molecular characterization development, and a deeper understanding of the tumor microenvironment, have raised new hypotheses to improve new therapeutic strategies, including immunotherapy.
4. PD-L1 expression in HER2-positive gastric cancer
Several studies have shown that the immune system plays a key role in the growth of malignant tumors [
32,
33].
PD-L1 is an inhibitory molecule expressed in a broad range of cancers, it is a ligand of the PD-1 receptor expressed on the T cell surface [
33].
The function of PD-1 is to downmodulate undesirable immune responses, and it has been shown to negatively regulate antigen receptor signaling by interacting with its ligand [
34,
35].
When PD-L1 is expressed on a tumor cell membrane, it interacts with the PD-1 receptor on the T cell. This interaction blocks T-cell proliferation and activity against the tumor, which allows cancer to escape from the host’s antitumor immunity [
36,
37,
38]. Additionally, the expression of PD-L1 on tumor cells leads to the apoptosis of specific CD8+ cytotoxic lymphocytes, which further decreases the antitumor immune response [
39]. These characteristics make PD-L1/PD-1 a potential treatment target in a wide range of malignant tumors, including gastroesophageal cancer.
The PD-L1 combined positive score (CPS) has been increasingly developed as a possible predictive biomarker of response to immunotherapy, and is being regularly used as a stratification marker in clinical trials [
40]. This predictive score has been defined as the number of PD-L1-positive cells (tumor cells, macrophages, and lymphocytes), divided by the total number of tumor cells and multiplied by 100. The score varies between CPS ≥ 1, CPS ≥ 5, and CPS ≥ 10. In gastric cancer, PD-L1 CPS ≥ 1 generally defines PD-L1-positive tumors [
41].
Some studies have reported significantly higher PD-L1 expression rates in HER2-negative tumors [
8,
42], while other studies reported opposite findings [
43,
44] or found no differences in PD-L1 expression between HER2-positive and negative tumors [
7,
45]. These discrepancies may be due to the heterogeneity of the populations studied, differences in the scoring methods, or in the monoclonal antibodies used for detection.
The relationship between molecular subtypes of gastric cancer and PD-L1, HER2, and combined HER2 and PD-L1 expression, requires further investigation.
4.1. Trastuzumab as an inducer of immunity
There is strong evidence based on preclinical and clinical studies, that the immune system contributes significantly to the therapeutic effects of trastuzumab in solid tumors [
46,
47].
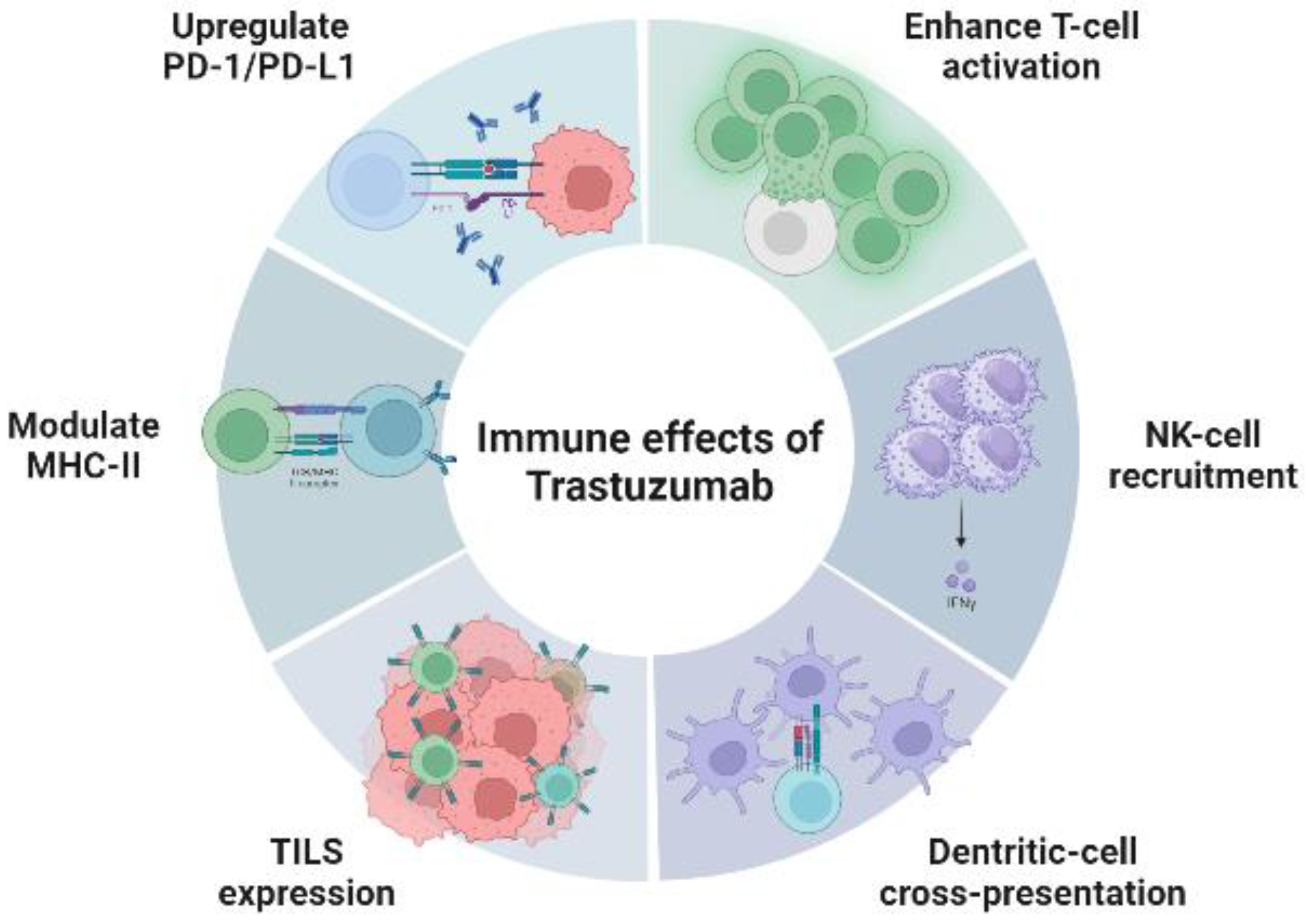
The precise mechanism by which trastuzumab acts in cancer cells is not completely understood. Trastuzumab seems not only to prevent the dimerization of HER2 with other HER family members and stimulate endocytosis (HER2 internalization), rather it also appears to play an important role in the tumor microenvironment.
HER2-positive cancers have high levels of T-cell infiltration [
48]. Several preclinical studies have shown that trastuzumab increases T-cell activation (antibody-dependent cellular cytotoxicity), recruitment of natural killer cells (degranulation and cytotoxicity), cross-presentation by dendritic cells, inhibits angiogenesis, induces the expression of tumor-infiltrating lymphocytes, and modulates the expression of the major histocompatibility complex class II, resulting in the enhancement of cell-mediated antitumor immunity [
22,
41,
49,
50,
51,
52,
53]. These effects are represented in
Figure 1.
Trastuzumab also upregulates the expression of PD-1 and PD-L1, which has been described as a mechanism of resistance in several studies [
51,
54].
This finding led to the hypothesis that combining trastuzumab with a second antibody that activated the host’s innate immune system (anti-PD-1/PD-L1), associated with standard cytotoxic chemotherapy, could enhance the therapeutic effects of anti-HER2 antibodies.
4.2. Synergistic activity: anti-HER2 and anti-PD-1/PD-L1
Preclinical studies show that a monoclonal antibody against PD-1/PD-L1 can substantially boost the efficacy of anti-HER2 treatment [
54,
55].
Stagg
et al., demonstrated the synergistic activity of anti-PD-1 and trastuzumab. Combining both antibodies, greater tumor regression was observed than with trastuzumab alone in a HER2-positive mouse model [
54].
Junttila
et al., observed that combining a trastuzumab-based bispecific HER2 antibody with anti-PD-L1, inhibited tumor growth, increasing the rates and durability of therapeutic responses [
56].
The association of an anti-PD-1/PD-L1 with an anti-HER2 antibody can enhance its effect by reducing the immune escape of tumor cells, increasing the effect of cytotoxic T cells, and facilitating the elimination of cancer cells by trastuzumab indirectly [
7,
41,
54,
57].
5. Targeting HER2 in gastric cancer
5.1. Standard therapies
In resectable locally advanced HER2-positive tumors, the treatment of choice is perioperative chemotherapy. Since 2019, with data from the FLOT4 clinical trial [
NCT01216644], chemotherapy based on the FLOT regimen (5FU, oxaliplatin, and docetaxel) constitutes the standard scheme, replacing ECF / ECX (epirubicin, cisplatin and 5FU / capecitabine)—the previous standard perioperative treatment since the results of the MAGIC trial [
NCT00002615] in 2006 [
58]. In the FLOT4 trial, median OS was 35 months (m) with ECF/ECX versus 50 months with FLOT chemotherapy (hazard ratio [HR] 0.77 [95% confidence interval [CI], 0.63-0.94]; p=0.012) [
59].
The association of an anti-HER2 therapy to perioperative chemotherapy has not demonstrated benefit so far in this setting, therefore it does not form part of standard treatment. However, different combinations of chemotherapy with several anti-HER2 agents are being clinically investigated.
In unresectable or metastatic disease, a platinum and fluoropyrimidine doublet chemotherapy regimen associated with trastuzumab constitutes the standard first-line treatment after the ToGa trial [
NCT01041404] in 2010. Its results showed an improvement in OS in the trastuzumab plus chemotherapy arm (cisplatin plus capecitabine or 5-fluorouracil [5-FU]), compared with chemotherapy-alone (13.8 m vs. 11.1 m, HR 0.74 [95% CI, 0.60-0.91],
p=0.0046). No clinically meaningful difference in toxicity between arms was observed [
31].
The choice of chemotherapy regimen is based on the patient’s general condition, comorbidities, and considering the toxicity profile of each drug. The most frequently prescribed chemotherapy combinations include cisplatin or oxaliplatin associated with 5-FU or capecitabine. There are only a few head-to-head comparisons between regimens, showing similar efficacy [
60,
61].
Since the addition of trastuzumab to chemotherapy, which represented a milestone in the treatment of HER2-positive disease, no further advance has been made in the first-line treatment setting.
As standard second-line treatment, paclitaxel in combination with ramucirumab demonstrated a significant improvement in OS when compared with paclitaxel alone (9.6 m vs. 7.4 m, HR 0.807 [95% CI, 0.68-0.96],
p=0.017), as reported in the RAINBOW trial [
NCT01170663] [
62].
Other second and further treatment lines include single-agent therapies such as taxanes (docetaxel in the COUGAR-02 trial [
NCT00978549] [
63] or paclitaxel in the WJOG4007 trial [
NCT01224652] [
64]), irinotecan [
NCT00144378] [
65], or ramucirumab in the REGARD trial [
NCT00917384] [
66].
Despite these treatments, the duration of clinical benefit with the current SoC is limited. The majority of patients develop treatment resistance within a year and second-line treatment options are scarce and of limited efficacy. Therefore, novel therapeutic approaches are warranted to improve survival outcomes.
5.2. Clinical research in the perioperative setting
5.2.1. Addition of anti-HER2 agents to perioperative chemotherapy
Data concerning the addition of an anti-HER2 therapy to standard perioperative chemotherapy are still scarce.
Several clinical trials are evaluating numerous combinations of chemotherapy with anti-HER2 alone or combined with immunotherapy.
One of the first studies to test this combination was the Asian phase II Trigger study [
jRCTs031180006], which analyzed the combination of S1/cisplatin plus trastuzumab or placebo, as preoperative treatment in 46 patients with extensive lymph node metastasis. The objective response rate (ORR) tended to be higher in the trastuzumab group than in the placebo group (66.7 % vs. 36.4%, respectively;
p=0.08). The proportion of patients downstaging to ypStages 0/I/II was also higher in the trastuzumab group (22.7% vs. 50.0%,
p=0.07). Survival outcomes are not available as yet [
67].
Another relevant study evaluating the combination of chemotherapy with an anti-HER2 is the HER-FLOT trial [
NCT01472029], a phase II study that evaluated FLOT plus trastuzumab in 56 patients. A pathological complete response (pCR) was achieved in 12 patients (21.4%) and 14 patients (25.0%) had near-complete responses. Median disease-free survival (DFS) was 42.5 months and the three-year OS rate was 82.1%. The primary endpoint (pCR > 20%) was reached. No unexpected safety issues were observed and long-term survival outcomes are promising [
68].
Similarly, the phase II-III PETRARCA trial [
NCT02581462] compared the standard chemotherapy regimen FLOT to FLOT plus trastuzumab in combination with pertuzumab. The release of negative results from the phase III JACOB trial [
NCT01774786]— evaluating the addition of pertuzumab to first-line HER2-positive standard treatment— resulted in the decision to terminate enrollment [
69].
Testing a different chemotherapy combination, the phase II NEOHX trial [
NCT01130337], evaluated the XELOX-T regimen (capecitabine, oxaliplatin, and trastuzumab) in 36 patients. Surgery was finally performed in 31 patients, of whom 28 had R0 resection and three presented pCR. After a median follow-up of 24.1 m, the 18-month DFS was 71% (95% CI, 53-83%). An update after 102 months of follow-up showed a median OS of 79.9 months and a 60-month OS of 58% (95% CI, 40-73%) [
70].
Also exploring the role of HER2-targeting in the perioperative setting, the phase II INNOVATION study [
NCT02205047] randomizes patients to receive chemotherapy alone (cisplatin plus 5FU/capecitabine or FLOT), chemotherapy plus trastuzumab or chemotherapy plus trastuzumab and pertuzumab. Results are not yet available [
71].
Until new results become available, additional HER2-targeted treatment should not be recommended outside clinical trials in the perioperative setting.
5.2.2. Addition of immunotherapy plus anti-HER2 agents to perioperative chemotherapy
Immune checkpoint inhibitors (anti-PD-1 and anti-PD-L1) are also a potential therapy under investigation in the perioperative HER2-positive setting.
In this scenario, there are several ongoing phase II studies analyzing different combinations that include immunotherapy. An Asian phase II trial [NCT04819971] is evaluating the association of tislelizumab (anti-PD-1), trastuzumab, and chemotherapy (docetaxel, S1, and oxaliplatin), while the single-arm phase II PHERFLOT [NCT02954536] will analyze pembrolizumab (anti-PD-1) in combination with trastuzumab and FLOT chemotherapy. Furthermore, an Asian phase II trial [NCT04661150] will randomize patients to receive atezolizumab (anti-PD-L1) plus trastuzumab, capecitabine, and oxaliplatin.
5.3. Clinical research in the advanced setting
After the approval of trastuzumab in the metastatic setting, other HER2-targeted agents have been evaluated, failing to demonstrate improved efficacy compared with standard chemotherapy. Some of the main randomized clinical trials are represented in
Table 1.
In spite of these unsatisfactory results, many other treatment strategies are being explored in numerous clinical trials, including chemotherapy, immunotherapy, and novel HER2-targeted therapy.
In this review, we summarize the results of the most remarkable studies and cite those that are currently ongoing.
5.3.1. First line
5.3.1.1. Addition of immunotherapy to standard first-line HER2-positive SoC
Exploring the role of immunotherapy in the first-line setting, an investigator-initiated single-arm phase II trial [
NCT02954536] was conducted between 2016 and 2019 by Janjigian
et al., [
76]. Pembrolizumab associated with standard first-line therapy was evaluated in 37 patients. Chemotherapy regimens included cisplatin or oxaliplatin plus capecitabine or 5-FU. The primary endpoint—progression-free survival (PFS) after six months—was reached in 70% of patients. The ORR was 91% (32/35 patients), with six patients (17%) achieving complete response, 26 (74%) partial response, and three stable disease as best response. Median duration of response (DoR) was 10 months. After 12 months, the OS rate was 80%. The combination appeared safe, without dose-limiting toxicities.
Similarly, a phase Ib/II trial [
NCT02901301] conducted by Lee
et al., [
77] evaluated the efficacy and safety of pembrolizumab associated with a first-line standard regimen in 43 patients. The chemotherapy regimen used was the combination of cisplatin and capecitabine. The primary endpoint was achieved, showing an ORR of 76.7% (complete responses in 14% and partial responses in 62.8% of patients). Median PFS was 8.6 m, median OS was 19.3 m, and DoR was 10.8 m. The toxicity profile was acceptable, with no patients discontinuing pembrolizumab due to severe toxicities. PD-L1 status was not related to survival.
The phase III KEYNOTE-811 study [NCT03615326] evaluated first-line SoC (trastuzumab plus chemotherapy) associated with pembrolizumab or placebo. The ORR improved by 22.7% in the pembrolizumab arm compared with the placebo group (77.4% vs. 51.9%, respectively; p=0.00006). Complete responses were 11.3% in the pembrolizumab group versus 3.1% in the placebo group. The median DoR was 10.6 months for patients treated with pembrolizumab and 9.5 months for those in the placebo arm. Grade 3 or higher adverse events occurred in 57.1% of the pembrolizumab group versus 57.4% in the placebo group. These interim analyses showed that the combination of pembrolizumab with trastuzumab and chemotherapy significantly improved the objective response rate and included complete responses in some participants.
These results led to the Food and Drug Administration (FDA) accelerating the approval of pembrolizumab in combination with trastuzumab and chemotherapy in the first-line treatment for patients with HER2-positive gastric cancer. The OS and PFS results are pending [
78].
In addition to pembrolizumab, other immunotherapy agents have been evaluated in this setting.
In the phase II INTEGA study [
NCT03409848], the immune checkpoint inhibitors nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) were tested in different combinations. The study assessed the combination of trastuzumab plus nivolumab plus ipilimumab in comparison with nivolumab plus trastuzumab plus FOLFOX chemotherapy. The median OS was 21.8 months in the FOLFOX arm compared with 16.4 months in the chemotherapy-free arm. The OS results obtained in the chemotherapy arm were significantly improved compared with the historic control from the ToGA trial [
79].
Another novel anti-PD-1, camrelizumab, has also been tested in combination with the first-line SoC [
ChiECRCT20220008]. Overall, 41 patients were included and primary endpoints were ORR, disease control rate (DCR), PFS, OS, and safety. ORRs were 75 % vs. 46.2 % (
p=0.032), showing benefit for the combination arm. Survival outcomes were favorable for the camrelizumab arm for DCR (96.4 vs. 69.2%;
p=0.003), PFS (3.78 vs. 1.74 months; HR: 0.416, CI 0.186-0.932;
p=0.027), and OS (18.4 vs. 13.2 months; HR: 0.343 (CI 0.151-0.783;
p=0.008). The combination was well tolerated, however, higher incidences of reactive cutaneous capillary endothelial proliferation and hypothyroidism were observed in the camrelizumab arm [
80].
Also evaluating camrelizumab in the first-line setting, is a single-arm phase II trial [NCT05070598] analyzing its combination with pyrotinib maleate, nab-paclitaxel, and tegafur (recruiting).
In earlier-phase trials, the anti-PD-L1 HLX10, will be tested in combination with first-line SoC in HER2-positive population (not recruiting yet) [NCT05311189].
5.3.1.2. Novel anti-HER2 and immunotherapy in first-line HER2-positive SoC
In the first-line scenario, novel anti-HER2 therapies are arising in combination with immunotherapy.
The phase II/III MAHOGANY trial [
NCT04082364] is evaluating the efficacy of several drug combinations including margetuximab (anti-HER2 specific for the Fc domain), retifanlimab (anti-PD-1), tebotelimab (anti-PD-1/anti-LAG3 antibody), trastuzumab, and chemotherapy. The study is structured into five cohorts testing combinations. The first results of the safety analysis of 43 PD-L1-positive (CPS ≥ 1), non-MSI patients treated with margetuximab plus retifanlimab (cohort A) were presented at ESMO 2021, reporting a tumor shrinkage of 85.7% (30/35 patients). After this first safety analysis, a randomized study design will evaluate the combination of margetuximab, immunotherapy with or without chemotherapy compared to the first-line standard therapy of trastuzumab plus chemotherapy [
81].
Also exploring the anti-HER2 and immunotherapy association, several ongoing studies are pending results or are in the recruitment phase.
The phase III HERIZON-GEA-01 [
NCT05152147] is an ongoing trial with three treatment arms: SoC treatment with chemotherapy and trastuzumab vs. standard chemotherapy with zanidatamab (bispecific anti-HER2) vs. standard chemotherapy with zanidatamab plus tislelizumab. PD-L1 expression was not required for enrollment but will be performed retrospectively [
82].
This trial was designed based on the promising results of the phase I trial in HER2-positive solid tumors where zanidatamab was evaluated. Zanidatamab was well tolerated and demonstrated an ORR >30% in advanced gastric cancer [
41,
83].
A phase Ib/II Destiny-gastric03 trial [
NCT04379596] investigates the efficacy of the antibody-drug conjugate against HER2, trastuzumab-deruxtecan (T-DXd), in several combinations including durvalumab (anti-PD-L1), pembrolizumab and chemotherapy. In the dose-escalation phase, patients with prior trastuzumab therapy received either T-DXd combined with 5-FU/capecitabine/durvalumab or capecitabine plus oxaliplatin/5-FU or capecitabine plus durvalumab. In the dose-expansion phase, therapy-naive metastatic patients are stratified by HER2 status and randomized into five study arms: T-DXd, trastuzumab plus 5-FU/capecitabine plus oxaliplatin/cisplatin, T-DXd plus 5-FU/capecitabine and oxaliplatin, T-DXd plus 5-FU/capecitabine and pembrolizumab or T-DXd plus pembrolizumab. Primary endpoints include safety, dose-finding, and ORR. Results presented at ASCO GI 2022 suggest the tolerability and feasibility of the recommended phase II doses for T-DXd plus 5-FU and T-DXd plus capecitabine. The ORR results of both arms are promising and patient recruitment is ongoing [
84].
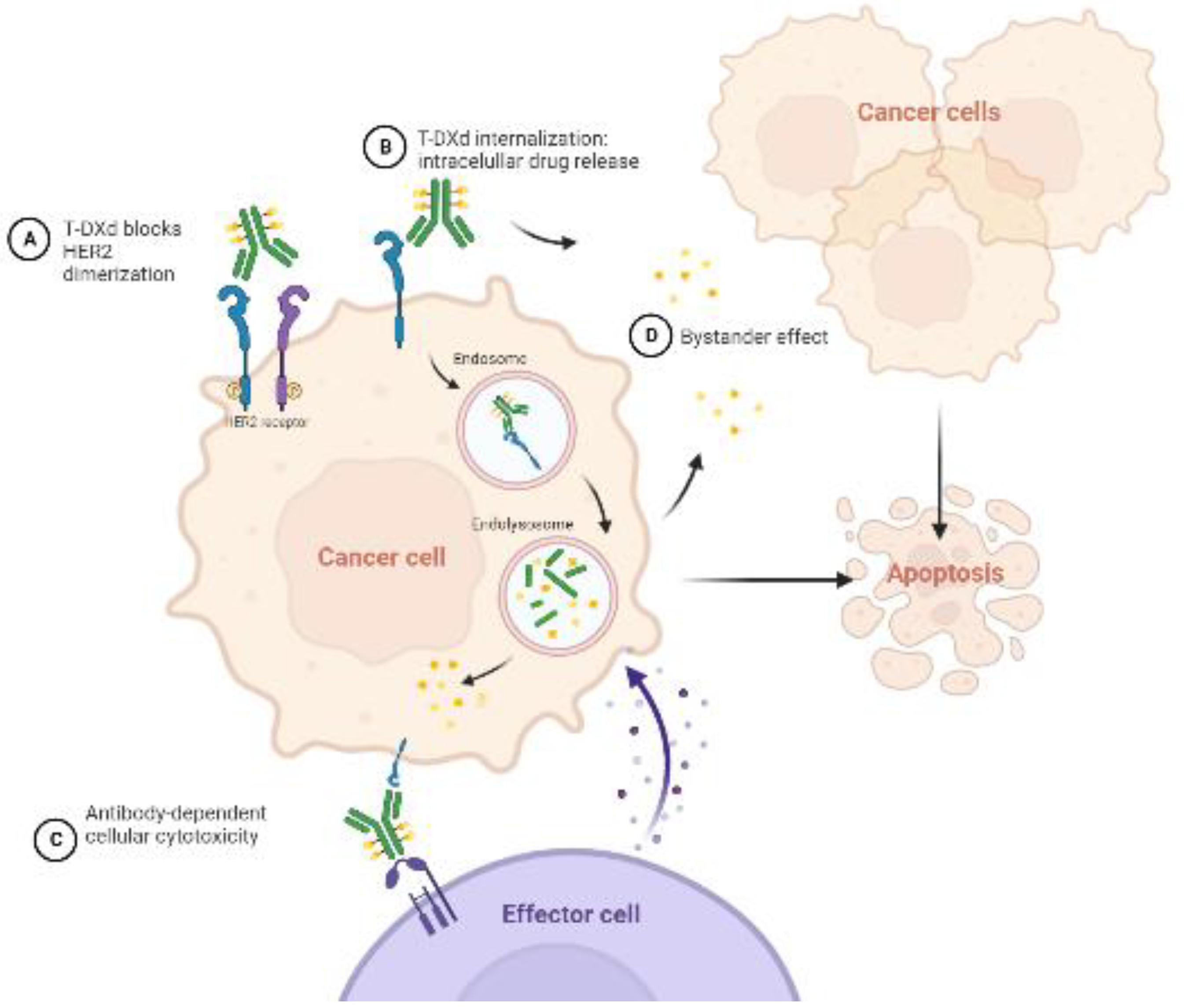
T-Dxd is an antibody-drug conjugate against HER2 with a complex mechanism of action. The antibody blocks HER2-receptor dimerization and is internalized via endocytosis. Once inside the cancer cell, the payload (cytotoxic topoisomerase I inhibitor) is released. This payload is also delivered in the extracellular matrix, acting against neighbor cancer cells (bystander effect). Moreover, it has been observed that it enhances antibody-dependent cellular cytotoxicity against the tumor. All these mechanisms are represented in
Figure 2.
Finally, IBI315, the novel recombinant fully human bispecific antibody against PD-1 and HER2, is being evaluated in an exploratory single-center phase Ib/II trial [NCT05608785] as first-line treatment in a HER2-positive cohort in combination with oxaliplatin and capecitabine.
5.3.2. Second and further lines
As second and further treatment lines, the therapeutic options available are still limited.
Trastuzumab-deruxtecan was approved by the FDA in January 2021 as a second-line therapy based on the results of the phase II Destiny-Gastric01 study [
NCT03329690]. T-DXd improved responses and increased OS (median OS 12.5 versus 8.9 months) compared with physician’s choice chemotherapy (irinotecan or paclitaxel). The ORRs were 51.3% vs. 14.3%, respectively. Median DoR was also improved, with the T-DXd arm being 12.5 vs. 3.9 months. The median PFS was 5.6 vs. 3.5 months [
85].
The phase III trial Destiny-Gastric04 [NCT04704934] is underway, where T-DXd will be compared with the current second-line SoC, paclitaxel plus ramucirumab.
In further treatment lines, pembrolizumab initially received accelerated approval from the FDA as a third-line and beyond treatment option in September 2017, based on the results of the phase II KEYNOTE-059 study [
NCT02335411]. Pembrolizumab was tested in monotherapy in a larger cohort of heavily pretreated patients (after two or more lines of systemic treatment). This cohort included HER2-negative and HER2-positive tumors that had previously received treatment with trastuzumab. Durable objective responses were observed in all the population, regardless of PD-L1 status with an ORR of 12%, while a higher response rate (16%) was observed in the PD-L1-positive population [
86].
Despite these data, the indication was finally withdrawn in April 2021, as the confirmatory phase III KEYNOTE-061 trial [
NCT02370498] did not show any clinically meaningful improvement in OS compared to paclitaxel [
87].
Several clinical trials with novel agents and combinations are ongoing. The phase II/III ASPEN-06 trial [NCT05002127] is testing evorpacept (ALX148) in combination with trastuzumab, ramucirumab, and paclitaxel; the KN026 study [NCT05427383] is evaluating the combination of KN26 (anti-HER2 agent) with chemotherapy. The phase II nextHERIZON trial [NCT05311176] is exploring the role of a vaccine against HER2 (IMU-131, named HER-Vaxx) in combination with chemotherapy or pembrolizumab. It is currently in the recruiting phase. Also recruiting, is the phase II K-Umbrella trial [NCT05270889], exploring the role of tislelizumab in combination with zanidatamab as second-line therapy.
In earlier-phase trials, the single-arm phase Ib/II HER-RAM trial [NCT04888663] is evaluating ramucirumab in combination with trastuzumab and paclitaxel in patients that had progressed with a previous trastuzumab-containing chemotherapy. Furthermore, the phase Ib/II CP-MGAH22-05 trial [NCT02689284] evaluated margetuximab and demonstrated favorable results when combined with pembrolizumab, with an ORR of 24% and a disease control rate of 62% in a second-line setting.
Finally, the phase II clinical trial ILUSTRO [
NCT03505320] is evaluating the novel antibody Zolbetuximab in different combinations: Zolbetuximab monotherapy or associated with immunotherapy and/or standard chemotherapy in gastric cancer patients (including HER2-positive disease) after at least two previous treatment lines. Zolbetuximab is a first-in-class monoclonal antibody that binds to the protein claudin 18.2. Targeting this protein has demonstrated promising results in HER2-negative patients, as observed in the clinical trials SPOTLIGHT [
NCT03504397] [
88] and GLOW [
NCT03653507] [
89], in which Zolbetuximab was tested associated with first-line chemotherapy. The ongoing ILUSTRO trial will provide information in pre-treated HER2-positive gastric cancer patients, with results still pending.
6. Conclusions
The treatment landscape of HER2-positive gastric cancer has evolved over recent years, especially with the introduction of novel anti-HER2 drugs and combination strategies. The use of immunotherapy in combination with anti-HER2 agents demonstrate a strong preclinical rationale and clinical trials have produced encouraging results. Additionally, the diverse therapeutic drugs under development mark an exciting era in the management of HER2-positive disease. Until ongoing research becomes available, it is crucial to consider treatment options within clinical trials, since these are patients with a current poor prognosis and limited therapies available.
Author Contributions
Concept and design, A.P. and C.B.; literature search, A.P. and C.B.; writing—original draft preparation, A.P. and C.B.; figure assembly, A.P. and C.B.; writing—review and editing, A.P., L.N., C.H., L.L. and C.B.; final approval of the article, A.P., L.N., C.H, L.L, and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable since the information is gathered from published articles.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN. Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209-249. [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.T; Lordick F. Gastric Cancer. Lancet 2020, 396, 635-648. [CrossRef]
- Lin, S.J.; Gagnon-Bartsch, J.A.; Tan, I.B.; Earle, S.; Ruff, L.; Pettinger, K.; Ylstra, B.; van Grieken, N.; Rha, S.Y.; Chung, H.C.; et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut 2015, 64, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines Version 2.2020 Gastric Cancer. Available from. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed: 14 Jan 2023).
- Okines, A.F.; Norman, A.R.; McCloud, P.; Kang, Y.K.; Cunningham, D. Metaanalysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009, 20, 1529-34. [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [CrossRef]
- Beer, A.; Taghizadeh, H.; Schiefer, A.I.; Puhr, H.C.; Karner, A.K.; Jomrich, G.; Schoppmann, S.F.; Kain, R.; Preusser, M.; Ilhan-Mutlu, A. PD-L1 and HER2 Expression in Gastroesophageal Cancer: a Matched Case Control Study. Pathol. Oncol. 2020, 26, 2225–2235. [Google Scholar] [CrossRef]
- Amirmoezi, F.; Geramizadeh, B. Molecular Classification of Gastric Cancer With Emphasis on PDL-1 Expression: The First Report From Iran. Clin Pathol. 2022, 15, 2632010X2210963. [CrossRef]
- Kelly, C.M.; Janjigian, Y.Y. The genomics and therapeutics of HER2-positive gastric cancer—from trastuzumab and beyond. J Gastrointest Oncol. 2016, 7, 750–762. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, G.; Hu, C. Molecular Classification of Gastric Adenocarcinoma. Gastroenterol Res Pract 2019, 12, 275–282. [Google Scholar] [CrossRef]
- Camargo, M.C.; Kim, W.H.; Chiaravalli, A.M.; Kim, K.M.; Corvalan, A.H.; Matsuo, K.; Yu, J.; Sung, J.J.; Herrera-Goepfert, R.; Meneses-Gonzalez, F.; et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut 2014, 63, 236–243. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Cecchini, M.; Shitara, K.; Enzinger, P.C.; Wainberg, Z.A.; Catenacci, D.V.; Chau, I.; Satoh, T.; Lee, J.; Loboda, A.; et al. Genomic landscape of late-stage gastric cancer. Ann Oncol. 2021, 32, S1062 - S1063. [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Lordick, F.; Janjigian, Y.Y. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat Rev Clin Oncol 2016, 13, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Gravalos, C.; Jimeno, A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2018, 19, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Mori, S.; Kawamoto, T.; Taniguchi, S.; Kobori, O.; Morioka, Y.; Kuroki, T.; Kano, K. Expression of epidermal growth factor receptors on normal human gastric epithelia and gastric carcinomas. J Natl Cancer Inst. 1986, 77, 1047–1052. PMID: 3464796.
- Bartley, A.N.; Washington, M.K.; Colasacco, C.; Ventura, C.B.; Ismaila, N.; Benson, A.; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 446–464. [Google Scholar] [CrossRef]
- Hierro, C.; Alsina, M.; Tabernero, J. Unraveling the hurdles in the development of HER2-targeted agents for metastatic gastro-esophageal cancer patients. Transl Cancer Res 2017, 6, S1035–S1039. [Google Scholar] [CrossRef]
- Ghosn, M.; Tabchi, S.; Kourie, H.R.; Tehfe, M. Metastatic gastric cancer treatment: Second line and beyond. World J Gastroenterol. 2016, 22, 3069–77. [Google Scholar] [CrossRef]
- Jørgensen, J.T.; Hersom, M. HER2 as a Prognostic Marker in Gastric Cancer - A Systematic Analysis of Data from the Literature. J. Cancer 2012, 3, 137–144. [Google Scholar] [CrossRef]
- He, C. Correlation of human epidermal growth factor receptor 2 expression with clinicopathological characteristics and prognosis in gastric cancer. World J Gastroenterol. 2013, 19, 2171. [Google Scholar] [CrossRef]
- Abrahao-Machado, L.F., Scapulatempo-Neto, C. HER2 testing in gastric cancer: An update. World J Gastroenterol. 2016, 22, 4619. [CrossRef]
- Akiyama, T.; Sudo, C.; Ogawara, H.; Toyoshima, K.; Yamamoto, T. The Product of the Human c- erb B-2 Gene: a 185-Kilodalton Glycoprotein with Tyrosine Kinase Activity. Science 1986, 232, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- Baykara, M.; Benekli, M.; Ekinci, O.; Irkkan, S.C.; Karaca, H.; Demirci. U.; Akinci, M.B.; Unal, O.U.; Dane, F.; Turkoz, F.P.; et al. Clinical Significance of HER2 Overexpression in Gastric and Gastroesophageal Junction Cancers. J. Gastrointest. Surg. 2015, 19, 1565–1571. [CrossRef]
- Allgayer, H.; Babic, R.; Gruetzner, K.U.; Tarabichi, A.; Schildberg, F.W.; Heiss, MM. C-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J. Clin. Oncol. 2000, 18, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, J.W.; Park, J.H.; Oh, S.J.; Kim, H.J.; Cho, Y.K.; Sohn, C.I.; Jeon, W.K.; Kim, B.I.; Yoo, C.H.; et al. HER-2/neu Amplification Is an Independent Prognostic Factor in Gastric Cancer. Dig. Dis. Sci. 2006, 51, 1371–1379. [Google Scholar] [CrossRef]
- Creemers, A.; Ebbing, E.A.; Hooijer, G.K.J.; Stap, L.; Jibodh-Mulder, R.A.; Gisbertz, S.S.; van Berge Henegouwen, M.I.; van Montfoort, M.L.; Hulshof, M.C.C.M.; Krishnadath, K.K.; et al. The dynamics of HER2 status in esophageal adenocarcinoma. Oncotarget 2018, 9, 26787–26799. [Google Scholar] [CrossRef]
- Gowryshankar, A.; Nagaraja, V.; Eslick, G.D. HER2 status in Barrett’s esophagus & esophageal cancer: a meta analysis. J Gastrointest Oncol. 2014, 5, 25–35. [Google Scholar] [CrossRef]
- Plum, P.S.; Gebauer, F.; Krämer, M.; Alakus, H.; Berlth, F.; Chon, S.; Schiffmann, L.; Zander, T.; Büttner, R.; Hölscher, A.H.; et al. HER2/neu (ERBB2) expression and gene amplification correlates with better survival in esophageal adenocarcinoma. BMC Cancer 2019, 19, 38. [Google Scholar] [CrossRef]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef]
- Bang, Y.J.; van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Williford, J.M.; Ishihara, J.; Ishihara, A.; Mansurov, A.; Hosseinchi, P.; Marchell, T.; Potin, L.; Swartz, M.A.; Hubbell, J.A. Recruitment of CD103 + dendritic cells via tumor-targeted chemokine delivery enhances efficacy of checkpoint inhibitor immunotherapy. Sci. Adv. 2019, 5, eaay1357. [Google Scholar] [CrossRef]
- Lian, J.; Zhang, G.; Zhang, Y.; Liu, H.; Zhang, J.; Nan, P.; Tian, W. PD-L1 and HER2 expression in gastric adenocarcinoma and their prognostic significance. Dig Liver Dis 2022, 54, 1419–1427. [Google Scholar] [CrossRef]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Maeda, A.; Nishimura, H.; Kurosaki, T.; Honjo, T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001, 98, 13866–13871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, W. Neutrophils diminish T-cell immunity to foster gastric cancer progression: the role of GM-CSF/PD-L1/PD-1 signalling pathway. Gut 2017, 66, 1878–1880. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Yao, H.; Li, C.; Fang, J.Y.; Xu, J. Regulation of PD-L1: Emerging Routes for Targeting Tumor Immune Evasion. Front. Pharmacol. 2018, 9, 536. [Google Scholar] [CrossRef]
- Buglioni, S.; Melucci, E.; Sperati, F.; Pallocca, M.; Terrenato, I.; de Nicola, F.; Goeman, F.; Casini, B.; Amoreo, C.A.; Gallo, E.; et al. The clinical significance of PD-L1 in advanced gastric cancer is dependent on ARID1A mutations and ATM expression. Oncoimmunology 2018, 7, e1457602. [Google Scholar] [CrossRef]
- Högner, A.; Moehler, M. Immunotherapy in Gastric Cancer. Curr Oncol 2022, 29, 1559–1574. [Google Scholar] [CrossRef]
- Takei, S.; Kawazoe, A.; Shitara, K. The New Era of Immunotherapy in Gastric Cancer. Cancers 2022, 14, 1054. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Ni, S.; Tan, C.; Cai, X.; Huang, D.; Sheng, W. Programmed death-ligand 1 expression in gastric cancer: correlation with mismatch repair deficiency and HER2-negative status. Cancer Med. 2018, 7, 2612–2620. [Google Scholar] [CrossRef]
- Li, Z.; Lai, Y.; Sun, L.; Zhang, X.; Liu, R.; Feng, G.; Zhou, L.; Jia, L.; Huang, X.; Kang, Q.; et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum. Pathol. 2016, 55, 182–189. [Google Scholar] [CrossRef]
- Oki, E.; Okano, S.; Saeki, H.; Umemoto, Y.; Teraishi, K.; Nakaji, Y.; Ando, K.; Zaitsu, Y.; Yamashita, N.; Sugiyama, M.; et al. Protein Expression of Programmed Death 1 Ligand 1 and HER2 in Gastric Carcinoma. Oncology 2017, 93, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Abd El Hafez, A.; Abdel-Aziz, A.; Elmetwaly, S.; Mokhtar, N. Prognostic Value of PD-L1 Immunohistochemical Marker in Gastric Carcinoma and Its Correlation with HER2 Status. Asian Pac. J. Cancer Prev. 2022, 23, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Bellati, F.; Napoletano, C.; Gasparri, M.L.; Panici, P.B.; Nuti, M. Immunologic Systemic Effect of Neoadjuvant Chemotherapy Requires Investigation Before Tumor-Associated Lymphocytes Can Be Introduced in Breast Cancer Treatment Algorithm. J. Clin. Oncol. 2021, 28, 471– 472. [CrossRef]
- Ferris, R.L.; Jaffee, E.M.; Ferrone, S. Tumor Antigen–Targeted, Monoclonal Antibody–Based Immunotherapy: Clinical Response, Cellular Immunity, and Immunoescape. J. Clin. Oncol. 2021, 28, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E.; et al. Prognostic and Predictive Value of Tumor-Infiltrating Lymphocytes in a Phase III Randomized Adjuvant Breast Cancer Trial in Node-Positive Breast Cancer Comparing the Addition of Docetaxel to Doxorubicin With Doxorubicin-Based Chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Varadan, V.; Gilmore, H.; Miskimen, K.L.S.; Tuck, D.; Parsai, S.; Awadallah, A.; Krop, I.E.; Winer, E.P.; Bossuyt, V.; Somlo, G.; et al. Immune Signatures Following Single Dose Trastuzumab Predict Pathologic Response to Preoperative Trastuzumab and Chemotherapy in HER2-Positive Early Breast Cancer. Clin. Cancer Res. 2016, 22, 3249–3259. [Google Scholar] [CrossRef]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases HER2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef]
- Chaganty, B.K.R.; Qiu, S.; Gest, A.; Lu, Y.; Ivan, C.; Calin, G.A.; Weiner, L.M.; Fan, Z. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett. 2018, 430, 47–56. [Google Scholar] [CrossRef]
- Hudis, C.A. Trastuzumab — Mechanism of Action and Use in Clinical Practice. N Engl J Med 2017, 357, 39–51. [Google Scholar] [CrossRef]
- Wolpoe, M.E.; Lutz, E.R.; Ercolini, A.M.; Murata, S.; Ivie, S.E.; Garrett, E.S.; Emens, L.A.; Jaffee, E.M.; Reilly, R.T. HER-2/neu-Specific Monoclonal Antibodies Collaborate with HER-2/neu-Targeted Granulocyte Macrophage Colony-Stimulating Factor Secreting Whole Cell Vaccination to Augment CD8 + T Cell Effector Function and Tumor-Free Survival in Her-2/ neu -Transgenic Mice. J Immunol. 2003, 171, 2161–2169. [Google Scholar] [CrossRef]
- Stagg, J.; Loi, S.; Divisekera, U.; Ngiow, S.F.; Duret, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti–ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti–PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A. 2011, 108, 7142–7147. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Gianni, L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014, 15, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Junttila, T.T.; Li, J.; Johnston, J.; Hristopoulos, M.; Clark, R.; Ellerman, D.; Wang, B.E.; Li, Y.; Mathieu, M.; Li, G.; et al. Antitumor Efficacy of a Bispecific Antibody That Targets HER2 and Activates T Cells. Cancer Res. 2014, 74, 5561–5571. [Google Scholar] [CrossRef]
- Ubago, J.M.; Blanco, L.Z.; Shen, T.; Siziopikou, K.P. The PD-1/PD-L1 Axis in HER2+ Ductal Carcinoma In Situ (DCIS) of the Breast. Am. J. Clin. Pathol. 2019, 152, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, WH.; Stenning, SP.; Thompson, J.N.; van de Velde, C.J.H.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef]
- Cassidy, J.; Saltz, L.; Twelves, C.; Van Cutsem, E.; Hoff, P.; Kang, Y.; Saini, J.P.; Gilberg, F.; Cunningham, D. Efficacy of capecitabine versus 5-fluorouracil in colorectal and gastric cancers: a meta-analysis of individual data from 6171 patients. Ann Oncol. 2011, 22, 2604–2609. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Y.; Xu, Y.; Zhu, Y.; Huang, J.; Liu, Y.; Zhao, L.; Li, Z.; Liu, H.; Wang, Q. L.; et al. Comparative effectiveness and safety between oxaliplatin-based and cisplatin-based therapy in advanced gastric cancer: A meta-analysis of randomized controlled trials. Oncotarget 2016, 7, 34824–34831. [Google Scholar] [CrossRef]
- Wilke, H.; Muro, K.; van Cutsem, E.; Oh, S.C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Ford, H.E.R.; Marshall, A.; Bridgewater, J.A.; Janowitz, T.; Coxon, F.Y.; Wadsley, J.; Mansoor, W.; Fyfe, D.; Madhusudan, S.; Middleton, G.W.; et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014, 15, 78–86. [Google Scholar] [CrossRef]
- Hironaka, S.; Ueda, S.; Yasui, H.; Nishina, T.; Tsuda, M.; Tsumura, T.; Sugimoto, N.; Shimodaira, H.; Tokunaga, S.; Moriwaki, T.; et al. Randomized, Open-Label, Phase III Study Comparing Irinotecan With Paclitaxel in Patients With Advanced Gastric Cancer Without Severe Peritoneal Metastasis After Failure of Prior Combination Chemotherapy Using Fluoropyrimidine Plus Platinum: WJOG 4007 Trial. J. Clin. Oncol. 2013, 31, 4438–4444. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Kretzschmar, A.; Bichev, D.; Deist, T.; Hinke, A.; Breithaupt, K.; Dogan, Y.; Gebauer, B.; Schumacher, G.; Reichardt, P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer – A randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur. J. Cancer 2011, 47, 2306–2314. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; Dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Kataoka, K.; Tokunaga, M.; Mizusawa, J.; Machida, N.; Katayama, H.; Shitara, K.; Tomita, T.; Nakamura, K.; Boku, N.; Sano, T.; et al. A randomized Phase II trial of systemic chemotherapy with and without trastuzumab followed by surgery in HER2-positive advanced gastric or esophagogastric junction adenocarcinoma with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1301 (Trigger Study). Jpn. J. Clin. Oncol. 2015, 45, 1082–1086. [Google Scholar] [CrossRef]
- Hofheinz, R.; Hegewisch-Becker, S.; Kunzmann, V.; Thuss-Patience, P.; Fuchs, M.; Homann, N.; Graeven, U.; Schulte, N.; Merx, K.; Pohl, M.; et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int. J. Cancer 2021, 149, 1322–1331. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Merx, K.; Haag, G.M.; Springfeld, C.; Ettrich, T.; Borchert, K.; Kretzschmar, A.; Teschendorf, C.; Siegler, G.; Ebert, M.P.; et al. FLOT Versus FLOT/Trastuzumab/Pertuzumab Perioperative Therapy of Human Epidermal Growth Factor Receptor 2–Positive Resectable Esophagogastric Adenocarcinoma: A Randomized Phase II Trial of the AIO EGA Study Group. J. Clin. Oncol. 2022, 40, 3750–3761. [Google Scholar] [CrossRef]
- Rivera, F.; Izquierdo-Manuel, M.; García-Alfonso, P.; Martínez de Castro, E.; Gallego, J.; Limón, M.L.; Alsina, M.; López, L.; Galán, M.; Falcó, E.; et al. Perioperative trastuzumab, capecitabine and oxaliplatin in patients with HER2-positive resectable gastric or gastro-oesophageal junction adenocarcinoma: NEOHX phase II trial. Eur. J. Cancer 2021, 145, 158–167. [Google Scholar] [CrossRef]
- Wagner, A.D.; Grabsch, H.I.; Mauer, M.; Marreaud, S.; Caballero, C.; Thuss-Patience, P; Mueller, L.; Elme, A.; Moehler, M.H.; Martens, U.; et al. EORTC-1203-GITCG - the “INNOVATION”-trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer 2019, 19, 494. [CrossRef]
- Hecht, J.R.; Bang, Y.J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC--A Randomized Phase III Trial. J Clin Oncol 2016, 34, 443–51. [Google Scholar] [CrossRef]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Siddiqui, A.; Heeson, S.; Kiermaier, A.; Macharia, H.; Restuccia, E.; et al. Pertuzumab, trastuzumab, and chemotherapy in HER2-positive gastric/gastroesophageal junction cancer: end-of-study analysis of the JACOB phase III randomized clinical trial. J Clin Oncol. 2023, 26, 123–131. [Google Scholar] [CrossRef]
- Satoh, T.; Xu, R.H.; Chung, H.C.; Sun, G.P.; Doi, T.; Xu, J.M.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.W.; et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014, 32, 2039–49. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017, 18, 640–53. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Maron, S.B.; Chatila, W.K.; Millang, B.; Chavan, S.S.; Alterman, C.; Chou, J.F.; Segal, M.F.; Simmons, M.Z.; Momtaz, P.; et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 821–831. [Google Scholar] [CrossRef]
- Lee, J.; Pang, K.; Kim, J.; Hong, E.; Lee, J.; Cho, H.J.; Park, J.; Son, M.; Park, S.; Lee, M.; et al. ESRP1-regulated isoform switching of LRRFIP2 determines metastasis of gastric cancer. Nat Commun 2022, 13, 6274. [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef]
- Stein, A.; Paschold, L.; Tintelnot, J.; Goekkurt, E.; Henkes, S.S.; Simnica, D.; Schultheiss, C.; Willscher, E.; Bauer, M.; Wickenhauser, C.; et al. Efficacy of Ipilimumab vs. FOLFOX in Combination With Nivolumab and Trastuzumab in Patients With Previously Untreated ERBB2 -Positive Esophagogastric Adenocarcinoma. JAMA Oncol. 2022, 8, 1150-1158. [CrossRef]
- Xu, M.; Meng, X.; Lu, Y.; Wang, F. Efficacy and safety of camrelizumab in combination with trastuzumab and chemotherapy as the first-line treatment for patients with HER2-positive advanced gastric cancer. J Gastrointest Oncol. 2022, 13, 548–558. [Google Scholar] [CrossRef]
- Catenacci, D.V.; Rosales, M.; Chung, H.C.; H Yoon, H.; Shen, L.; Moehler, M.; Kang, Y.K. MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol 2021, 17, 1155–1164. [Google Scholar] [CrossRef]
- Tabernero, J.; Shen, L.; Elimova, E.; Ku, G.; Liu, T.; Shitara, K.; Lin, X.; Boyken, L.; Li, H.; Grim, J.; et al. HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol 2022, 18, 3255–3266. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hanna, D.L.; El-Khoueiry, A.B.; Kang, Y.K.; Oh, D.Y.; Chaves, J.M.; Rha, S.Y.; Hamilton, E.P.; Pant, S.; Javle, M.M.; et al. Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): Results from a phase I study. J Clin. Oncol. 2021, 39, 299. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Oh, D.Y.; Rha, S.Y.; Lee, K.W.; Steeghs, N.; Chao, Y.; Di Bartolomeo, M.; Díez-García, M.; Haj Mohammad, N.; Stein, A.; et al. Dose-escalation and dose-expansion study of trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients (pts) with advanced/metastatic HER2+ gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): DESTINY-Gastric03. J. Clin. Oncol. 2022, 40, 295–295. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Shitara, K.; Özgüroğlu, M.; Bang, Y. J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M. H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Chung, H. C.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.J.; Enzinger, P.; Ilson, D.; Shah, M.A.; van Cutsem, E.; Xu, R.H.; Aprile, G.; Xu, J.; et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401, 1655–1668. [Google Scholar] [CrossRef]
- Xu, R.H.; Shitara, K.; Ajani, J.A.; Bang, Y.J.; Enzinger, P.C.; Ilson, D.H.; Lordick, F.; Van Cutsem, E.; Gallego, J.; Huang, J.; et al. Zolbetuximab + CAPOX in 1L claudin-18.2+ (CLDN18.2+)/HER2− locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary phase 3 results from GLOW. J Clin. Oncol. 2023, 41, (suppl 36; abstr 405736). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).