1. Introduction
Human breath analysis has become a promising research field that has recently attracted significant interest owing to advances in analytical techniques. Exhaled human breath typically consists of nitrogen (78%), oxygen (16%), carbon dioxide (4−5%), hydrogen (5%), inert gases (0.9%), and volatile organic compounds (VOCs) [
1]. VOCs can vary depending on a person's metabolism, which changes when someone becomes sick.
For decades, humans have used the superior sense of smell of dogs to identify illicit drugs or explosives. Sniffing animals have also been trained to detect human diseases, such as cancers, tuberculosis, or even coronavirus disease 2019 [
2,
3]
. In all cases, animals are presumed to detect chemicals emitted by humans through their body odors or breath.
Researchers do not know exactly which components the animals smell; however, it is understood that these diseases cause the human body to release the characteristic patterns of VOCs in scents.
The kidneys remove waste products and extra water from the body and maintain homeostasis for the normal functioning of cells and organs. Chronic kidney disease (CKD) is a long-term condition of gradual loss of filtering function that results in the progressive accumulation of more than hundred uremic retention solutes [
4,
5]. In patients with CKD, the compositional profile of breath VOCs can change because of the limited ability of kidneys to eliminate metabolic products from the blood. Metabolites produced in the body are transported to the alveoli, and trace amounts of volatile substances are exhaled via respiration. Breath VOCs can be analyzed using mass spectrometry, and changes in the VOC profile according to health status can be used as a breathprint for human diseases. Evidence suggests that patients with CKD have higher levels of VOCs in their breath than those of healthy individuals [
6,
7,
8,
9,
10]. CKD is clinically silent and asymptomatic in many cases and is often detected during health checkups or is not detected until the late stage.
The severity of CKD can be quantified by low estimated glomerular filtration rate (eGFR) and increased urinary albumin levels, both of which require blood sampling and/or urine collection [
11]
. Breath VOC analysis is a noninvasive tool for assessing health status information. Exhaled breath is one of the most easily collected samples and contains profound but unknown information related to human diseases. Olfactory evaluation can provide diagnostic clues and guide further evaluation.
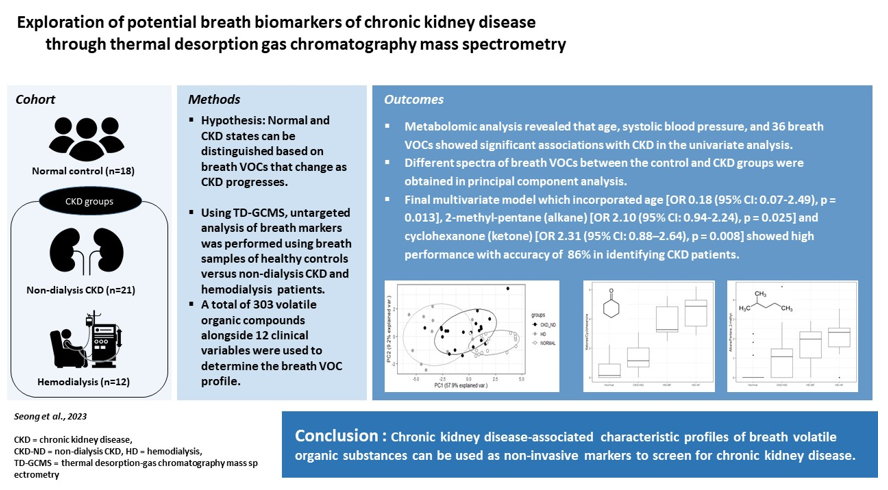
In this study, we analyzed more than 300 VOCs in the breath of 51 participants (18 normal healthy controls, 21 patients with CKD, and 12 patients with CKD undergoing hemodialysis) using thermal desorption gas chromatography/mass spectrometry (TD-GCMS) along with clinical variables. This dataset allowed us to test the hypothesis that the normal and CKD states can be distinguished based on breath VOCs that change as CKD progresses.
2. Materials and Methods
2.1. Study Design and Participants
To address our hypothesis, we designed a cross-sectional study to compare the compositional profile of breath VOCs between normal healthy controls and CKD patients along with confounding clinical variables. A total of 51 participants were included in this study; 18 healthy controls, 21 non-dialysis outpatients with CKD (CKD-ND), and 12 hemodialysis patients (HD). All participants were adults aged 19 years or more, and patients with CKD-ND and HD were recruited from Kangdong Sacred Heart Hospital between March and September 2021.
CKD was defined as the presence of kidney damage or decreased kidney function for three or more months, irrespective of the cause [
11]. The inclusion criterion for CKD-ND was stable renal function for >3 months, without acute kidney injury or hospitalization. Hemodialysis patients underwent regular hemodialysis three times a week for more than three months and had no acute diseases. Normal healthy controls were adults who were confirmed to be free of kidney disease through general health checkup and had CKD-EPI eGFR of ≥ 80 ml/min/1.73 m
2, with no proteinuria. This study was approved by the Institutional Review Board of Kangdong Sacred Heart Hospital (IRB no. 2019-10-001) and was conducted in compliance with the Helsinki Congress and Declaration of Istanbul. Written informed consent was obtained from all participants. Laboratory data were collected to determine the biochemical parameters.
2.2. Breath Sample Collection and Preparation
Pre-concentration methods can detect trace concentrations of VOCs in exhaled breath samples. Sorbent-containing thermal desorption stainless steel tubes are most frequently used to adsorb and enrich VOCs in breath [
12]. Prior to sample collection, each thermal desorption tube (Tenax TA, 3.5 inch, 60−80 mesh, 230−250 mg, KNR, Namyangju, Korea) was heated and sealed to ensure absence of any residual gas in the tube. Tedlar bags are difficult to fill due to their low gas flow conductance, and hence a 10 L polyester bag (Top-Trading, Seoul, Korea), which is a disposable sampling device, was used for breath collection. Exhaled breath samples were collected from participants in the morning. Breath samples from normal controls and non-dialysis CKD patients were collected once, and breath samples from hemodialysis patients were collected twice, before and after hemodialysis. Breath collection was conducted in the same outpatient clinic room of our hospital, in a clean and quiet environment, which was dedicated for breath collection on the sampling day. The participants were asked to breath deeply and inflate a 10 L polyester bag. A Gilair Plus pump (Sensidyne, St. Petersburg, USA) was used to force ambient air to flow at a rate of 250 mL/min through the desorption tubes (active sampling). The thermal desorption tube was connected and VOCs were adsorbed for 12 min from the 3 L aliquot flow from the polyester bag. Three consecutive samples were collected from each patient. To collect the background air, 3 L of air from the sampling room was concentrated in a thermal desorption tube in duplicate using a Gilair Plus pump at a pumping speed of 250 mL/min for 12 min. The VOC-adsorbed thermal desorption tube was immediately sealed and stored in an icebox to avoid deterioration of collected samples.
2.3. VOC Analysis Using TD-GCMS
The adsorbed VOCs were vaporized and analyzed by TD-GCMS (Turbomatrix 650, Perkin Elmer, Waltham, USA; 7890B-GC and 5977A-MS, Agilent Technologies, Santa Clara, USA). A fused silica capillary GC column, Elite-5 ms column (60 m × 0.32 mm × 1 μm, Perkin Elmer, Waltham, USA) was used to analyze the desorbed VOCs in the breath. Helium with a chromatographic purity (220 kPa) was used as the carrier gas. The VOCs were desorbed for 15 min from the thermal desorption tube to which breath VOCs were adsorbed. Maintaining oven, cold trap, and transmission line temperatures of 250ºC, -30 to 300ºC (10 min) at 33ºC/min, and 250 ºC, respectively, gas chromatography column temperature was held at 30°C for 10 minutes, following by gradual ramping (3.4°C/min to 50°C and 5°C/min to 100°C with a hold for 10 minutes, and at 3.5°C/min to 150°C and at 100°C/min to 250°C with a hold at 250°C for 10 minutes) [
12,
13]. The electron impact (EI) voltage in the mass spectrometer was set to 70 eV. The mass detection range varied from 30 to 350 m/z. Compound identification was performed based on the retention time in gas chromatography and ion fragmentation patterns using Wiley and NIST GC-MS libraries (
Wiley Spectral Libraries, Wiley Science Solutions, Hoboken, USA) and compared with previously reported retention time data in the literature [
14,
15].The relative concentration of each compound was calculated from the peak areas of the selected ion chromatograms. Most of the VOCs observed in the TD-GCMS experiment have been reported previously [
13,
14,
15]. Untargeted metabolomics using TD-GCMS was performed on the VOCs observed in breath samples.
2.4. Statistical Analysis
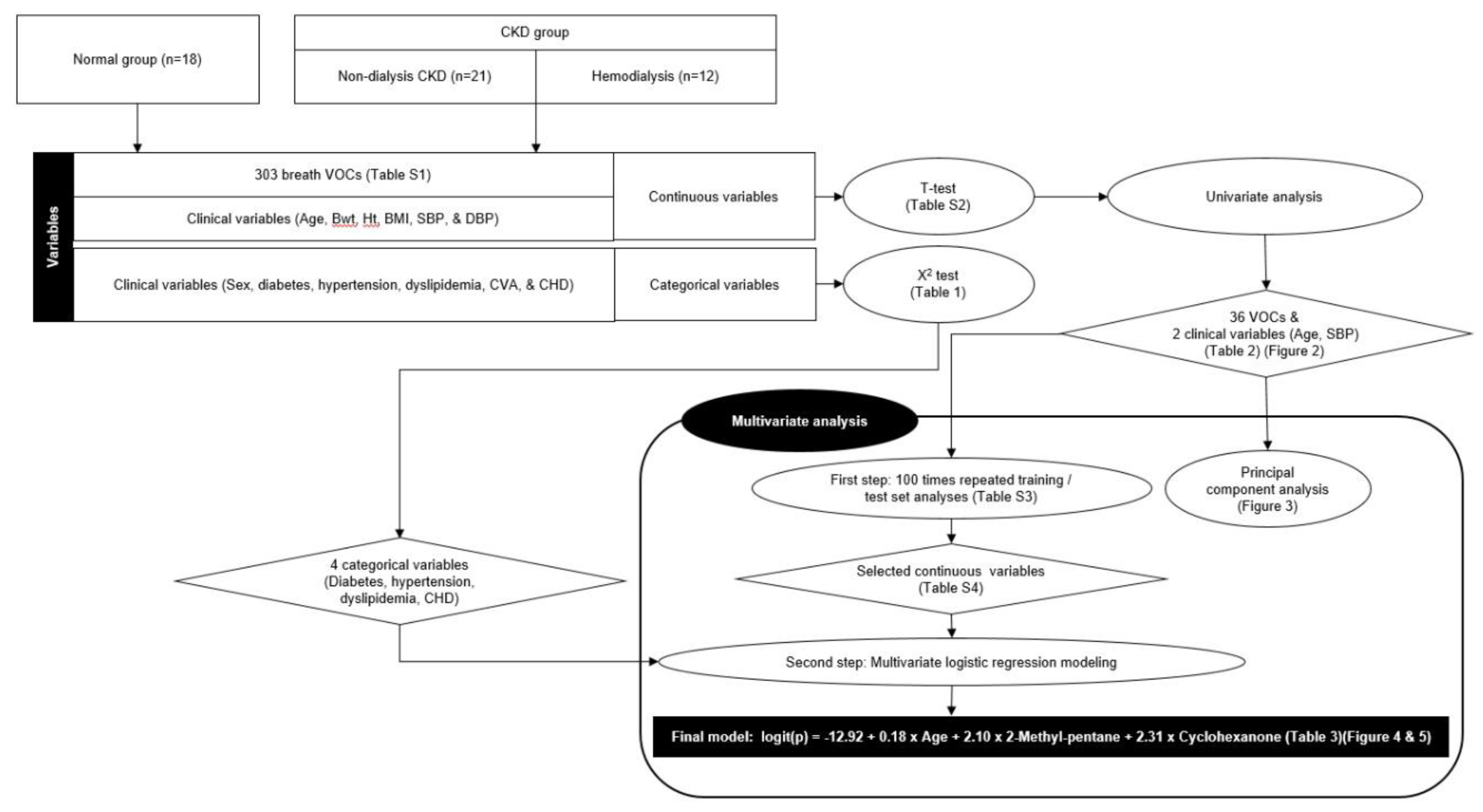
We compared the levels of 303 breath VOCs and 12 clinical variables between the healthy control and CKD groups, which included both non-dialysis CKD and hemodialysis patients. The VOC values before hemodialysis were used to investigate the relationship between renal dysfunction and breath VOCs. For descriptive analysis, continuous variables are expressed as mean ± standard deviation (SD), and categorical variables are expressed as frequencies (percentages). Continuous variables included six clinical variables (age, body weight, height, body mass index, and systolic and diastolic blood pressure) and 303 breath VOCs. Because most of the measured VOC values ranged between 106 and 108, these were scaled down as follows: SQRT (breath VOC value - background room air VOC value)/1000. The categorical variables included six clinical variables: sex, diabetes, hypertension, dyslipidemia, cerebrovascular accident (CVA), and coronary heart disease (CHD).
Categorical variables were compared using the χ2 test between groups as appropriate. For continuous variables, we conducted Student t test and univariate logistic regression to select significant VOCs and clinical variables that could distinguish between the normal and CKD (non-dialysis CKD + HD) groups.
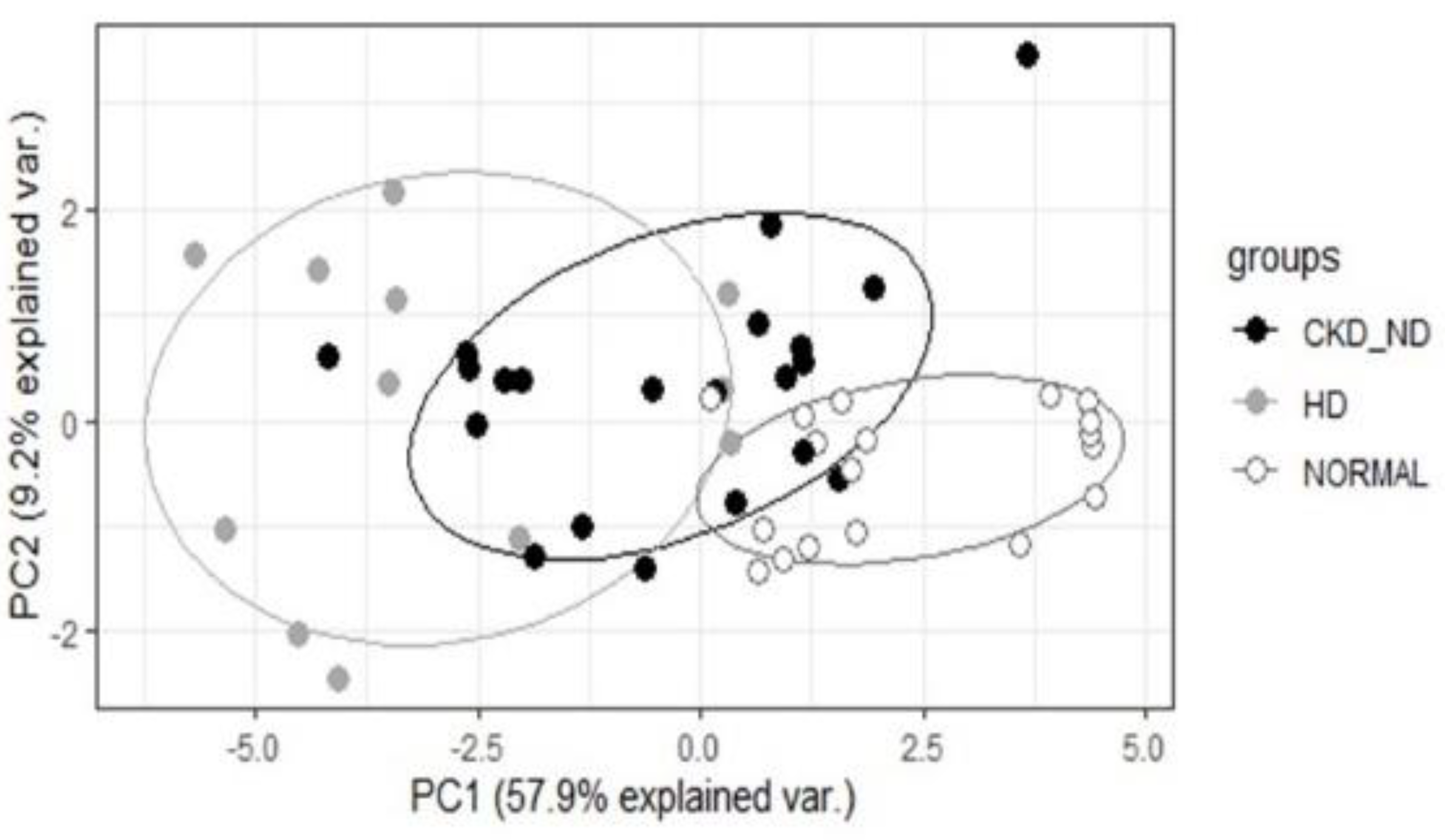
Principal component analysis (PCA) was performed with breath VOCs and clinical variables that showed P values of odds ratio <0.05 in the univariate analysis. PCA was used to determine the possibility of separating patients with CKD from healthy controls by analyzing breath VOCs.
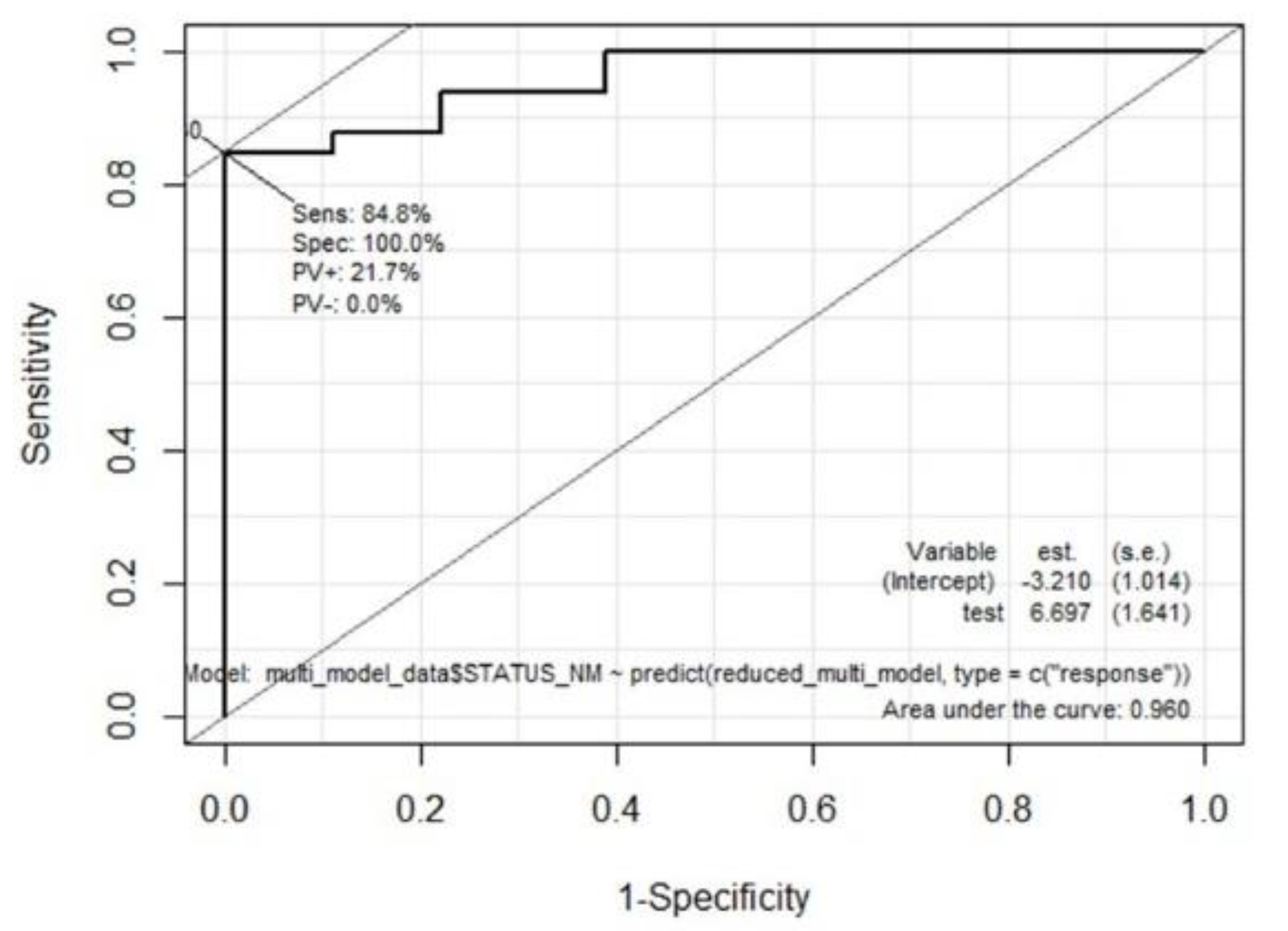
Next, multivariate analysis was performed in two steps. First, significant continuous variables were selected from 100 repeated training and test sets. Briefly, 51 participants were randomly sampled into the training set (67%) and the test set (33%), and a multivariate model was constructed with the training dataset; further, the accuracy was derived by applying this model to the corresponding test set. This process was repeated hundred times and the variables used in each multivariate model were listed based on the number of times they were used. The most frequently used variables were determined as the selected continuous variables. In the second step, the final multivariate logistic regression model was built by integrating the selected continuous variables in the first step and four significant categorical variables selected using the χ2 test. We used stepwise regression with forward selection and backward elimination to obtain a model that minimized the Akaike information criterion (AIC) while avoiding overfitting. Receiver operating characteristic (ROC) curve analysis was performed by applying the final multivariate model to all participants. The software package R version 4.1.1 (
www.r-project.org; The R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analyses, and a
P-value of < 0.05 was considered significant. A flowchart of the statistical analysis is shown in
Figure 1.
3. Results
3.1. Characteristics of Study Participants
All participants were confirmed eligible and included for analysis in the study.
Table 1 summarizes the demographic and clinical characteristics of the study participants. The mean age of normal, CKD-ND and HD groups was 46.0 ± 8.9, 63.3 ± 16.4, and 56.1 ± 16.0 years, respectively. Patients in the CKD-ND and HD groups were older, had higher systolic blood pressures, and more comorbidities than the control group. None of the participants had any lung disease. Six participants had a history of cancer, but none reported cancer recurrence at the time of breath sampling. The mean eGFR of normal, CKD-ND, and HD groups was 100.4 ± 12.0, 50.8 ± 26.9, and 4.4 ± 1.0 mL/min/1.73 m
2, respectively. We divided the study participants into two groups for metabolomic analysis: a normal group (normal healthy controls) and a CKD group, which included patients with CKD-ND and HD.
3.2. Overview of the Breath VOCs
A total of 324 VOCs with molecular weights ranging from 40 to 400 u were measured and classified into the following 16 VOC groups: volatile sulfur compounds (VSCs) 20, ketones 25, alcohols 43, halo-hydrocarbons 16, alkenes 13, alkyne 1, alkanes 68, terpenes 22, aromatics 26, acetates 25, acids 9, furans 3, steroids 1, aldehydes 12, nitrogen compounds 7, others 12, and siloxanes 21 (
Table S1). We excluded 21 VOCs belonging to the siloxane group from the analysis because they were assumed to be derived from methylpolysiloxane, which was coated onto a GC-MS column (Elite-5ms column, Perkin Elmer, Waltham, USA) [
16]. Before excluding them, we conducted an analysis that included 21 VOCs from the siloxane group; however, no significant differences were observed. After excluding 21 VOCs, 303 VOCs were included in the analysis to identify the breath markers of CKD.
3.3. Potential Breath Markers for CKD
We used an untargeted metabolomic strategy to identify marker breath VOCs associated with CKD and used a step-by-step approach to identify significant VOCs that could distinguish patients with CKD from normal controls. We analyzed 12 clinical variables to correct for confounding variables caused by the clinical characteristics or underlying comorbidities of the participants.
3.3.1. Selection of Significant Breath VOCs and Clinical Variables
Among the six categorical variables, the χ2 test showed that diabetes, hypertension, dyslipidemia, and CHD were significantly higher in the CKD group compared with the control group (
Table 1). Further, t-test analysis revealed significant differences in 58 breath VOCs and two clinical variables (age and SBP) between the CKD and normal groups (
Table S2).
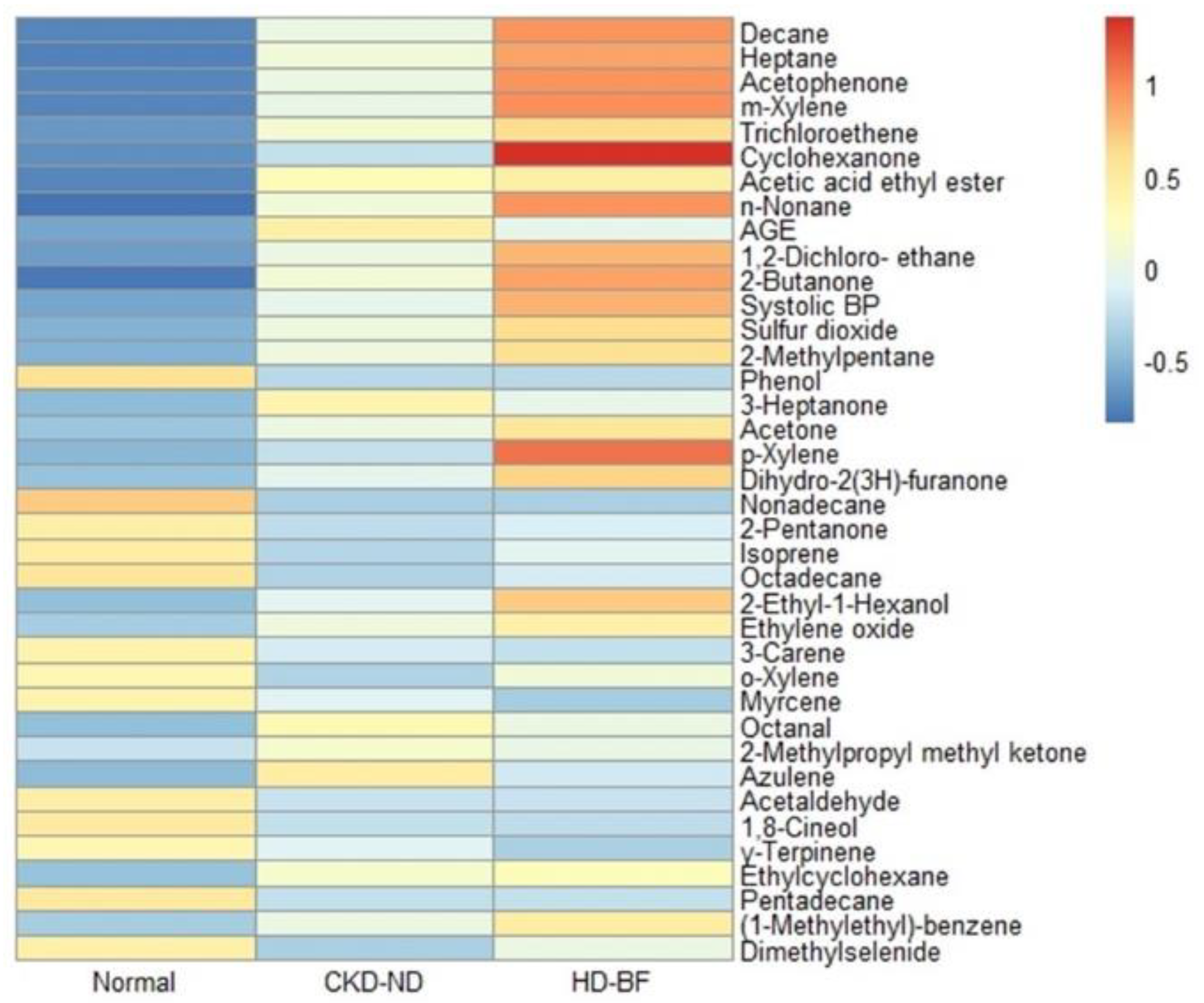
In the univariate analysis, 36 VOCs and two clinical variables (age and SBP) were significantly associated with the CKD state: VSC 1, ketones 8, alcohols 2, halo-hydrocarbon 2, alkanes 8, terpenes 4, aromatics 5, acetate 1, aldehydes 2, and others 3 (10 VOC groups)
(Table 2). In general, the heat plot analysis showed a greater and more distinct increase in breath VOCs as CKD progressed (Figure 2).
4. Discussion
In this untargeted breath analysis study using TD-GCMS, we identified and measured 303 VOCs from human breath, excluding 21 contaminant siloxane compounds. We showed that breath VOCs are a good marker for discriminating between patients with CKD and healthy controls, even after correcting for many clinical confounding variables. Thirty-six breath VOCs were significantly different between patients with CKD and normal controls in the univariate analysis. We also derived a final multivariate model that incorporated age and breath 2-methyl-pentane and cyclohexanone, producing a high accuracy of 86.3% in predicting CKD.
Considering the accumulation of putative uremic retention metabolites as a result of progressive kidney dysfunction, it is reasonable to assume that CKD leads to a characteristic chemical profile of breath VOCs. The list of uremic retention solutes is constantly evolving as new compounds are discovered [
5]. In this context, an untargeted approach to breath analysis is more appropriate than focusing on specific breath VOCs to identify CKD breath markers. To our knowledge, this is the first study to provide a general overview of breath VOCs in patients with CKD as well as ESRD. TD-GCMS is a powerful analytical technique that is often used to analyze VOCs in air and human breath. One of the main advantages of TD-GCMS is its ability to separate and identify a wide range of VOCs. TD-GDMS is considered one of the most sensitive and selective techniques available [
17]. We controlled for the effect of VOCs in the room air by subtracting the VOC value of the room air from the VOC values of the participants. In this study, we showed that 36 breath VOCs were significantly related to CKD in the univariate analysis, and the heat plot of these VOCs revealed that many of these breath VOCs increased with decreasing renal function. Changes in breath VOCs associated with decreased renal function were also confirmed using PCA. As renal dysfunction progressed from the normal group to the hemodialysis group, the spectrum of breath VOC patterns shifted accordingly.
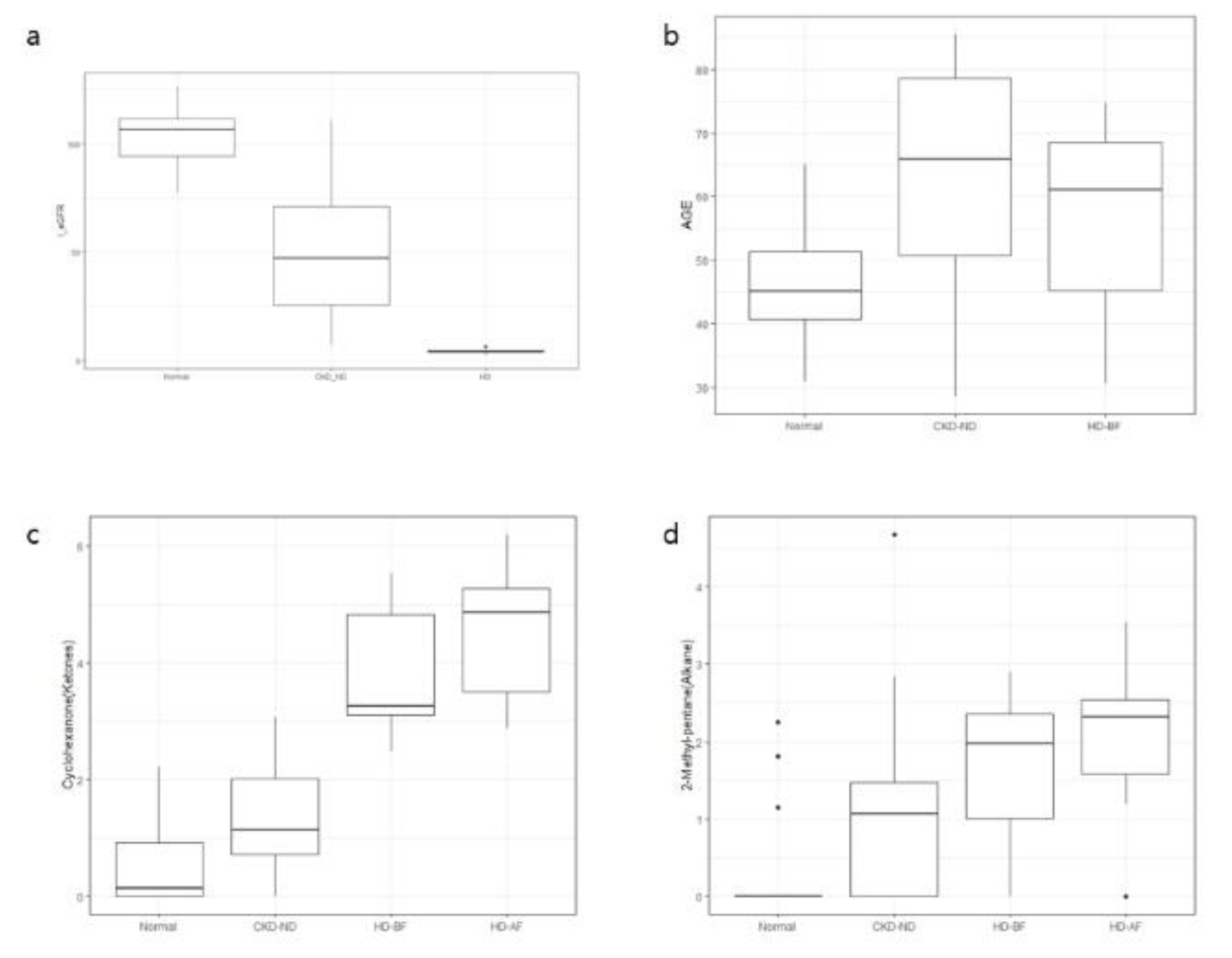
In previous animal and human studies, cyclohexanone was used as a solvent in the production of extracorporeal circuits and intravenous bags and was considered one of the contaminants from extracorporeal materials, for example, during hemodialysis. The increase in breath cyclohexanone levels after hemodialysis (
Figure 5c) was in good agreement with the results of previous reports [
18,
19]. However, in our study, cyclohexanone was detected in the breath of normal participants and non-dialysis patiwnts with CKD and hemodialysis and showed an increasing trend with decreasing renal function, with the highest level in hemodialysis patients after adjusting for room-air cyclohexanone. Cyclohexanone (CAS No. 108-94-1), a six-carbon cyclic compound with a ketone group, is an organic compound with the chemical formula
(CH2)5CO that is miscible with water. This colorless liquid had a sweet and pungent smell reminiscent of acetone. One of the most common uses of cyclohexanone is the production of nylon,
which is used in various end-use industries, including automotive, construction, consumer goods, and electronics [
20]
. Cyclohexanone is a synthetic compound that is not produced by the human body. However, limited information is available regarding the role of cyclohexanone in human metabolism.
Cyclohexanone is well absorbed through the skin, respiratory tract, and alimentary tract and metabolized to cyclohexanol, which is conjugated with glucuronic acid and excreted mainly in the urine [
21,
22,
23]. However, cyclohexanone toxicity has rarely been documented in humans or experimental animals [
24]. Subacute tubular nephrotoxicity of intraperitoneally injected cyclohexane has been reported in female Sprague-Dawley rats, as evidenced by a significant increase in ß
2-microglobulinuria [
25]. Ong et al. studied occupational exposure to cyclohexanone by analyzing the breath and urine of 59 workers [
26]. The possible source of breath cyclohexanone detected in normal controls and non-dialysis patients with CKD in this study is not certain and may be exposure through the use of products or inhalation of gases that contain cyclohexanone-related chemicals. However, a decrease in kidney function resulted in significantly increased levels of breath cyclohexanone. Cyclohexanone was retained after hemodialysis as shown in
Figure 5c because it is a soluble chemical and may be relatively slowly removed from the blood by exhalation in hemodialysis patients in whom urinary excretion of its metabolites is almost absent. Mochalski et al. reported that uremic breath is affected by contaminants from extracorporeal circuits, and cyclohexanone, one of those contaminants, can play a role as a uremic toxin because it is retained after hemodialysis [
19].
2-methyl-pentane (C6H14, CAS NO. 107-83-5), also known as isohexane (an isomer of hexane), is a branched-chain alkane (subclassification of hydrocarbons) with
a gasoline-like odor that floats on water. It is a colorless, flammable liquid commonly used as a solvent in industry. Solvents containing hexane are primarily used to extract vegetable oils from crops such as soybeans. These solvents are used as cleaning agents in the printing, textile, furniture, and shoe-making industries [
27]. It is also used in several consumer products, such as gasoline, quick-drying glues, and rubber cement [
28].
2-methyl-pentane is distributed throughout the body in the blood and metabolized by mixed-function oxidases in the liver into a number of metabolites [
29]
. It has been detected in the feces, breath, saliva, and blood of healthy humans [
30,
31]. Further, it has also been reported that 2-methyl-pentane increases the breathing of preschool asthmatic children [
32]. 2-methyl-pentane was detected in the blood and breath of hemodialysis patients and was classified as a contaminant from dialyzers and bloodlines [
19]. We do not know the origin and metabolic fate of the 2-methyl-pentane detected in this study. It was rarely detected in the breath of normal controls, except in three patients but was increased in the breath of non-dialysis patients with CKD, was higher in those of hemodialysis patients, and was not removed after hemodialysis (
Figure 5d).
This study had several limitations. The results were obtained from the analysis of VOCs in a small number of breath samples in a hospital environment. Therefore, these results require further validation. The mean age of patients with CKD was higher than that of normal participants. We did not measure the blood levels of the corresponding breath VOCs, and hence the significance of VOCs needs to be explored in the context of internal and external metabolism. Unexpectedly, some chemicals exposed to the environment were measured through exhalation, and they accumulated as kidney function decreased, even if a person did not receive hemodialysis. This study and earlier studies have shown that as kidney function deteriorates, various uremic retention metabolites accumulate in the body, which is reflected in increased breath VOCs. Further research is needed to fully understand the relationship between CKD and breath VOCs and to determine whether breath VOCs can be used as a diagnostic tool for CKD. However, the presence of certain VOCs in the breath of patients with CKD may provide valuable information regarding disease progression and treatment efficacy.
5. Conclusions
We determined the breath metabolomic signatures of patients with CKD. Knowledge of these specific breath VOC profiles may enable the transition from blood to breath analysis and the development of non-invasive point-of-care tests for CKD screening.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Supplementary Table S1. A total of 324 volatile organic compounds (VOCs) were measured in the breath of participants using TD-GCMS. Supplementary Table S2. Fifty-eight breath volatile organic compounds (VOCs) and two clinical variables were significantly different between patients with chronic kidney disease (non-dialysis CKD + hemodialysis) and normal healthy control groups in the t-test. Supplementary Table S3. Training/test set analysis:100 randomly repeated samplings (training vs. test set) and logistic regression multivariate modeling with the training set, with an average accuracy of 0.714. Supplementary Table S4. Significant continuous variables were selected from the first step of multivariate analysis through a hundred-times repeated training and test sets. The variables are listed in order of frequency as shown in Table S3.
Author Contributions
HSK, YML, JSK and JO designed and set up the study. SSH, JSK, and JO were involved in sample collection and analysis. SSH, YML and HSK performed TD-GCMS and measured and identified the breath VOCs. SP and JO performed statistical analysis. SSH and JO wrote the first draft of the paper. The submitted version was approved by all authors.
Funding
This research was supported by "Regional Innovation Strategy (RIS)" through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-001) (to YM Lee).
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Kangdong Sacred Heart Hospital (IRB no. 2019-10-001) and was conducted in compliance with the Helsinki Congress and Declaration of Istanbul.
Informed Consent Statement
Written informed consent was obtained from all participants.
Data Availability Statement
The datasets of the current study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy.
Acknowledgments
We are sincerely grateful for the commitment of our participants. We acknowledge the help of research nurse Young-Kyeong Lee with the breath sample and data collection. We also thank Seunghee Kim for correcting the reference format in this paper. The abstract of this paper was submitted as a poster for Korean Society of Nephrology 2023.
Conflicts of Interest
All the authors declared no competing interests.
References
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Jendrny, P.; Schulz, C.; Twele, F.; Meller, S.; von Köckritz-Blickwede, M.; Osterhaus, A.D.M.E.; Ebbers, J.; Pilchová, V.; Pink, I.; Welte, T.; et al. Scent dog identification of samples from COVID-19 patients – a pilot study. BMC Infect. Dis. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Coronel Teixeira R, Rodríguez M, Jiménez de Romero N, et al. The potential of a portable, point-of-care electronic nose to diagnose tuberculosis. J Infect. 2017;75:441-447.
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Demirjian, S.; Paschke, K.M.; Wang, X.; Grove, D.; Heyka, R.J.; A Dweik, R. Molecular breath analysis identifies the breathprint of renal failure. J. Breath Res. 2017, 11, 026009. [Google Scholar] [CrossRef]
- Pagonas, N.; Vautz, W.; Seifert, L.; Slodzinski, R.; Jankowski, J.; Zidek, W.; Westhoff, T.H. Volatile Organic Compounds in Uremia. PLOS ONE 2012, 7, e46258. [Google Scholar] [CrossRef]
- Simenhoff, M.L.; Burke, J.F.; Saukkonen, J.J.; Ordinario, A.T.; Doty, R.; Dunn, S. Biochemical Profile of Uremic Breath. New Engl. J. Med. 1977, 297, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Grabowska-Polanowska, B.; Faber, J.; Skowron, M.; Miarka, P.; Pietrzycka, A.; Śliwka, I.; Amann, A. Detection of potential chronic kidney disease markers in breath using gas chromatography with mass-spectral detection coupled with thermal desorption method. J. Chromatogr. A 2013, 1301, 179–189. [Google Scholar] [CrossRef]
- Narasimhan, L.R.; Goodman, W.; Patel, C.K.N. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc. Natl. Acad. Sci. 2001, 98, 4617–4621. [Google Scholar] [CrossRef]
- Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150.
- Westphal, K.; Dudzik, D.; Waszczuk-Jankowska, M.; Graff, B.; Narkiewicz, K.; Markuszewski, M.J. Common Strategies and Factors Affecting Off-Line Breath Sampling and Volatile Organic Compounds Analysis Using Thermal Desorption-Gas Chromatography-Mass Spectrometry (TD-GC-MS). Metabolites 2022, 13, 8. [Google Scholar] [CrossRef]
- De Vietro, N.; Aresta, A.M.; Picciariello, A.; Altomare, D.F.; Lucarelli, G.; Di Gilio, A.; Palmisani, J.; De Gennaro, G.; Zambonin, C. Optimization of a Breath Analysis Methodology to Potentially Diagnose Transplanted Kidney Rejection: A Preclinic Study. Appl. Sci. 2023, 13, 2852. [Google Scholar] [CrossRef]
- Phillips, M.; Herrera, J.; Krishnan, S.; Zain, M.; Greenberg, J.; Cataneo, R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B: Biomed. Sci. Appl. 1999, 729, 75–88. [Google Scholar] [CrossRef]
- Vinaixa, M.; Schymanski, E.L.; Neumann, S.; Navarro, M.; Salek, R.M.; Yanes, O. Mass spectral databases for LC/MS- and GC/MS-based metabolomics: State of the field and future prospects. TrAC Trends Anal. Chem. 2016, 78, 23–35. [Google Scholar] [CrossRef]
- Doran, S.L.F.; Romano, A.; Hanna, G.B. Optimisation of sampling parameters for standardised exhaled breath sampling. J. Breath Res. 2017, 12, 016007. [Google Scholar] [CrossRef]
- Davies, R. The metabolomic quest for a biomarker in chronic kidney disease. Clin. Kidney J. 2018, 11, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Torgerson, C.S.; Champion, H.C.; Santhanam, L.; Harris, Z.L.; Shoukas, A.A. Cyclohexanone contamination from extracorporeal circuits impairs cardiovascular function. Am. J. Physiol. Circ. Physiol. 2009, 296, H1926–H1932. [Google Scholar] [CrossRef]
- Mochalski, P.; King, J.; Haas, M.; Unterkofler, K.; Amann, A.; Mayer, G. Blood and breath profiles of volatile organic compounds in patients with end-stage renal disease. BMC Nephrol. 2014, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia; Cyclohexanone. Accessed January 29, 2023. https://en.wikipedia.org/wiki/Cyclohexanone. 28 January.
- International Agency for Research on Cancer (IARC). Cyclohexanone. Some organic solvents, resin monomers and related compounds, pigments and occupational exposures in paint manufacture and painting, vol. 47. World Health Organiztion, 1989, pp 157-169.
- Martis, L.; Tolhurst, T.; Koeferl, M.T.; Miller, T.R.; Darby, T.D. Disposition kinetics of cyclohexanone in beagle dogs*1. Toxicol. Appl. Pharmacol. 1980, 55, 545–553. [Google Scholar] [CrossRef]
- Human Metabolome DataBase (HMDB); Cyclohexanone. Accessed , 2023. https://hmdb.ca/metabolites/HMDB0003315.
- Lee, Y.-H.; Chung, Y.H.; Kim, H.-Y.; Shin, S.H.; Lee, S.B. Subacute Inhalation Toxicity of Cyclohexanone in B6C3F1 Mice. Toxicol. Res. 2018, 34, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.M.; de Russis, R.; Normand, J.-C.; Lauwerys, R.R. Evaluation of the subacute nephrotoxicity of cyclohexane and other industrial solvents in the female Sprague-Dawley rat. Toxicol. Lett. 1989, 45, 271–280. [Google Scholar] [CrossRef]
- Ong, C.; Chia, S.; Phoon, W.H.; Tan, K.T.; Kok, P.W. Monitoring of exposure to cyclohexanone through the analysis of breath and urine. Scand. J. Work. Environ. Heal. 1991, 17, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Brugnone F, Perbellini L, Grigolini L, et al. Solvent exposure in a shoe upper factory. II. Methylcyclopentane, 2-methylpentane, and 3-methylpentane concentration in alveolar and in environmental air and in blood. Int Arch Occup Environ Health. 1979;42:355-363.
- National Center for Biotechnology Information : PubChem; 2-Methylpentane. Accessed January 29, 2023. https://pubchem.ncbi.nlm.nih.gov/compound/2-Methylpentane.
- Human Metabolome DataBase (HMDB); 2-Methylpentane. Accessed , 2023. https://hmdb.ca/metabolites/HMDB0061884#spectra. 29 January.
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 14001. [Google Scholar] [CrossRef] [PubMed]
- Garner, C.E.; Smith, S.; de Lacy Costello, B.; White, P.; Spencer, R.; Probert, C.S.J.; Ratcliffem, N.M. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007, 21, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, A.; Klaassen, E.M.M.; Dallinga, J.W.; van de Kant, K.D.G.; Jobsis, Q.; Moonen, E.J.C.; van Schayck, O.C.P.; Dompeling, E.; van Schooten, F.J. Profiling of Volatile Organic Compounds in Exhaled Breath As a Strategy to Find Early Predictive Signatures of Asthma in Children. PLOS ONE 2014, 9, e95668. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).