1. Introduction
Hodgkin’s lymphoma (HL) is a lymphoproliferative syndrome frequently associated with patients with human immunodeficiency virus (HIV) infection, although it is not currently recognized as a defining entity of human immunodeficiency syndrome (AIDS) [
1]. Extranodal involvement is common in this situation, although it is usually accompanied by lymph node involvement [
2,
3]. Co-infection with the esptein-Baar virus (EBV) may play a role in the etiology of this type of lymphoma in this population, although the relationship is not well established [
4]. The combination of the three previous entities have been defined as causing the development of hemophagocytic lymphohistiocytosis (HLH), requiring specific management. We present a clinical case presenting Hodgkin’s lymphoma as an initial manifestation of HIV infection and EBV coinfection associated with HLH and severe complications during treatment [
5].
2. Case presentation
A 50-year-old Uruguayan man with no personal history of interest presented to the emergency department with 4-months history asthenia, anorexia, profuse nocturnal diaphoresis, weight loss of up to 10 kilograms and intermittent fever with associated dystrophic sensation. The patient reported risky sexual relations with the last screening serology for HIV infection 3 years ago being negative.
Relevant clinical findings on admission included a striking masseteric and temporal atrophy, oral thrush, bilateral laterocervical subcentimeteric lymphadenopathy predominantly on the left side, residual lesions of metameric herpes zoster on the left side and palpable hepatosplenomegaly. In addition, the patient had a body temperature of 39 °C.
The hemogram showed pancytopenia (leukocytes (Leu) 3.16×/103 µl (0.9 × 103/µl lymphocytes and 1.9 × 103/µl neutrophils), hemoglobin (Hb) 10.8 g/dl, platelets (Pts) 134000 × 103 µl as well as an ultrasensitive C-reactive protein of 19 mg/dl.
A full-body computed tomography (CT) scan showed hepatomegaly of approximately 23 centimeters (cm), without focal lesions, as well as lymphadenopathies in the upper abdomen, hepatic hilum up to 29 millimeters (mm), retroperitoneal and mesenteric up to 11 mm and in subcentimeter iliac and inguinal regions.
Flow cytometry was requested due to alteration in the hemogram showing severe lymphopenia at the expense of all NK, B and T lymphocyte subpopulations, the last one presenting a significant decrease in the TCD4+ cell count (<100 cells/µl) with a preserved TCD8+ cell count. In addition, circulating lymphoplasmoid cells are identified without light chain restriction suggesting normality.
Based on these findings, blood and urine cultures were requested and were normal. A serology for multiple viruses showed positive HIV1 and HIV2 antibodies and p24 antigen. The confirmatory Western blot test showed positive antibodies against HIV1 gp160, gp41 and p24 proteins. Regarding viral load, quantification of HIV-1 viral RNA was 72100 copies/ml (4.86 log) (Undetectable values < 20).
In addition, anti-core antibodies for hepatitis B virus were positive, being the viral load measured by DNA as well as the rest of the study negative, presenting the patient a carrier of this virus within the study. The rest of the study showed no significant findings.
At that time, the patient started highly active antiretroviral therapy (HAART) (Bictegravir 50 mg, Emtricitabine 200 mg and Tenofovir alafenamide 25 mg per day) and empiric antibiotherapy started at emergency department was suspended due to the low clinical suspicion of bacterial infection.
Bone marrow (BM) aspirate was performed and showed no cytological data neither dysplasia, infiltration by a lymphoproliferative syndrome or plasma cell dyscrasia. Marrow blood immunophenotyping was normal, as well as marrow cultures.
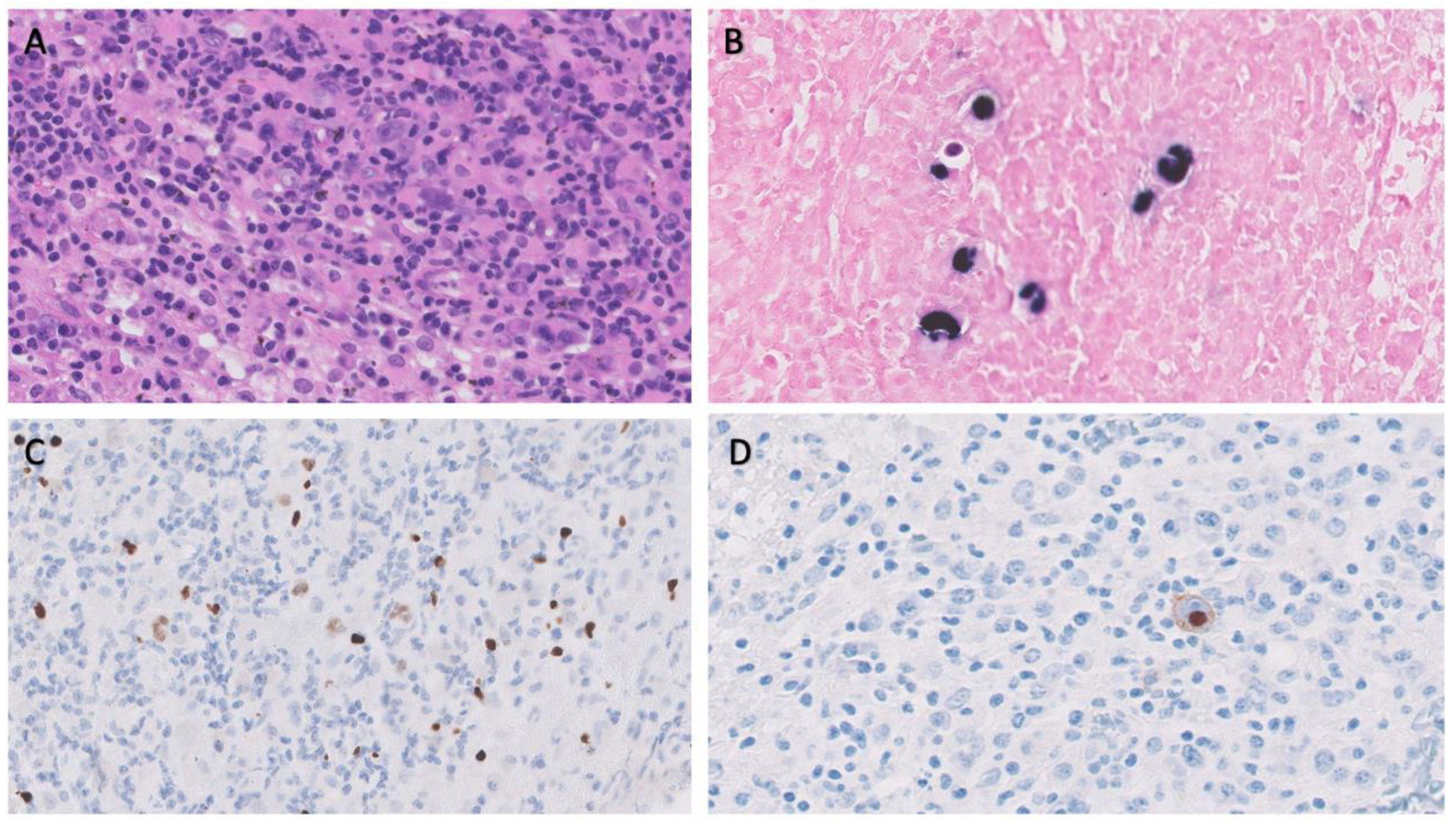
BM biopsy revealed isolated ample cytoplasmic cells with one or more nuclei with prominent nucleoli expressing CD15, CD30, PAX5 and EBER as well as images of hemophagocytosis with hardly any residual normal hematopoietic tissue. Reed-Sternberg were observed. In addition, large numbers of polytypic plasma cells and a striking increase in CD3/CD8 positive small T lymphocytes and abundant loose epithelioid histiocytes are also shown, with no granuloma formation. The observed cells do not express CD20 or CD79a, and no positive Human Herpes Virus 8 (HHV8) cells or increased number of blasts were observed (
Image 1).
These findings make the diagnosis more likely as Hodgkin’s lymphoma (HL)
In order to confirm and stage the disease, positron emission tomography-CT (PET-CT) scan was requested, revealing multiple adenopathies, most of them with discrete-moderate fluorodeoxyglucose (FDG) uptake (some with intense activity), supra and infradiaphragmatic, being the most accessible for biopsy in the right inguinal region.
In addition, FDG uptake is revealed in gastric fundus, requesting gastroscopy showing only data of gastritis, hepatomegaly without significant alterations of metabolism and a discrete diffuse hypermetabolism in BM. No alterations were found in the rest of the study.
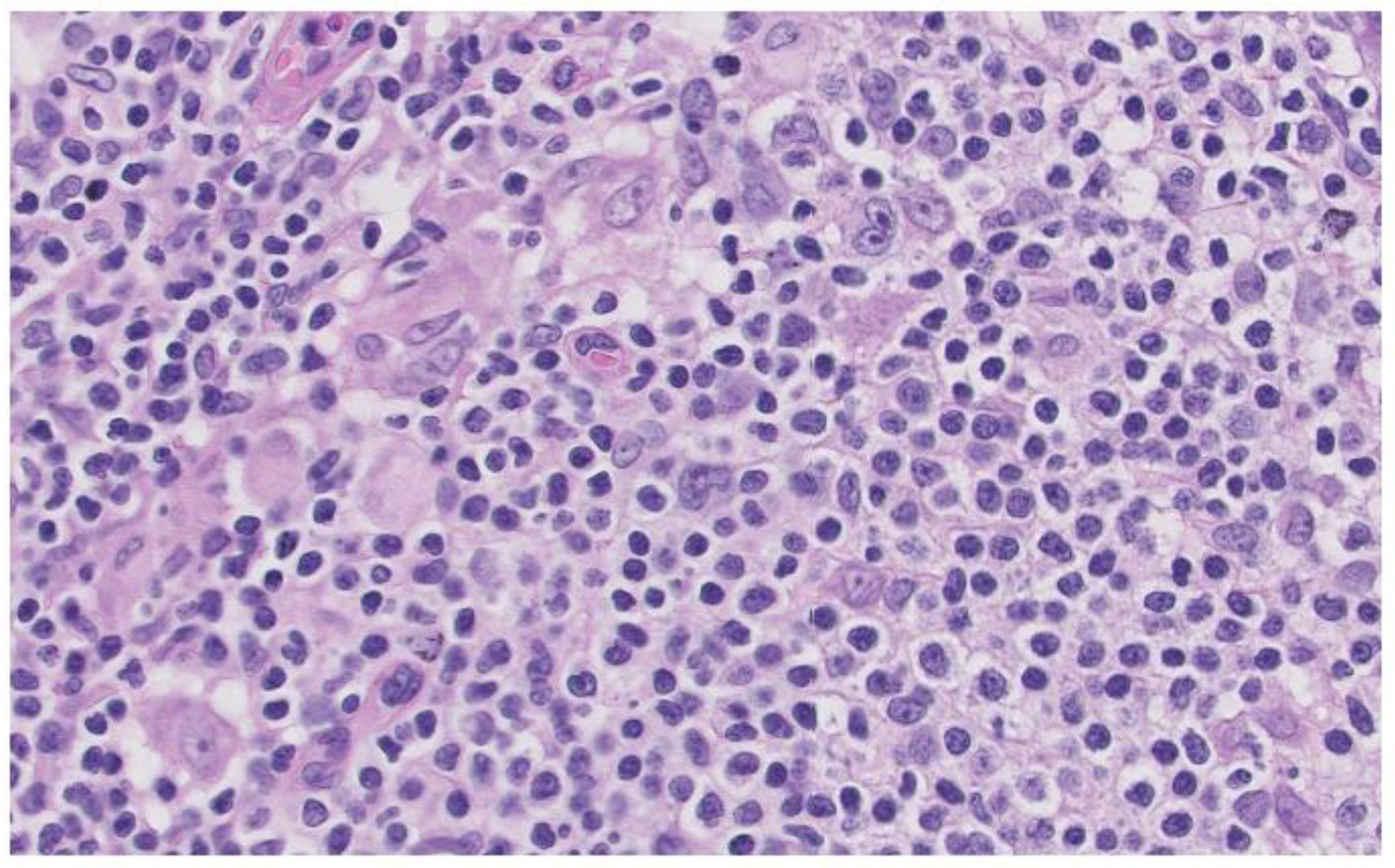
A lymph node biopsy was performed showing hyperplasia of germinal centers with increased vascularization in centers and interfollicular area with expansion and fibrosis of the capsule. Monocytic cells are revealed in subcapsular sinusoids, histiocyte clusters and numerous plasma cells in centers and perifollicular area that do not show light chain restriction. Abundant CD30 cells are revealed in centers and interfollicular area with numerous EBV (EBER) positive cells in centers, although in smaller numbers, surrounding them. These findings are compatible with the histologic diagnosis of HIV-related lymphoid hyperplasia associated with possible EBV reactivation. (
Image 2)
As part of the etiological study, viral load of Epstein-Barr virus measured by DNA polymerase chain reaction (PCR) in peripheral blood using probes that hybridize in the EBNA-1 protein gene was requested, being 47406 copies/mL.
With these findings, the striking clinical improvement after initiation of antiretroviral therapy and the rarity of Hodgkin’s disease isolated to marrow, made us take the results of the first marrow biopsy with caution, delaying systemic treatment with chemotherapy.
Later, 4 weeks after initiation of HAART, the patient’s clinical condition worsened, making HL the likely diagnosis.
Subsequently, the patient developed more pronounced pancytopenia associated (Leu 0.61 × 103/µl, Hb 6.6 g/dL, Pts 42000/µL), recurrence of fever, persistent splenomegaly on physical examination, hyperferritinemia (8190 ng/mL, (elevated CD25 soluble interleukin receptor (>7500 U/mL), hypertriglycidemia (275 mg/dL), which associated with the phenomena of hemophagocytosis observed in the histological study, suggests HLH.
With respect to the trigger of the HLH; HIV infection, EVB infection, Hodgkin’s lymphoma or a combination of several of the above, could be causing the current clinical situation.
Once the diagnosis of HL limited to the marrow with HLH associated with lymphoproliferative syndrome, HIV infection and EBV infection, after the decision of the multidisciplinary committee of lymphoproliferative neoplasms, treatment was started according to the protocol of the Histiocyte Society (HLH-94) which included descending doses of dexamethasone, etoposide. Lumbar puncture was performed and showed no disease, for which reason triple intrathecal therapy was excluded from the treatment.
In order to control EBV infection as possible underlying triggers of HLH, monoclonal antibody Rituximab was added to the treatment.
Finally, for the treatment of HLH, the ABVD scheme (anthracycline, bleomycin, etoposide, dacarbazine) was decided upon for six cycles.
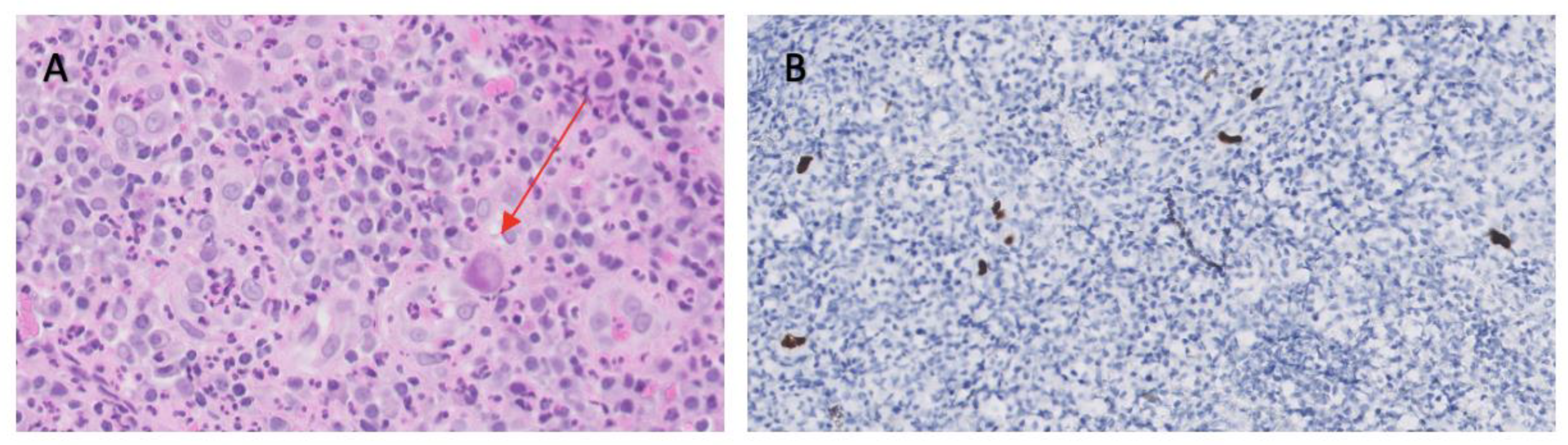
During treatment, the patient presented multiple clinical complications, mainly hematologic toxicity and cytomegalovirus infection that caused esophageal ulcers (
Image 3) leading to a stenosis that required enteral nutrition.
Currently, the patient is being monitored and is in complete remission with all secondary complications resolved with a current follow-up of 27 months.
3. Discussion
As previously described, we present the case of a patient with primary HIV and EBV co-infection with a diagnosis of HL with exclusive involvement of the BM and with development of HLH secondary to the above.
In addition, during treatment, the patient develops severe complications such as CMV esophagitis. The treatment is successful with the patient being alive and disease free at 27 months of follow-up.
Regarding the initial presentation, the clinical findings, pancytopenia, and both analytical and CT imaging findings, made it essential to perform a BM workup to reach a definitive diagnosis.
In patients with HL and HIV infection, extensive extranodal involvement is frequent, including BM infiltration, although exclusive involvement of the BM is infrequent. This fact is probably explained by the underdiagnosis of this entity [
1].
The incidence of HL is high mainly in immunocompromised populations such as HIV positive, although HL is not currently considered an AIDS-defining event and is under debate [
1].
On another note, as part of the diagnosis, a lymph node biopsy was performed which, although the incidence of lymph node involvement in this population is high, the histological findings were compatible with related lymphoid hyperplasia associated.
The reasons for this conclusion were that these findings in inguinal lymph node biopsy can occur in diseases other than HL, such as peripheral B- and T-cell lymphomas, infectious mononucleosis EBV relataed and follicular hyperplasia [
6].
Importanly, the prevalence of EBV infection in the world is estimated to be around 90% contributing to Hodgkin/Reed-Sternberg cell survival (HRS) [
7].
This fact, added to the high viral load of EBV demonstrated by PCR in peripheral blood, made us consider related lymphoid hyperplasia associated as a diagnosis and not another cause contemplated in the differential diagnosis [
3,
7,
8].
Finally, with respect to diagnostic features, the diagnosis of HLH is of exclusion. It is a life-threatening entity that has to be considered in patients with cytopenias, increased transaminases, triglycerides and ferritin, coagulopathy, elevated IL-2 receptor and decreased or absent NK cells, as well as the appearance of fever and splenomegaly [
9]. Demonstration of hemogagocytosis in lymph nodes or marrow alone is not diagnostic of this pathology. HLH is a potentially fatal condition. It can present in a primary or pediatric form, caused by genetic defects, or in a secondary form. Both have in common an uncontrolled activation of CD8þ cytotoxic T cells is the root abnormality in most forms of HLH [
5].
Regarding the secondary causes that can trigger this condition, autoimmune diseases, infections, malignant diseases and drugs, among others, may be involved [
10].
In the presented patient’s clinical situation, HIV infection, EVB infection, HL or a combination of several of the above, could be causing the current clinical status.
Treatment was started according to the protocol of the Histiocyte Society (HLH-94) [
11] which included descending doses of dexamethasone, etoposide. This protocol is derived from the primary or pediatric HLH. Lumbar puncture was performed and showed no disease, for which reason triple intrathecal therapy was excluded from the treatment. A new protocol of this same society is being carried out (HLH-2004) which includes cyclophosphamide as induction therapy, although to date we still do not have solid data [
5].
Regarding treatment and referring to HL, the treatment of advanced HL defined as the accumulation of different criteria of poor prognosis such as age over 45 years, stage IV, decreased serum albumin levels, male sex, anemia and lymphocytosis or lymphopenia, is based on intensive BEACCOP type treatments (bleomycin, adriamycin male sex, anemia and lymphocytosis or lymphopenia, is recommended for the treatment of fit patients [
12].
The rationale for choosing a less intensive ABVD-type regimen was based on the decision of the multidisciplinary lymphoma committee not to increase the added toxicity to the regimen for the treatment of HLH [
13].
In addition, in patients with HL associated with lymphoproliferative neoplasms, initiation of HAART therapy is indicated [
14].
Despite HAART, HL’s incidence has not decreased since its implementation and this could be explained in part by the dependence of HRS on CD4+ T lymphocytes [
15].
Chronic anti-genic stimulation and co-infection with other oncogenic viruses appear to be key factors for lymphomagenesis in HIV patients. This may be the reason why even in the HAART era the incidence of neoplasms is higher in patients living with HIV than in general population [
16].
Finally, in order to control EBV infection as possible underlying triggers of HLH, monoclonal antibody Rituximab was added to the treatment as it is thought that it can eliminate EBV-infected B cells [
17].
As a consequence of all the treatment administered, the patient developed a picture of retrosternal pain, dysphagia, performing a gastroscopy, where the biopsy was compatible with CMV infection. In addition, the patient developed cytopenias that required transfusion support and delays in the treatment to be resolved. In spite of the above, with a follow-up of almost 2 and a half years, the patient remains alive and in remission with symptoms derived from CMV esophagitis. Close observation in consultations continues.
4. Conclusions
The incidence of HL in the HIV population is increased and its presentation is mainly advanced, with exclusive medullary involvement being less frequent. EBV co-infection may play an important role in the etiopathogenesis of this type of lymphoma, with an increased incidence even in the HAART era. The three previous entities can produce HLH, a potentially fatal entity that requires specific treatment for its management. Multidisciplinary patient management is fundamental for the treatment of these entities.
Author Contributions
Conceptualization, A.L.G., R.C. and M.R.P.; methodology, A.L.G. and M.R.P.; software, A.L.G. and J.C.C.; validation, M.R.P., R.C. and L.S.; formal analysis, A.L.G.; investigation, L.P., A.L.G., J.C., J.C.C., F.J.D.P. and B.A.; resources, B.A., F.J.D.P. and M.R.P.; data curation, L.S.; writing—original draft preparation, A.L.G.; writing—review and editing, all autrhos; visualization, X.X.; supervision, M.R.P. and R.C.; project administration, R.C. and A.L.G.; funding acquisition, R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this case report.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Temple, J.J.; Andes, W.A. AIDS and Hodgkin’s disease. Lancet 1986, 2, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Diehl, V.; Thomas, R.K.; Re, D. Part II: Hodgkin’s lymphoma—diagnosis and treatment. The Lancet Oncology 2004, 5, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, M.; Fumagalli, L.; Rossi, G.; Freschi, M.; Re, A.; Viganò, M.G.; et al. Isolated bone marrow manifestation of HIV-associated Hodgkin lymphoma. Mod Pathol 2002, 15, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Shindiapina, P.; Ahmed, E.H.; Mozhenkova, A.; Abebe, T.; Baiocchi, R.A. Immunology of EBV-Related Lymphoproliferative Disease in HIV-Positive Individuals. Frontiers in Oncology 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatric Blood & Cancer 2007, 48, 124–131. [Google Scholar]
- Knecht, H.; Mai, S. LMP1 and Dynamic Progressive Telomere Dysfunction: A Major Culprit in EBV-Associated Hodgkin’s Lymphoma. Viruses 2017, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Dunmire, S.K.; Verghese, P.S.; Balfour, H.H. Primary Epstein-Barr virus infection. J Clin Virol 2018, 102, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.G.; Young, L.S. An etiological role for the Epstein-Barr virus in the pathogenesis of classical Hodgkin lymphoma. Blood 2019, 134, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Griffin, G.; Shenoi, S.; Hughes, G.C. Hemophagocytic lymphohistiocytosis: An update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol 2020, 34, 101515. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.B.; Allen, C.E.; Weitzman, S.; Filipovich, A.H.; McClain, K.L. How I treat hemophagocytic lymphohistiocytosis. Blood 2011, 118, 4041–4052. [Google Scholar] [CrossRef] [PubMed]
- Hasenclever, D.; Diehl, V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med 1998, 339, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Eichenauer, D.A.; Aleman, B.M.P.; André, M.; Federico, M.; Hutchings, M.; Illidge, T.; et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018, 29 (Suppl. 4), iv19–iv29. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Lu, H. Malignancies in HIV-Infected and AIDS Patients. In Infectious Agents Associated Cancers: Epidemiology and Molecular Biology; Cai, Q., Yuan, Z., Lan, K., Eds.; Springer: Singapore, 2017; pp. 167–179. [Google Scholar] [CrossRef]
- Carbone, A.; Vaccher, E.; Gloghini, A. Hematologic cancers in individuals infected by HIV. Blood 2022, 139, 995–1012. [Google Scholar] [CrossRef] [PubMed]
- Pantanowitz, L.; Carbone, A.; Dolcetti, R. Microenvironment and HIV-related lymphomagenesis. Semin Cancer Biol 2015, 34, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.C.; Tsai, D.E.; Hodinka, R.L.; Silverman, L.B.; Malbran, A.; Wasik, M.A.; et al. Treatment of primary Epstein-Barr virus infection in patients with X-linked lymphoproliferative disease using B-cell-directed therapy. Blood 2005, 105, 994–996. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).