Submitted:
12 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
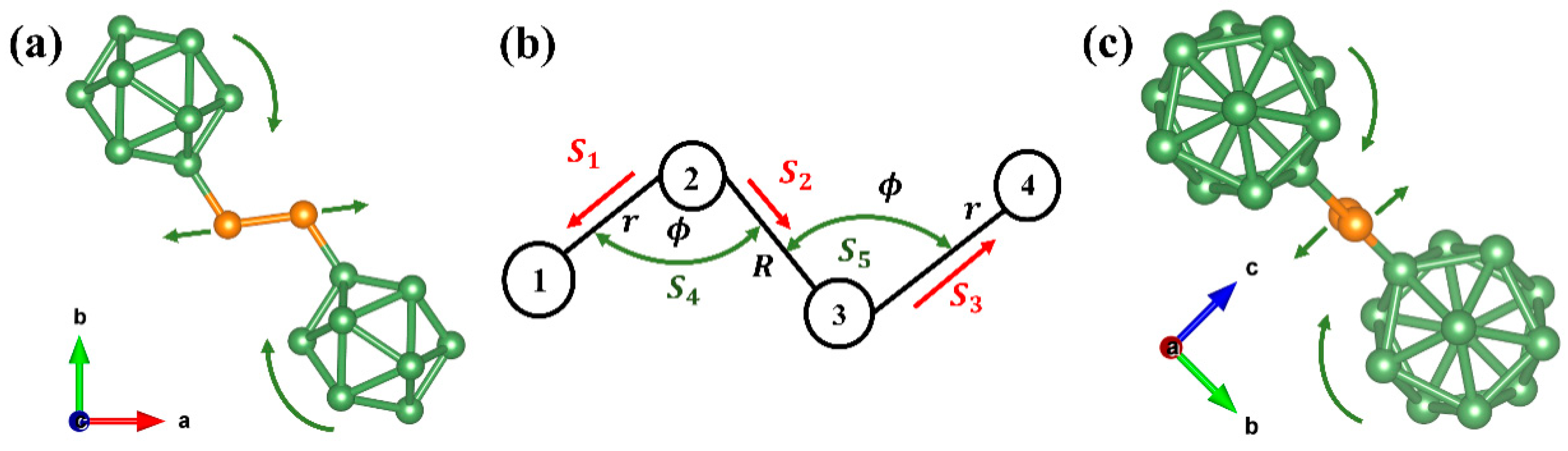
Experimental details
First principle calculations
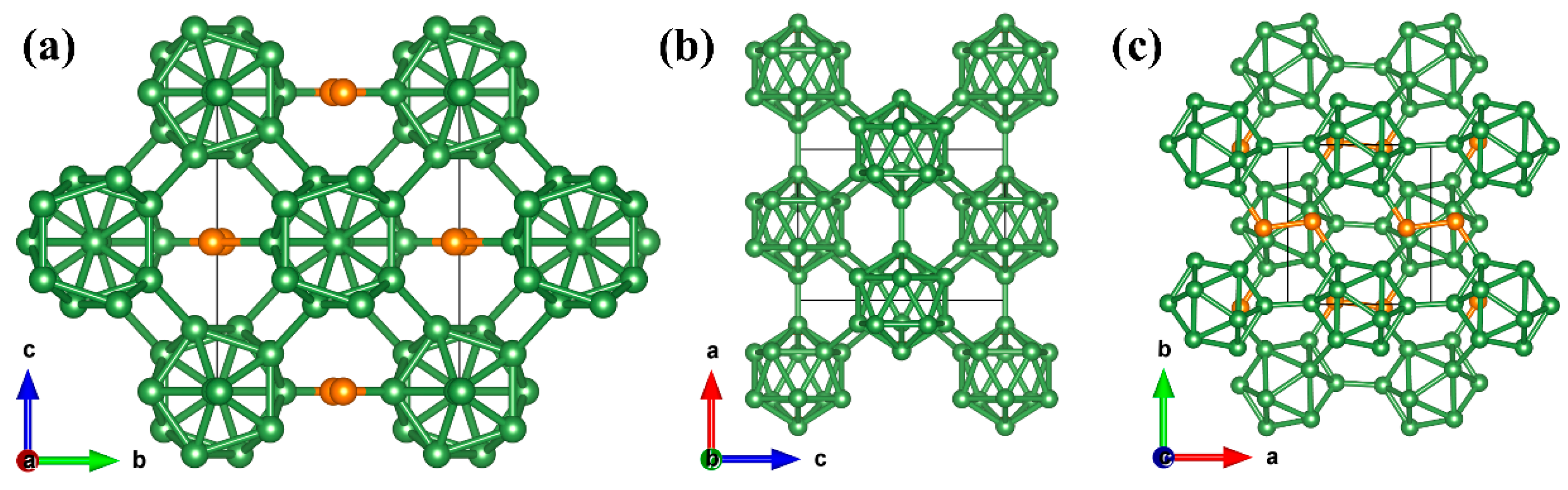
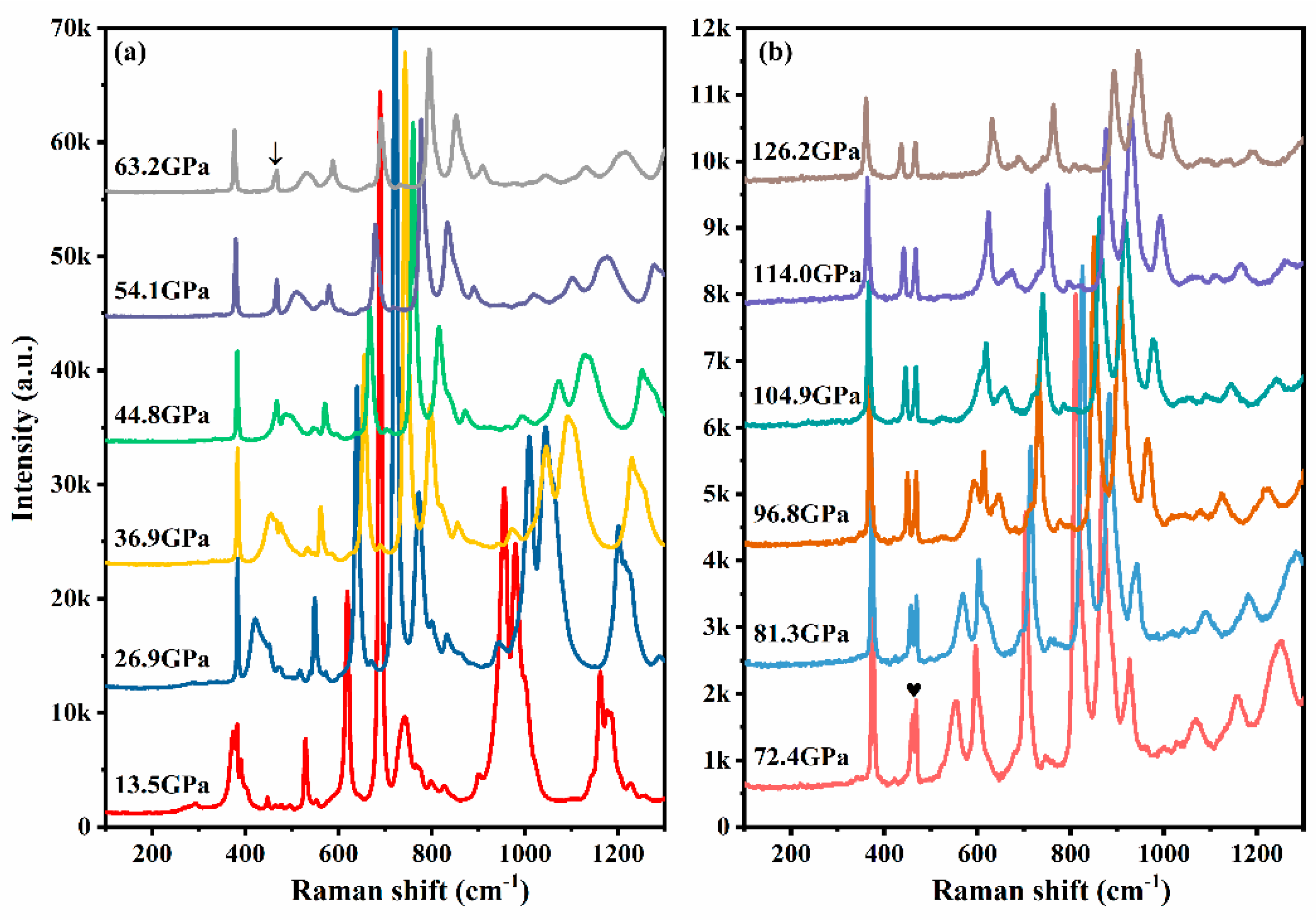
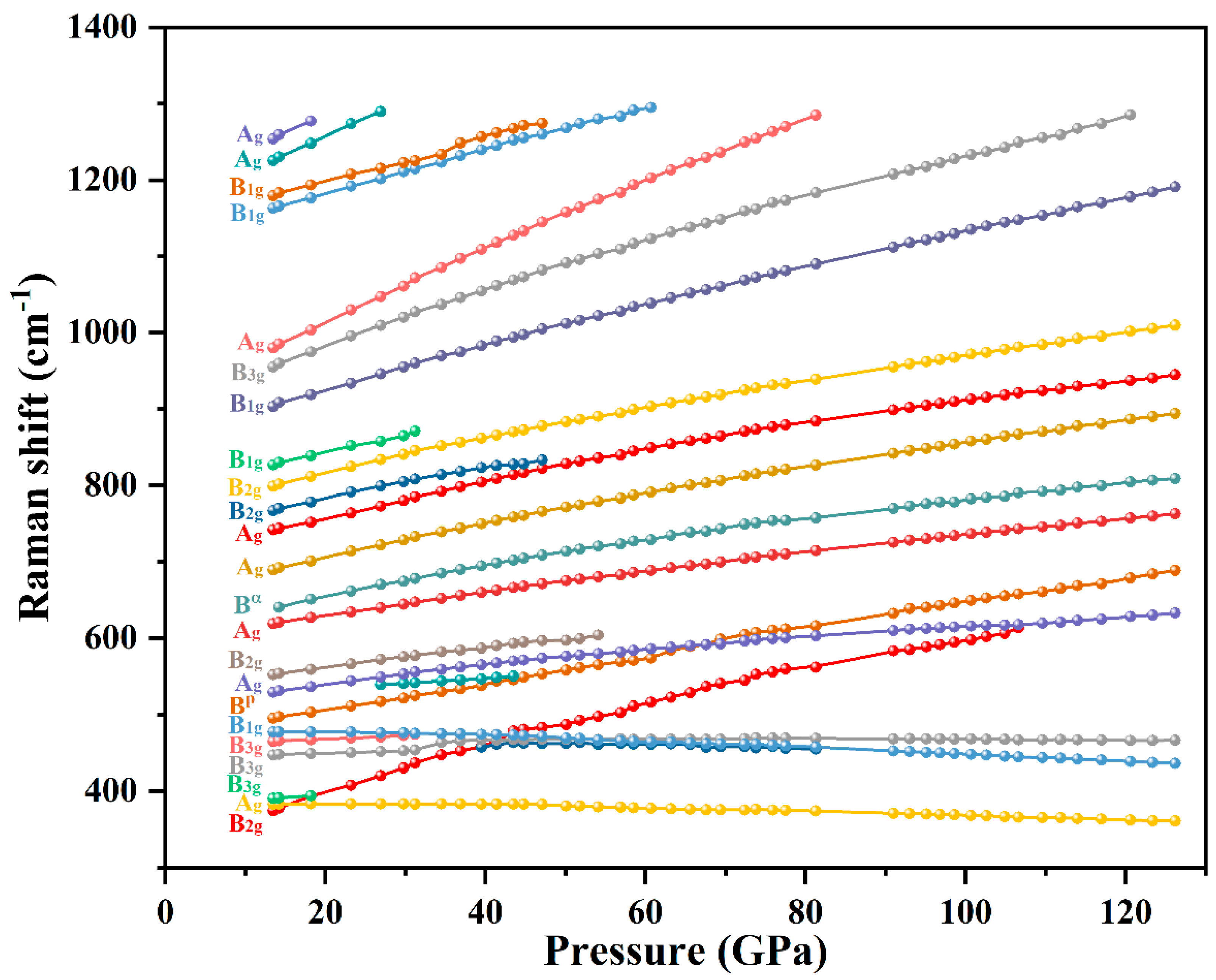
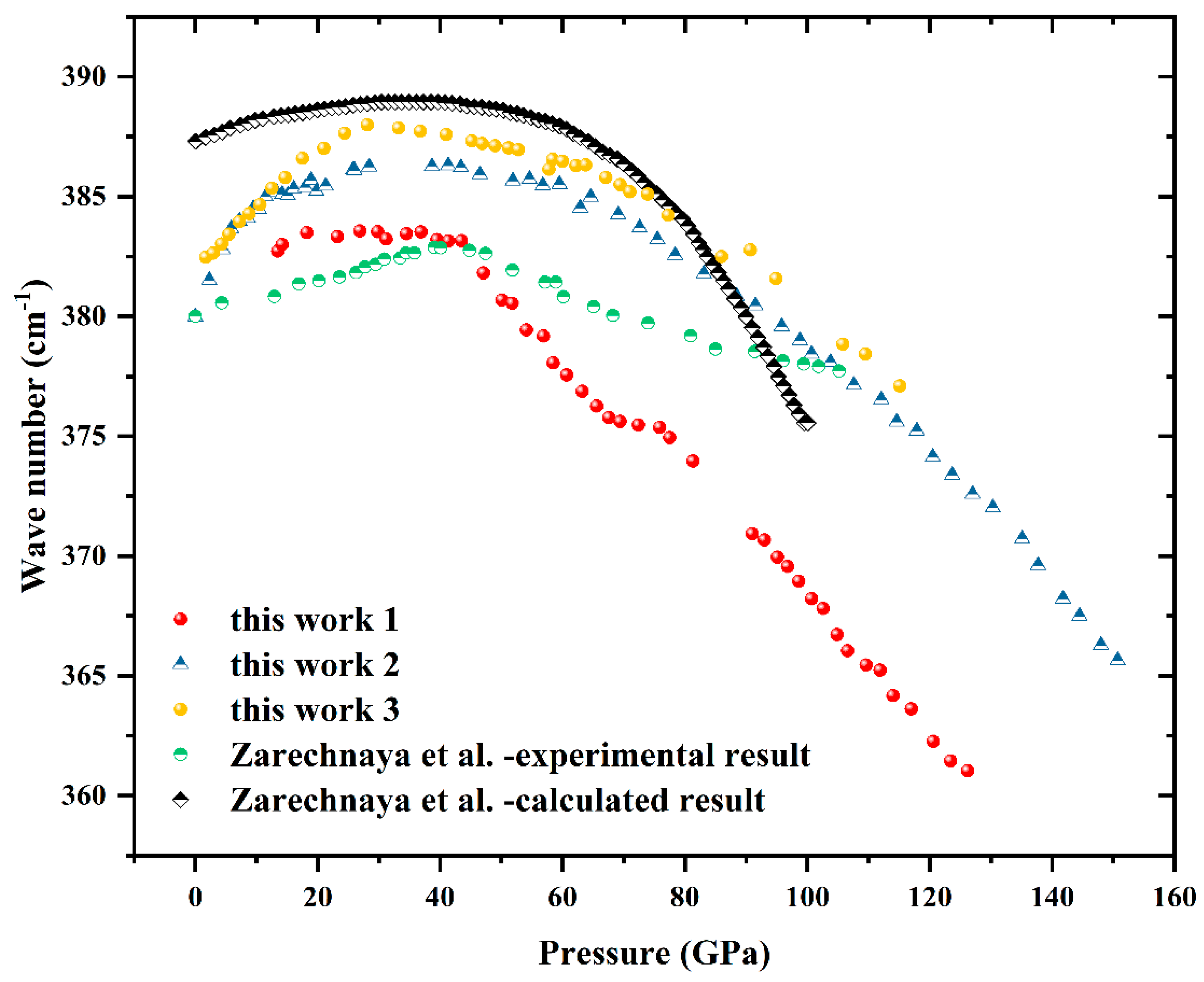
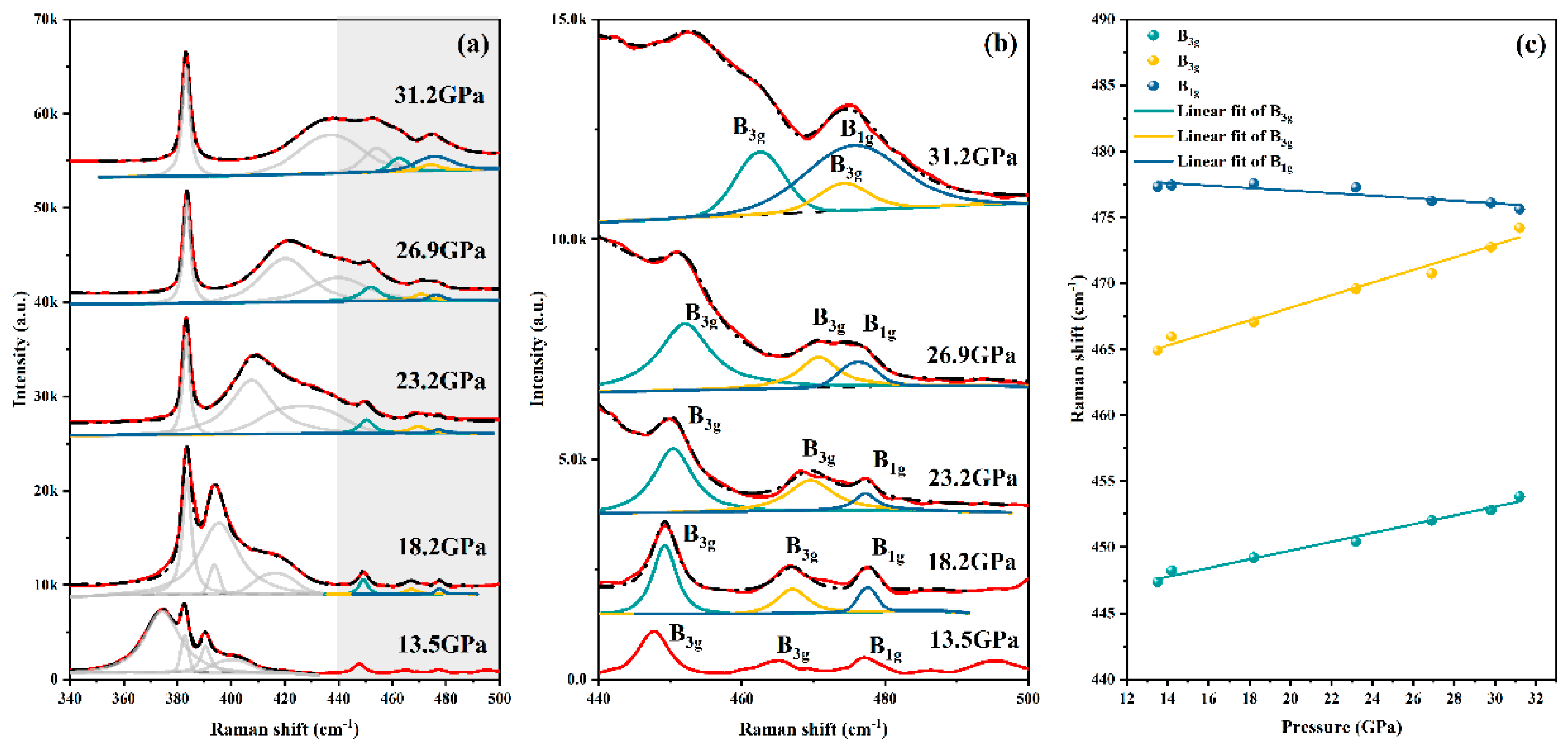
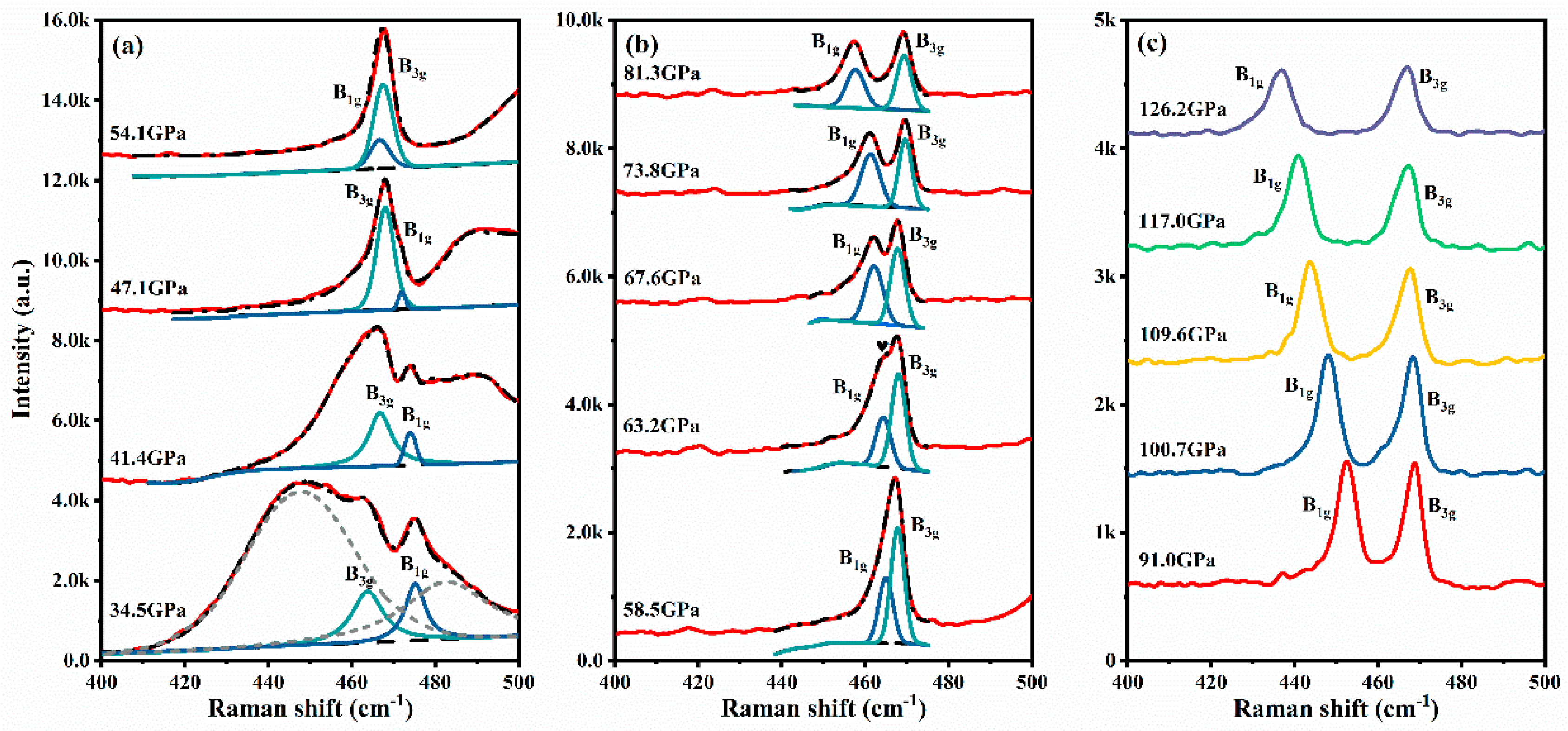
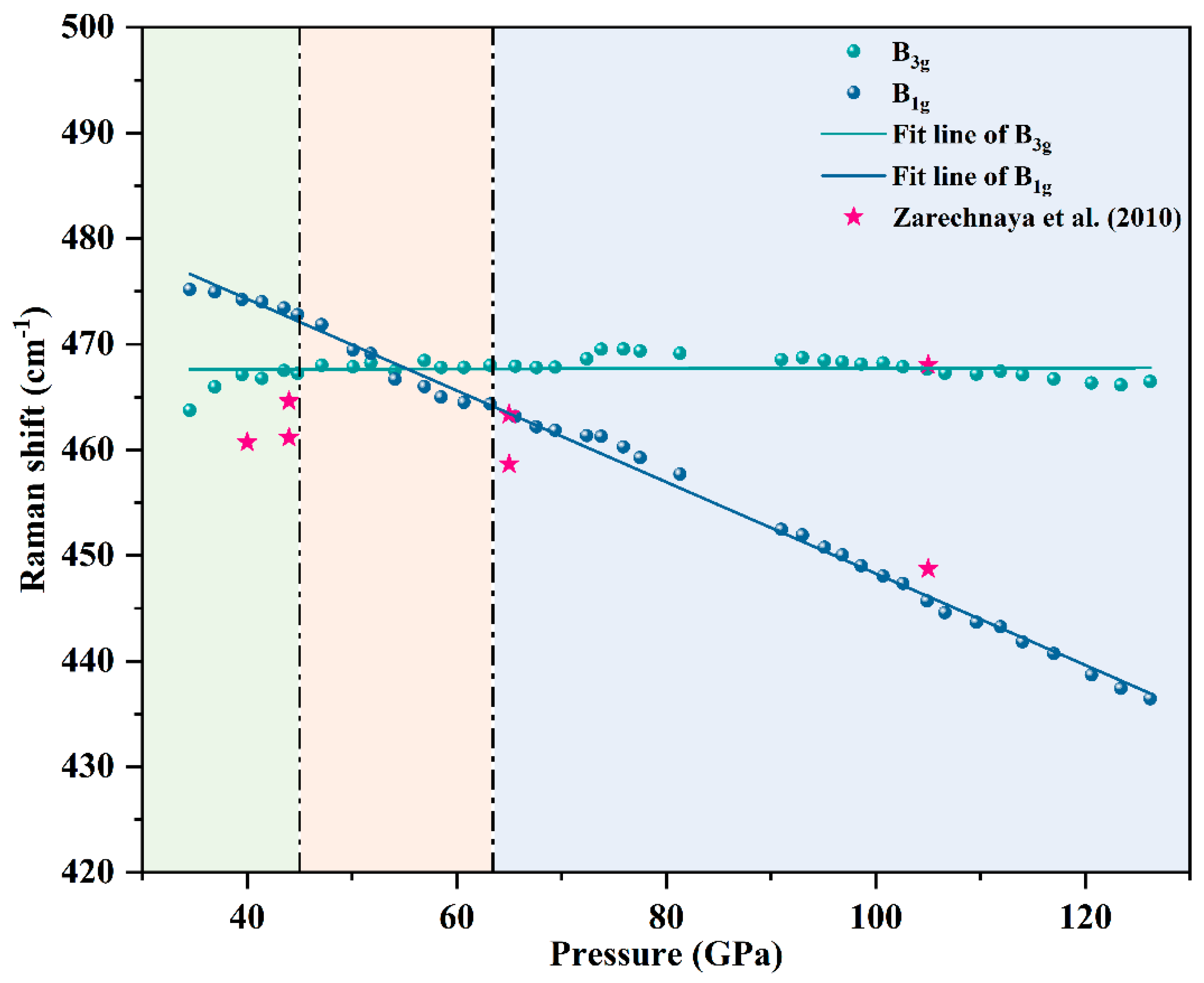
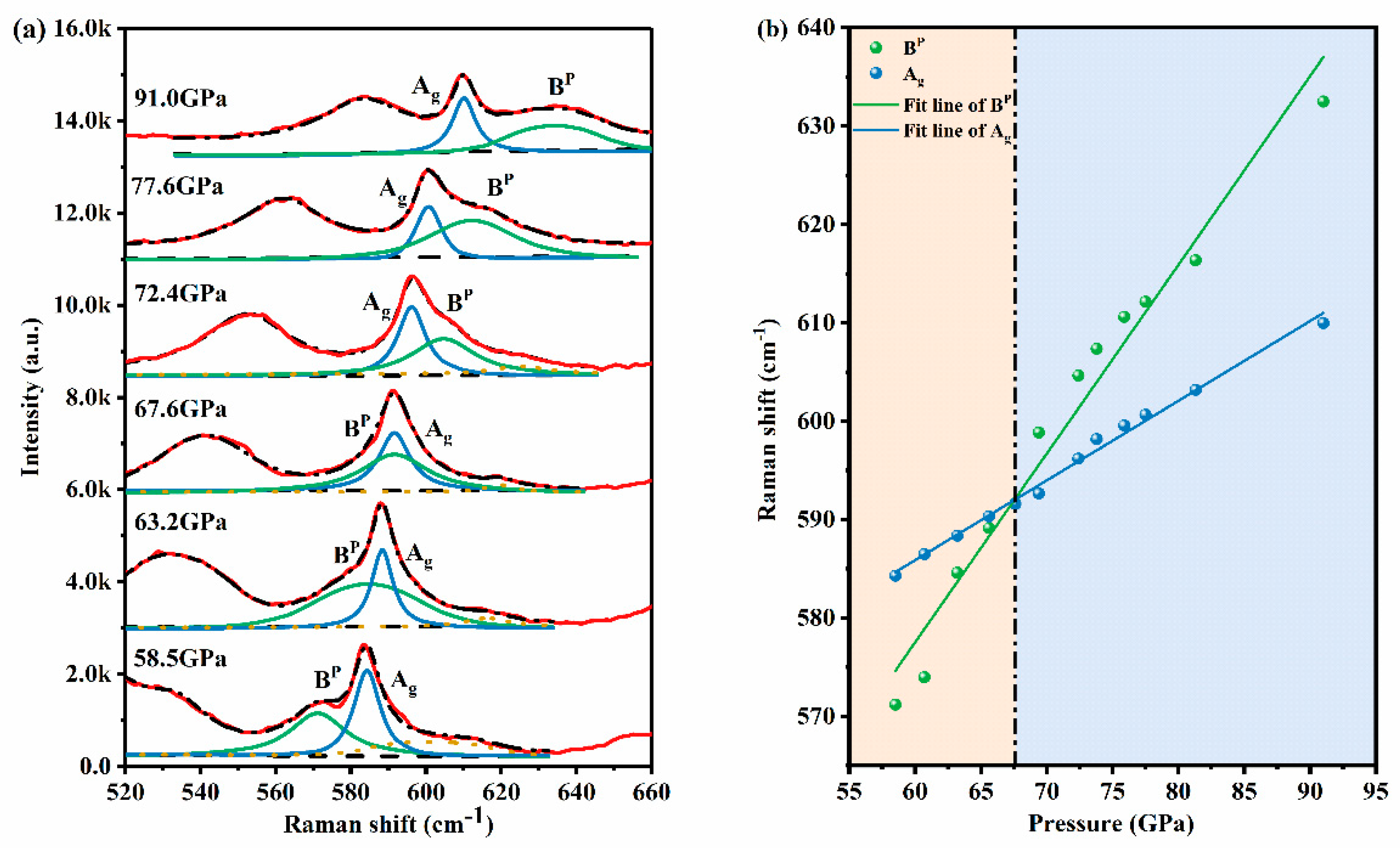
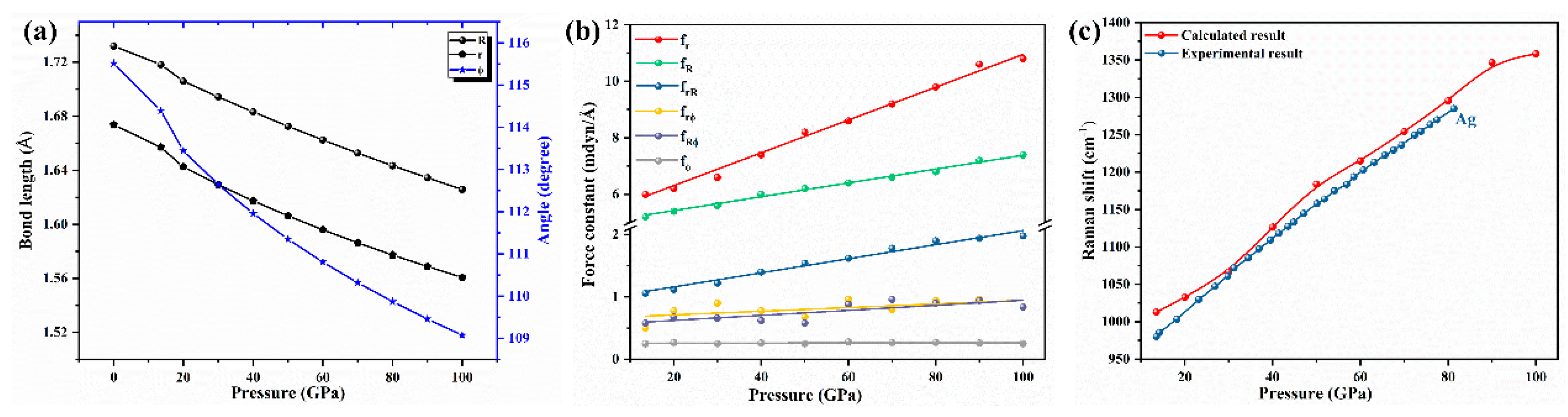
Results and Discussion
Conclusion
Acknowledgements
References
- Douglas, B.; Shih-MingHo. Structure and Chemistry of Crystalline Solids; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hughes, R.E.; Kennard, C.H.L.; Sullenger, D.B.; Weakliem, H.A.; Sands, D.E.; Hoard, J.L. The Structure of β-Rhombohedral Boron. J. Am. Chem. Soc. 1963, 85, 361–362. [Google Scholar] [CrossRef]
- Oganov, A.R.; Chen, J.; Gatti, C.; Ma, Y.; Ma, Y.; Glass, C.W.; Liu, Z.; Yu, T.; Kurakevych, O.O.; Solozhenko, V.L. Ionic high-pressure form of elemental boron. Nature 2009, 457, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, J.P. Pressure-induced metallization in solid boron. Phys. Rev. B 2002, 66. [Google Scholar] [CrossRef]
- Haussermann, U.; Simak, S.I.; Ahuja, R.; Johansson, B. Metal-nonmetal transition in the boron group elements. Phys Rev Lett 2003, 90, 065701. [Google Scholar] [CrossRef]
- Shirai, K.; Dekura, H.; Yanase, A. Electronic Structure and Electrical Resistivity of α-Boron under High Pressure. J. Phys. Soc. Jpn. 2009, 78. [Google Scholar] [CrossRef]
- Eremets, M.I.; Struzhkin, V.V.; Mao, H.; Hemley, R.J. Superconductivity in boron. Science 2001, 293, 272–274. [Google Scholar] [CrossRef]
- Kaneshige, M.; Hirayama, S.; Yabuuchi, T.; Matsuoka, T.; Shimizu, K.; Mita, Y.; Hyoudo, H.; Kimura, K. Measurement of Electrical Resistance and Raman Spectrum of α-Boron under High Pressure. J. Phys. Soc. Jpn. 2007, 76, 19–20. [Google Scholar] [CrossRef]
- Shimizu, K.; Kaneshige, M.; Hashimoto, Y.; Nagatochi, T.; Hyodo, H.; Kimura, K. Superconductivity in α-boron at Mbar pressure. Phys. C Supercond. Its Appl. 2010, 470, S631–S632. [Google Scholar] [CrossRef]
- Vast, N.; Baroni, S.; Zerah, G.; Besson, J.M.; Polian, A.; Grimsditch, M.; Chervin, J.C. Lattice Dynamics of Icosahedralα-Boron under Pressure. Phys. Rev. Lett. 1997, 78, 693–696. [Google Scholar] [CrossRef]
- Masago, A.; Shirai, K.; Katayama-Yoshida, H. Crystal stability ofα- andβ-boron. Phys. Rev. B 2006, 73. [Google Scholar] [CrossRef]
- Shang, S.; Wang, Y.; Arroyave, R.; Liu, Z.-K. Phase stability inα- andβ-rhombohedral boron. Phys. Rev. B 2007, 75. [Google Scholar] [CrossRef]
- Polian, A.; Chervin, J.C.; Munsch, P.; Gauthier, M. α-boron at very high pressure: structural and vibrational properties. J. Phys. Conf. Ser. 2008, 121. [Google Scholar] [CrossRef]
- Parakhonskiy, G.; Vtech, V.; Dubrovinskaia, N.; Caracas, R.; Dubrovinsky, L. Raman spectroscopy investigation of alpha boron at elevated pressures and temperatures. Solid State Commun. 2013, 154, 34–39. [Google Scholar] [CrossRef]
- Chuvashova, I.; Bykova, E.; Bykov, M.; Svitlyk, V.; Gasharova, B.; Mathis, Y.-L.; Caracas, R.; Dubrovinsky, L.; Dubrovinskaia, N. High-pressure behavior of α-boron studied on single crystals by X-ray diffraction, Raman and IR spectroscopy. J. Solid State Chem. 2017, 245, 50–60. [Google Scholar] [CrossRef]
- Pokatashkin, P.A.; Korotaev, P.Y.; Yanilkin, A.V. Amorphization inα-boron: A molecular dynamics study. Phys. Rev. B 2017, 95. [Google Scholar] [CrossRef]
- Sanz, D.N.; Loubeyre, P.; Mezouar, M. Equation of state and pressure induced amorphization of beta-boron from X-ray measurements up to 100 GPa. Phys Rev Lett 2002, 89, 245501. [Google Scholar] [CrossRef]
- Ma, Y.; Prewitt, C.T.; Zou, G.; Mao, H.-k.; Hemley, R.J. High-pressure high-temperature x-ray diffraction of β-boron to 30 GPa. Phys. Rev. B 2003, 67. [Google Scholar] [CrossRef]
- Siberchicot, B. Ab initioequation of state ofα- andβ-boron: Possible amorphization ofβ-boron under high pressure. Phys. Rev. B 2009, 79. [Google Scholar] [CrossRef]
- An, Q.; Morozov, S.I. Brittle failure of β- and τ-boron: Amorphization under high pressure. Phys. Rev. B 2017, 95. [Google Scholar] [CrossRef]
- Shirai, K.; Masago, A.; Katayama-Yoshida, H. High-pressure properties and phase diagram of boron. physica status solidi (b) 2007, 244, 303–308. [Google Scholar] [CrossRef]
- Parakhonskiy, G.; Dubrovinskaia, N.; Bykova, E.; Wirth, R.; Dubrovinsky, L. Experimental pressure-temperature phase diagram of boron: resolving the long-standing enigma. Sci Rep 2011, 1, 96. [Google Scholar] [CrossRef]
- Qin, J.; Irifune, T.; Dekura, H.; Ohfuji, H.; Nishiyama, N.; Lei, L.; Shinmei, T. Phase relations in boron at pressures up to 18 GPa and temperatures up to 2200∘C. Phys. Rev. B 2012, 85. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Kurakevych, O.O. Equilibrium p-T phase diagram of boron: experimental study and thermodynamic analysis. Sci Rep 2013, 3, 2351. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K. Phase diagram of boron crystals. Jpn. J. Appl. Phys. 2017, 56. [Google Scholar] [CrossRef]
- Wentorf, R.H., Jr. Boron: Another Form. Science 1965, 147, 49–50. [Google Scholar] [CrossRef]
- Ma, Y.; Tse, J.S.; Klug, D.D.; Ahuja, R. Electron-phonon coupling ofα−Gaboron. Phys. Rev. B 2004, 70. [Google Scholar] [CrossRef]
- Jiang, C.; Lin, Z.; Zhang, J.; Zhao, Y. First-principles prediction of mechanical properties of gamma-boron. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Zarechnaya, E.Y.; Dubrovinsky, L.; Dubrovinskaia, N.; Filinchuk, Y.; Chernyshov, D.; Dmitriev, V.; Miyajima, N.; El Goresy, A.; Braun, H.F.; Van Smaalen, S.; et al. Superhard semiconducting optically transparent high pressure phase of boron. Phys Rev Lett 2009, 102, 185501. [Google Scholar] [CrossRef]
- Isaev, E.I.; Simak, S.I.; Mikhaylushkin, A.S.; Vekilov, Y.K.; Zarechnaya, E.Y.; Dubrovinsky, L.; Dubrovinskaia, N.; Merlini, M.; Hanfland, M.; Abrikosov, I.A. Impact of lattice vibrations on equation of state of the hardest boron phase. Phys. Rev. B 2011, 83. [Google Scholar] [CrossRef]
- Godec, Y.L.; Kurakevych, O.O.; Munsch, P.; Garbarino, G.; Solozhenko, V.L. Equation of state of orthorhombic boron, -B28. Solid State Commun. 2009, 149, 1356–1358. [Google Scholar] [CrossRef]
- Zarechnaya, E.; Dubrovinskaia, N.; Caracas, R.; Merlini, M.; Hanfland, M.; Filinchuk, Y.; Chernyshov, D.; Dmitriev, V.; Dubrovinsky, L. Pressure-induced isostructural phase transformation inγ-B28. Phys. Rev. B 2010, 82. [Google Scholar] [CrossRef]
- Oganov, A.R.; Solozhenko, V.L.; Gatti, C.; Kurakevych, O.O.; Le Godec, Y. The high-pressure phase of boron, γ-B28: Disputes and conclusions of 5 years after discovery. J. Superhard Mater. 2011, 33, 363–379. [Google Scholar] [CrossRef]
- Yu Zarechnaya, E.; Dubrovinsky, L.; Dubrovinskaia, N.; Miyajima, N.; Filinchuk, Y.; Chernyshov, D.; Dmitriev, V. Synthesis of an orthorhombic high pressure boron phase. Sci Technol Adv Mater 2008, 9, 044209. [Google Scholar] [CrossRef] [PubMed]
- Zarechnaya, E.Y.; Dubrovinskaia, N.; Dubrovinsky, L. Polarized Raman spectroscopy of high-pressure orthorhombic boron phase. High Press. Res. 2009, 29, 530–535. [Google Scholar] [CrossRef]
- Silvera, I.F.; Jeon, S.J.; Lorenzana, H.E. Pressure dependence of the vibron modes in solid hydrogen and deuterium. Phys Rev B Condens Matter 1992, 46, 5791–5794. [Google Scholar] [CrossRef]
- Wilson, E.B. Some Mathematical Methods for the Study of Molecular Vibrations. J. Chem. Phys. 1941, 9, 76–84. [Google Scholar] [CrossRef]
- Decius, J.C. A Tabulation of General Formulas for Inverse Kinetic Energy Matrix Elements in Acyclic Molecules. J. Chem. Phys. 1948, 16, 1025–1034. [Google Scholar] [CrossRef]
- Wilson, E.B. A Method of Obtaining the Expanded Secular Equation for the Vibration Frequencies of a Molecule. J. Chem. Phys. 1939, 7, 1047–1052. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




