1. Introduction
Periodontitis or periodontal disease (PD) is an inflammatory immune condition caused by bacterial biofilms developed in the subgingival space that causes the destruction of the connective tissue, the alveolar ligament, and ultimately, bone resorption resulting in the loss of teeth [
1]. Therefore, the persistence of oral microorganisms at the level of dental structures causes an imbalance in bone metabolism resulting in the release of pro-inflammatory mediators, growth mediators, and signaling molecules. Periodontitis is the most common cause of tooth loss among the human population, and it is associated with atherosclerosis, carotid stenosis, premature birth, low birth weight fetus, etc. [
2,
3].
Among the frequent causes associated with periodontitis, we find smoking, diabetes, stress, age, social status, or genetic factors [
4]. All of them affect the homeostasis of the oral cavity, ultimately leading to disturbances in the oral microbiome [
5]. Consequently, inflammation and the formation of biofilms appear on the dental surface, as well as the invasion of gingival tissues [
2]. In dental plaque, bacteria such as
Streptococcus spp, frequently found in the mouth of all people, which express adhesins, provide the necessary support for other bacterial colonizers.
Since the pathogenesis of periodontal disease has been studied for a long time, today there are data that characterize the microbial flora, both in healthy and affected patients [
6].
Porphyromonas gingivalis (P.g),
Tannerella forsythia (T.f),
Treponema denticola (T.d) and
Aggregatibacter actinomycetemcomitans (A.a) are the most incriminated pathogens that trigger periodontitis.
Fusobacterium nucleatum (F.n) was also involved in periodontal health, due to its frequent detection in subgingival plaque samples, having an important role in the organization of biofilms as a result of the expression of multiple adhesins [
7]. PD symptomatology varies according to the patient's age, thus, in the case of young people, it is mainly triggered by A.a., and in adults, the triggering agents of PD are P.g., T.d or spirochetes. In an anaerobic environment, as we find in the subgingival space, periodontal pockets are formed where the growth of pathogens and pathobionts that express virulence factors and lead to imbalances in the host's inflammatory response is favored [
8].
A.a is a non-motile Gram-facultative anaerobe belonging to the Pasteurellaceae family [
9] that contributes to the occurrence of PD due to several virulence factors it expresses cytolethal distention toxin, leucotoxin A, and collagenase [
10]. In the case of juvenile periodontitis, leukotoxin A is the most studied virulence factor because it kills PMNs and macrophages, important components of host defense [
11]. In the conditions of oral existence of commensals such as F.n and
Streptococcus oralis (S.o), A.a has a devastating action because in the early stages of periodontitis, A.a uses the lactic acid produced by S
treptococcus spp. as a nutrient to increase its number. The production of H
2O
2 by
Streptococcus spp. causes A.a to migrate deeper into the gingival pocket, where the bacterial cells are exposed to the host's immune response. The release of cytolethal distension toxin in this environment inhibits phagocytosis, and the release of leukotoxin by A.a will promote neutrophil degranulation or death, which will lead to the promotion of bone resorption [
12].
The purpose of our research was to study the potential trigger of the periodontal disease in a rat model using the bacterial species incriminated in the pathology of human periodontitis and to establish their optimal concentration capable of reproducing the disease, with the idea of subsequently developing innovative treatments for the condition.
2. Materials and Methods
Ethics statement
The animal experiments were carried out at the Baneasa Animal Facility (BAF) of the Bucharest Cantacuzino National Medical-Military Institute for Research and Development (IC). The study was approved by the Ethics Committee of the Faculty of Veterinary Medicine Bucharest no 25/15.06.2022 and by the veterinary health authority, in accordance with EU Directive 63/2010 on the care, use, and protection of animals used for scientific purposes.
Processing of bacterial strains selected for study
A.a, F.n, and S.o originating from the collection of bacterial strains of IC were inoculated on Schadler broth medium, 24h, at 37oC under anaerobiosis conditions (5%CO2), daily, for 4 weeks. The density of the bacterial suspension was measured daily using the densitometer (Densitometer McFarland Biosan DEN-1), for oral contamination we established 3 concentrations of each strain, namely 108, 109, and 1010 CFU/ml, the dose of inoculum established being 0.6 ml, the dose in which the 3 bacteria were found in equal volumes and which were administered by gavage, 5 days/week, 4 weeks.
Periodontitis rat model protocol
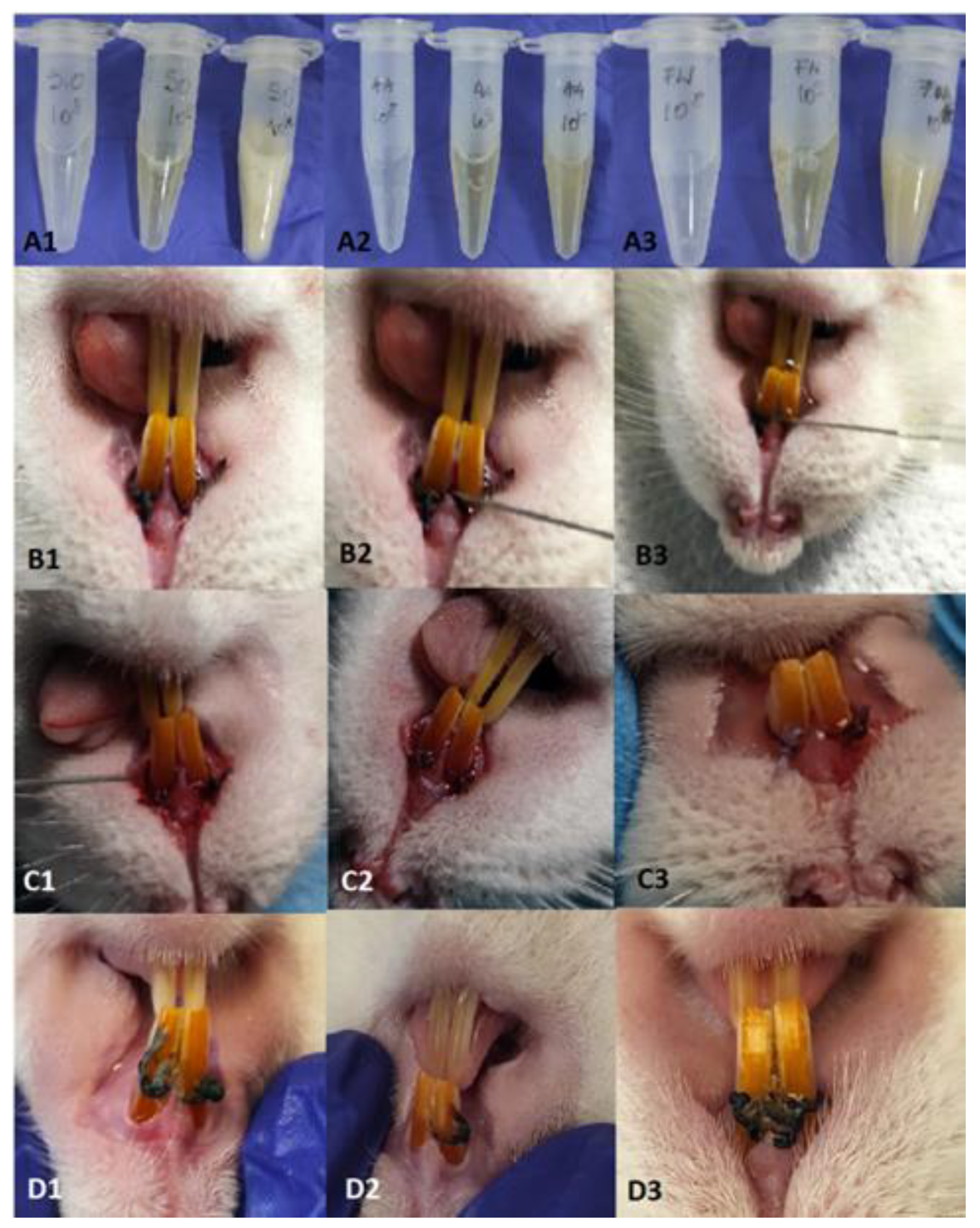
The procedures developed to create the animal model for PD were performed on 15 Wistar rats, aged 20 weeks, from the SPF (Specific Pathogen Free) of BAF. Throughout the experiment, the animals were housed, 5 each, in conventional conditions at a temperature of 20-22°C, a 12H:12H light-dark cycle, and received water and feed ad libitum. Rats were given 20 mg/ml kanamycin and 20 mg/ml ampicillin in their drinking water for five days to suppress the resident flora. At the end of the treatment cotton swabs with saliva were taken from the mouths of the animals to determine the effectiveness of the decontamination but also of the flora left unaffected by the treatment. In order to induce periodontitis, we resorted to the application of ligatures on the upper incisors, using a gingival retraction thread (Ultrapak, UltraDent, Romania). Thus, the rats were deeply anesthetized with Ketamine (0.5mg/kg, Pasteur Institute, Romania) and Medetomidine (0.5mg/kg, Biotur, Romania), in a weight-dependent dose. The animals were positioned in ventrodorsal recumbency on the operating table. By applying a mouth spacer, the incisors were isolated. With a dental curette, the gum was detached, and a ligature was applied around each incisor in the bag thus created. The animals were divided into 3 groups depending on the bacterial concentration used (group 10
8, group 10
9, and group 10
10). At the end of the procedure, the fresh 24-h inoculum, consisting of the three bacteria, was used to impregnate the thread but also to wash the gingival pocket (
Figure 1), then the animals received Atipamezole (0.02mg/kg, Biotur, Romania) to reverse the effect of anesthesia.
At each intervention, the body weight, and the periodontal pocket were monitored and also, and blood samples were taken from the retroorbital sinus to perform the hematological examination. At the end of the study, the animals were euthanized by an overdose of anesthetic, and samples were collected for the microbiological examination as well as the incisors for the histopathological analysis.
Statistical analysis
Analyzes were performed using Prism 9 for Windows software (GraphPad LLC, USA). To compare the data, the One-way ANOVA function was used, and a value of p < 0.05 was considered statistically significant. Regarding the analysis of the data obtained after the hematological examination, we compared the results obtained from each work group (108,109, 1010) comparing them with day 0, using the One-way ANOVA function, multiple comparisons, comparing the data from days 7, 24, and 28 with day 0, using the Dunnet test.
3. Results
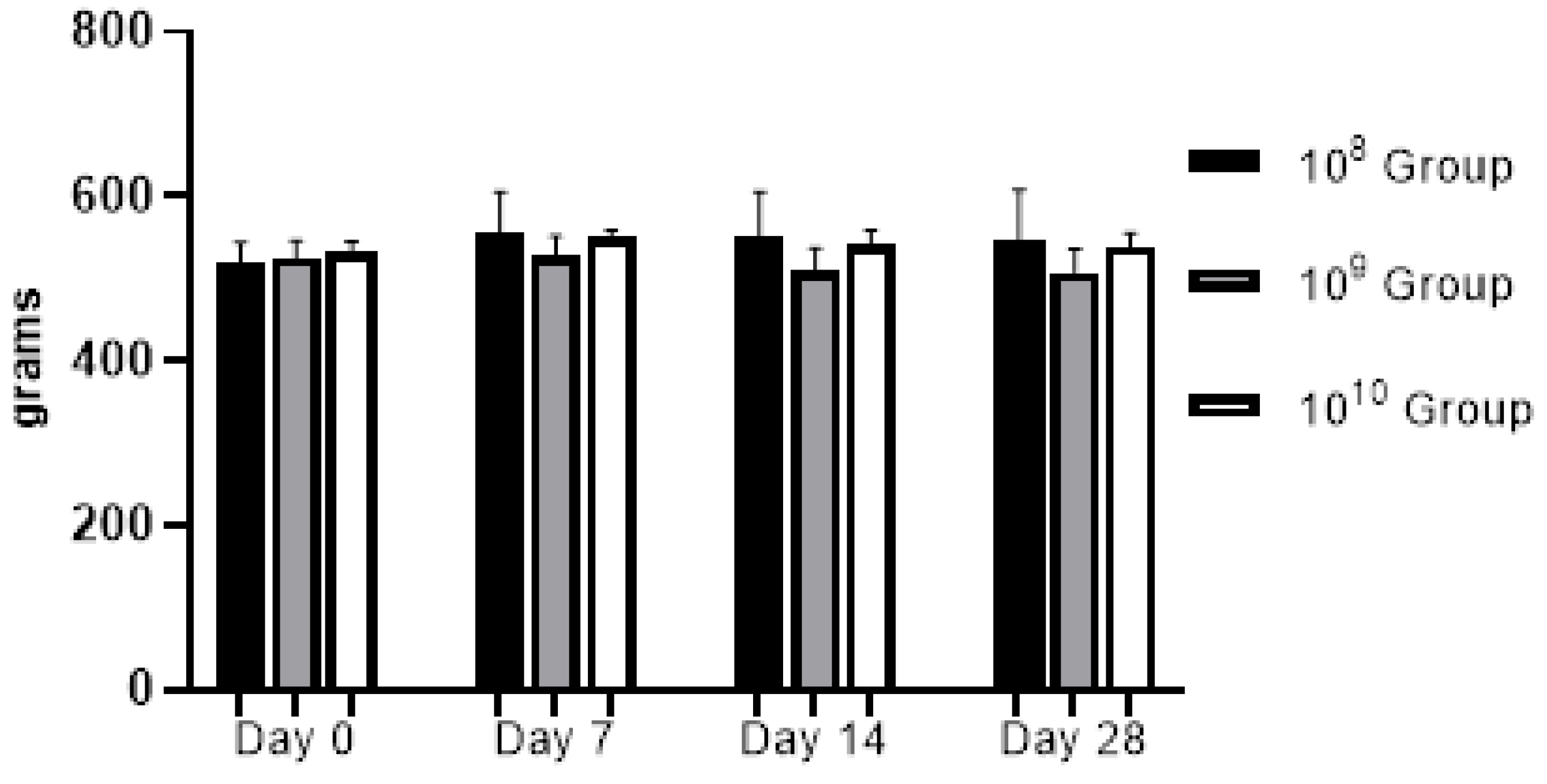
From a clinical point of view, the animals did not present discomfort during mastication, and through the weekly monitoring of body weight we could observe a relatively upward trend in the first three weeks, in the last week a slight weight decrease was recorded (
Figure 2). Gingival bleeding and periodontal pocket formation were clinically visible starting in the 2nd week after contamination.
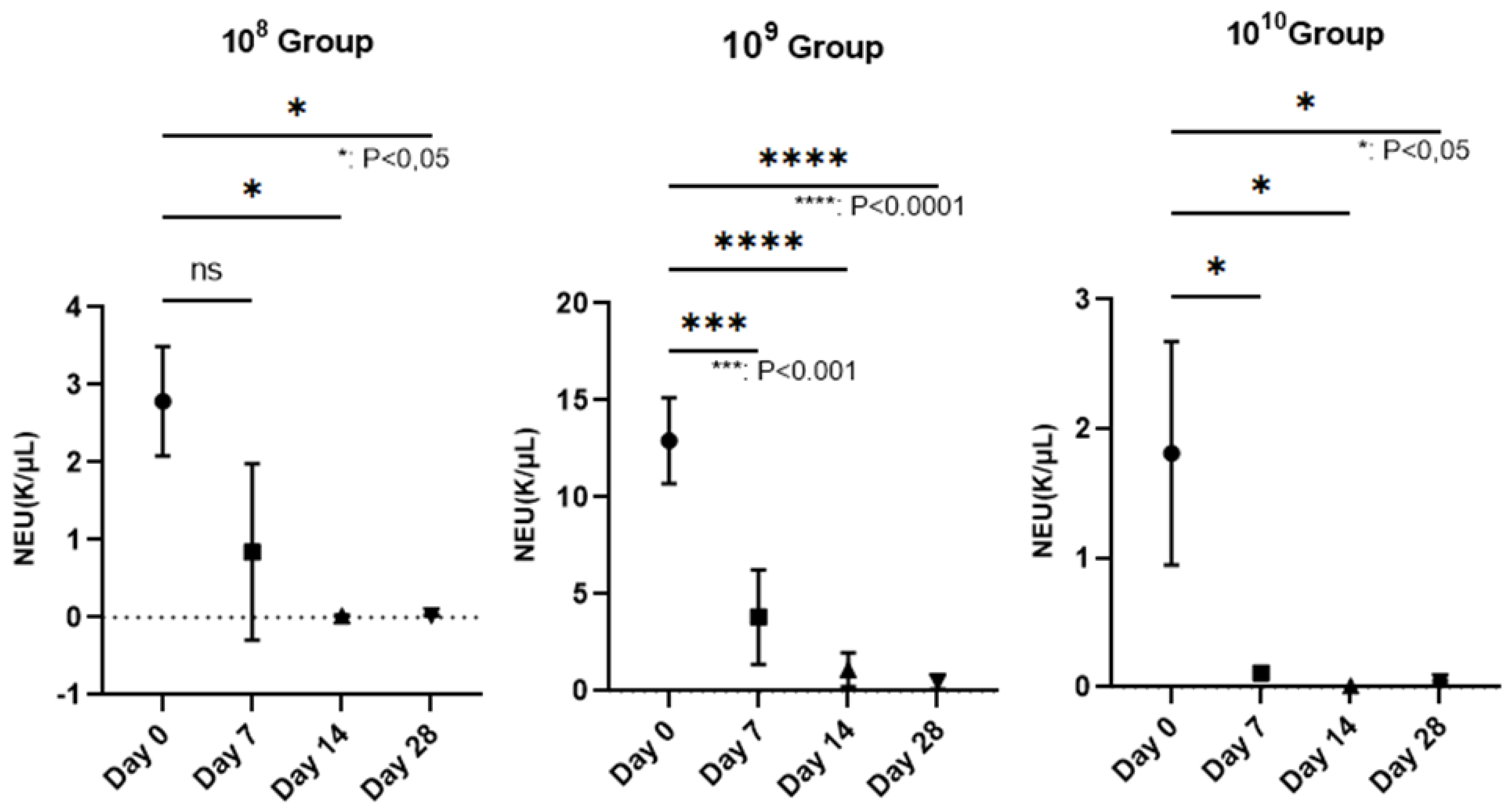
The hematological examination was performed on the Idexx Procyte 5diff analyzer from blood collected in EDTA vacutainers (KIMA Vacutest, Italy). We followed the polymorphonuclear cells (PMN), since in PD they are the cells responsible for the annihilation of pathogens. Regarding the number of neutrophils, which represent 50-70% of the total PMN, a decrease in their number could be observed as periodontitis was installed, in the case of all groups, but a strongly statistically significant relevance was recorded in group 10
9, where p< 0.0001 (
Figure 3), in the last 2 weeks of the study. Lymphocytes, the elements involved in the immune response of the host, did not provide statistically significant results except in the case of group 10
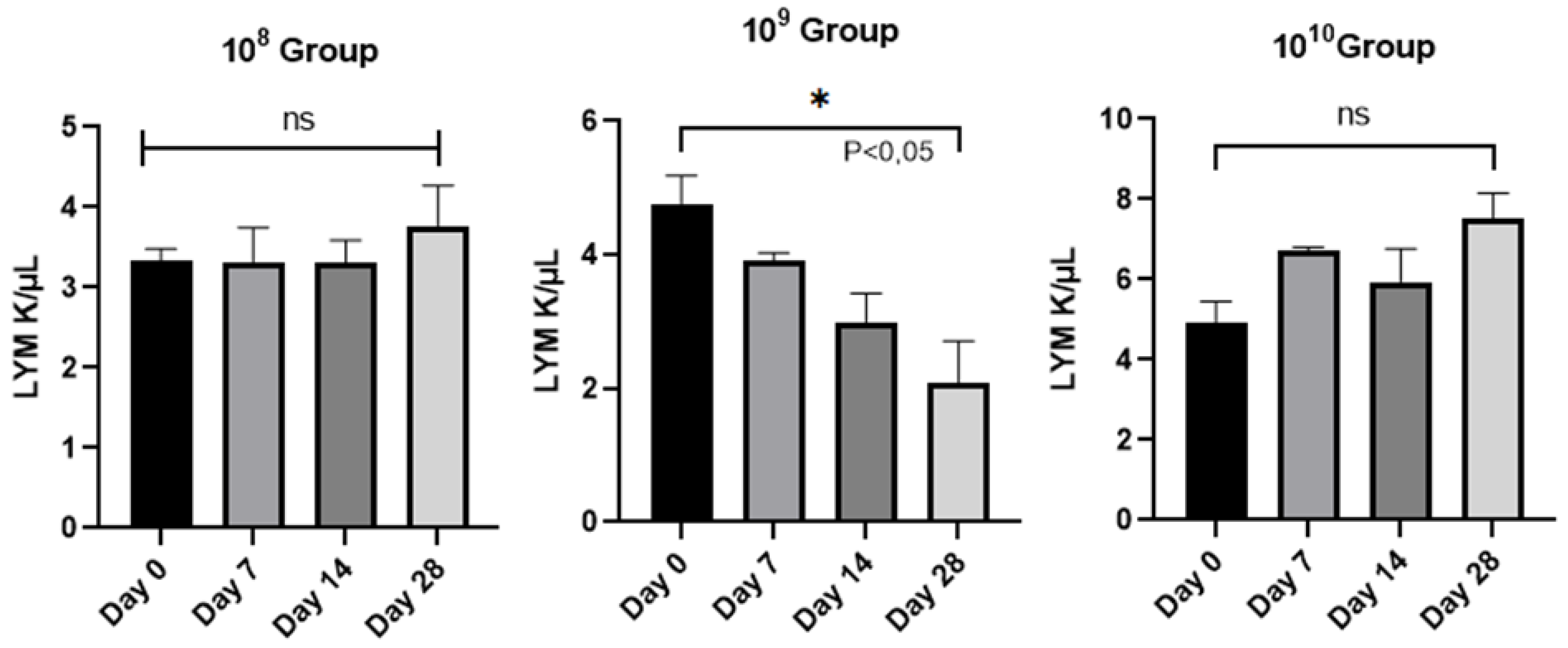
9 (
Figure 4), between day 0 and the final day of the study (P<0.05), a sign that in the case of this group, it is maintained in an active phase, an aspect strengthened by the activity of neutrophils.
Histological analysis - The samples represented by the maxillary incisors with the ligature together with the related gum were collected, fixed in 10% neutral buffered formalin, and demineralized in 5% nitric acid for 14 days. After decalcification, the specimens were dehydrated and embedded in paraffin. Four μm thick sections were obtained in the transverse plane. Sections were stained with hematoxylin and eosin (H.E) using standard protocols [
13]. The sections were evaluated by light microscopy (4X magnification) and parameters such as the influx of inflammatory cells, and the integrity of the alveolar bone and cement were monitored (
Figure 5 and
Figure 6).
The microbiological examination consisted of taking samples from the gum and even the ligatures, the samples were seeded in a liquid Schadler medium and incubated under anaerobic conditions, 24 hours, after each renewal of the ligatures. From the 24 h suspension, plates were inoculated with Shadler Agar but also Columbia 5% ram blood and Columbia 7% ram blood. A 24-hour reincubation of the plates followed, then smears were made from the grown colonies to identify the bacterial strains used. From the first week after contamination, in the case of all groups, Gram-specific A.a coccobacilli, F.n Gram-bacilli or S.o Gram+ chains were identified in the smears, together with the oral microflora of rats represented by Staphyococcus sciuri, Staphylococcus xylosus, Proteus mirabilis or Enterococcus faecium, the analysis of the smears being completed by the one performed at MaldiTof (Bruker MALDI Biotyper).
4. Discussion
The term periodontitis is used to express the presence and multiplication of microorganisms at the level of the oral cavity, more precisely, at the level of the gum, ligament, and alveolar bone [
14]. Incriminated in the development of the periodontal disease are Gram-anaerobes, and the most widespread in the subgingival space seem to be A.a, P.g,
Prevotella intermedia (P.i), and T.f. Through an immunopathogenic mechanism, they are involved in the development of the disease from the beginning by multiplying, resulting in the periodontal pocket. The body responds by forming the inflammatory infiltrate represented by macrophages and lymphocytes and they will produce cytokines and biological mediators [
15].
Dental plaque is a favorable environment for the multiplication of microorganisms and the formation of biofilms, so PD is associated with them and although it has a wide etiology, the most studied are microbial and immunological causes [
20,
21]. In the oral environment, bacteria grow in complex polymicrobial associations, with more than 700 bacterial species living in the oral cavity [
16]. Species of the genus Streptococcus are early colonizers of the mouth that actively recruit bacteria such as P.g through several genetic mechanisms [
17], contributing to the general functional heterogeneity of the biofilm. This heterogeneity gives the biofilm new characteristics such as easy adhesion to surfaces, metabolic cooperation, in which the waste product of one bacterial species serves as a food source for another [
18], increased antibiotic resistance or the ability of biofilms to evade the host's immune system. Recent research has shown that A.a is in association with
Streptocoocus spp. stimulates resistance to the host's innate immunity [
22] generating imbalances in the normal flora of the oral cavity. This phenomenon is translated by the term dysbiosis, and as periodontitis develops, the oral microbiota changes from one consisting mainly of Gram+ aerobes to a constant one mainly of Gram - anaerobes [
23] ultimately resulting in the clinical expression of the disease. Simultaneously, a succession of microbial complexes takes place, the first being the so-called "orange complex", which consists of anaerobic Gram - species, among which we find F.n [
24,
25]. F.n is a binder for other bacterial species responsible for PD development and here we are talking about strains belonging to the "red complex" which includes bacteria such as P.g., T.f, T.d, and more recently, A.a [
26]. In this sense, the objective of the study was to induce periodontitis through the oral contamination of rats with bacteria that are directly responsible for this condition in humans, precisely in order to better understand the pathogenesis and also to try therapeutic schemes afterward.
The analysis of periodontitis in the rat model has provided remarkable insights into the pathogenesis of the disease, recapitulating the clinical or histological characteristics [
27,
28]. The rat resembles humans when developing periodontitis, the composition of the dental plaque, and the appearance of histopathological lesions specific to this disease being similar to humans. In rats, PD appears within a few weeks when it is induced by ligatures and in an even shorter period of 7-15 days when the pathogenic bacterial flora intervenes [
29].
In most studies, placing a silk thread around the bundle of maxillary or mandibular premolars stimulates bacterial colonization and biofilm formation, resulting in apical epithelial migration and bone loss like those observed in a clinical setting [
30]. In our study, we placed these ligatures with gingival retraction wire around the maxillary incisors for the advantage of easy access, and the injury to the gum to apply the ligatures contributed to the rapid establishment of PD, an aspect also mentioned by other researchers who associate traumatic injuries with the pathogenesis of PD induced by ligation in rodents [
31]. Simple ligature, without bacterial involvement, does not cause significant bone loss in rats as shown by Bezerra et al [
33] in their study, unlike other researchers who conclude that the accumulation of bacteria around the ligature thread plays an important role in inducing and PD progression [
32]. Following these considerations for the periodontal disease induction, we chose the ligation model completed by oral contamination with 3 of the most representative bacteria for periodontitis. The clinical signs observed since the first week of contamination (bleeding when palpating the gums) suggested the onset of PD installation. A disadvantage of placing the ligature on the maxillary incisors was the loss of the ligatures within 4-5 days of application, even if we also tried the ligature in ”8” [
34], thus requiring their renewal every week.
Biological mediators involved in periodontitis provide valuable information about host-microbial interactions and inflammation [
35]. The diseased periodontal tissue constantly guides neutrophils and leukocytes [
36] to the junctional epithelium that borders the oral microflora causing the activation of immune cells such as lymphocytes. The latter trigger the release of prostaglandins, interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and IL-6, culminating in the triggering of osteoclastogenesis and bone destruction by direct stimulation of osteoclasts or by the release of enzymes tissue destruction by inflammatory cells [
37]. Using the ligature-induced periodontitis model periodically impregnated with A.a, F.n, and S.o, in rats, we addressed the host response at a clinical, biological, and histological level from the onset of the disease until the end of the 28-day experimental period, and our results provide evidence of the PD installation and the fact that the disease is in full evolutionary process in the group where the bacterial strains were tested at a concentration of 10
9.
From a histopathological point of view, the results indicate that the bacterially contaminated ligation pattern leads to progressive alveolar bone resorption suggesting two distinct phases: an acute phase (in group 10
9) and a chronic phase (in the case of group 10
10). Molon et al. divided the process of ligation-induced periodontitis in rats into two successive processes concluding that in the acute process of periodontitis, inflammatory cell infiltration was obvious, and alveolar bone resorption was rapid. While in the chronic phase, the number of infiltrating inflammatory cells decreased, and alveolar bone resorption slowed [
34]. In fact, in the case of all the tested groups, different stages of the periodontitis installation could be observed, but the need to differentiate the PD installation as a shoulder of the ligature placement versus the bacterial action is imperative. Since bone resorption in the ligature model is dependent on the presence of oral microorganisms [
31], in our study we could observe the presence of bacteria in the periodontal sac or attached to the ligature wire regardless of the concentration used. Clinical periodontitis is mainly an inflammatory disease caused by bacteria as the initiating factor. Through the formation of bacterial plaque, inflammatory cells infiltrate the local periodontal tissue, and the differentiation of osteoclasts occurs, resulting in alveolar bone resorption [
39]. Similar to the traditional rat model of periodontitis, this model induced local periodontitis by simulating bacterial aggregation in the periodontal tissue and causing alveolar bone resorption [
40]. Bone resorption occurred only in the case of concentrations 10
9 and 10
10, complemented by a pyogranulomatous reaction specific to bacterial aggressiveness on the periodontal tissues, as mentioned by Bascones-Martínez in his research [
15]. Comparing the histological effects produced by the 3 bacterial concentrations, we mention that the severity of the disease depends on the concentration in the sense of exacerbating its intensity, as suggested by Yuan et al. [
38]. So, for oral contamination over a period of 4 weeks, the bacterial action is necessary for a concentration of 10
9 or 10
10, through the PD model thus created, and an advanced bone loss can be expected in a relatively short time.
Figure 1.
A1-A3: inocula of S.o, A.a and F.n prepared for oral contamination. B1-B3: clinical appearance at the time of attaching the first ligatures (Day 0), C1-C3: clinical appearance on day 14, D1-D3: clinical appearance on day 28.
Figure 1.
A1-A3: inocula of S.o, A.a and F.n prepared for oral contamination. B1-B3: clinical appearance at the time of attaching the first ligatures (Day 0), C1-C3: clinical appearance on day 14, D1-D3: clinical appearance on day 28.
Figure 2.
Evolution of body weight during the study (regardless of the bacterial concentration used, no statistically significant changes were recorded).
Figure 2.
Evolution of body weight during the study (regardless of the bacterial concentration used, no statistically significant changes were recorded).
Figure 3.
Reduction of the number of neutrophils on the wall of the PD installation, depending on the bacterial concentration used (In group 108, neutrophils decreased starting with the 3rd week after contamination, P<0.05. The most relevant decrease of neutrophils was observed in group 109, where, starting with the second week, their number began to decrease, more dramatically than in the case of group 108, registering lower and lower values until the end of the study when P<0.0001. In group 1010, the number of neutrophils decreased constantly, during the study, the value P<0.05).
Figure 3.
Reduction of the number of neutrophils on the wall of the PD installation, depending on the bacterial concentration used (In group 108, neutrophils decreased starting with the 3rd week after contamination, P<0.05. The most relevant decrease of neutrophils was observed in group 109, where, starting with the second week, their number began to decrease, more dramatically than in the case of group 108, registering lower and lower values until the end of the study when P<0.0001. In group 1010, the number of neutrophils decreased constantly, during the study, the value P<0.05).
Figure 4.
Evolution of the number of lymphocytes/group analyzed from day 0 to day 28.
Figure 4.
Evolution of the number of lymphocytes/group analyzed from day 0 to day 28.
Figure 5.
A, B, C and D: images with cross-sections through the collected part, E: the part collected from a control rat, uncontaminated and without ligature showing intact alveolar bone and continuous cementum, F: the part from the rat that belongs to group 108, which presents hypertrophy, granulation tissue with nucleated cells that peel off, abundant neutrophils, coccobacilli, inflammation, hyperemia, hyperplasia of the gingival epithelium, periodontal lysis. ligature thread present, G: rat teeth from group 109 - Granuloma, alveolar bone lysis, pulp tissue with necrotic bone fragments, around which there is pyogranulomatous inflammation, neutrophilic infiltrate, abundant granulation tissue, bacteria (coccobacilli), fodder in the space periodontally invaded with bacteria. H: group 1010 rat teeth with hyperplasia, intact alveolodental ligament, abundant granulation tissue, fewer inflammatory cells, bacteria in the alveolar sac, and anucleated desquamation cells on the alveolodental ligament area. Sections were stained with H&E. Original magnification 4X. Scale bars = 200 μm.
Figure 5.
A, B, C and D: images with cross-sections through the collected part, E: the part collected from a control rat, uncontaminated and without ligature showing intact alveolar bone and continuous cementum, F: the part from the rat that belongs to group 108, which presents hypertrophy, granulation tissue with nucleated cells that peel off, abundant neutrophils, coccobacilli, inflammation, hyperemia, hyperplasia of the gingival epithelium, periodontal lysis. ligature thread present, G: rat teeth from group 109 - Granuloma, alveolar bone lysis, pulp tissue with necrotic bone fragments, around which there is pyogranulomatous inflammation, neutrophilic infiltrate, abundant granulation tissue, bacteria (coccobacilli), fodder in the space periodontally invaded with bacteria. H: group 1010 rat teeth with hyperplasia, intact alveolodental ligament, abundant granulation tissue, fewer inflammatory cells, bacteria in the alveolar sac, and anucleated desquamation cells on the alveolodental ligament area. Sections were stained with H&E. Original magnification 4X. Scale bars = 200 μm.
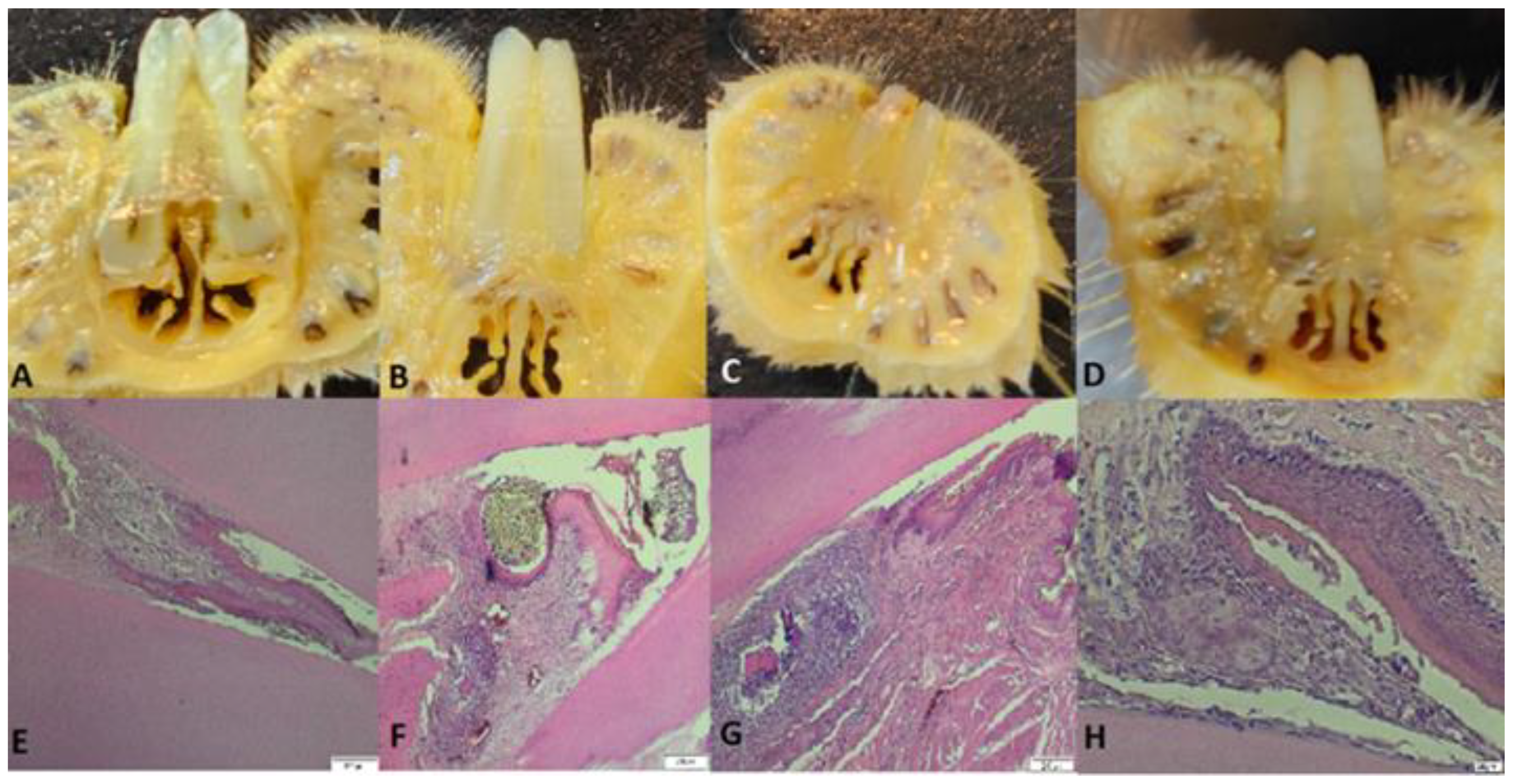
Figure 6.
A: Detail of a rat tooth from group 108, partially desquamated alveolodental ligament, B: strong reaction of hyperemia and neutrophilic infiltrates in a rat from group 109, C: overview with the highlight of the periodontal sac in a rat from group 1010.
Figure 6.
A: Detail of a rat tooth from group 108, partially desquamated alveolodental ligament, B: strong reaction of hyperemia and neutrophilic infiltrates in a rat from group 109, C: overview with the highlight of the periodontal sac in a rat from group 1010.