1. Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS), and its pathological targets are thought to be myelin proteins or oligodendrocytes. More than 80% of MS patients demonstrate a relapsing remitting clinical course, but some proportion of patients undergo a secondary progressive phase, and worsening of neurologic function is observed independent from relapses [
1,
2]. Connexin (Cx) is a component of gap junctions, which connect the cytoplasm between cells. In the CNS, oligodendroglia express Cx29, Cx32, and Cx47 [
3,
4,
5,
6,
7], while astroglia express Cx26, Cx30, and Cx43 [
8,
9,
10,
11,
12]. We previously reported loss of oligodendroglial Cx32 and Cx47 expression in most active and chronic lesions from all analyzed cases of MS and neuromyelitis optica spectrum disorder [
13]. Recently, we reported a novel model of secondary progressive multiple sclerosis (SPMS) induced by immunization with myelin oligodendrocyte glycoprotein peptide 35-55 (MOG
35-55) in oligodendroglia-specific
Cx47-inducible conditional knockout (
Cx47 icKO) mice [
14]. This new progressive experimental autoimmune encephalomyelitis (pEAE) model demonstrated clinical exacerbation during the acute phase followed by a progressive course with more pronounced demyelination than that in
Cx47 floxed/floxed (
fl/fl) mice and is considered to be a suitable model for SPMS. In pEAE mice, infiltration of T helper 17 (Th17) cells into lumbar lesions was enhanced, and proinflammatory “A1” astroglia and injury-related microglia were increased in the spinal cord during the chronic phase [
14,
15].
Iguratimod (IGU), a selective cyclooxygenase-2 (COX-2) inhibitor [
16], is in clinical use for treatment of rheumatoid arthritis (RA) with low risk of progressive multifocal leukoencephalopathy and other adverse effects. Recently, several studies have shown that IGU exerts various effects on the immune system. For instance, IGU can inhibit interleukin (IL)-6 or IL-8 in RA synovial fibroblast-like cells via interference with nuclear factor (NF)-κB [
17,
18], IL-17-mediated signaling by disrupting Act1 or migration inhibitory factor (MIF) [
19], and regulation of B cell differentiation by disrupting the protein kinase C (PKC) pathway [
20]. We previously reported that IGU ameliorates acute and chronic EAE by suppressing microglia and macrophages through inhibition of the NF-κB pathway [
21]. Astroglia also activate microglia [
15], but the direct effect of IGU on astroglia is unclear. Our aim is to clarify whether IGU ameliorates the clinical course of pEAE and elucidate the biological mechanisms of IGU.
2. Materials and Methods
2.1. Ethics statement
All efforts were made to minimize the number and suffering of mice based on the guidelines for the proper conduct of animal experiments published by the Science Council of Japan and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines 2.0 for animal research [
42,
43]. The Animal Care and Use Committee of Kyushu University granted ethical approval for the study on January, 28 2021 (#A21–183) and permitted an extension of the experimental period until March, 31 2023 (#A23–059).
2.2. Generation of PLP/CreERT;Cx47fl/fl mice
To generate
Cx47 conditional floxed mice (
Cx47fl/fl mice), targeting vectors for the “knockout-first allele” [
44] were obtained from the European Conditional Mouse Mutagenesis Program (ID: 45393). The targeting vector consisted of an
En2SA-IRES-LacZ reporter cassette, a neomycin resistance cassette, and
loxP sites flanking an exon of the
Cx47 gene (NCBI Gene ID: 118454). The targeting vector was linearized and introduced into RENKA embryonic stem (ES) cells (C57BL/6) by electroporation. After selection with geneticin (G418 sulfate), the resistant clones were isolated, and their deoxyribonucleic acid (DNA) was screened for homologous recombination by polymerase chain reaction (PCR) assays using the following primer set:
sc_3GR3n, 5′-CTT ATA GGC TGG GAC TTG TGG ATG GC-3′ and
neo_G02, 5′-ATC AGG ACA TAG CGT TGG CTA C-3′. The PCR-positive ES clones were expanded, and their isolated DNA was further analyzed by PCR amplification using the following primer sets:
sc_5GF1, 5′-GCT TCA TGG ATC AGG GTA ATT CCA G-3′ and
LAR3, 5′-CAC AAC GGG TTC TGT TAG TCC-3′ for 5′ amplification;
sc_3GR4n, 5′-TCC TGA GGT AAC CCT AAC AAA CAC G-3′ and
neo_MS02, 5′-TTC GCA GCG CAT CGC CTT CTA TCG-3′ for 3′ amplification; and
loxF3, 5′-GAG GCT GAG ATG GCG CAA CG-3′ and
loxR2, 5′-TTC AGT CAT CCG CGC TAC ACA CC-3′ for amplification of the 3′
loxP region. Homologous recombination of the clones was confirmed by genomic Southern hybridization with probing for the neomycin resistance gene. Homologous recombinant ES cell clones were aggregated with eight-cell ICR mouse embryos to generate chimeric mice. To obtain the floxed allele by removing the reporter gene and neomycin resistance cassette, the chimeric mice were crossed with
CAG-Flp transgenic mice (CARD, Kumamoto, Japan) that expressed Flp in their germ cells [
45]. The floxed allele was identified by PCR using the following primer set:
F14989, 5′-TGG TGC TGG AAT TGG AAG C-3′ and
R15381, 5′-TCT GCA TTC ACA TCC TAT GTG C-3′.
Cx47fl/fl mice and
PLP/CreERT mice (Jackson Laboratory, Bar Harbor, ME), which is a useful mouse line for inducible ablation of target genes in mature oligodendroglia [
46], were bred to generate
PLP/CreERT;Cx47fl/fl mice. The following primer sets were used for mouse genotyping:
mGjc2-F3, 5′-TGG GCT CAA TGC AAC CTC TC-3′ and
mGjc2- R1, 5′-CAG GGT TTG GTC TCC AGC TT-3′ were used for WT mice and yielded a 1172-bp band;
loxF3, 5′-GAG GCT GAG ATG GCG CAA CG-3′ and
loxR2, 5′-TTC AGT CAT CCG CGC TAC ACA CC-3′ were used for
Cx47fl/fl mice and yielded a 202-bp band;
olMR1084, 5′-GCG GTC TGG CAG TAA AAA CTA TC-3′ and
olMR1085, 5′-GTG AAA CAG CAT TGC TGT CAC TT-3′ were used for
PLP/CreERT mice and yielded a 100-bp band.
2.3. Tamoxifen injection
At 8 –10 weeks of age, PLP/CreERT;Cx47fl/fl mice were intraperitoneally injected with 1 mg of tamoxifen (Sigma-Aldrich, Steinheim, Germany) in 100 µl of corn oil twice per day for 5 days.
2.4. Induction and clinical evaluation of EAE
EAE was induced by subcutaneous injection of MOG35-55 peptide (4 mg/ml, S-PEP; Scrum, Tokyo, Japan) emulsified in complete Freund’s adjuvant containing 10 mg/ml Mycobacterium tuberculosis H37RA (#7027; Chondrex Inc., Woodinville, WA, USA) at a dose of 200 µg (100 µl) per mouse, followed by intraperitoneal injection with pertussis toxin (300 ng per mouse, # 168-22471; Wako, Osaka, Japan) on days 0 and 2. Mice were examined daily for signs of EAE and scored as follows: 0, no disease; 1, limp tail; 2, abnormal gait and hind limb weakness (shaking); 3, paralysis of the two hind limbs; 4, tetraplegia; 5, moribund (death).
2.5. IGU treatment in vivo
IGU (Toyama Chemical Co. Ltd., Tokyo, Japan) was suspended in 100 µl of 0.5% (w/v) methylcellulose solution and orally administered twice daily (50 mg/kg/day) from 17 to 50 dpi for therapeutic treatment experiments. The same amount of methylcellulose solution was administered to vehicle-treated mice.
2.6. Tissue preparations
Mice were deeply anesthetized with isoflurane (Pfizer Japan Inc., Tokyo, Japan) and transcardially perfused with phosphate-buffered saline (PBS) and then with 4% paraformaldehyde (PFA) in 0.1M PBS. The spinal cords were then carefully dissected. The tissues were fixed overnight in 4% PFA at 4°C and then processed into paraffin sections (5 µm). To prepare frozen sections, spinal cords were harvested and fixed overnight in 4% PFA using the same protocol and sequentially incubated with 20% and 30% sucrose in PBS solution for 24 h each at 4°C. The resulting tissues were embedded in Tissue-Tek optimal cutting temperature compound (4583, SAKURA, USA) and stored at −80°C.
2.7. Histopathological and immunohistochemical analyses
A portion of the paraffin-embedded axial L4–5 spinal cord sections were examined by immunostaining using an indirect immunoperoxidase method. After deparaffinization, endogenous peroxidase was quenched with 0.3% hydrogen peroxide in absolute methanol for 30 min. The sections were washed using Tris-HCl for 5 min, dipped in 10 mM citrate buffer, and then autoclaved (120°C, 10 min). All sections were cooled to room temperature (RT) and incubated overnight at 4°C with primary antibodies. The dilution solution contained 5% normal goat serum and 1% bovine serum albumin in 50 mM Tris-HCl (pH 7.6). After rinsing the slides on the next day, the sections were labeled with a streptavidin-biotin complex or an enhanced indirect immunoperoxidase method using Envision (K4003, Dako, Glostrup, Denmark). 3,3-Diaminobenzidine tetrahydrochloride (D5637, Sigma- Aldrich, Tokyo, Japan) was used for color reactions. Finally, the sections were counterstained with hematoxylin. Other paraffin sections were used for immunofluorescence analysis. Lumbar spinal cord sections were deparaffinized. After washing and autoclaving, the sections were incubated with primary antibodies overnight at 4°C. The sections were then washed, incubated with secondary antibodies conjugated with Alexa Fluor 488 or 594 (1:1000; Thermo Fisher, Rockford, IL, USA) and 4,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, Tokyo, Japan), and mounted with PermaFluor (#TA-030-FM; Thermo Scientific, Fremont, CA). Frozen spinal cord sections were cut on a Leica CM 1850 cryostat (Leica Microsystems GmbH, Wetzlar, Germany), incubated for 2 h at RT in blocking solution (PBS-T with 10% normal goat serum), and incubated at 4°C with primary antibodies overnight. Next, the sections were incubated with secondary antibodies conjugated with Alexa Fluor 488 or 594 (1:1000; Thermo Fisher, Rockford, IL, USA) and DAPI (Sigma-Aldrich, Tokyo, Japan) and mounted with PermaFluor (#TA-030-FM; Thermo Scientific). Images were captured using a confocal laser microscope system (Nikon A1; Nikon, Tokyo, Japan). Detailed information regarding the primary antibodies is provided in
Supplementary Table S1.
2.8. Quantification of immunohistochemical images
Immunohistochemically stained sections were automatically scanned and quantified using ImageJ Analysis 1.51h software (
https://imagej.nih.gov/ij/index.html). To quantify immunostaining, except for Iba-1 staining, transverse spinal cord sections were divided by horizontal and vertical lines passing through the central canal (solid lines in
Figure S5a), and the central gray matter area was manually excluded to define the white matter region of interest (the area encircled by a dashed line in
Figure S5a). The positive-stained areas in this region were automatically measured and expressed as a percentage of the white matter area of interest. To quantify Iba-1 staining, transverse spinal cord sections were divided by horizontal and vertical lines passing through the central canal (solid lines in
Figure S5b), and the region of interest consisting of the central gray matter and white matter (the area encircled by a dashed line in
Figure S5b) was defined. The positive-stained areas in this region were measured. Quantification was also performed on L4–5 spinal cord sections immunostained with each antibody. These stained areas were expressed as a percentage of the total region of interest.
2.9. Microglial circularity analysis
The circularity of microglial cells (circularity = 4πS/L2) was calculated automatically using ImageJ software. Cells with circularity close to 1 were considered to have round morphology, which indicated an activated state [
47].
2.10. Flow cytometry
Spinal cord and brain cells were isolated using a density gradient technique, as previously described [
48]. Briefly, spinal cord and brain tissues were minced with a tissue homogenizer to obtain a single-cell suspension. Stock isotonic Percoll (10-fold dilution in 10× Hanks’ balanced salt solution (HBSS) without Ca
2+ and Mg
2+) was added to the cell suspension to produce a 30% Percoll cell solution. A 70% Percoll gradient was pipetted underneath the 30% Percoll cell solution, and the mixture was centrifuged at 800 × g for 40 min. Thereafter, the myelin layer was discarded, and the mononuclear cell interphase was isolated and resuspended in FACS buffer. Three principal CD4
+ T cell subsets (Th1, Th17, and Th1/17) were measured using monoclonal antibodies against surface chemokine receptors, as previously described [
49]. For surface marker staining, cells were incubated with fluorochrome-conjugated antibodies against CD4, CXCR3, and CCR6 for 30 min at 4°C and analyzed using a FACS Verse™ Flow Cytometer (Becton Dickinson, Tokyo, Japan). The percentages of CXCR3
+ CCR6
− CD4
+ T cells corresponding to Th1 cells, CXCR3
− CCR6
+ CD4
+ T cells corresponding to Th17 cells, and CXCR3
+ CCR6
+ CD4
+ T cells corresponding to Th1/Th17 cells in CD4
+ T cells were measured.
2.11. Isolation of splenocytes
Spleens were aseptically removed from acute-phase EAE mice at 17 dpi and dissociated into single cells as described previously [
14]. Briefly, splenocytes were removed from EAE mice and filtered through a 100-µm cell strainer. The cells were then centrifuged at 300 × g for 5 min. After removal of the supernatant, PBS was added, and the cells were centrifuged as described above. Next, the cells were resuspended in red blood cell lysis buffer, incubated for 10 min, and centrifuged for 5 min at 300 × g. Viable cells were counted using a hemocytometer and 0.4% trypan blue staining.
2.12. T cell proliferation assay
Mouse spleens were collected near the peak of acute EAE. Splenocyte suspensions (7.5 × 105 cells/100 µl/well) were placed in a 96-well plate, and 100 µl of Roswell Park Memorial Institute 1640 medium containing 0 (negative control), 2.5, 12.5, or 25 µg/ml MOG35–55 was added. The plates were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 72 h. T cell proliferation was assessed using a bromodeoxyuridine (BrdU) kit (ab126556; BrdU cell proliferation enzyme-linked immunosorbent assay (ELISA) kit, Abcam) according to the manufacturer’s instructions. Briefly, during the last 24 h of incubation, 20 µl of 1× BrdU solution was added to each well. A background control without BrdU was included in the plate. After 72 h of incubation, the cells were fixed with 200 µl of fixing solution for 30 min and incubated with 100 µl of anti-BrdU monoclonal detector antibody for 1 h at RT. The cells were washed and incubated with 100 µl of 1× peroxidase-conjugated goat anti-mouse IgG filtered through a 0.22-µM syringe filter for 30 min at RT. The cells were then incubated with 100 µl of tetramethylbenzidine peroxidase in the dark for 30 min. After addition of 100 µl of the stop reaction solution, the absorbances of the solutions in the wells were measured using a microplate reader (MTP-800AFC; Corona Electric, Hitachinaka, Japan).
2.13. Glial cell cultures
Primary mixed glial cell cultures were prepared from the brains of newborn C57BL/6 J mice according to a previously described method [
50,
51]. Briefly, brains were removed under sterile conditions, and the meninges were carefully removed using tweezers. The tissue was dissociated by passing it through a nylon mesh in HBSS (Sigma-Aldrich, Saint Louis, MO, USA) containing 50 U/ml penicillin and 50 µg/ml streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) to prevent contamination. After washing with HBSS, the cell suspension was plated in 75-cm
2 culture flasks at a density of one to two brains per flask in 10 ml of culture medium, consisting of Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich, Tokyo, Japan) supplemented with 10% fetal bovine serum (Equitech-Bio, Kerrville, TX, USA), 5 µg/ml bovine insulin (Sigma-Aldrich, Tokyo, Japan), and 0.2% glucose. The cells were maintained at 37°C in a humidified atmosphere containing 5% CO
2 with three medium changes during the first week and no medium change during the second week to induce proliferation of microglia. At confluency (12–15 days), mixed glial cells were detached by Accutase (Innovative Cell Technologies, San Diego, CA, USA) treatment and replated. After 5–10 days in culture, mixed glial cell cultures that had reached 100% confluence were used for experiments.
2.14. Harvesting of glial cell culture supernatant
To measure cytokine or chemokine production, glial cells were incubated with stimulants including 1 µg/ml LPS, 3 ng/ml IL-1α, 30 ng/ml TNF-α, and 400 ng/ml C1q at 37°C in a humidified atmosphere containing 5% CO2 for 24 h. Glial cells were simultaneously treated with or without IGU. During the incubation, 50 µl of each supernatant was harvested at 2, 4, 6, 8, and 24 h, centrifuged, and stored at −80°C.
2.15. Multiplexed fluorescence immunoassay for cytokines
Cytokine levels in the supernatant were measured using a Bio-plex Pro™ Assay (M60- 009RDPD; Bio-Rad, Tokyo, Japan) according to the manufacturer’s instructions. The concentrations of cytokines/chemokines {IL-1α, IL-1β, IL-4, IL-6, IL-10, IL-17, IFN-γ, CCL2/monocyte chemotactic protein (MCP)-1, CCL3/MIP-1α, CCL5/regulated on activation normal T cell expressed and secreted (RANTES)} in the supernatants were measured using a multiplexed fluorescence bead-based immunoassay as described previously [
51]. The cytokine/chemokine levels were calculated by reference to a standard curve generated using standards assayed by the same procedure used for supernatant samples. The limit of detection for each molecule was determined by the recovery of the corresponding standard, and the lowest value with > 70% recovery was set as the lower limit of detection. Some samples were beyond the upper limit of detection, and other samples were below the lower limit of detection.
2.16. Chemokine analysis by ELISA
Chemokine (CCL-20/MIP-3α) levels in mixed glial cell culture supernatants were measured using a Mouse CCL-20/MIP-3 alpha Quantikine ELISA Kit (MCC200, R&D Systems, Minneapolis, USA) according to the manufacturer’s instructions. Briefly, 100 µl of the samples were added to each well and incubated for 2 h. After washing, 200 µl of human MIP-3α conjugate was added to each well and incubated for 2 h. The wells were washed, and 200 µl of substrate solution was added to each well and incubated. After addition of 100 µl of the stop reaction solution, the absorbances of the solutions in the wells were measured using a microplate reader.
2.17. Migration assay
Transwell chambers (Corning, Corning, USA) consisted of an upper chamber and a lower chamber with or without mixed glial cells (1.0 × 10
6 cells/well), stimulants {IL-1α (3 ng/ml), C1q (400 ng/ml), TNF-α (30 ng/ml), LPS (1 µg/ml)}, and IGU (10 µg/ml). CD4
+ T cells (5.0×10
5 cells/well) from acute EAE mice were loaded in the upper chamber. After 24 h of incubation, cells that had migrated were counted using flow cytometry as described above. Three principal CD4
+ T cell subsets (Th1, Th17, and Th1/17) were measured using monoclonal antibodies against surface chemokine receptors, as previously described [
49]. For surface marker staining, cells were incubated with fluorochrome-conjugated antibodies against CD4, CXCR3, and CCR6 for 30 min at 4°C and analyzed using a FACS Verse™ Flow Cytometer (Becton Dickinson). As described above, the percentages of CXCR3
+ CCR6
− CD4
+ T cells corresponding to Th1 cells, CXCR3
+ CCR6
+ CD4
+ T cells corresponding to Th1/Th17 cells, and CXCR3
− CCR6
+ CD4
+ T cells corresponding to Th17 cells among CD4
+ T cells were measured.
2.18. Incubation of CD4+ T cells with IGU
CD4+ T cells from acute EAE mice were incubated with or without IGU for 24 h. CD4+ T cells were counted by flow cytometry using the same procedure as described above.
2.19. Statistical analysis
All data are presented as the mean ± standard error of the mean. The statistical significance of differences between values was determined using an unpaired t-test or one-way analysis of variance. Values of p < 0.05 were considered statistically significant.
4. Discussion
We used IGU to treat pEAE mice, which is a novel mouse model of SPMS that presents an aggravated disease course with pronounced glial inflammation, and examined its effects and mechanisms in vivo and in vitro. The main findings in these experiments were as follows. First, IGU was sufficiently effective in mitigating the clinical signs of pEAE and producing a diminished inflammatory astroglial and microglial phenotype of lesions during the chronic phase of pEAE. Second, IGU suppressed Th17 infiltration in the pEAE mouse spinal cord, and we showed that IGU inhibited Th17 migration in vitro by performing a migration assay using CD4+ T cells and pro-inflammatory mixed glial cells under conditions similar to those of glial cells in pEAE mice. Third, IGU reduced CSF IL-6 levels in pEAE mice. Moreover, we revealed a reduction of the IL-6 level in the mixed glial cell culture media after IGU treatment.
The main mechanism of IGU was initially reported to be COX-2 inhibition [
16]. However, recent studies have shown that IGU modifies the immune system via several mechanisms. For instance, IGU can inhibit IL-6 and IL-8 in RA synovial fibroblast-like cells by interfering with NF-κB [
17,
18], IL-17-mediated signaling by disrupting Act1 or MIF [
19], and B cell differentiation by disrupting the PKC pathway [
20]. Several studies have examined IGU application to EAE models. IGU treatment ameliorated clinical signs of EAE, and in
in vitro studies using myelin basic protein-specific T cells, IGU inhibited production of pro-inflammatory cytokines such as IFN-γ, IL-6, and TNF in T cells [
23]. IGU ameliorated acute and chronic EAE by suppressing infiltration and activation of immune cells, especially microglia and macrophages, through inhibition of the NF-κB pathway [
21].
We previously reported that oligodendroglial
Cx47 icKO mice demonstrated exacerbation of acute and chronic relapsing EAE. In the CNS tissue of
Cx47 icKO EAE mice (pEAE mice in this manuscript), A1 astroglia and M1-like microglia were pathologically increased in both the acute and chronic phases. Moreover, Th17 cells isolated from the CNS tissue were increased in
Cx47 icKO EAE mice [
14]. Therefore, we considered that IGU was the most suitable candidate for the treatment of
Cx47 icKO pEAE mice, which demonstrated enhanced infiltration of Th17 cells and activated glial inflammation in lesions. Therapeutic administration of IGU significantly ameliorated the clinical signs of pEAE mice. Immunohistochemical examinations revealed that IGU inhibited A1 astoroglial and M1-like microglial activation in pEAE mice during the chronic phase. Immunostaining and flow cytometry also revealed that IGU decreased the infiltration of pathogenic Th17 cells in pEAE mice during the chronic phase. An
in vivo mouse CSF cytokine assay revealed that IGU decreased IL-6 levels during the chronic phase of pEAE. IL-6, together with transforming growth factor-β, induces differentiation of Th17 cells from naïve T cells [
24,
25]. Thus, IL-6 blockade by IGU treatment might impede Th17 differentiation and accumulation in the spinal cord.
We performed an
in vitro primary mixed glial cell culture experiment to determine the direct effects of IGU on glial cells. However, glial cells need to be harvested from newborn mice, and it is difficult to use
Cx47 icKO mice, which require tamoxifen treatment. Therefore, we prepared a mixed glial cell culture containing astroglia and microglia from newborn WT mice. To obtain mixed glial cell culture containing pro-inflammatory A1 astroglia and M1-like microglia that mimic the conditions in
Cx47 icKO EAE (pEAE) mice, we added stimulating cytokines (IL-1α, C1q, TNF-α, and LPS) to the culture media [
15]. Moreover, we added IGU directly to the pro-inflammatory mixed glial cell culture, which is appropriate because a previous report showed that orally administered IGU levels in the CNS reach approximately 10% of the peripheral blood levels [
26]. We found that IGU could reduce Th17 cell migration toward the mixed glial cell culture in the
in vitro migration assay. In the natural course of MOG
35-55-induced conventional EAE, the number of Th17 cells in the CNS peaks earlier than that of Th1 cells [
27]. Thus, Th17 cells may play an essential role in autoimmune CNS inflammation, perhaps mainly during the initial phase of the disease [
28]. Because in this study IGU was administered immediately after the peak of clinical sign (17 dpi), it is unknown whether IGU might affect glial cells in the earlier chronic phase. In fact, the clinical scores of the IGU-treated group improved immediately after starting IGU treatment. Furthermore, in MS patients, Th17 cells increased in the CSF during clinical relapse [
29]. Moreover, in peripheral blood from SPMS patients, IL-17-producing peripheral blood mononuclear cells were increased compared with those in healthy controls, and Th17 cells are considered candidate therapeutic targets in SPMS [
30]. CCL20 was reported to strongly induce Th17 migration [
31]. IGU did not affect the CCL20 level in our mixed glial culture supernatant. However, IGU treatment reduced CCL2 production in mixed glial cells. Although CCL2 is not a Th17 cell-specific chemokine, CCR2, the CCL2 receptor, is expressed in Th17 cells [
32]. Therefore, IGU might secondarily suppress Th17 cell migration.
We also found that IL-6 levels in mixed glial cell culture were significantly decreased by IGU treatment. This result was comparable to the decrease in CSF IL-6 levels produced by IGU treatment. Astroglia are a primary source of IL-6 in the CNS [
33]. Meanwhile, previous reports have shown that neurons can produce IL-6 [
33], and IGU can reduce IL-6 expression in T cells [
23]. Therefore, factors that influence CSF IL-6 levels other than glial cells should be considered. However,
in vitro experiments revealed that IGU directly affected glial cells by inhibiting glial cell-induced inflammation and IL-6 release. Although IL-6 could have been produced by both astroglia and microglia in this study, we assumed that IGU treatment affected IL-6 production in astroglia more than that in microglia. This assumption is not only based on a previous study reporting astroglia as a major source of IL-6, but also from our finding that CCL3, which was mainly produced by microglia in the supernatant of mixed glial cell culture, was not affected by IGU treatment [
35]. This result was compatible with the decrements of IL-1β and CCL2, both of which were produced by cultured astroglia stimulated by LPS [
36,
37], in the mixed glial cell culture supernatant after IGU treatment. In this pEAE model, astroglia might have a strong effect on inflammation because Cx47 on oligodendroglia forms oligodendroglia-astroglia gap junction channels with Cx43 on astroglia, and loss of Cx47 increases astroglial Cx43 hemichannels that secrete various bioactive molecules, including proinflammatory cytokines and chemokines [
38,
39]. Therefore, IGU dramatically ameliorated the severity of EAE by affecting astroglia rather than microglia.
A possible mechanism of action of IGU on pEAE based on the results obtained in this study is summarized in
Figure S4. In the acute phase of EAE, pathogenic T cells, including Th17 cells, infiltrate the blood–brain barrier {1}. Then, activated T cells produce chemoattractants and induce a pro-inflammatory M1-like phenotype in microglia [
40,
41] {2}. These M1-like microglia induce an A1 phenotype in astroglia [
14,
15] {3}. In pEAE mice, astroglia and microglia kept activated as A1 and M1-like phenotypes, even during the chronic phase [
14] {4}, and IGU can suppress microglial and macrophage activation through the NF-κB pathway [
21]. Additionally, we found that IGU can suppress not only pro-inflammatory M1-like microglia but also A1 astroglia. Moreover, IGU can suppress the production of chemoattractants, especially IL-6 and CCL2, in activated glial cells {5}. Therefore, IGU can decrease the migration of pathogenic T cells, Th17 cells, into the CNS {6}, which ameliorates the clinical signs of pEAE.
This study has several limitations. First, vascular endothelial cells were not used in the migration assay. Generally, vascular endothelial cells are needed to form typical blood–brain barrier models in migration assays. However, we decided to omit these cells to simplify assays and focus on the direct effects of IGU on glial cells. Moreover, we did not examine the effect of IGU on sheer stress; we used a static model, instead of a flow model, in the migration assay. Additionally, we used a mixed glial cell culture containing astroglia and microglia in this study, but we did not examine each type of glial cell culture. We considered it sufficient to use only mixed glial cell culture to examine the direct effect of IGU on glial cell culture and CD4+ T cell migration. However, further in vitro functional assays of single glial cells will complement the present findings.
Figure 1.
Iguratimod (IGU) ameliorated the severity of progressive experimental autoimmune encephalomyelitis (pEAE) in mice and suppressed demyelination during the chronic phase. (a) EAE clinical scores (mean ± standard error of the mean, SEM) of IGU-treated (filled squares, n = 10) or vehicle-treated mice (filled circles, n = 6). Horizontal bars in the graph indicate the periods with significant differences between the groups. **26–29 days postimmunization (dpi), 31 dpi; *34–50 dpi. (b) Comparison of the area under the curve between IGU-treated and vehicle-treated mice. (c) Myelin basic protein (MBP) immunostaining in paraffin sections of the lumbar spinal cord of IGU-treated and vehicle-treated mice at 50 dpi. Scale bar: 100 µm. (d) The graph shows the percentages of the MBP-positive areas in the spinal cord of IGU-treated and vehicle-treated mice (n = 5 per group). Scale bars: 100 µm. Significant differences were determined using an unpaired t-test. * p < 0.05; ** p < 0.01; **** p < 0.0001.
Figure 1.
Iguratimod (IGU) ameliorated the severity of progressive experimental autoimmune encephalomyelitis (pEAE) in mice and suppressed demyelination during the chronic phase. (a) EAE clinical scores (mean ± standard error of the mean, SEM) of IGU-treated (filled squares, n = 10) or vehicle-treated mice (filled circles, n = 6). Horizontal bars in the graph indicate the periods with significant differences between the groups. **26–29 days postimmunization (dpi), 31 dpi; *34–50 dpi. (b) Comparison of the area under the curve between IGU-treated and vehicle-treated mice. (c) Myelin basic protein (MBP) immunostaining in paraffin sections of the lumbar spinal cord of IGU-treated and vehicle-treated mice at 50 dpi. Scale bar: 100 µm. (d) The graph shows the percentages of the MBP-positive areas in the spinal cord of IGU-treated and vehicle-treated mice (n = 5 per group). Scale bars: 100 µm. Significant differences were determined using an unpaired t-test. * p < 0.05; ** p < 0.01; **** p < 0.0001.
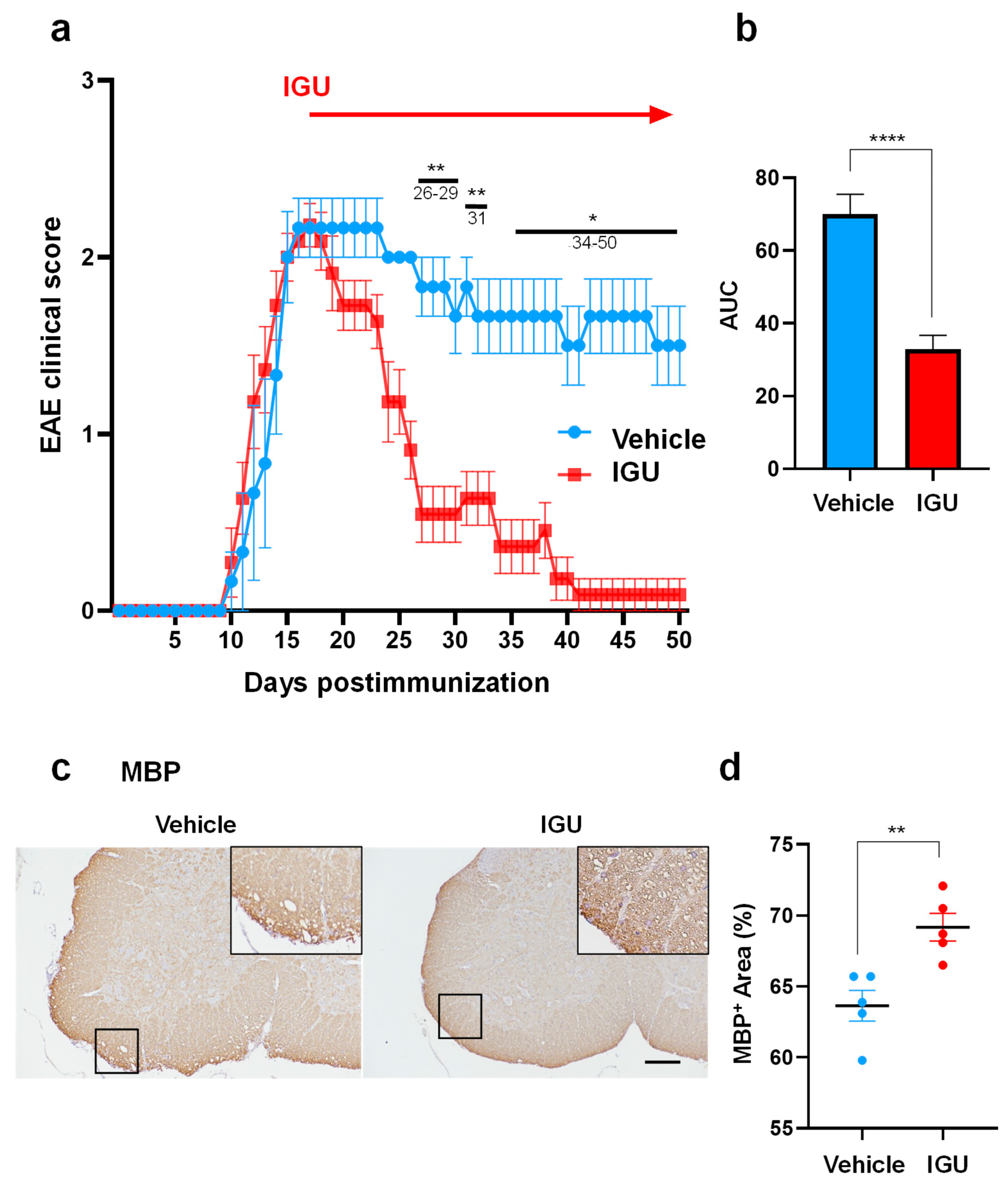
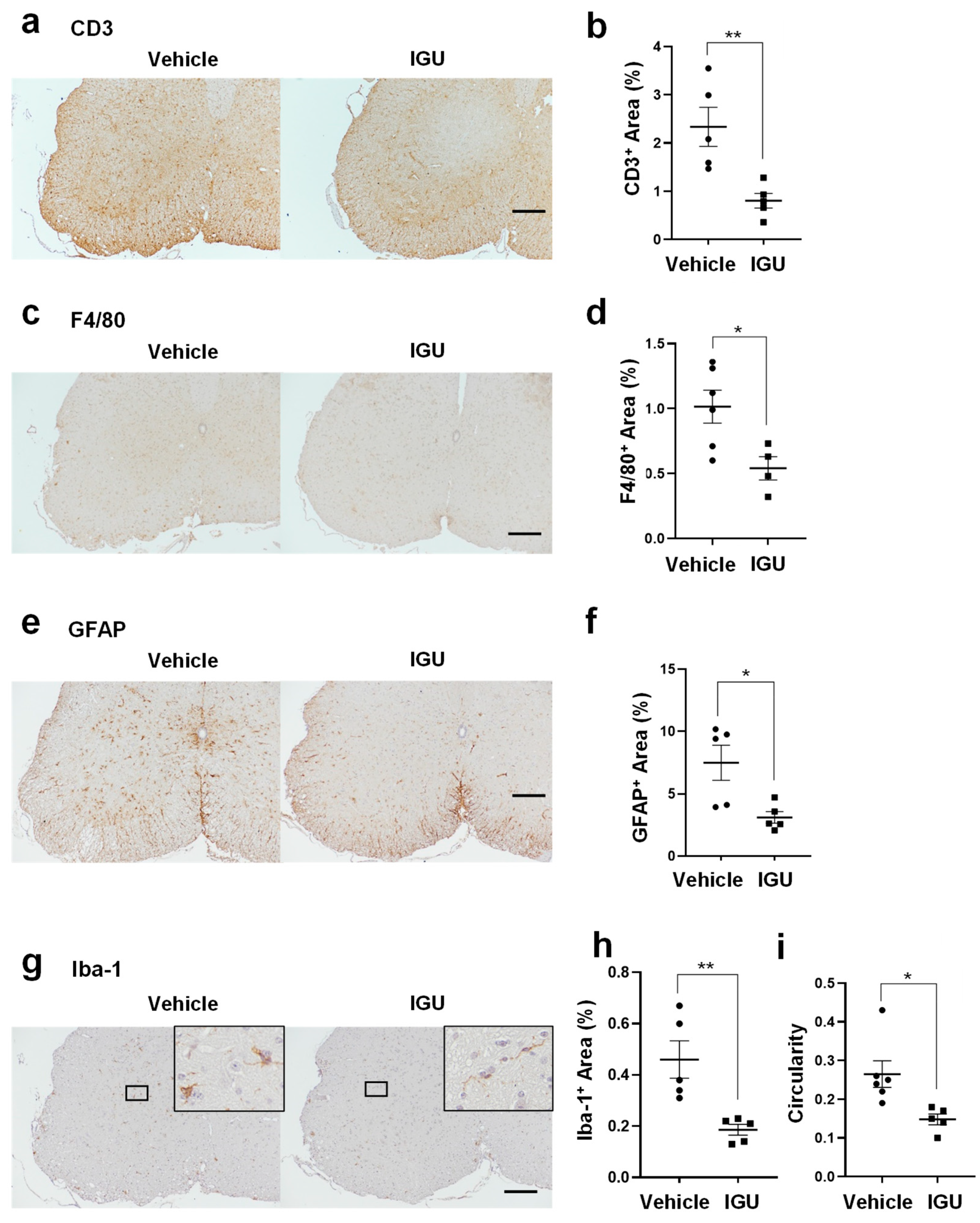
Figure 2.
IGU inhibited inflammatory cell infiltration of the lumbar spinal cord during the chronic phase. (a, c, e, g) CD3 (a), F4/80 (c), glial fibrillary acidic protein (GFAP) (e), and Iba-1 (g) immunostaining in paraffin sections of the lumbar spinal cord of IGU-treated and vehicle-treated mice at 50 dpi. Scale bar: 100 µm. (b, d, f, h) The graphs show the percentages of CD3- (n = 5 per group) (b), F4/80- (n = 6, vehicle group; n = 4, IGU group) (d), GFAP- (n = 5 per group) (f), and Iba-1- (n = 5 per group) (h) positive areas in the spinal cord of IGU-treated and vehicle-treated mice. (i) Microglial cell circularity quantified at 50 dpi (n = 6, vehicle group; n = 5, IGU group). Scale bar: 100 µm. Significant differences were determined using an unpaired t-test. * p < 0.05; ** p < 0.01.
Figure 2.
IGU inhibited inflammatory cell infiltration of the lumbar spinal cord during the chronic phase. (a, c, e, g) CD3 (a), F4/80 (c), glial fibrillary acidic protein (GFAP) (e), and Iba-1 (g) immunostaining in paraffin sections of the lumbar spinal cord of IGU-treated and vehicle-treated mice at 50 dpi. Scale bar: 100 µm. (b, d, f, h) The graphs show the percentages of CD3- (n = 5 per group) (b), F4/80- (n = 6, vehicle group; n = 4, IGU group) (d), GFAP- (n = 5 per group) (f), and Iba-1- (n = 5 per group) (h) positive areas in the spinal cord of IGU-treated and vehicle-treated mice. (i) Microglial cell circularity quantified at 50 dpi (n = 6, vehicle group; n = 5, IGU group). Scale bar: 100 µm. Significant differences were determined using an unpaired t-test. * p < 0.05; ** p < 0.01.
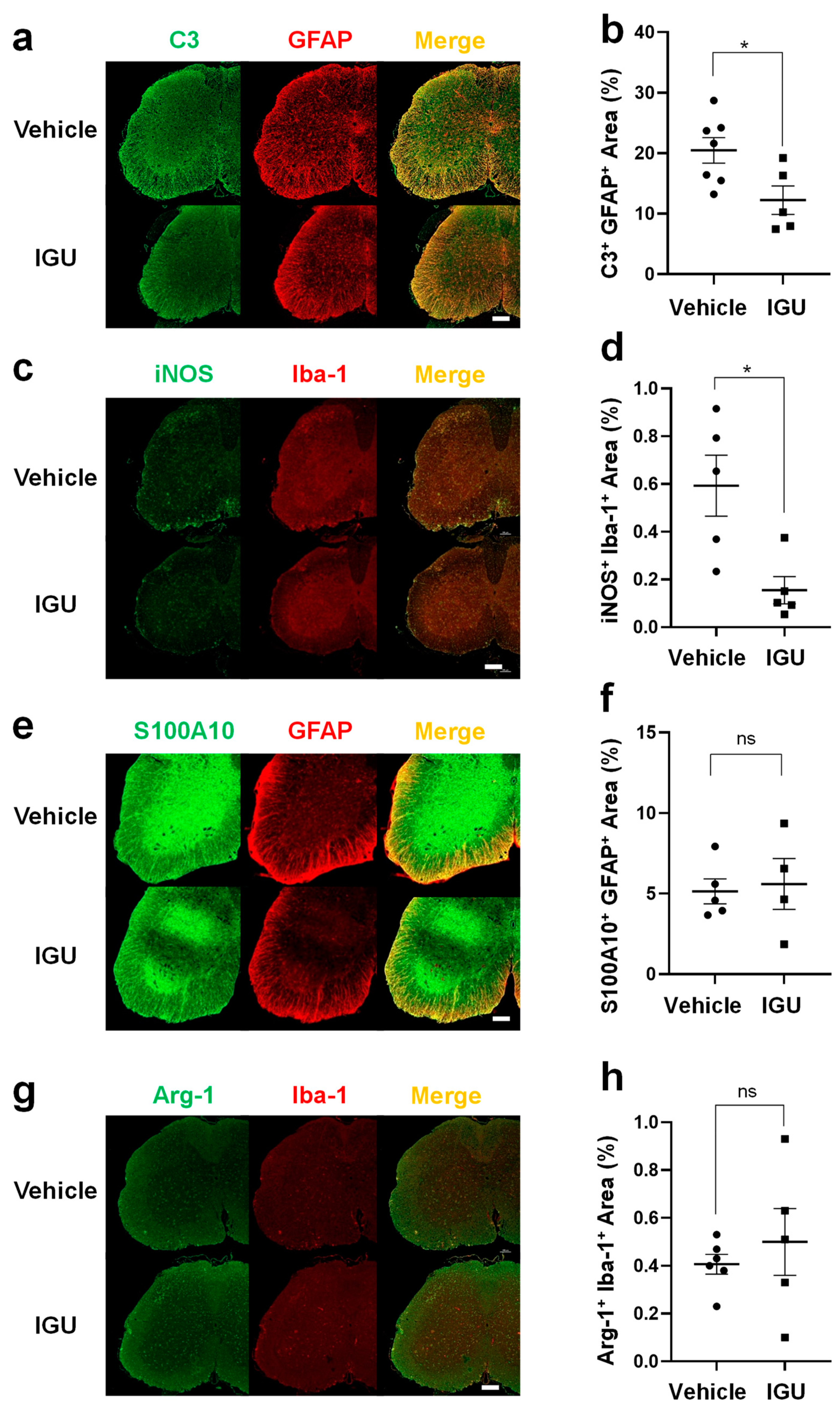
Figure 3.
IGU reduced A1 astroglia and M1-like microglia during the chronic phase. (a, c, e, g) Double immunofluorescence staining of the lumbar spinal cord for C3 and GFAP (a), inducible nitric oxide synthase (iNOS) and Iba-1 (c), S100A10 and GFAP (e), and Arg-1 and Iba-1 (g) at 50 dpi. Scale bar: 100 µm. (b, d, f, h) The graphs show the percentages of C3 and GFAP (n = 7, vehicle group; n = 5, IGU group) (b), iNOS and Iba-1 (n = 5 per group) (d), S100A10 and GFAP (n = 5, vehicle group; n = 4, IGU group) (f), and Arg-1 and Iba-1 (n = 6, vehicle group; n = 5, IGU group) (h) double-positive areas in the spinal cord of IGU-treated and vehicle-treated mice. Significant differences were determined using an unpaired t-test. * p < 0.05; ns, not significant.
Figure 3.
IGU reduced A1 astroglia and M1-like microglia during the chronic phase. (a, c, e, g) Double immunofluorescence staining of the lumbar spinal cord for C3 and GFAP (a), inducible nitric oxide synthase (iNOS) and Iba-1 (c), S100A10 and GFAP (e), and Arg-1 and Iba-1 (g) at 50 dpi. Scale bar: 100 µm. (b, d, f, h) The graphs show the percentages of C3 and GFAP (n = 7, vehicle group; n = 5, IGU group) (b), iNOS and Iba-1 (n = 5 per group) (d), S100A10 and GFAP (n = 5, vehicle group; n = 4, IGU group) (f), and Arg-1 and Iba-1 (n = 6, vehicle group; n = 5, IGU group) (h) double-positive areas in the spinal cord of IGU-treated and vehicle-treated mice. Significant differences were determined using an unpaired t-test. * p < 0.05; ns, not significant.
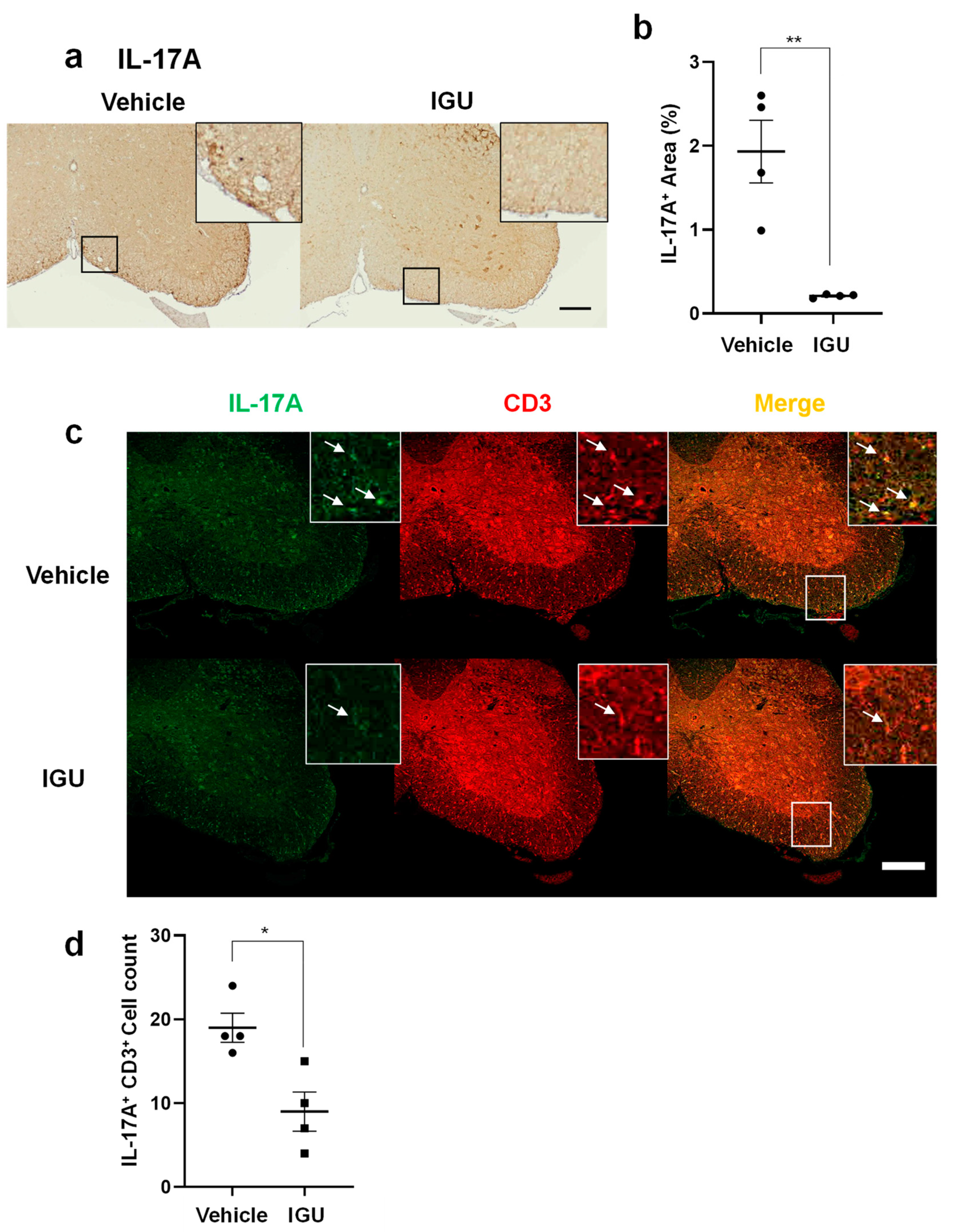
Figure 4.
IGU suppressed T helper 17 (Th17) cell migration in the spinal cord during the chronic phase. (a) Interleukin (IL)-17A immunostaining in paraffin sections of the lumbar spinal cord of IGU-treated and vehicle-treated mice at 50 dpi. Scale bar: 100 µm. (b) The graph shows the percentages of IL-17A-positive areas in the spinal cord of IGU-treated and vehicle-treated mice (n = 4 per group). (c) Double immunofluorescence of the lumbar spinal cord for IL-17A and CD3 at 50 dpi. White arrows indicate IL-17A+ CD3+ Th17 cells. (d) The graph shows the number of IL-17A and CD3 double-positive cells in the spinal cord of IGU-treated and vehicle-treated mice (n = 4 per group). Scale bars: 100 µm. Significant differences were determined using an unpaired t-test. * p < 0.05; ** p < 0.01.
Figure 4.
IGU suppressed T helper 17 (Th17) cell migration in the spinal cord during the chronic phase. (a) Interleukin (IL)-17A immunostaining in paraffin sections of the lumbar spinal cord of IGU-treated and vehicle-treated mice at 50 dpi. Scale bar: 100 µm. (b) The graph shows the percentages of IL-17A-positive areas in the spinal cord of IGU-treated and vehicle-treated mice (n = 4 per group). (c) Double immunofluorescence of the lumbar spinal cord for IL-17A and CD3 at 50 dpi. White arrows indicate IL-17A+ CD3+ Th17 cells. (d) The graph shows the number of IL-17A and CD3 double-positive cells in the spinal cord of IGU-treated and vehicle-treated mice (n = 4 per group). Scale bars: 100 µm. Significant differences were determined using an unpaired t-test. * p < 0.05; ** p < 0.01.
Figure 5.
IGU inhibited cerebrospinal fluid (CSF) cytokine production and CD4+ T cell infiltration into the central nervous system (CNS) tissue (spinal cord and brain) of pEAE mice during the chronic phase. (a) Representative flow cytometry plots showing the gating strategy for T cells isolated from the CNS tissue of vehicle-treated (left side) and IGU-treated (right side) pEAE mice. (b) The percentages of CD4+ T cells among lymphocytes in the CNS tissue of pEAE mice at 50 dpi were measured by flow cytometry (n = 4 per group). (c–e) The percentages of CXCR3− CCR6+ CD4+ Th17 (c), CXCR3+ CCR6+ CD4+ Th1/Th17 (d), and CXCR3+ CCR6− CD4+ Th1 (e) cells among CD4+ T cells were measured by flow cytometry (n = 4 per group). (f–i) CSF cytokine levels in the chronic phase of pEAE. IL-6 (n = 4 per group) (f), IL-1β (n = 4, vehicle group; n = 5, IGU group) (g), IL-4 (n = 5 per group) (h), and interferon (IFN)-γ (n = 4 per group) (i) were analyzed using a multiplexed fluorescence immunoassay. Significant differences were determined using an unpaired t-test. * p < 0.05; ** p < 0.01; ns, not significant.
Figure 5.
IGU inhibited cerebrospinal fluid (CSF) cytokine production and CD4+ T cell infiltration into the central nervous system (CNS) tissue (spinal cord and brain) of pEAE mice during the chronic phase. (a) Representative flow cytometry plots showing the gating strategy for T cells isolated from the CNS tissue of vehicle-treated (left side) and IGU-treated (right side) pEAE mice. (b) The percentages of CD4+ T cells among lymphocytes in the CNS tissue of pEAE mice at 50 dpi were measured by flow cytometry (n = 4 per group). (c–e) The percentages of CXCR3− CCR6+ CD4+ Th17 (c), CXCR3+ CCR6+ CD4+ Th1/Th17 (d), and CXCR3+ CCR6− CD4+ Th1 (e) cells among CD4+ T cells were measured by flow cytometry (n = 4 per group). (f–i) CSF cytokine levels in the chronic phase of pEAE. IL-6 (n = 4 per group) (f), IL-1β (n = 4, vehicle group; n = 5, IGU group) (g), IL-4 (n = 5 per group) (h), and interferon (IFN)-γ (n = 4 per group) (i) were analyzed using a multiplexed fluorescence immunoassay. Significant differences were determined using an unpaired t-test. * p < 0.05; ** p < 0.01; ns, not significant.
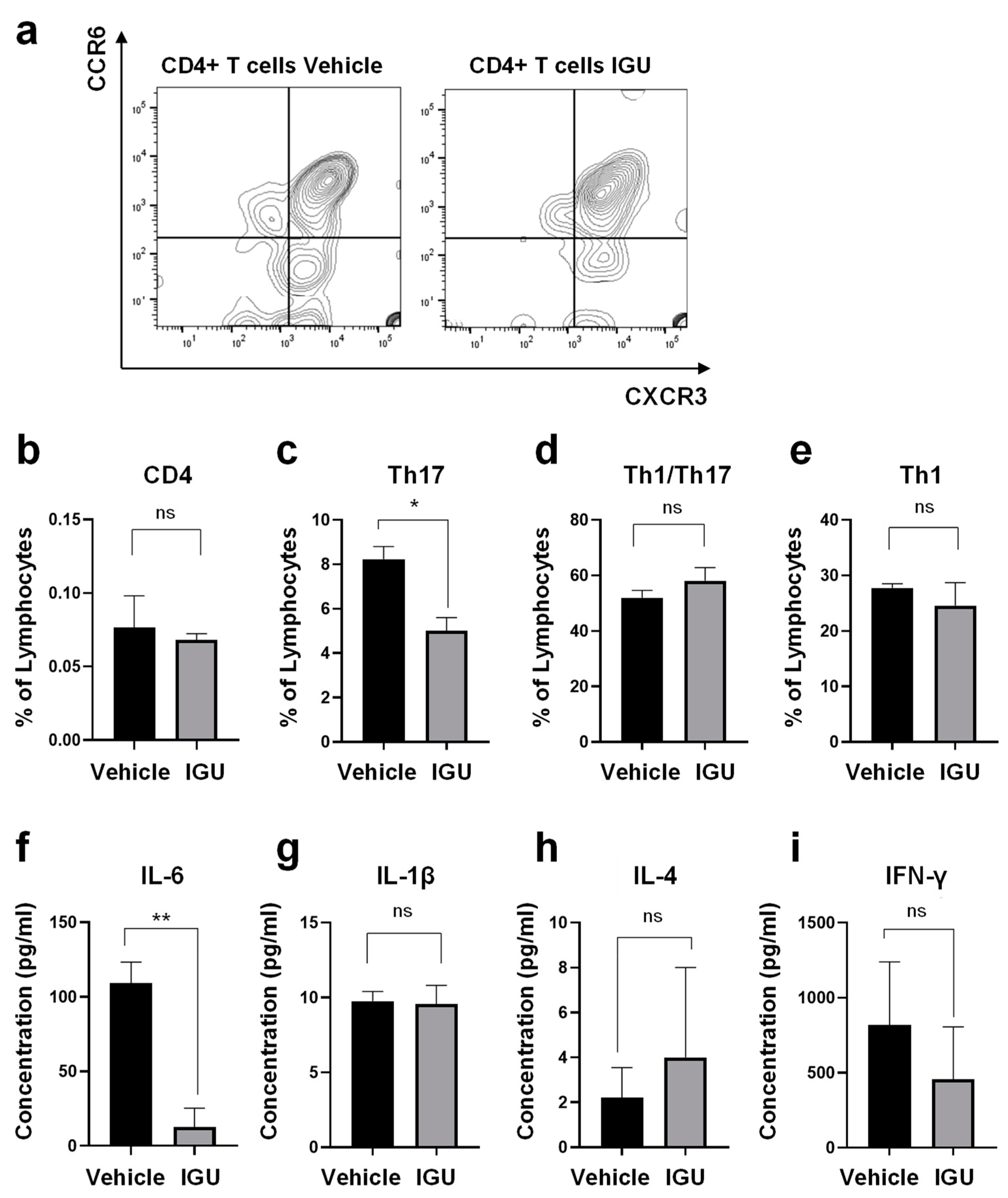
Figure 6.
IGU inhibited cytokine/chemokine release from glial cells in vitro in an activated glial inflammation model. Cytokine levels in the culture supernatant were collected at each time point. (a) IL-1α levels (n = 4 per group). (b) IL-1β levels (n = 4 per group). (c) IL-4 levels (n = 4 per group). (d) IL-6 levels (n = 3 per group). (e) IL-10 levels (n = 4 per group). (f) IFN-γ levels (n = 4 per group). (g) CCL2 (MCP-1) levels (n = 5 per group). (h) CCL3 (MIP-1α) levels (n = 4 per group). Concentrations between 4 and 24 h were saturated (> 4289 pg/ml). (i) CCL5 (RANTES) levels (n = 4 per group). Concentrations between 8 and 24 h were saturated (> 62,700 pg/ml). (j) CCL20 (MIP-3α) levels (n = 4 per group). Significant differences were determined by two-way analysis of variance (ANOVA), followed by Sidak’s multiple comparisons test. * p < 0.05; ** p < 0.01; **** p < 0.0001; Signs for not “significant (ns)” are omitted when there is no significant difference in the groups for each hour. Black bar: vehicle group. Gray bar: IGU group.
Figure 6.
IGU inhibited cytokine/chemokine release from glial cells in vitro in an activated glial inflammation model. Cytokine levels in the culture supernatant were collected at each time point. (a) IL-1α levels (n = 4 per group). (b) IL-1β levels (n = 4 per group). (c) IL-4 levels (n = 4 per group). (d) IL-6 levels (n = 3 per group). (e) IL-10 levels (n = 4 per group). (f) IFN-γ levels (n = 4 per group). (g) CCL2 (MCP-1) levels (n = 5 per group). (h) CCL3 (MIP-1α) levels (n = 4 per group). Concentrations between 4 and 24 h were saturated (> 4289 pg/ml). (i) CCL5 (RANTES) levels (n = 4 per group). Concentrations between 8 and 24 h were saturated (> 62,700 pg/ml). (j) CCL20 (MIP-3α) levels (n = 4 per group). Significant differences were determined by two-way analysis of variance (ANOVA), followed by Sidak’s multiple comparisons test. * p < 0.05; ** p < 0.01; **** p < 0.0001; Signs for not “significant (ns)” are omitted when there is no significant difference in the groups for each hour. Black bar: vehicle group. Gray bar: IGU group.
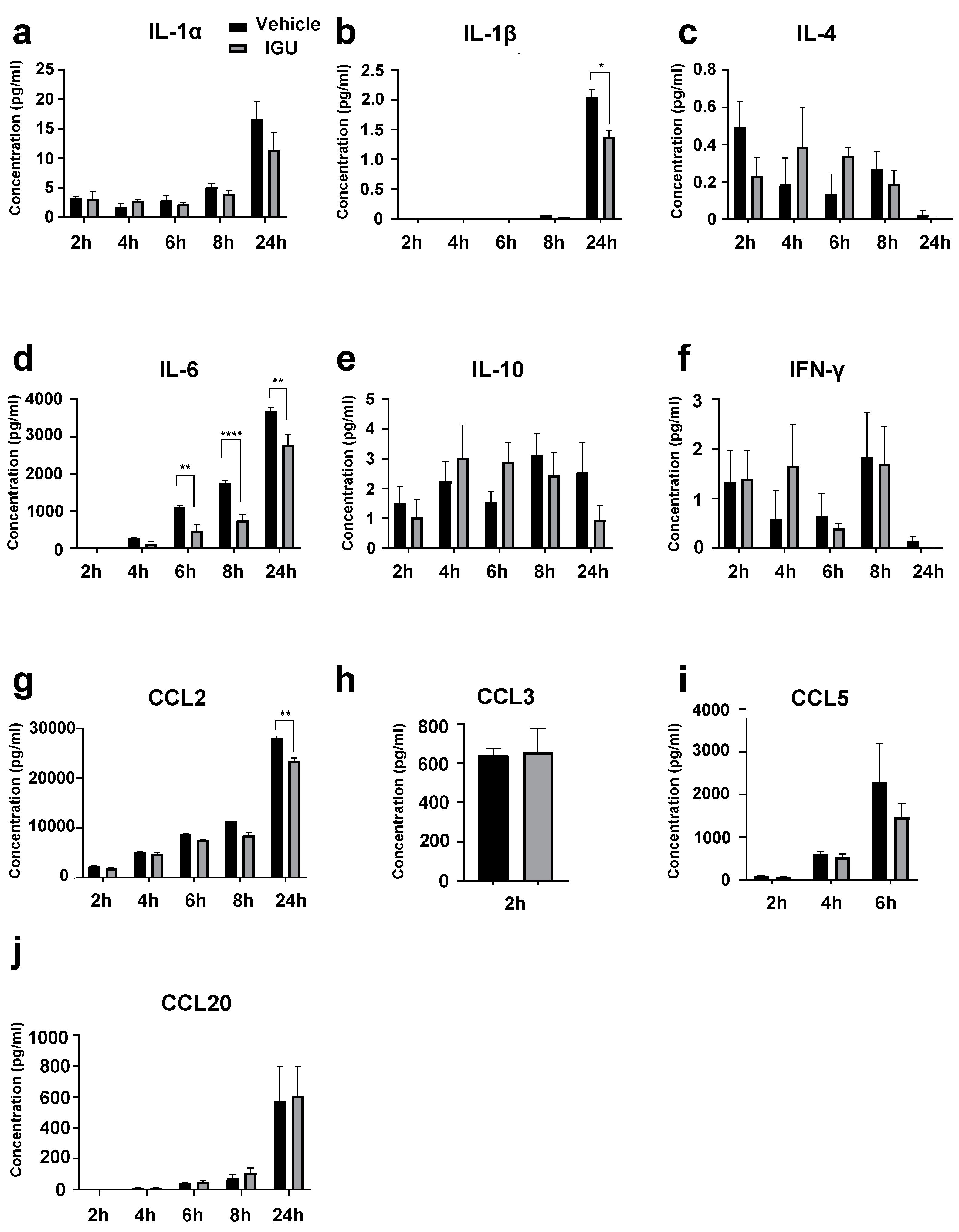
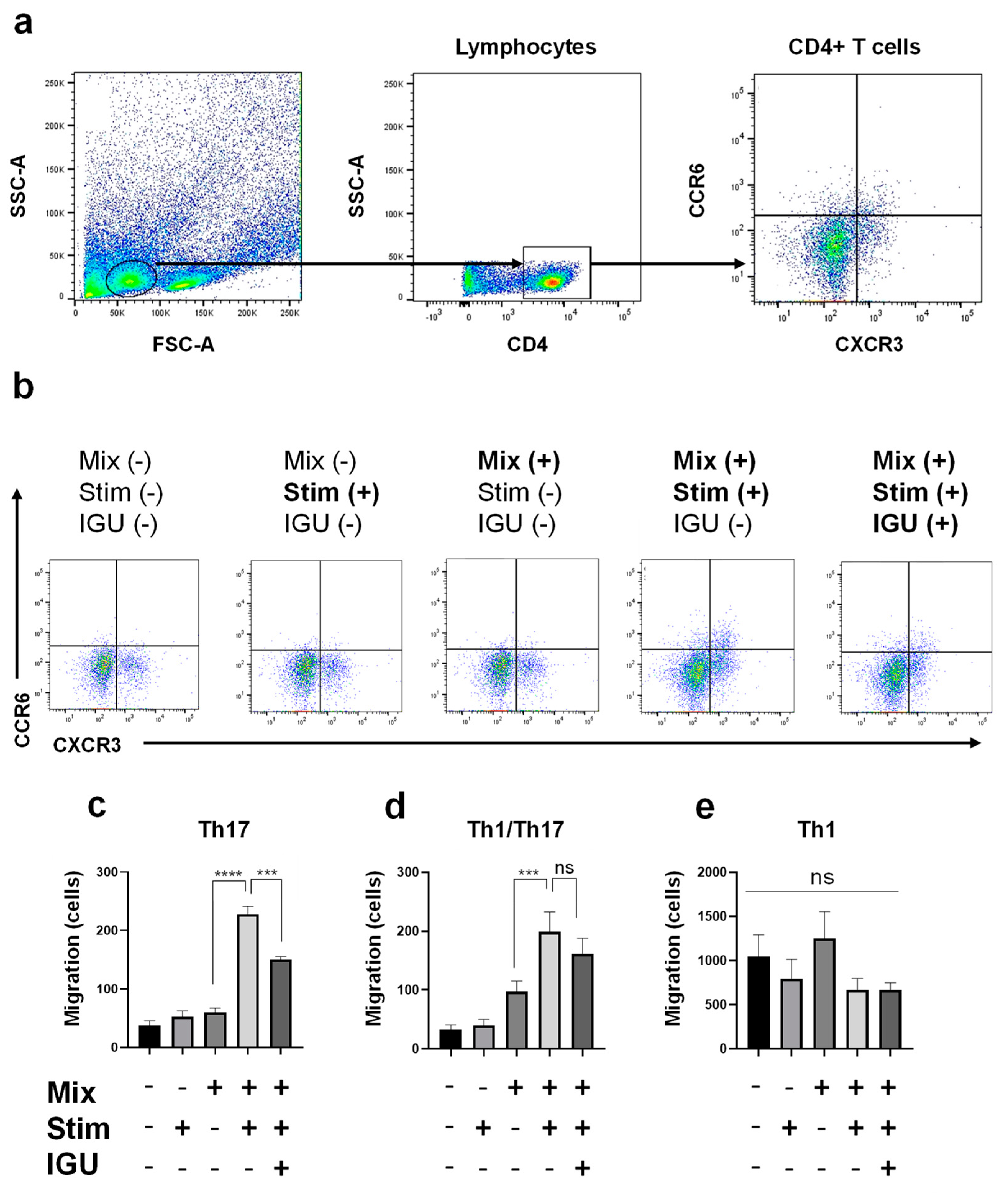
Figure 7.
IGU decreased the number of migrating Th17 and Th1/Th17 cells in an in vitro migration assay. (a) Gating strategy for the evaluation of CD4+ T cells migrated from the upper chamber to the lower chamber. (b) The numbers of CXCR3− CCR6+ CD4+ Th17 cells, CXCR3+ CCR6+ CD4+ Th1/Th17 cells, and CXCR3+ CCR6− CD4+ Th1 cells that migrated from the upper chamber to the lower chamber with or without mixed glial cells (1.0 × 106 cells/well), stimulation {IL-1α, C1q, tumor necrosis factor (TNF)-α, lipopolysaccharide (LPS)}, and IGU were counted using flow cytometry. (c–e) Bar graphs showing the numbers of Th17 cells (c), Th1/Th17 cells (d), and Th1 cells (e) that migrated in each lower chamber (n = 4 per group). For clarity, in the graphs of Th17 cells and Th1/Th17 cells, signs of significance were used only for comparisons between Mix(+) Stim(−) IGU(−) and Mix(+) Stim(+) IGU(−) and between Mix(+) Stim(+) IGU(−) and Mix(+) Stim(+) IGU(−). Significant differences were determined by one-way ANOVA, followed by Tukey’s multiple comparisons test. *** p < 0.001; **** p < 0.0001; ns, not significant. Mix: mixed glial cell culture. Stim: stimulation. IGU: iguratimod.
Figure 7.
IGU decreased the number of migrating Th17 and Th1/Th17 cells in an in vitro migration assay. (a) Gating strategy for the evaluation of CD4+ T cells migrated from the upper chamber to the lower chamber. (b) The numbers of CXCR3− CCR6+ CD4+ Th17 cells, CXCR3+ CCR6+ CD4+ Th1/Th17 cells, and CXCR3+ CCR6− CD4+ Th1 cells that migrated from the upper chamber to the lower chamber with or without mixed glial cells (1.0 × 106 cells/well), stimulation {IL-1α, C1q, tumor necrosis factor (TNF)-α, lipopolysaccharide (LPS)}, and IGU were counted using flow cytometry. (c–e) Bar graphs showing the numbers of Th17 cells (c), Th1/Th17 cells (d), and Th1 cells (e) that migrated in each lower chamber (n = 4 per group). For clarity, in the graphs of Th17 cells and Th1/Th17 cells, signs of significance were used only for comparisons between Mix(+) Stim(−) IGU(−) and Mix(+) Stim(+) IGU(−) and between Mix(+) Stim(+) IGU(−) and Mix(+) Stim(+) IGU(−). Significant differences were determined by one-way ANOVA, followed by Tukey’s multiple comparisons test. *** p < 0.001; **** p < 0.0001; ns, not significant. Mix: mixed glial cell culture. Stim: stimulation. IGU: iguratimod.