Submitted:
13 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
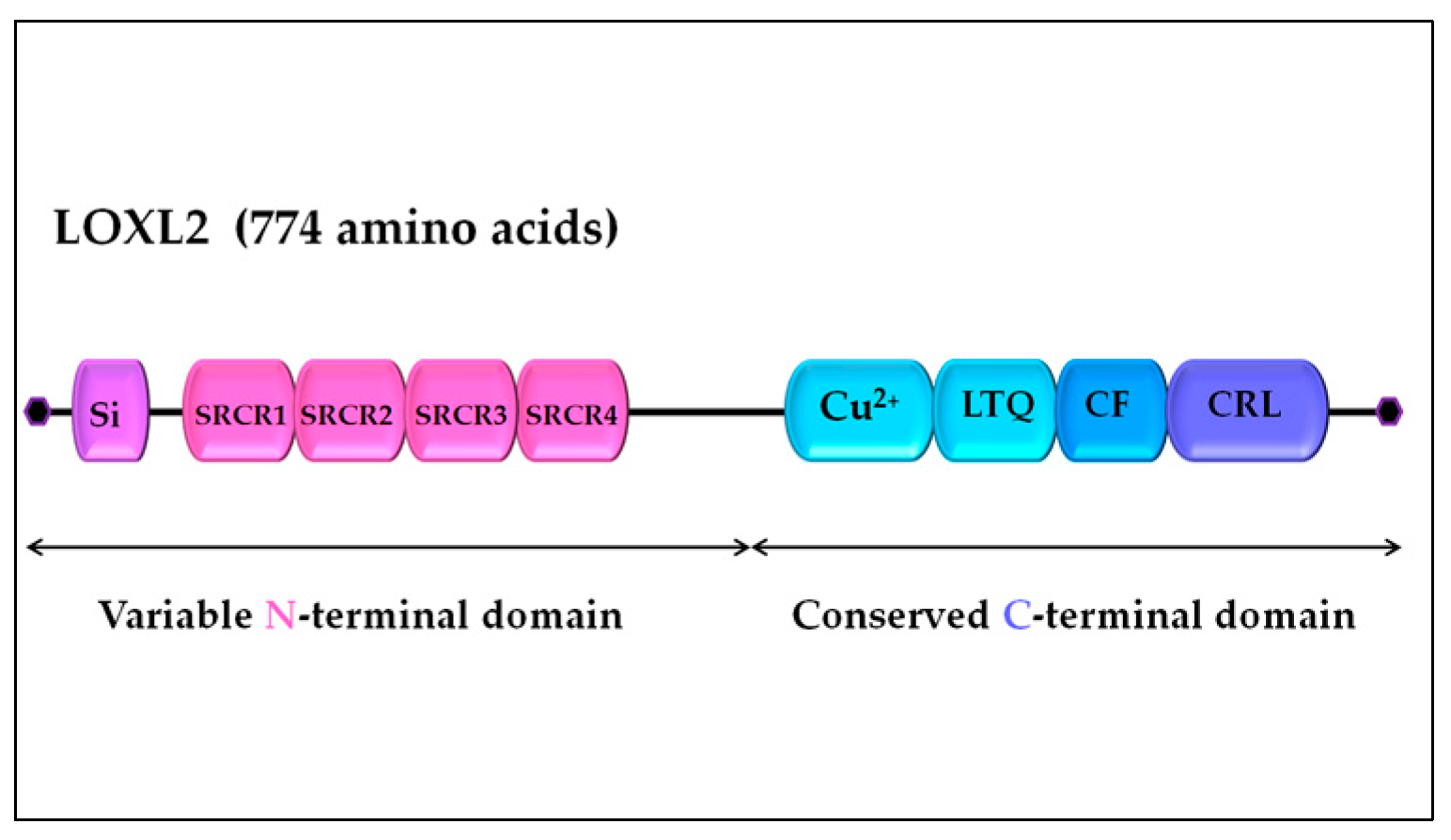
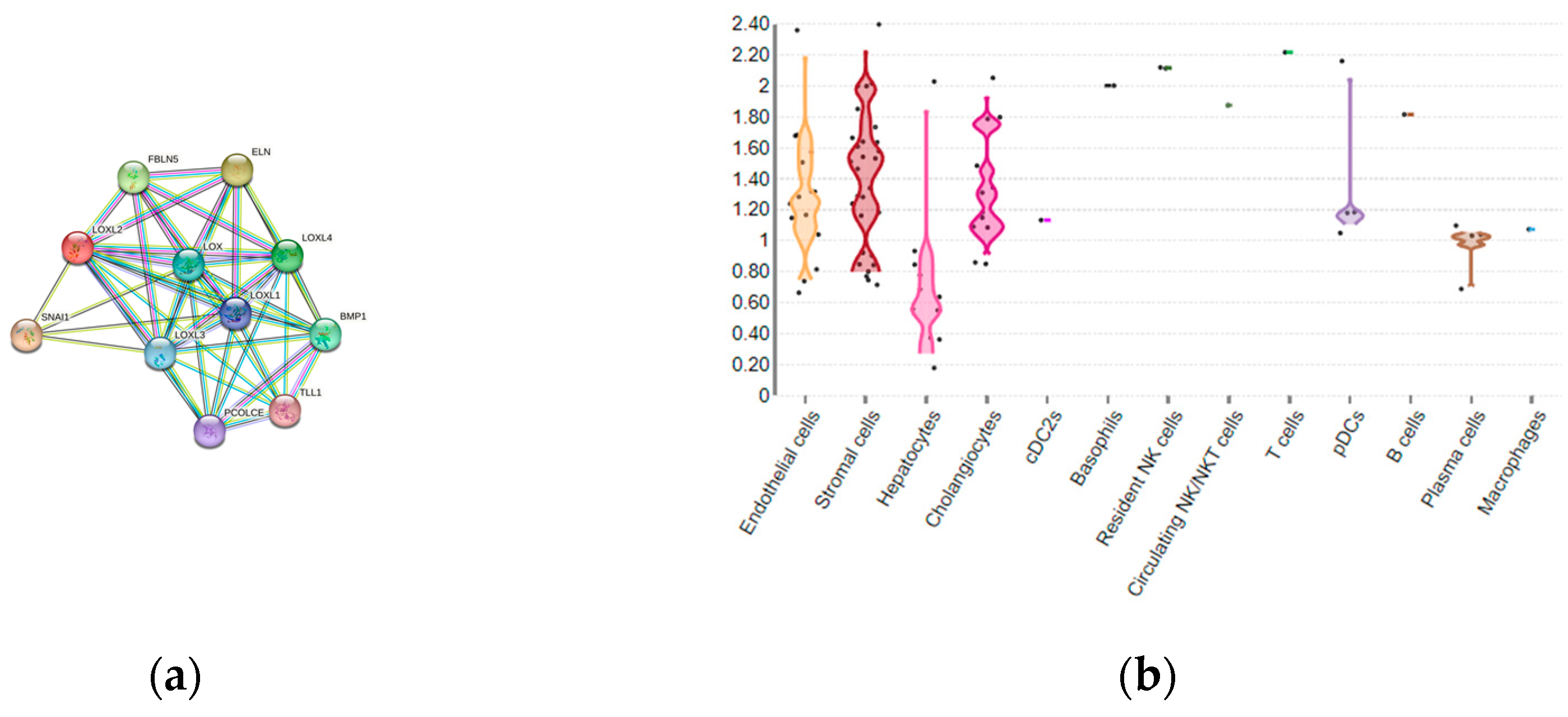
2. LOXL2 introduction: LOX family, structure and function of LOXL2
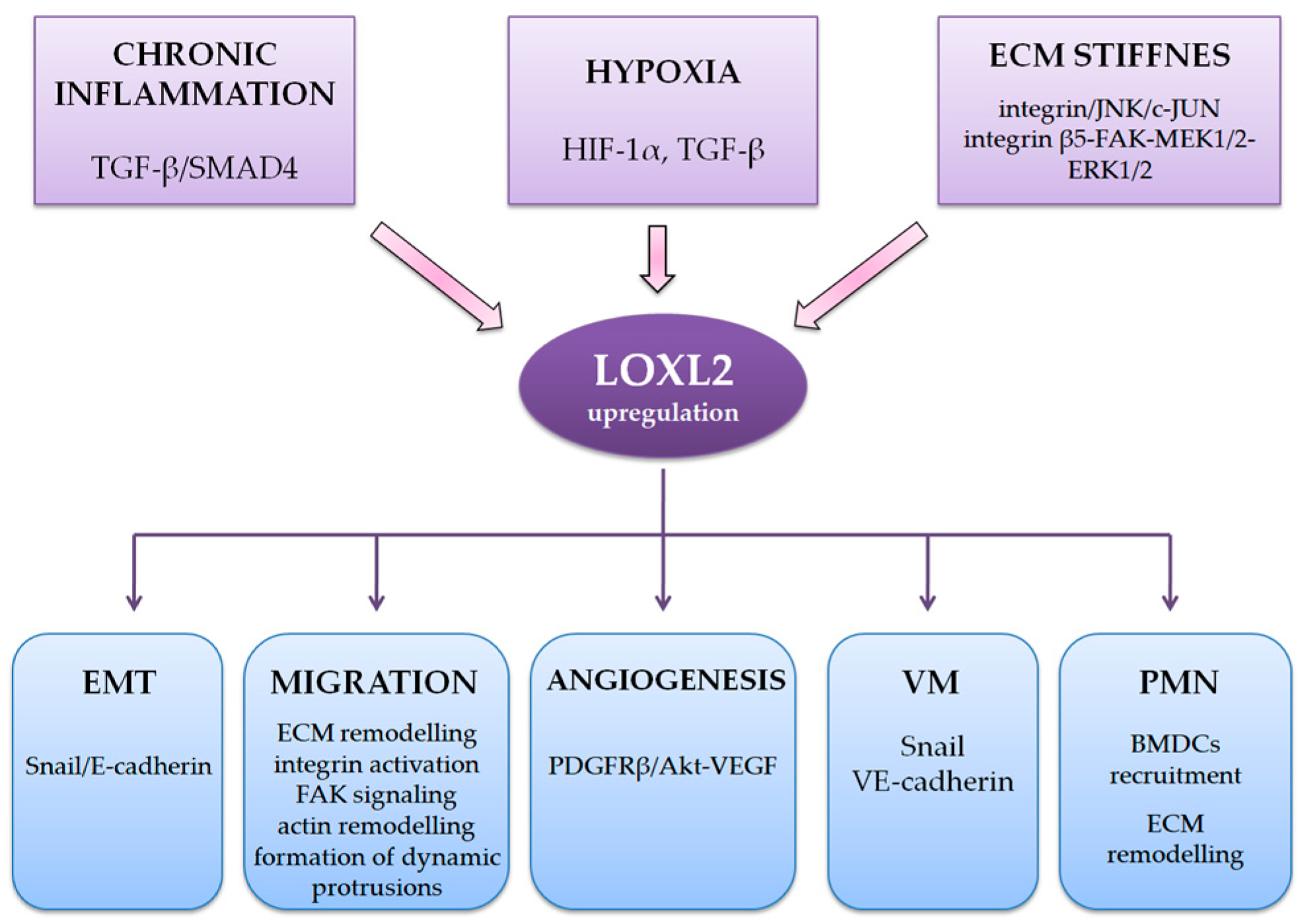
3. LOXL2 expression in HCC and correlation with clinical parameters
4. LOXL2 in the regulation of tumor microenvironment and formation of pre-metastatic niches
4.1. LOXL2 and CAFs
4.2. LOXL2 and TAMs
4.3. LOXL2 in the formation of pre-metastatic niches
5. LOXL2 role in EMT
6. LOXL2 and hypoxia, angiogenesis and vasculogenic mimicry
7. LOXL2 and micro-RNAs in HCC
8. LOXL2 as potential target for HCC treatment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular Carcinoma |
| LOXL2 | Lysyl Oxidase-like 2 |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| NAFLD | Nonalcoholic Fatty Liver Disease |
| TACE | Transarterial Chemoembolization |
| VEGF | Vascular Endothelial Growth Factor |
| TME | Tumor Microenvironment |
| EMT | Epithelial-Mesenchcymal Transition |
| CAF | Cancer-associated Fibroblasts |
| ECM | Extracellular Matrix |
| LTQ | Lysine tyrosylquinone |
| ELN | Elastin |
| FBLN5 | fibulin-5 |
| PCOLCE | procollagen C-endopeptidase enhancer 1 |
| TLL1 | Tolloid-like protein 1 |
| BMP | Bone morphogenic protein 1 |
| OS | Overall Survival |
| DFS | Desease Free Durvival |
| DSS | Disease Specific Survival |
| EHRFS | Extrahepatic Reccurence-free Survival |
| CAIX | Carbon Anhydrase IX |
| HSC | Hepatic Stellate Cells |
| BMDC | Bone Marrow-derived Cells |
| TGF-ß | Transforming Growth Factor Beta |
| HIF-1α | Hypoxia-inducible Factor 1 Alpha |
| JNK-c | Jun N-terminal Kinase |
| 5FU | 5-Fluorouracil |
| FAK | Fokal Adhesion Kinase |
| ROCK | Rho-associated Protein Kinase |
| CCL5 | Chemokine ligand 5 |
| ZEB1 | Zinc finger E-box-binding Homeobox 1 |
| MEK1/2 | Mitogen-activated Protein Kinase 1/2 |
| ERK 1/2 | Extracellular Signal-regulated Kinase 1/2 |
| TAM | Tumor-associated Macrophage |
| PMN | Pre-metastatic Nichae |
| HRE | hypoxia responsive element |
| FBP1 | Fructose-1,6-biphosphatase Protein 1 |
| PDGFRß | Platelt-derived Growth Factor Receptor Beta |
| VM | Vasculogenic Mimicry |
| BAPN | ß-aminopropionitrile. |
References
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatol. Baltim. Md 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A Global View of Hepatocellular Carcinoma: Trends, Risk, Prevention and Management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Affo, S.; Yu, L.-X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. 2017, 12, 153–186. [Google Scholar] [CrossRef]
- Sangiovanni, A.; Prati, G.M.; Fasani, P.; Ronchi, G.; Romeo, R.; Manini, M.; Del Ninno, E.; Morabito, A.; Colombo, M. The Natural History of Compensated Cirrhosis Due to Hepatitis C Virus: A 17-Year Cohort Study of 214 Patients. Hepatology 2006, 43, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Wege, H.; Li, J.; Ittrich, H. Treatment Lines in Hepatocellular Carcinoma. Visc. Med. 2019, 35, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivas, A.N.; Prashant, A.; Harikumar, K.B.; Kumar, D.P. Therapeutic Options in Hepatocellular Carcinoma: A Comprehensive Review. Clin. Exp. Med. 2023. [Google Scholar] [CrossRef]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular Carcinoma Incidence, Mortality, and Survival Trends in the United States From 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef]
- Bao, M.H.-R.; Wong, C.C.-L. Hypoxia, Metabolic Reprogramming, and Drug Resistance in Liver Cancer. Cells 2021, 10, 1715. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region with Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primer 2021, 7, 6. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Yang, Y.; Yan, Q.; Ren, J.; Luo, J.; Tiu, Y.C.; Fang, X.; Liu, B.; Lam, R.H.W.; et al. Inhibition of Lysyl Oxidase-like 2 Overcomes Adhesion-dependent Drug Resistance in the Collagen-enriched Liver Cancer Microenvironment. Hepatol. Commun. 2022, 6, 3194–3211. [Google Scholar] [CrossRef] [PubMed]
- Hernandez–Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the Microenvironment in the Pathogenesis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef]
- Wong, C.C.-L.; Tse, A.P.-W.; Huang, Y.-P.; Zhu, Y.-T.; Chiu, D.K.-C.; Lai, R.K.-H.; Au, S.L.-K.; Kai, A.K.-L.; Lee, J.M.-F.; Wei, L.L.; et al. Lysyl Oxidase-like 2 Is Critical to Tumor Microenvironment and Metastatic Niche Formation in Hepatocellular Carcinoma. Hepatology 2014, 60, 1645–1658. [Google Scholar] [CrossRef]
- Payne, S.L.; Hendrix, M.J.C.; Kirschmann, D.A. Paradoxical Roles for Lysyl Oxidases in Cancer—A Prospect. J. Cell. Biochem. 2007, 101, 1338–1354. [Google Scholar] [CrossRef]
- Zhan, X.; Jiao, J.; Zhang, H.; Li, C.; Zhao, J.; Liao, L.; Wu, J.; Wu, B.; Wu, Z.; Wang, S.; et al. A Three-Gene Signature from Protein-Protein Interaction Network of LOXL2 - and Actin-Related Proteins for Esophageal Squamous Cell Carcinoma Prognosis. Cancer Med. 2017, 6, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Bueno, G.; Salvador, F.; Martín, A.; Floristán, A.; Cuevas, E.P.; Santos, V.; Montes, A.; Morales, S.; Castilla, M.A.; Rojo-Sebastián, A.; et al. Lysyl Oxidase-like 2 (LOXL2), a New Regulator of Cell Polarity Required for Metastatic Dissemination of Basal-like Breast Carcinomas. EMBO Mol. Med. 2011, 3, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, J.; Lee, Y.S.; Kim, J.K.; Dong, S.M.; Yoon, D.S. Emerging Role of LOXL2 in the Promotion of Pancreas Cancer Metastasis. Oncotarget 2106, 7, 42539–42552. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Garcia-Palmero, I.; Herrera, M.; Bartolomé, R.A.; Peña, C.; Fernandez-Aceñero, M.J.; Padilla, G.; Peláez-García, A.; Lopez-Lucendo, M.; Rodriguez-Merlo, R.; et al. LOXL2 Is Highly Expressed in Cancer-Associated Fibroblasts and Associates to Poor Colon Cancer Survival. Clin. Cancer Res. 2015, 21, 4892–4902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Xu, J. Prognostic Utility and Clinical Significance of Lysyl Oxidase-like 2 Protein Expression in Digestive System Cancers. J. Cell. Physiol. 2019, 234, 20713–20720. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Li, C.-J.; Yang, Y.-L.; Huang, Y.-H.; Hsiau, Y.-T.; Chu, P.-Y. Roles of Lysyl Oxidase Family Members in the Tumor Microenvironment and Progression of Liver Cancer. Int. J. Mol. Sci. 2020, 21, 9751. [Google Scholar] [CrossRef]
- Wen, B.; Xu, L.-Y.; Li, E.-M. LOXL2 in Cancer: Regulation, Downstream Effectors and Novel Roles. Biochim. Biophys. Acta BBA - Rev. Cancer 2020, 1874, 188435. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhu, Y. The Function and Mechanisms of Action of LOXL2 in Cancer (Review). Int. J. Mol. Med. 2015, 36, 1200–1204. [Google Scholar] [CrossRef]
- Peng, T.; Deng, X.; Tian, F.; Li, Z.; Jiang, P.; Zhao, X.; Chen, G.; Chen, Y.; Zheng, P.; Li, D.; et al. The Interaction of LOXL2 with GATA6 Induces VEGFA Expression and Angiogenesis in Cholangiocarcinoma. Int. J. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, S.; Tian, Y.; Ju, A.; Hou, Q.; Liu, J.; Fu, Y.; Luo, Y. Lysyl Oxidase-Like Protein 2 Promotes Tumor Lymphangiogenesis and Lymph Node Metastasis in Breast Cancer. Neoplasia 2019, 21, 413–427. [Google Scholar] [CrossRef]
- Shao, B.; Zhao, X.; Liu, T.; Zhang, Y.; Sun, R.; Dong, X.; Liu, F.; Zhao, N.; Zhang, D.; Wu, L.; et al. LOXL2 Promotes Vasculogenic Mimicry and Tumour Aggressiveness in Hepatocellular Carcinoma. J. Cell. Mol. Med. 2019, 23, 1363–1374. [Google Scholar] [CrossRef]
- Barker, H.E.; Bird, D.; Lang, G.; Erler, J.T. Tumor-Secreted LOXL2 Activates Fibroblasts through FAK Signaling. Mol. Cancer Res. 2013, 11, 1425–1436. [Google Scholar] [CrossRef]
- Mäki, J.M.; Kivirikko, K.I. Cloning and Characterization of a Fourth Human Lysyl Oxidase Isoenzyme. Biochem. J. 2001, 355, 381–387. [Google Scholar] [CrossRef]
- Molnar, J.; Fong, K.S.K.; He, Q.P.; Hayashi, K.; Kim, Y.; Fong, S.F.T.; Fogelgren, B.; Molnarne Szauter, K.; Mink, M.; Csiszar, K. Structural and Functional Diversity of Lysyl Oxidase and the LOX-like Proteins. Biochim. Biophys. Acta BBA - Proteins Proteomics 2003, 1647, 220–224. [Google Scholar] [CrossRef]
- Kenyon, K.; Modi, W.S.; Contente, S.; Friedman, R.M. A Novel Human CDNA with a Predicted Protein Similar to Lysyl Oxidase Maps to Chromosome 15q24-Q25. J. Biol. Chem. 1993, 268, 18435–18437. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Boyd, C.D.; Csiszar, K. A New Gene with Sequence and Structural Similarity to the Gene Encoding Human Lysyl Oxidase. J. Biol. Chem. 1995, 270, 7176–7182. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Papaconstantinou, J.; Sato, H.; Goldstein, S. Regulation of a Novel Gene Encoding a Lysyl Oxidase-Related Protein in Cellular Adhesion and Senescence. J. Biol. Chem. 1997, 272, 8157–8160. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. Cloning and Characterization of a Human Lysyl Oxidase-like 3 Gene (HLOXL3). Matrix Biol. 2001, 20, 153–157. [Google Scholar] [CrossRef]
- Siegel, R.C.; Pinnell, S.R.; Martin, G.R. Cross-Linking of Collagen and Elastin. Properties of Lysyl Oxidase. Biochemistry 1970, 9, 4486–4492. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R.; Martin, G.R. The Cross-Linking of Collagen and Elastin: Enzymatic Conversion of Lysine in Peptide Linkage to Alpha-Aminoadipic-Delta-Semialdehyde (Allysine) by an Extract from Bone. Proc. Natl. Acad. Sci. 1968, 61, 708–716. [Google Scholar] [CrossRef]
- Jourdan-Le Saux, C.; Le Saux, O.; Donlon, T.; Boyd, C.D.; Csiszar, K. The Human Lysyl Oxidase-Related Gene (LOXL2) Maps between Markers D8S280 and D8S278 on Chromosome 8p21.2–P21.3. Genomics 1998, 51, 305–307. [Google Scholar] [CrossRef]
- Csiszar, K. Lysyl Oxidases: A Novel Multifunctional Amine Oxidase Family. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier, 2001; Vol. 70, pp. 1–32 ISBN 978-0-12-540070-1.
- Lucero, H.A.; Kagan, H.M. Lysyl Oxidase: An Oxidative Enzyme and Effector of Cell Function. Cell. Mol. Life Sci. 2006, 63, 2304–2316. [Google Scholar] [CrossRef]
- Xiao, Q.; Ge, G. Lysyl Oxidase, Extracellular Matrix Remodeling and Cancer Metastasis. Cancer Microenviron. 2012, 5, 261–273. [Google Scholar] [CrossRef]
- Meier, A.A.; Kuczera, K.; Mure, M. A 3D–Predicted Structure of the Amine Oxidase Domain of Lysyl Oxidase–Like 2. Int. J. Mol. Sci. 2022, 23, 13385. [Google Scholar] [CrossRef]
- Boufraqech, M.; Zhang, L.; Nilubol, N.; Sadowski, S.M.; Kotian, S.; Quezado, M.; Kebebew, E. Lysyl Oxidase (LOX) Transcriptionally Regulates SNAI2 Expression and TIMP4 Secretion in Human Cancers. Clin. Cancer Res. 2016, 22, 4491–4504. [Google Scholar] [CrossRef] [PubMed]
- Hornstra, I.K.; Birge, S.; Starcher, B.; Bailey, A.J.; Mecham, R.P.; Shapiro, S.D. Lysyl Oxidase Is Required for Vascular and Diaphragmatic Development in Mice. J. Biol. Chem. 2003, 278, 14387–14393. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Meroni, M.; Baselli, G.A.; Bassani, G.A.; Rametta, R.; Pietrelli, A.; Maggioni, M.; Facciotti, F.; Trunzo, V.; Badiali, S.; et al. Insulin Resistance Promotes Lysyl Oxidase Like 2 Induction and Fibrosis Accumulation in Non-Alcoholic Fatty Liver Disease. Clin. Sci. 2017, 131, 1301–1315. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, A.; Chen, W.; Wang, P.; Liu, T.; Cong, M.; Xu, A.; Yan, X.; Jia, J.; You, H. Inhibition of Lysyl Oxidase-like 1 (LOXL1) Expression Arrests Liver Fibrosis Progression in Cirrhosis by Reducing Elastin Crosslinking. Biochim. Biophys. Acta BBA - Mol. Basis Dis. 2018, 1864, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, N.; Peng, Z.-W.; Vaid, K.A.; Liu, S.B.; Yoshida, S.; Sverdlov, D.Y.; Mikels-Vigdal, A.; Smith, V.; Schuppan, D.; Popov, Y.V. Selective Targeting of Lysyl Oxidase-like 2 (LOXL2) Suppresses Hepatic Fibrosis Progression and Accelerates Its Reversal. Gut 2017, 66, 1697–1708. [Google Scholar] [CrossRef]
- Guilliams, M.; Bonnardel, J.; Haest, B.; Vanderborght, B.; Wagner, C.; Remmerie, A.; Bujko, A.; Martens, L.; Thoné, T.; Browaeys, R.; et al. Spatial Proteogenomics Reveals Distinct and Evolutionarily Conserved Hepatic Macrophage Niches. Cell 2022, 185, 379–396. [Google Scholar] [CrossRef]
- Liver Cell Atlas. Available online: https://www.livercellatlas.org/umap-humanAll.php (accessed on 10.06.2023).
- String. Available online: https://string-db.org/cgi/network?taskId=b4sTPzQtSCz5&sessionId=bljZKLXOKXys (accessed on 10.06.2023).
- Wang, T.-H.; Hsia, S.-M.; Shieh, T.-M. Lysyl Oxidase and the Tumor Microenvironment. Int. J. Mol. Sci. 2016, 18, 62. [Google Scholar] [CrossRef]
- Xu, L.; Go, E.P.; Finney, J.; Moon, H.; Lantz, M.; Rebecchi, K.; Desaire, H.; Mure, M. Post-Translational Modifications of Recombinant Human Lysyl Oxidase-like 2 (RhLOXL2) Secreted from Drosophila S2 Cells*. J. Biol. Chem. 2013, 288, 5357–5363. [Google Scholar] [CrossRef]
- Luo, W.; Chang, R.; Zhong, J.; Pandey, A.; Semenza, G.L. Histone Demethylase JMJD2C Is a Coactivator for Hypoxia-Inducible Factor 1 That Is Required for Breast Cancer Progression. Proc. Natl. Acad. Sci. 2012, 109. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, M.-X.; Zhang, X.-D.; Xu, X.-E.; Wu, Z.-Y.; Liao, L.-D.; Li, L.-Y.; Xie, Y.-M.; Wu, J.-Y.; Zou, H.-Y.; et al. SMYD3 Stimulates EZR and LOXL2 Transcription to Enhance Proliferation, Migration, and Invasion in Esophageal Squamous Cell Carcinoma. Hum. Pathol. 2016, 52, 153–163. [Google Scholar] [CrossRef]
- Liu, C.; Guo, T.; Sakai, A.; Ren, S.; Fukusumi, T.; Ando, M.; Sadat, S.; Saito, Y.; Califano, J.A. A Novel Splice Variant of LOXL2 Promotes Progression of Human Papillomavirus–Negative Head and Neck Squamous Cell Carcinoma. Cancer 2020, 126, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.-Q.; Zou, H.-Y.; Liao, L.-D.; Cao, H.-H.; Zeng, F.-M.; Wu, B.-L.; Xie, J.-J.; Fang, W.-K.; Xu, L.-Y.; Li, E.-M. Identification of a Novel Lysyl Oxidase-like 2 Alternative Splicing Isoform, LOXL2 Δe13, in Esophageal Squamous Cell Carcinoma. Biochem. Cell Biol. 2014, 92, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Xiao, H.; Xiao, W.; Xiong, Z.; Hu, W.; Gao, Y.; Ru, Z.; Wang, C.; Bao, L.; Wang, K.; et al. Upregulation of MIAT Regulates LOXL2 Expression by Competitively Binding MiR-29c in Clear Cell Renal Cell Carcinoma. Cell. Physiol. Biochem. 2018, 48, 1075–1087. [Google Scholar] [CrossRef]
- Fukumoto, I.; Kikkawa, N.; Matsushita, R.; Kato, M.; Kurozumi, A.; Nishikawa, R.; Goto, Y.; Koshizuka, K.; Hanazawa, T.; Enokida, H.; et al. Tumor-Suppressive MicroRNAs (MiR-26a/b, MiR-29a/b/c and MiR-218) Concertedly Suppressed Metastasis-Promoting LOXL2 in Head and Neck Squamous Cell Carcinoma. J. Hum. Genet. 2016, 61, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, J.; Guo, T.; Pan, X. MiR-504 Inhibits Cell Proliferation and Invasion by Targeting LOXL2 in Non Small Cell Lung Cancer. Biomed. Pharmacother. 2018, 97, 1289–1295. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the Extracellular Matrix: Drivers of Tumour Metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, Q.; Xing, X.; Dong, Y.; Wang, Y.; You, Y.; Chen, R.; Hu, C.; Chen, J.; Gao, D.; et al. Matrix Stiffness-Upregulated LOXL2 Promotes Fibronectin Production, MMP9 and CXCL12 Expression and BMDCs Recruitment to Assist Pre-Metastatic Niche Formation. J. Exp. Clin. Cancer Res. 2018, 37, 99. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, X.; Zhu, D.; Liu, T.; Liang, X.; Liu, F.; Zhang, Y.; Dong, X.; Sun, B. HIF-1α Promoted Vasculogenic Mimicry Formation in Hepatocellular Carcinoma through LOXL2 up-Regulation in Hypoxic Tumor Microenvironment. J. Exp. Clin. Cancer Res. 2017, 36, 60. [Google Scholar] [CrossRef]
- Zhao, N.; Chen, C.; Guo, Y.; Liu, T.; Che, N.; Zhang, D.; Liang, X.; Zhang, Y.; Zhao, X. LOXL2 Serves as a Prognostic Biomarker for Hepatocellular Carcinoma by Mediating Immune Infiltration and Vasculogenic Mimicry. Dig. Liver Dis. 2023, 55, 661–672. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Tsai, M.-C.; Chang, Y.-H.; Wang, C.-C.; Chu, P.-Y.; Lin, H.-Y.; Huang, Y.-H. MIR29A Impedes Metastatic Behaviors in Hepatocellular Carcinoma via Targeting LOX, LOXL2, and VEGFA. Int. J. Mol. Sci. 2021, 22, 6001. [Google Scholar] [CrossRef]
- Umezaki, N.; Nakagawa, S.; Yamashita, Y.; Kitano, Y.; Arima, K.; Miyata, T.; Hiyoshi, Y.; Okabe, H.; Nitta, H.; Hayashi, H.; et al. Lysyl Oxidase Induces Epithelial-mesenchymal Transition and Predicts Intrahepatic Metastasis of Hepatocellular Carcinoma. Cancer Sci. 2019, cas.14010. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Chung, T.; Rhee, H.; Kim, Y.-J.; Jeon, Y.; Yoo, J.E.; Noh, S.; Han, D.H.; Park, Y.N. Increased Expression of the Matrix-Modifying Enzyme Lysyl Oxidase-Like 2 in Aggressive Hepatocellular Carcinoma with Poor Prognosis. Gut Liver 2019, 13, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Y.; Zhu, Y.; Cong, Q.; Xiang, Y.; Fu, L. The Effect of LOXL2 in Hepatocellular Carcinoma. Mol. Med. Rep. 2016, 14, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Li, C.-J.; Yang, Y.-L.; Huang, Y.-H.; Hsiau, Y.-T.; Chu, P.-Y. Roles of Lysyl Oxidase Family Members in the Tumor Microenvironment and Progression of Liver Cancer. Int. J. Mol. Sci. 2020, 21, 9751. [Google Scholar] [CrossRef]
- Sas, Z.; Cendrowicz, E.; Weinhäuser, I.; Rygiel, T.P. Tumor Microenvironment of Hepatocellular Carcinoma: Challenges and Opportunities for New Treatment Options. Int. J. Mol. Sci. 2022, 23, 3778. [Google Scholar] [CrossRef] [PubMed]
- Satilmis, B.; Sahin, T.T.; Cicek, E.; Akbulut, S.; Yilmaz, S. Hepatocellular Carcinoma Tumor Microenvironment and Its Implications in Terms of Anti-Tumor Immunity: Future Perspectives for New Therapeutics. J. Gastrointest. Cancer 2021, 52, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Guizhen, Z.; Guanchang, J.; Liwen, L.; Huifen, W.; Zhigang, R.; Ranran, S.; Zujiang, Y. The Tumor Microenvironment of Hepatocellular Carcinoma and Its Targeting Strategy by CAR-T Cell Immunotherapy. Front. Endocrinol. 2022, 13, 918869. [Google Scholar] [CrossRef]
- Lu, C.; Rong, D.; Zhang, B.; Zheng, W.; Wang, X.; Chen, Z.; Tang, W. Current Perspectives on the Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Challenges and Opportunities. Mol. Cancer 2019, 18, 130. [Google Scholar] [CrossRef]
- Yu, L.-X.; Ling, Y.; Wang, H.-Y. Role of Nonresolving Inflammation in Hepatocellular Carcinoma Development and Progression. Npj Precis. Oncol. 2018, 2, 6. [Google Scholar] [CrossRef]
- Lo, C.-M.; Wang, H.-B.; Dembo, M.; Wang, Y. Cell Movement Is Guided by the Rigidity of the Substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Liburkin-Dan, T.; Toledano, S.; Neufeld, G. Lysyl Oxidase Family Enzymes and Their Role in Tumor Progression. Int. J. Mol. Sci. 2022, 23, 6249. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Gordon-Walker, T.T.; Aucott, R.L.; Van Deemter, M.; Quaas, A.; Walsh, S.; Benten, D.; Forbes, S.J.; Wells, R.G.; Iredale, J.P. Matrix Stiffness Modulates Proliferation, Chemotherapeutic Response, and Dormancy in Hepatocellular Carcinoma Cells. Hepatology 2011, 53, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Tachi, Y.; Hirai, T.; Kojima, Y.; Ishizu, Y.; Honda, T.; Kuzuya, T.; Hayashi, K.; Ishigami, M.; Goto, H. Liver Stiffness Measurement Predicts Hepatocellular Carcinoma Development in Patients Treated with Direct-Acting Antivirals: Liver Stiffness and Cancer Development. JGH Open 2017, 1, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, J.; Chen, B.; Wang, K.; Tang, Y.; Liang, X. Plasticity of Cancer Cell Invasion: Patterns and Mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Condeelis, J. Regulation of the Actin Cytoskeleton in Cancer Cell Migration and Invasion. Biochim. Biophys. Acta BBA - Mol. Cell Res. 2007, 1773, 642–652. [Google Scholar] [CrossRef]
- Ezzoukhry, Z.; Henriet, E.; Piquet, L.; Boyé, K.; Bioulac-Sage, P.; Balabaud, C.; Couchy, G.; Zucman-Rossi, J.; Moreau, V.; Saltel, F. TGF-Β1 Promotes Linear Invadosome Formation in Hepatocellular Carcinoma Cells, through DDR1 up-Regulation and Collagen I Cross-Linking. Eur. J. Cell Biol. 2016, 95, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhu, E.; Zhang, Y. Advances of Cancer-Associated Fibroblasts in Liver Cancer. Biomark. Res. 2022, 10, 59. [Google Scholar] [CrossRef]
- Kubo, N.; Araki, K.; Kuwano, H.; Shirabe, K. Cancer-Associated Fibroblasts in Hepatocellular Carcinoma. World J. Gastroenterol. 2016, 22, 6841. [Google Scholar] [CrossRef]
- Brenner, D.A.; Waterboer, T.; Choi, S.K.; Lindquist, J.N.; Stefanovic, B.; Burchardt, E.; Yamauchi, M.; Gillan, A.; Rippe, R.A. New Aspects of Hepatic Fibrosis. J. Hepatol. 2000, 32, 32–38. [Google Scholar] [CrossRef]
- Török, N.J. Recent Advances in the Pathogenesis and Diagnosis of Liver Fibrosis. J. Gastroenterol. 2008, 43, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Luedde, T.; Trautwein, C. Inflammatory Pathways in Liver Homeostasis and Liver Injury. Clin. Rev. Allergy Immunol. 2009, 36, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-Y.; Chuang, Y.-H.; Chuang, W.-L. Cancer-Associated Fibroblasts up-Regulate CCL2, CCL26, IL6 and LOXL2 Genes Related to Promotion of Cancer Progression in Hepatocellular Carcinoma Cells. Biomed. Pharmacother. 2012, 66, 525–529. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, J.; Li, J.; Zhu, Z.; Cui, Z.; Liu, R.; Lu, R.; Yao, Z.; Xu, Q. Cancer Associated Fibroblast–Derived CCL5 Promotes Hepatocellular Carcinoma Metastasis through Activating HIF1α/ZEB1 Axis. Cell Death Dis. 2022, 13, 478. [Google Scholar] [CrossRef]
- Xing, X.; Wang, Y.; Zhang, X.; Gao, X.; Li, M.; Wu, S.; Zhao, Y.; Chen, J.; Gao, D.; Chen, R.; et al. Matrix Stiffness-mediated Effects on Macrophages Polarization and Their LOXL2 Expression. FEBS J. 2021, 288, 3465–3477. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Shi, Y.; Zhang, M.; Goswami, S.; Afridi, S.; Meng, L.; Ma, J.; Chen, Y.; Lin, Y.; Zhang, J.; et al. Global Immune Characterization of HBV/HCV-Related Hepatocellular Carcinoma Identifies Macrophage and T-Cell Subsets Associated with Disease Progression. Cell Discov. 2020, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Zhang, S.; He, G.; Liu, J.; Fan, Q.; et al. Targeting Tumor-Associated Macrophages in Hepatocellular Carcinoma: Biology, Strategy, and Immunotherapy. Cell Death Discov. 2023, 9, 65. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-Metastatic Niches: Organ-Specific Homes for Metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Park, H.J.; Gusarova, G.; Wang, Z.; Carr, J.R.; Li, J.; Kim, K.; Qiu, J.; Park, Y.; Williamson, P.R.; Hay, N.; et al. Deregulation of FoxM1b Leads to Tumour Metastasis. EMBO Mol. Med. 2011, 3, 21–34. [Google Scholar] [CrossRef]
- Polyak, K.; Weinberg, R.A. Transitions between Epithelial and Mesenchymal States: Acquisition of Malignant and Stem Cell Traits. Nat. Rev. Cancer 2009, 9, 265–273. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Mashita, N.; Yamada, S.; Nakayama, G.; Tanaka, C.; Iwata, N.; Kanda, M.; Kobayashi, D.; Fujii, T.; Sugimoto, H.; Koike, M.; et al. Epithelial to Mesenchymal Transition Might Be Induced via CD44 Isoform Switching in Colorectal Cancer: EMT and CD44 in Colorectal Cancer. J. Surg. Oncol. 2014, 110, 745–751. [Google Scholar] [CrossRef]
- Yamada, S.; Fuchs, B.C.; Fujii, T.; Shimoyama, Y.; Sugimoto, H.; Nomoto, S.; Takeda, S.; Tanabe, K.K.; Kodera, Y.; Nakao, A. Epithelial-to-Mesenchymal Transition Predicts Prognosis of Pancreatic Cancer. Surgery 2013, 154, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Yamada, S.; Fuchs, B.C.; Fujii, T.; Nakayama, G.; Sugimoto, H.; Koike, M.; Fujiwara, M.; Tanabe, K.K.; Kodera, Y. Epithelial-to-Mesenchymal Transition Predicts Prognosis in Clinical Gastric Cancer: EMT in Clinical Gastric Cancer. J. Surg. Oncol. 2014, 109, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Christofori, G.; Semb, H. The Role of the Cell-Adhesion Molecule E-Cadherin as a Tumour-Suppressor Gene. Trends Biochem. Sci. 1999, 24, 73–76. [Google Scholar] [CrossRef]
- Peinado, H.; Del Carmen Iglesias-de La Cruz, M.; Olmeda, D.; Csiszar, K.; Fong, K.S.K.; Vega, S.; Nieto, M.A.; Cano, A.; Portillo, F. A Molecular Role for Lysyl Oxidase-like 2 Enzyme in Snail Regulation and Tumor Progression. EMBO J. 2005, 24, 3446–3458. [Google Scholar] [CrossRef]
- Cuevas, E.P.; Moreno-Bueno, G.; Canesin, G.; Santos, V.; Portillo, F.; Cano, A. LOXL2 Catalytically Inactive Mutants Mediate Epithelial-to-Mesenchymal Transition. Biol. Open 2014, 3, 129–137. [Google Scholar] [CrossRef]
- Ninomiya, G.; Yamada, S.; Hayashi, M.; Takeda, S.; Suenaga, M.; Takami, H.; Kanda, M.; Iwata, N.; Niwa, Y.; Tanaka, C.; et al. Significance of Lysyl Oxidase-like�2 Gene Expression on the Epithelial-mesenchymal Status of Hepatocellular Carcinoma. Oncol. Rep. 2018. [Google Scholar] [CrossRef]
- Postovit, L.-M.; Abbott, D.E.; Payne, S.L.; Wheaton, W.W.; Margaryan, N.V.; Sullivan, R.; Jansen, M.K.; Csiszar, K.; Hendrix, M.J.C.; Kirschmann, D.A. Hypoxia/Reoxygenation: A Dynamic Regulator of Lysyl Oxidase-Facilitated Breast Cancer Migration. J. Cell. Biochem. 2008, 103, 1369–1378. [Google Scholar] [CrossRef]
- Schietke, R.; Warnecke, C.; Wacker, I.; Schödel, J.; Mole, D.R.; Campean, V.; Amann, K.; Goppelt-Struebe, M.; Behrens, J.; Eckardt, K.-U.; et al. The Lysyl Oxidases LOX and LOXL2 Are Necessary and Sufficient to Repress E-Cadherin in Hypoxia. J. Biol. Chem. 2010, 285, 6658–6669. [Google Scholar] [CrossRef]
- Tse, A.P.-W.; Sze, K.M.-F.; Shea, Q.T.-K.; Chiu, E.Y.-T.; Tsang, F.H.-C.; Chiu, D.K.-C.; Zhang, M.S.; Lee, D.; Xu, I.M.-J.; Chan, C.Y.-K.; et al. Hepatitis Transactivator Protein X Promotes Extracellular Matrix Modification through HIF/LOX Pathway in Liver Cancer. Oncogenesis 2018, 7, 44. [Google Scholar] [CrossRef]
- Fan, Z.; Zheng, W.; Li, H.; Wu, W.; Liu, X.; Sun, Z.; Hu, H.; Du, L.; Jia, Q.; Liu, Q. LOXL2 Upregulates Hypoxia-inducible Factor-1α Signaling through Snail-FBP1 Axis in Hepatocellular Carcinoma Cells. Oncol. Rep. 2020. [Google Scholar] [CrossRef]
- Pang, R.W.C.; Joh, J.W.; Johnson, P.J.; Monden, M.; Pawlik, T.M.; Poon, R.T.P. Biology of Hepatocellular Carcinoma. Ann. Surg. Oncol. 2008, 15, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhou, H.; Zhang, C.; Shang, L.; Zhang, L.; Xu, J.; Zheng, L.; Yuan, Y.; Guo, R.; Jia, W.; et al. A Novel Vascular Pattern Promotes Metastasis of Hepatocellular Carcinoma in an Epithelial–Mesenchymal Transition–Independent Manner. Hepatology 2015, 62, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. Tumor Angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.-M.; Bird, D.; Welti, J.C.; Gourlaouen, M.; Lang, G.; Murray, G.I.; Reynolds, A.R.; Cox, T.R.; Erler, J.T. Lysyl Oxidase Plays a Critical Role in Endothelial Cell Stimulation to Drive Tumor Angiogenesis. Cancer Res. 2013, 73, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Annese, T.; Ruggieri, S.; Tamma, R.; Crivellato, E. Limitations of Anti-Angiogenic Treatment of Tumors. Transl. Oncol. 2019, 12, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-D.; Sun, H.-C. Emerging Agents and Regimens for Hepatocellular Carcinoma. J. Hematol. Oncol.J Hematol Oncol 2019, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of Resistance to Anti-Angiogenic Therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Zheng, N.; Zhang, S.; Wu, W.; Zhang, N.; Wang, J. Regulatory Mechanisms and Therapeutic Targeting of Vasculogenic Mimicry in Hepatocellular Carcinoma. Pharmacol. Res. 2021, 166, 105507. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, D.; Zhao, N.; Zhao, X. Epithelial-to-Endothelial Transition and Cancer Stem Cells: Two Cornerstones of Vasculogenic Mimicry in Malignant Tumors. Oncotarget 2017, 8, 30502–30510. [Google Scholar] [CrossRef]
- Sun, T.; Sun, B.; Zhao, X.; Zhao, N.; Dong, X.; Che, N.; Yao, Z.; Ma, Y.; Gu, Q.; Zong, W.; et al. Promotion of Tumor Cell Metastasis and Vasculogenic Mimicry by Way of Transcription Coactivation by Bcl-2 and Twist1: A Study of Hepatocellular Carcinoma. Hepatology 2011, 54, 1690–1706. [Google Scholar] [CrossRef]
- Wong, C.C.-L.; Tse, A.P.-W.; Huang, Y.-P.; Zhu, Y.-T.; Chiu, D.K.-C.; Lai, R.K.-H.; Au, S.L.-K.; Kai, A.K.-L.; Lee, J.M.-F.; Wei, L.L.; et al. Lysyl Oxidase-like 2 Is Critical to Tumor Microenvironment and Metastatic Niche Formation in Hepatocellular Carcinoma. Hepatology 2014, 60, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Lian, W.-S.; Wang, F.-S.; Wang, P.-W.; Lin, H.-Y.; Tsai, M.-C.; Yang, Y.-L. MiR-29a Curbs Hepatocellular Carcinoma Incidence via Targeting of HIF-1α and ANGPT2. Int. J. Mol. Sci. 2022, 23, 1636. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Yang, Y.; Zhao, J. LncRNA CARMN Affects Hepatocellular Carcinoma Prognosis by Regulating the MiR-192-5p/LOXL2 Axis. Oxid. Med. Cell. Longev. 2022, 2022, 1–24. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, B.; Wang, H.; Chen, L. New Knowledge of the Mechanisms of Sorafenib Resistance in Liver Cancer. Acta Pharmacol. Sin. 2017, 38, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Hajdú, I.; Kardos, J.; Major, B.; Fabó, G.; Lőrincz, Z.; Cseh, S.; Dormán, G. Inhibition of the LOX Enzyme Family Members with Old and New Ligands. Selectivity Analysis Revisited. Bioorg. Med. Chem. Lett. 2018, 28, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Rodríguez-Sinovas, A.; Martínez-González, J. Lysyl Oxidase as a Potential Therapeutic Target. Drug News Perspect. 2008, 21, 218–224. [Google Scholar] [CrossRef]
- Barry-Hamilton, V.; Spangler, R.; Marshall, D.; McCauley, S.; Rodriguez, H.M.; Oyasu, M.; Mikels, A.; Vaysberg, M.; Ghermazien, H.; Wai, C.; et al. Allosteric Inhibition of Lysyl Oxidase–like-2 Impedes the Development of a Pathologic Microenvironment. Nat. Med. 2010, 16, 1009–1017. [Google Scholar] [CrossRef]
- Rodriguez, H.M.; Vaysberg, M.; Mikels, A.; McCauley, S.; Velayo, A.C.; Garcia, C.; Smith, V. Modulation of Lysyl Oxidase-like 2 Enzymatic Activity by an Allosteric Antibody Inhibitor. J. Biol. Chem. 2010, 285, 20964–20974. [Google Scholar] [CrossRef]
- Verstovsek, S.; Savona, M.R.; Mesa, R.A.; Dong, H.; Maltzman, J.D.; Sharma, S.; Silverman, J.; Oh, S.T.; Gotlib, J. A Phase 2 Study of Simtuzumab in Patients with Primary, Post-Polycythaemia Vera or Post-Essential Thrombocythaemia Myelofibrosis. Br. J. Haematol. 2017, 176, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Abdelmalek, M.F.; Caldwell, S.; Shiffman, M.L.; Diehl, A.M.; Ghalib, R.; Lawitz, E.J.; Rockey, D.C.; Schall, R.A.; Jia, C.; et al. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Benson, A.B.; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma. The Oncologist 2017, 22, 243-e23. [Google Scholar] [CrossRef] [PubMed]
- Pharos. Available online: https://pharos.nih.gov/targets/LOXL2 (accessed on 10.06.2023.).
- Sampath Narayanan, A.; Siegel, R.C.; Martin, G.R. On the Inhibition of Lysyl Oxidase by β-Aminopropionitrile. Biochem. Biophys. Res. Commun. 1972, 46, 745–751. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, N.; Liu, H.; Zhao, L.; Liu, J.; Wan, J.; Wu, W.; Lei, H.; Liu, R.; Han, M. Lysyl Oxidase Inhibition via β-Aminoproprionitrile Hampers Human Umbilical Vein Endothelial Cell Angiogenesis and Migration In�vitro. Mol. Med. Rep. 2018. [Google Scholar] [CrossRef]
- Kirschmann, D.A.; Seftor, E.A.; Fong, S.F.T.; Nieva, D.R.C.; Sullivan, C.M.; Edwards, E.M.; Sommer, P.; Csiszar, K.; Hendrix, M.J.C. A Molecular Role for Lysyl Oxidase in Breast Cancer Invasion. Cancer Res. 2002, 62, 4478–4483. [Google Scholar] [PubMed]
- Abourbih, D.A.; Di Cesare, S.; Orellana, M.E.; Antecka, E.; Martins, C.; Petruccelli, L.A.; Burnier, M.N. Lysyl Oxidase Expression and Inhibition in Uveal Melanoma. Melanoma Res. 2010, 20, 97–106. [Google Scholar] [CrossRef]
- Bondareva, A.; Downey, C.M.; Ayres, F.; Liu, W.; Boyd, S.K.; Hallgrimsson, B.; Jirik, F.R. The Lysyl Oxidase Inhibitor, β-Aminopropionitrile, Diminishes the Metastatic Colonization Potential of Circulating Breast Cancer Cells. PLoS ONE 2009, 4, e5620. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Li, W.; Chen, J.; Xiao, X.; Wang, Y.; Yan, G.; Chen, L. Inactivation of Lysyl Oxidase by β-Aminopropionitrile Inhibits Hypoxia-Induced Invasion and Migration of Cervical Cancer Cells. Oncol. Rep. 2013, 29, 541–548. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, C.-C.; Ni, B.; Zhang, Z.-Z.; Jiang, S.-H.; Hu, L.-P.; Wang, X.; Zhang, X.-X.; Huang, P.-Q.; Yang, Q.; et al. Lysyl Oxidase Promotes Liver Metastasis of Gastric Cancer via Facilitating the Reciprocal Interactions between Tumor Cells and Cancer Associated Fibroblasts. eBioMedicine 2019, 49, 157–171. [Google Scholar] [CrossRef]
- Liu, S.B.; Ikenaga, N.; Peng, Z.; Sverdlov, D.Y.; Greenstein, A.; Smith, V.; Schuppan, D.; Popov, Y. Lysyl Oxidase Activity Contributes to Collagen Stabilization during Liver Fibrosis Progression and Limits Spontaneous Fibrosis Reversal in Mice. FASEB J. 2016, 30, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Findlay, A.; Turner, C.; Schilter, H.; Deodhar, M.; Zhou, W.; Perryman, L.; Foot, J.; Zahoor, A.; Yao, Y.; Hamilton, R.; et al. An Activity-Based Bioprobe Differentiates a Novel Small Molecule Inhibitor from a LOXL2 Antibody and Provides Renewed Promise for Anti-Fibrotic Therapeutic Strategies. Clin. Transl. Med. 2021, 11, e572. [Google Scholar] [CrossRef] [PubMed]
- Schilter, H.; Findlay, A.D.; Perryman, L.; Yow, T.T.; Moses, J.; Zahoor, A.; Turner, C.I.; Deodhar, M.; Foot, J.S.; Zhou, W.; et al. The Lysyl Oxidase like 2/3 Enzymatic Inhibitor, PXS-5153A, Reduces Crosslinks and Ameliorates Fibrosis. J. Cell. Mol. Med. 2019, 23, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.H.; Rowbottom, M.W.; Lonergan, D.; Darlington, J.; Prodanovich, P.; King, C.D.; Evans, J.F.; Bain, G. Small Molecule Lysyl Oxidase-like 2 (LOXL2) Inhibitors: The Identification of an Inhibitor Selective for LOXL2 over LOX. ACS Med. Chem. Lett. 2017, 8, 423–427. [Google Scholar] [CrossRef]
| Type | Agent | Target | References |
|---|---|---|---|
| monoclonal antibody |
AB0023 | LOXL2 | [46,122,123] |
| AB0024 | LOXL2 | [124,125,126] | |
| small-molecule inhibitor |
BAPN | LOX/LOXL1-4 | [101,134,135] |
| LOXL2-IN-1 | LOXL2 | [105] | |
| PXS-5338 | LOXL2 | [136] | |
| PXS-5382 | LOXL2 | [136] | |
| PXS-5878 | LOXL2 | [136] | |
| PXS-5153A | LOXL2/LOXL3 | [137] | |
| (2-chloropyridin-4-yl) methanamine | LOXL2 | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





