Submitted:
14 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
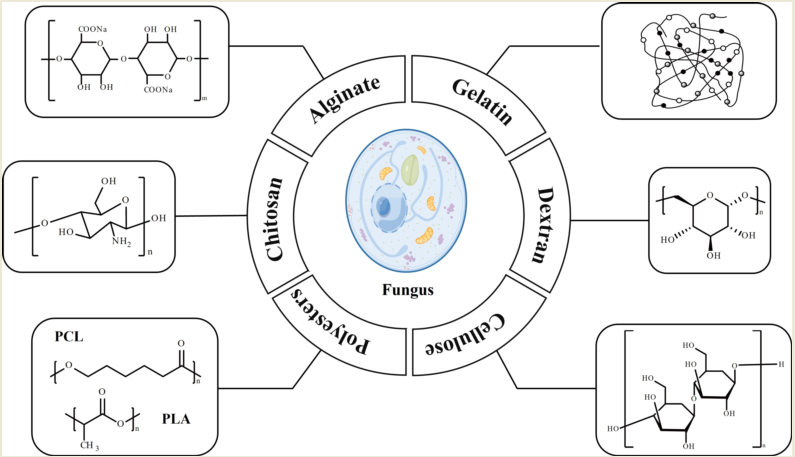
2. Polymers for Antifungal Drug Delivery Nanosystems
2.1. Chitosan
2.2. Alginate
2.3. Gelatin
2.4. Dextran
2.5. Cellulose
2.6. Polyesters
| Polymers | Other components | Loaded drugs | Fungal | Zeta potential(mV) | Diameters | Loading content (LC) | Encapsulation efficiency (EE) | Drug release | PDI | Antifungal efficacy | In vivo study | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLA | / | Bovine Lactoferrin | Aspergillus nidulans | / | 495 ± 127 nm | 20wt% | / | 17.7±4.4% (7weeks) | / | Significantly inhibit mycelium growth | N | [131] |
| PLA | Mesoporous silica nanoparticles | Levofloxacin | Candida albicans | / | 5.4nm | 33.3wt% | 0.9832 | 92% (280min) | / | ZOI: 43 mm at 72 h | N | [140] |
| PLA | Polyacrylonitrile/cellulose | Chitin | Aspergillus niger | -10.5±1.3 | 350-400nm | 15 wt% | / | / | / | > 99% for fungal spores (> 2 µm) | N | [132] |
| PLA | Cellulose nanofibrils | Silver nanoparticles | Fusarium/Aspergillus/ Curvularia | / | 1.44±0.32μm | <0.1wt% | / | / | / | inhibition > 95% | N | [129] |
| PLA | Poly(lactic-co-glycolic acid) | Amp B | Candida albicans | -10.9 ± 1.9 | 343.17 ± 24.74nm | 0.057 | 0.85 | 45.6% (48h) | / | inhibition: 99.65% | Y | [125] |
| PLA | Poly(lactic-co-glycolic acid) | Amp B | Candida albicans | -10.9 ± 1.9 | 287.8 ± 8.64 | 5.7 ± 0.12% | 85 ± 2.4% | / | 85 ± 2.4 | diffusion distance: 1.55 ± 0.11 μm | Y | [135] |
| PLA | / | Carvacrol | Candida albicans | 1.54 ± 1.07 μm | 28 wt% | / | 90% (150h) | / | inhibition: 92–96% | N | [128] | |
| PLA | PEG | Amp B | Candida albicans | / | 25.3 ± 2.7 nm | 40mg/batch | 56.5 ± 3.9% | 59.4 ± 5.7% (24h) | / | inhibition: 90.8% | Y | [136] |
| PLA | Poly(lactic-co-glycolic acid) | Butenafine | Candida albicans, Aspergillus niger | -20.3 | 267.21 ± 3.54 nm | 0.01 | 72.43 ± 3.11% | 42.76 ± 2.87% (48h) | 0.227 | ZOI: 20.54 ± 1.8 mm at 48 h | N | [134] |
| PLA | Cashew gum | Amp B | Candida albicans | -24.3 ± 2.3 | 1025 ± 143 nm | 0.091 | 0.897 | 52.2 ± 3.9% (168h) | 0.307 | MIC: 0.25 μg/mL | N | [133] |
| PLA | / | Hypocrellin A | Candida auris | / | 699 nm | 0.02 | / | / | inhibition: 99.9% | Y | [130] | |
| PCL | Squalene | Squalene | Candida albicans | -48 ± 2.00 | 254 ± 6.81 nm | 30.98 ± 2.20% | 86.09 ± 0.28% | 85% (4 h) | 0.23 ± 3.03 | inhibition: 92.47% | Y | [141] |
| PCL | / | Peppermint oil | Candida albicans/ Aspergillus niger | / | / | / | / | / | / | ZOI: 20.6 mm at 48 h | N | [142] |
| PCL | / | Essential oils | Candida albicans | -11±1 | 200 nm | 52±3% | 84±6 | / | 0.09±0.02 | inhibition: 89% | N | [143] |
| PCL | / | 4-Nerolidylcatechol | Microsporum canis | -9.30 ± 0.17 | 143.5 ± 1.36 nm | / | 1 | / | 0.232±0.00 | MIC: 0.625 μg/mL. MFC: 0.625 μg/mL. | Y | [138] |
| PCL | / | Miconazole nitrate | Candida albicans | –31.22 ± 2.1 | 89 ± 3.63 nm | 24.1 ± 0.65% | 98 ± 5.21% | 90% (48h) | 0.35 | MIC: 0.75 μg/mL | N | [144] |
| PCL | / | Diphenyl diselenide | Candida albicans | -10.1 ± 2.21 | 240 ± 52 nm | 5.07±0.14mg/g | 0.98 | / | 0.17± 0.08 | MIC: 0.5 μg/mL | Y | [145] |
| PCL | / | Amp B | / | 0 | 183 nm | 5mg/mL | 0.86 | 78% (48h) | 0.211 | / | N | [146] |
| PCL | Pluronic | Chloramphenicol | Candida | -22.4 | 123.5 nm | / | 0.983 | 88% (96h) | / | MIC: 2 μg/mL | Y | [147] |
| PCL | Polyethyleglicol | Am B | Albicans/ Glabrata/ Auris | -8.8±0.1 | 226 nm | 16.40 ± 0.18 wt% | / | 38% (100h) | 0.25 | MIC: 0.11 μg/mL | N | [148] |
3. Summary and Conclusions
Acknowledgment
References
- Köhler, J.R.; Casadevall, A.; Perfect, J. The spectrum of fungi that infects humans. Cold Spring Harb Perspect Med 2014, 5, a019273. [CrossRef]
- American Academy of Microbiology Colloquia Reports. In One Health: Fungal Pathogens of Humans, Animals, and Plants: Report on an American Academy of Microbiology Colloquium held in Washington, DC, on October 18, 2017; American Society for Microbiology.Copyright 2019 American Academy of Microbiology.: Washington (DC), 2019.
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. The Lancet Infectious Diseases 2018, 18, e339-e347. [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Emergence of Candida auris: An International Call to Arms. Clinical Infectious Diseases 2016, 64, 141-143. [CrossRef]
- Chan, J.F.; Lau, S.K.; Yuen, K.Y.; Woo, P.C. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 2016, 5, e19. [CrossRef]
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev 2019, 33. [CrossRef]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infectious Disease Clinics of North America 2016, 30, 103-124. [CrossRef]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Mucormycosis – from the pathogens to the disease. Clinical Microbiology and Infection 2014, 20, 60-66. [CrossRef]
- Rokas, A. Evolution of the human pathogenic lifestyle in fungi. Nature Microbiology 2022, 7, 607-619. [CrossRef]
- Du, W.; Gao, Y.; Liu, L.; Sai, S.; Ding, C. Striking Back against Fungal Infections: The Utilization of Nanosystems for Antifungal Strategies. International Journal of Molecular Sciences 2021, 22. [CrossRef]
- Dellenbach, P.; Thomas, J.L.; Guerin, V.; Ochsenbein, E.; Contet-Audonneau, N. Topical treatment of vaginal candidosis with sertaconazole and econazole sustained-release suppositories. International Journal of Gynecology & Obstetrics 2000, 71, 47-52. [CrossRef]
- Gayam, V.; Khalid, M.; Dahal, S.; Garlapati, P.; Gill, A. Hyperacute liver injury following intravenous fluconazole: A rare case of dose-independent hepatotoxicity. Journal of Family Medicine and Primary Care 2018, 7.
- Young, G.A.R.; Bosly, A.; Gibbs, D.L.; Durrant, S. A double-blind comparison of fluconazole and nystatin in the prevention of candidiasis in patients with leukaemia. European Journal of Cancer 1999, 35, 1208-1213. [CrossRef]
- Torre, P.d.l.; Meyer, D.K.; Reboli, A.C. Anidulafungin: A novel echinocandin for candida infections. Future Microbiology 2008, 3, 593-601. [CrossRef]
- Singal, A. Butenafine and superficial mycoses: Current status. Expert Opinion on Drug Metabolism & Toxicology 2008, 4, 999-1005. [CrossRef]
- Gupta, A.K.; Stec, N.; Summerbell, R.C.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. Onychomycosis: A review. Journal of the European Academy of Dermatology and Venereology 2020, 34, 1972-1990. [CrossRef]
- Ryder, N.S. Squalene epoxidase as a target for the allylamines. Biochemical Society Transactions 1991, 19, 774-777. [CrossRef]
- Crawford, F.; Hollis, S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database of Systematic Reviews 2007. [CrossRef]
- Mohd-Assaad, N.; McDonald, B.A.; Croll, D. Multilocus resistance evolution to azole fungicides in fungal plant pathogen populations. Mol Ecol 2016, 25, 6124-6142. [CrossRef]
- Mohr, J.; Johnson, M.; Cooper, T.; Lewis, J.S.; Ostrosky-Zeichner, L. Current Options in Antifungal Pharmacotherapy. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2008, 28, 614-645. [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 2014, 4. [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J Environ Pathol Toxicol Oncol 2018, 37, 209-230. [CrossRef]
- Mu, L.; Feng, S.S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol®): PLGA nanoparticles containing vitamin E TPGS. Journal of Controlled Release 2003, 86, 33-48. [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology 2007, 2, 751-760. [CrossRef]
- Choi, H.; Lee, D.G. Lycopene induces apoptosis in Candida albicans through reactive oxygen species production and mitochondrial dysfunction. Biochimie 2015, 115, 108-115. [CrossRef]
- Erdoğar, N.; Akkın, S.; Bilensoy, E. Nanocapsules for Drug Delivery: An Updated Review of the Last Decade. Recent Pat Drug Deliv Formul 2018, 12, 252-266. [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: Synthesis, characterization and biomedical applications. Artificial Cells, Nanomedicine, and Biotechnology 2017, 45, 788-799. [CrossRef]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. International Journal of Biological Macromolecules 2018, 114, 426-433. [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chemical Research in Toxicology 2012, 25, 2265-2284. [CrossRef]
- Frank, L.A.; Contri, R.V.; Beck, R.C.; Pohlmann, A.R.; Guterres, S.S. Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2015, 7, 623-639. [CrossRef]
- Liu, R.; Zhao, J.; Han, Q.; Hu, X.; Wang, D.; Zhang, X.; Yang, P. One-Step Assembly of a Biomimetic Biopolymer Coating for Particle Surface Engineering. Advanced Materials 2018, 30, 1802851. [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Marine Drugs 2015, 13, 5156-5186.
- Zhang, H.; Wu, X.; Quan, L.; Ao, Q. Characteristics of Marine Biomaterials and Their Applications in Biomedicine. Marine Drugs 2022, 20, 372.
- Chan, L.C.; Cox, B.G. Kinetics of Amide Formation through Carbodiimide/N-Hydroxybenzotriazole (HOBt) Couplings. The Journal of Organic Chemistry 2007, 72, 8863-8869. [CrossRef]
- de Carvalho, S.Y.B.; Almeida, R.R.; Pinto, N.A.R.; de Mayrinck, C.; Vieira, S.S.; Haddad, J.F.; Leitão, A.A.; Guimarães, L.G.d.L. Encapsulation of essential oils using cinnamic acid grafted chitosan nanogel: Preparation, characterization and antifungal activity. International Journal of Biological Macromolecules 2021, 166, 902-912. [CrossRef]
- El Rabey, H.A.; Almutairi, F.M.; Alalawy, A.I.; Al-Duais, M.A.; Sakran, M.I.; Zidan, N.S.; Tayel, A.A. Augmented control of drug-resistant Candida spp. via fluconazole loading into fungal chitosan nanoparticles. International Journal of Biological Macromolecules 2019, 141, 511-516. [CrossRef]
- Hassan, Y.A.; Khedr, A.I.M.; Alkabli, J.; Elshaarawy, R.F.M.; Nasr, A.M. Co-delivery of imidazolium Zn(II)salen and Origanum Syriacum essential oil by shrimp chitosan nanoparticles for antimicrobial applications. Carbohydrate Polymers 2021, 260, 117834. [CrossRef]
- Liu, Q.; Gao, L.; Qin, Y.; Ji, N.; Dai, L.; Xiong, L.; Sun, Q. Incorporation of oxidized debranched starch/chitosan nanoparticles for enhanced hydrophobicity of corn starch films. Food Packaging and Shelf Life 2023, 35, 101032. [CrossRef]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Ling, C.C.S.; Ambu, S.P.; Davamani, F. Cationic chitosan-propolis nanoparticles alter the zeta potential of S. epidermidis, inhibit biofilm formation by modulating gene expression and exhibit synergism with antibiotics. PLoS ONE 2019, 14, e0213079. [CrossRef]
- Biao, L.; Tan, S.; Wang, Y.; Guo, X.; Fu, Y.; Xu, F.; Zu, Y.; Liu, Z. Synthesis, characterization and antibacterial study on the chitosan-functionalized Ag nanoparticles. Materials Science and Engineering: C 2017, 76, 73-80. [CrossRef]
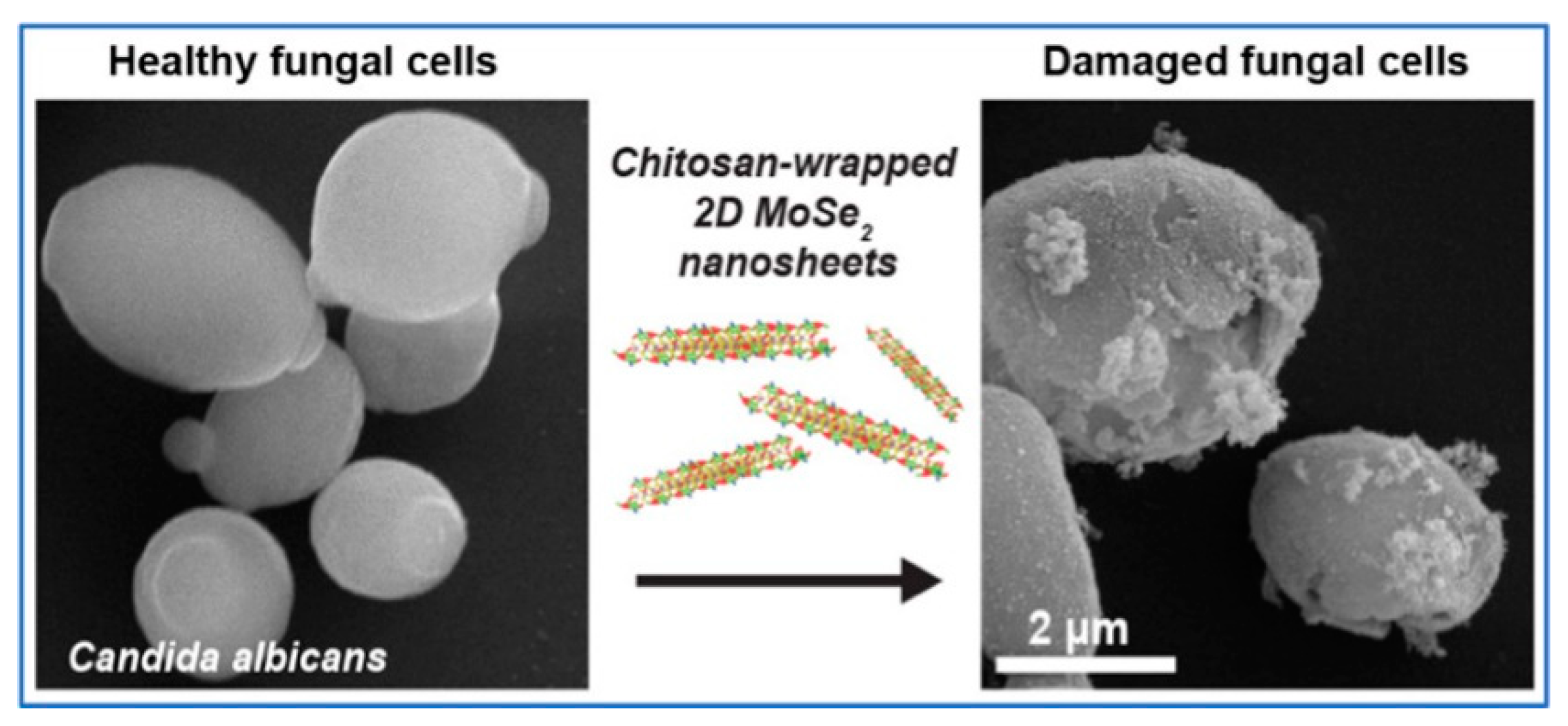
- Saha, S.; Gilliam, M.S.; Wang, Q.H.; Green, A.A. Eradication of Fungi Using MoSe2/Chitosan Nanosheets. ACS Applied Nano Materials 2022, 5, 133-148. [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Dehghan, S.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. International Journal of Biological Macromolecules 2013, 62, 582-588. [CrossRef]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.M.; Sagis, L.M.C. Preparation methods of alginate nanoparticles. Advances in Colloid and Interface Science 2014, 209, 163-171. [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Marine Drugs 2021, 19, 264.
- Wei, Q.; Zhou, J.; An, Y.; Li, M.; Zhang, J.; Yang, S. Modification, 3D printing process and application of sodium alginate based hydrogels in soft tissue engineering: A review. International Journal of Biological Macromolecules 2023, 232, 123450. [CrossRef]
- Wang, Y.; Zhao, Y.; He, J.; Sun, C.; Lu, W.; Zhang, Y.; Fang, Y. Doubling growth of egg-box structure during Calcium-mediated molecular assembly of alginate. Journal of Colloid and Interface Science 2023, 634, 747-756. [CrossRef]
- Zheng, J.; Han, Y.; Wei, L.; Li, M.; Zhu, L. Sludge-derived biopolymers for in-situ synthesis of magnetic ALE-Fe-Zr composites for phosphate removal. Chemical Engineering Journal 2023, 456, 140842. [CrossRef]
- Augustine, R.; Rajarathinam, K. Synthesis and characterization of silver nanoparticles and its immobilization on alginate coated sutures for the prevention of surgical wound infections and the in vitro release studies. International Journal of Nanodimensions 2012, 2, 205-212.
- Maestrelli, F.; Jug, M.; Cirri, M.; Kosalec, I.; Mura, P. Characterization and microbiological evaluation of chitosan-alginate microspheres for cefixime vaginal administration. Carbohydrate Polymers 2018, 192, 176-183. [CrossRef]
- Abid, S.; Uzair, B.; Niazi, M.B.K.; Fasim, F.; Bano, S.A.; Jamil, N.; Batool, R.; Sajjad, S. Bursting the Virulence Traits of MDR Strain of Candida albicans Using Sodium Alginate-based Microspheres Containing Nystatin-loaded MgO/CuO Nanocomposites. Int J Nanomedicine 2021, 16, 1157-1174. [CrossRef]
- Ma, S.; Moser, D.; Han, F.; Leonhard, M.; Schneider-Stickler, B.; Tan, Y. Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida albicans and Staphylococcus aureus. Carbohydrate Polymers 2020, 241, 116254. [CrossRef]
- Andersen, T.; Mishchenko, E.; Flaten, G.E.; Sollid, J.U.E.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Marine Drugs 2017, 15, 64.
- Alshubaily, F.A.; Al-Zahrani, M.H. Appliance of fungal chitosan/ceftriaxone nano-composite to strengthen and sustain their antimicrobial potentiality against drug resistant bacteria. International Journal of Biological Macromolecules 2019, 135, 1246-1251. [CrossRef]
- Khan, M.K.; Khan, B.A.; Uzair, B.; Iram Niaz, S.; Khan, H.; Hosny, K.M.; Menaa, F. Development of Chitosan-Based Nanoemulsion Gel Containing Microbial Secondary Metabolite with Effective Antifungal Activity: In vitro and in vivo Characterizations. Int J Nanomedicine 2021, 16, 8203-8219. [CrossRef]
- Tan, Y.; Ma, S.; Ding, T.; Ludwig, R.; Lee, J.; Xu, J. Enhancing the Antibiofilm Activity of β-1,3-Glucanase-Functionalized Nanoparticles Loaded With Amphotericin B Against Candida albicans Biofilm. Frontiers in Microbiology 2022, 13. [CrossRef]
- Martín, M.J.; Calpena, A.C.; Fernández, F.; Mallandrich, M.; Gálvez, P.; Clares, B. Development of alginate microspheres as nystatin carriers for oral mucosa drug delivery. Carbohydrate Polymers 2015, 117, 140-149. [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res 2007, 24, 2198-2206. [CrossRef]
- Bhosale, V.A.; Srivastava, V.; Valamla, B.; Yadav, R.; Singh, S.B.; Mehra, N.K. Preparation and Evaluation of Modified Chitosan Nanoparticles Using Anionic Sodium Alginate Polymer for Treatment of Ocular Disease. Pharmaceutics 2022, 14. [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10. [CrossRef]
- Spadari, C.C.; de Bastiani, F.; Lopes, L.B.; Ishida, K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. Int J Nanomedicine 2019, 14, 5187-5199. [CrossRef]
- Ma, X.; Zhang, S.; Yang, Y.; Tong, Z.; Shen, T.; Yu, Z.; Xie, J.; Yao, Y.; Gao, B.; Li, Y.C.; et al. Development of multifunctional copper alginate and bio-polyurethane bilayer coated fertilizer: Controlled-release, selenium supply and antifungal. International Journal of Biological Macromolecules 2023, 224, 256-265. [CrossRef]
- Spadari, C.d.C.; de Bastiani, F.W.M.d.S.; Lopes, L.B.; Ishida, K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. International Journal of Nanomedicine 2019, 14, 5187-5199. [CrossRef]
- Ahmed, T.A.; Alzahrani, M.M.; Sirwi, A.; Alhakamy, N.A. Study the Antifungal and Ocular Permeation of Ketoconazole from Ophthalmic Formulations Containing Trans-Ethosomes Nanoparticles. Pharmaceutics 2021, 13, 151.
- Abdelghany, S.; Alkhawaldeh, M.; AlKhatib, H.S. Carrageenan-stabilized chitosan alginate nanoparticles loaded with ethionamide for the treatment of tuberculosis. Journal of Drug Delivery Science and Technology 2017, 39, 442-449. [CrossRef]
- Germovsek, E.; Barker, C.I.; Sharland, M. What do I need to know about aminoglycoside antibiotics? Archives of disease in childhood - Education & practice edition 2017, 102, 89. [CrossRef]
- Zavascki, A.P.; Goldani, L.Z.; Li, J.; Nation, R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. Journal of Antimicrobial Chemotherapy 2007, 60, 1206-1215. [CrossRef]
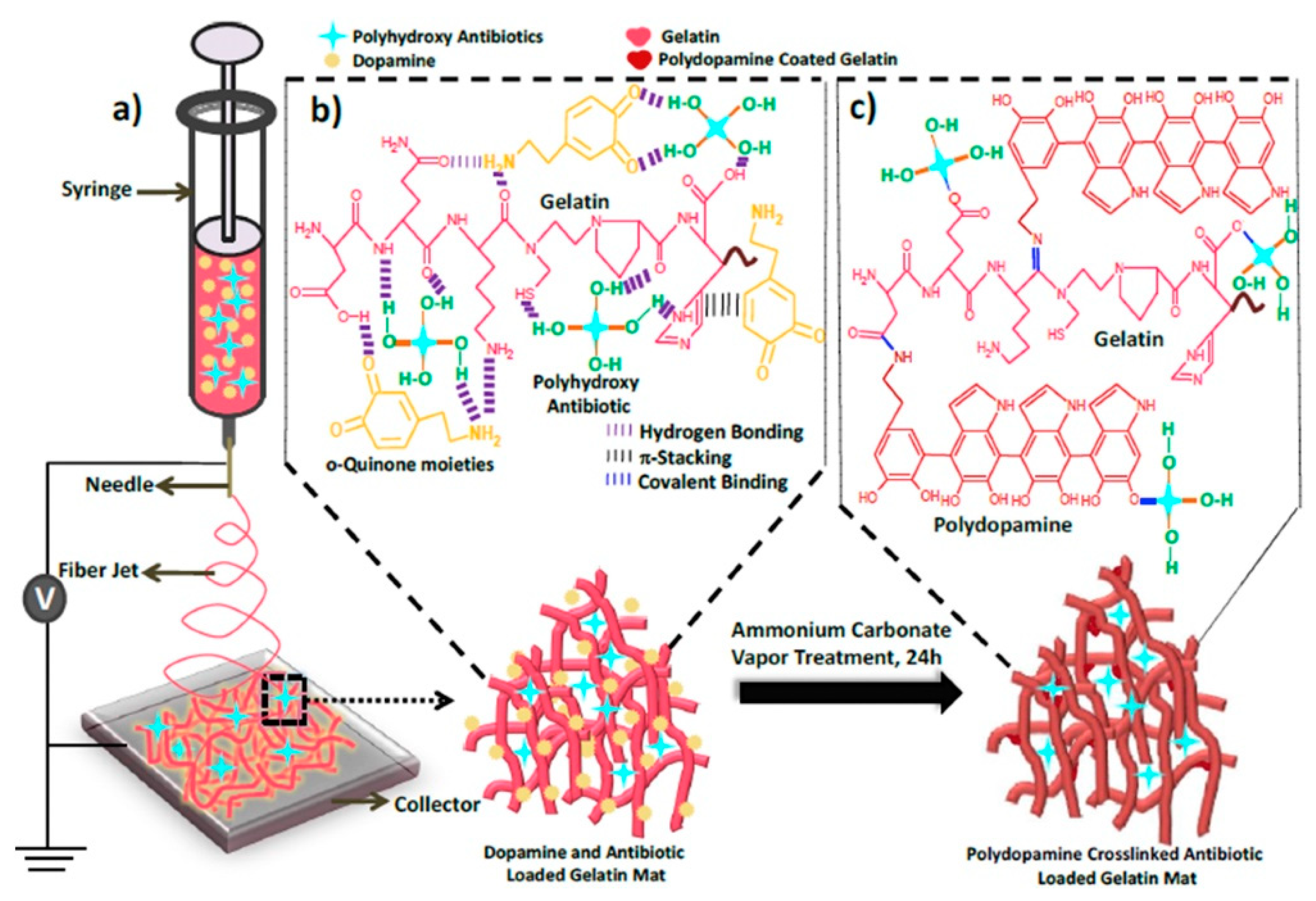
- Dhand, C.; Venkatesh, M.; Barathi, V.A.; Harini, S.; Bairagi, S.; Goh Tze Leng, E.; Muruganandham, N.; Low, K.Z.W.; Fazil, M.H.U.T.; Loh, X.J.; et al. Bio-inspired crosslinking and matrix-drug interactions for advanced wound dressings with long-term antimicrobial activity. Biomaterials 2017, 138, 153-168. [CrossRef]
- Ibrahim, H.M.; Taha, G.M.; El-Alfy, E.A.; El-Bisi, M.K. Enhancing antibacterial action of gauze by adding gelatin nanoparticles loaded with spectinomycin and chloramphenicol. Cellulose 2022, 29, 5677-5688. [CrossRef]
- Aparna, V.; Melge, A.R.; Rajan, V.K.; Biswas, R.; Jayakumar, R.; Gopi Mohan, C. Carboxymethylated ɩ-carrageenan conjugated amphotericin B loaded gelatin nanoparticles for treating intracellular Candida glabrata infections. International Journal of Biological Macromolecules 2018, 110, 140-149. [CrossRef]
- Ilhan, E.; Cesur, S.; Sulutas, R.B.; Pilavci, E.; Dalbayrak, B.; Kaya, E.; Arisan, E.D.; Tinaz, G.B.; Sengor, M.; Kijeńska-Gawrońska, E.; et al. The Role of Multilayer Electrospun Poly(Vinyl Alcohol)/Gelatin nanofibers loaded with Fluconazole and Cinnamaldehyde in the Potential Treatment of Fungal Keratitis. European Polymer Journal 2022, 176, 111390. [CrossRef]
- Asgari, Q.; Alishahi, M.; Davani, F.; Caravan, D.; Khorram, M.; Enjavi, Y.; Barzegar, S.; Esfandiari, F.; Zomorodian, K. Fabrication of amphotericin B-loaded electrospun core–shell nanofibers as a novel dressing for superficial mycoses and cutaneous leishmaniasis. International Journal of Pharmaceutics 2021, 606, 120911. [CrossRef]
- Ambrosio, J.A.R.; Pinto, B.C.d.S.; Godoy, D.d.S.; Carvalho, J.A.; Abreu, A.d.S.; da Silva, B.G.M.; Leonel, L.d.C.; Costa, M.S.; Beltrame Junior, M.; Simioni, A.R. Gelatin nanoparticles loaded methylene blue as a candidate for photodynamic antimicrobial chemotherapy applications in Candida albicans growth. Journal of Biomaterials Science, Polymer Edition 2019, 30, 1356-1373. [CrossRef]
- Wang, S.; Fontana, F.; Shahbazi, M.A.; Santos, H.A. Acetalated dextran based nano- and microparticles: Synthesis, fabrication, and therapeutic applications. Chem Commun (Camb) 2021, 57, 4212-4229. [CrossRef]
- Hernández-Rivas, M.; Guzmán, E.; Fernández-Peña, L.; Akanno, A.; Greaves, A.; Léonforte, F.; Ortega, F.; G. Rubio, R.; Luengo, G.S. Deposition of Synthetic and Bio-Based Polycations onto Negatively Charged Solid Surfaces: Effect of the Polymer Cationicity, Ionic Strength, and the Addition of an Anionic Surfactant. Colloids and Interfaces 2020, 4, 33.
- P, S.; C.R, R.; Sundaran, S.P.; Binoy, A.; Mishra, N.; A, S. In-vitro evaluation on drug release kinetics and antibacterial activity of dextran modified polyurethane fibrous membrane. International Journal of Biological Macromolecules 2019, 126, 717-730. [CrossRef]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Molecular Nutrition & Food Research 2021, 65, 1901071. [CrossRef]
- Abid, M.; Naveed, M.; Azeem, I.; Faisal, A.; Faizan Nazar, M.; Yameen, B. Colon specific enzyme responsive oligoester crosslinked dextran nanoparticles for controlled release of 5-fluorouracil. International Journal of Pharmaceutics 2020, 586, 119605. [CrossRef]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int J Biol Macromol 2019, 140, 255-264. [CrossRef]
- Delvart, A.; Moreau, C.; D'Orlando, A.; Falourd, X.; Cathala, B. Dextran-based polyelectrolyte multilayers: Effect of charge density on film build-up and morphology. Colloids Surf B Biointerfaces 2022, 210, 112258. [CrossRef]
- Vercauteren, R.; Bruneel, D.; Schacht, E.; Duncan, R. Effect of the Chemical Modification of Dextran on the Degradation by Dextranase. Journal of Bioactive and Compatible Polymers 1990, 5, 4-15. [CrossRef]
- Fu, Y.; Zhang, J.; Wang, Y.; Li, J.; Bao, J.; Xu, X.; Zhang, C.; Li, Y.; Wu, H.; Gu, Z. Reduced polydopamine nanoparticles incorporated oxidized dextran/chitosan hybrid hydrogels with enhanced antioxidative and antibacterial properties for accelerated wound healing. Carbohydrate Polymers 2021, 257, 117598. [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Haghi, M.; MacLoughlin, R.; Chellappan, D.K.; Gupta, G.; Paudel, K.R.; Hansbro, P.M.; George Oliver, B.G.; et al. Advances and applications of dextran-based nanomaterials targeting inflammatory respiratory diseases. Journal of Drug Delivery Science and Technology 2022, 74, 103598. [CrossRef]
- Chen, H.; Wang, H.; Wei, Y.; Hu, M.; Dong, B.; Fang, H.; Chen, Q. Super-resolution imaging reveals the subcellular distribution of dextran at the nanoscale in living cells. Chinese Chemical Letters 2022, 33, 1865-1869. [CrossRef]
- Chircov, C.; Ștefan, R.-E.; Dolete, G.; Andrei, A.; Holban, A.M.; Oprea, O.-C.; Vasile, B.S.; Neacșu, I.A.; Tihăuan, B. Dextran-Coated Iron Oxide Nanoparticles Loaded with Curcumin for Antimicrobial Therapies. Pharmaceutics 2022, 14, 1057.
- Cakić, M.; Glišić, S.; Nikolić, G.; Nikolić, G.M.; Cakić, K.; Cvetinov, M. Synthesis, characterization and antimicrobial activity of dextran sulphate stabilized silver nanoparticles. Journal of Molecular Structure 2016, 1110, 156-161. [CrossRef]
- Anusuya, S.; Sathiyabama, M. Preparation of β-d-glucan nanoparticles and its antifungal activity. International Journal of Biological Macromolecules 2014, 70, 440-443. [CrossRef]
- Tuchilus, C.G.; Nichifor, M.; Mocanu, G.; Stanciu, M.C. Antimicrobial activity of chemically modified dextran derivatives. Carbohydrate Polymers 2017, 161, 181-186. [CrossRef]
- Finbloom, J.A.; Raghavan, P.; Kwon, M.; Kharbikar, B.N.; Yu, M.A.; Desai, T.A. Codelivery of synergistic antimicrobials with polyelectrolyte nanocomplexes to treat bacterial biofilms and lung infections. Science Advances 2023, 9, eade8039, doi:doi:10.1126/sciadv.ade8039.
- Tran, T.-T.; Hadinoto, K. Ternary nanoparticle complex of antibiotic, polyelectrolyte, and mucolytic enzyme as a potential antibiotic delivery system in bronchiectasis therapy. Colloids and Surfaces B: Biointerfaces 2020, 193, 111095. [CrossRef]
- Sakima, V.T.; Barbugli, P.A.; Cerri, P.S.; Chorilli, M.; Carmello, J.C.; Pavarina, A.C.; Mima, E.G. Antimicrobial Photodynamic Therapy Mediated by Curcumin-Loaded Polymeric Nanoparticles in a Murine Model of Oral Candidiasis. Molecules 2018, 23. [CrossRef]
- Tiyaboonchai, W.; Limpeanchob, N. Formulation and characterization of amphotericin B–chitosan–dextran sulfate nanoparticles. International Journal of Pharmaceutics 2007, 329, 142-149. [CrossRef]
- Cheow, W.S.; Kiew, T.Y.; Yang, Y.; Hadinoto, K. Amorphization Strategy Affects the Stability and Supersaturation Profile of Amorphous Drug Nanoparticles. Molecular Pharmaceutics 2014, 11, 1611-1620. [CrossRef]
- Tian, W.; Gao, X.; Zhang, J.; Yu, J.; Zhang, J. Cellulose nanosphere: Preparation and applications of the novel nanocellulose. Carbohydrate Polymers 2022, 277, 118863. [CrossRef]
- Chen, S.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Disassembly of lignocellulose into cellulose, hemicellulose, and lignin for preparation of porous carbon materials with enhanced performances. Journal of Hazardous Materials 2021, 408, 124956. [CrossRef]
- Lehrhofer, A.F.; Goto, T.; Kawada, T.; Rosenau, T.; Hettegger, H. The in vitro synthesis of cellulose – A mini-review. Carbohydrate Polymers 2022, 285, 119222. [CrossRef]
- Ahmad, H. Celluloses as support materials for antibacterial agents: A review. Cellulose 2021, 28, 2715-2761. [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. Journal of Materials Research and Technology 2021, 10, 935-952. [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Graft copolymers from cellulose: Synthesis, characterization and evaluation. Carbohydrate Polymers 2013, 97, 18-25. [CrossRef]
- Li, Y.; Tian, Y.; Zheng, W.; Feng, Y.; Huang, R.; Shao, J.; Tang, R.; Wang, P.; Jia, Y.; Zhang, J.; et al. Composites of Bacterial Cellulose and Small Molecule-Decorated Gold Nanoparticles for Treating Gram-Negative Bacteria-Infected Wounds. Small 2017, 13, 1700130. [CrossRef]
- Esmaeili, A.; Haseli, M. Electrospinning of thermoplastic carboxymethyl cellulose/poly(ethylene oxide) nanofibers for use in drug-release systems. Materials Science and Engineering: C 2017, 77, 1117-1127. [CrossRef]
- Henschen, J.; Li, D.; Ek, M. Preparation of cellulose nanomaterials via cellulose oxalates. Carbohydrate Polymers 2019, 213, 208-216. [CrossRef]
- Singh, K.; Arora, J.K.; Sinha, T.J.M.; Srivastava, S. Functionalization of nanocrystalline cellulose for decontamination of Cr(III) and Cr(VI) from aqueous system: Computational modeling approach. Clean Technologies and Environmental Policy 2014, 16, 1179-1191. [CrossRef]
- Yuan, Z.; Cheng, J.; Lan, G.; Lu, F. A cellulose/Konjac glucomannan–based macroporous antibacterial wound dressing with synergistic and complementary effects for accelerated wound healing. Cellulose 2021, 28, 5591-5609. [CrossRef]
- Forero-Doria, O.; Polo, E.; Marican, A.; Guzmán, L.; Venegas, B.; Vijayakumar, S.; Wehinger, S.; Guerrero, M.; Gallego, J.; Durán-Lara, E.F. Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydrate Polymers 2020, 242, 116383. [CrossRef]
- Božič, M.; Liu, P.; Mathew, A.P.; Kokol, V. Enzymatic phosphorylation of cellulose nanofibers to new highly-ions adsorbing, flame-retardant and hydroxyapatite-growth induced natural nanoparticles. Cellulose 2014, 21, 2713-2726. [CrossRef]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-Based Antibacterial Materials. Advanced Healthcare Materials 2018, 7, 1800334. [CrossRef]
- Rimpy; Ahuja, M. Fluconazole-loaded TEOS-modified nanocellulose 3D scaffolds – Fabrication, characterization and its application as vaginal drug delivery system. Journal of Drug Delivery Science and Technology 2022, 75, 103646. [CrossRef]
- Doghish, A.S.; Hashem, A.H.; Shehabeldine, A.M.; Sallam, A.-A.M.; El-Sayyad, G.S.; Salem, S.S. Nanocomposite based on gold nanoparticles and carboxymethyl cellulose: Synthesis, characterization, antimicrobial, and anticancer activities. Journal of Drug Delivery Science and Technology 2022, 77, 103874. [CrossRef]
- Thakkar, M.; Islam, M.S.; Railkar, A.; Mitra, S. Antisolvent precipitative immobilization of micro and nanostructured griseofulvin on laboratory cultured diatom frustules for enhanced aqueous dissolution. Colloids and Surfaces B: Biointerfaces 2020, 196, 111308. [CrossRef]
- Vilela, C.; Oliveira, H.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Nanocellulose-based antifungal nanocomposites against the polymorphic fungus Candida albicans. Carbohydrate Polymers 2019, 217, 207-216. [CrossRef]
- Bellmann, T.; Luber, R.; Kischio, L.; Karl, B.; Pötzinger, Y.; Beekmann, U.; Kralisch, D.; Wiegand, C.; Fischer, D. Bacterial nanocellulose patches as a carrier for hydrating formulations to improve the topical treatment of nail diseases. International Journal of Pharmaceutics 2022, 628, 122267. [CrossRef]
- Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Jodeh, S.; Hamed, O.; Berrabah, M.; Jerdioui, S.; Salghi, R.; Akartasse, N.; Errich, A.; et al. Preparation and characterization of biodegradable nanocomposites derived from carboxymethyl cellulose and hydroxyapatite. Carbohydrate Polymers 2017, 167, 59-69. [CrossRef]
- Kaur, K.; Kumar, P.; Kush, P. Amphotericin B loaded ethyl cellulose nanoparticles with magnified oral bioavailability for safe and effective treatment of fungal infection. Biomedicine & Pharmacotherapy 2020, 128, 110297. [CrossRef]
- Kapileshwari, G.R.; Barve, A.R.; Kumar, L.; Bhide, P.J.; Joshi, M.; Shirodkar, R.K. Novel drug delivery system of luliconazole - Formulation and characterisation. Journal of Drug Delivery Science and Technology 2020, 55, 101302. [CrossRef]
- Robles, E.; Salaberria, A.M.; Herrera, R.; Fernandes, S.C.M.; Labidi, J. Self-bonded composite films based on cellulose nanofibers and chitin nanocrystals as antifungal materials. Carbohydrate Polymers 2016, 144, 41-49. [CrossRef]
- Schmücker, C.; Stevens, G.W.; Mumford, K.A. Liquid marble formation and solvent vapor treatment of the biodegradable polymers polylactic acid and polycaprolactone. Journal of Colloid and Interface Science 2018, 514, 349-356. [CrossRef]
- Gupta, P.K.; Gahtori, R.; Govarthanan, K.; Sharma, V.; Pappuru, S.; Pandit, S.; Mathuriya, A.S.; Dholpuria, S.; Bishi, D.K. Recent trends in biodegradable polyester nanomaterials for cancer therapy. Materials Science and Engineering: C 2021, 127, 112198. [CrossRef]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renewable and Sustainable Energy Reviews 2017, 79, 1346-1352. [CrossRef]
- Ajioka, M.; Enomoto, K.; Suzuki, K.; Yamaguchi, A. The basic properties of poly(lactic acid) produced by the direct condensation polymerization of lactic acid. Journal of environmental polymer degradation 1995, 3, 225-234. [CrossRef]
- Darie-Niță, R.N.; Râpă, M.; Frąckowiak, S. Special Features of Polyester-Based Materials for Medical Applications. Polymers 2022, 14, 951.
- Liu, J.-Y.; Zhang, L.-M. Preparation of a polysaccharide–polyester diblock copolymer and its micellar characteristics. Carbohydrate Polymers 2007, 69, 196-201. [CrossRef]
- Wu, J.; Zhang, Z.; Gu, J.g.; Zhou, W.; Liang, X.; Zhou, G.; Han, C.C.; Xu, S.; Liu, Y. Mechanism of a long-term controlled drug release system based on simple blended electrospun fibers. Journal of Controlled Release 2020, 320, 337-346. [CrossRef]
- Duygulu, N.E.; Ciftci, F.; Ustundag, C.B. Electrospun drug blended poly(lactic acid) (PLA) nanofibers and their antimicrobial activities. Journal of Polymer Research 2020, 27, 232. [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Advanced Drug Delivery Reviews 2016, 107, 333-366. [CrossRef]
- Yang, M.; Xie, S.; Adhikari, V.P.; Dong, Y.; Du, Y.; Li, D. The synergistic fungicidal effect of low-frequency and low-intensity ultrasound with amphotericin B-loaded nanoparticles on C. albicans in vitro. International Journal of Pharmaceutics 2018, 542, 232-241. [CrossRef]
- Li, Z.; Liu, L.; Chen, B.; Zhao, T.; Ran, L.; Yuan, X.; Cao, Z.; Wu, T. Structure and antimicrobial properties of long-chain branched poly (lactic acid). Journal of Biomedical Materials Research Part A 2019, 107, 2458-2467. [CrossRef]
- Yin, J.; Xu, L.; Ahmed, A. Batch Preparation and Characterization of Electrospun Porous Polylactic Acid-Based Nanofiber Membranes for Antibacterial Wound Dressing. Advanced Fiber Materials 2022, 4, 832-844. [CrossRef]
- Scaffaro, R.; Lopresti, F.; D’Arrigo, M.; Marino, A.; Nostro, A. Efficacy of poly(lactic acid)/carvacrol electrospun membranes against Staphylococcus aureus and Candida albicans in single and mixed cultures. Applied Microbiology and Biotechnology 2018, 102, 4171-4181. [CrossRef]
- Yan, D.; Yao, Q.; Yu, F.; Chen, L.; Zhang, S.; Sun, H.; Lin, J.; Fu, Y. Surface modified electrospun poly(lactic acid) fibrous scaffold with cellulose nanofibrils and Ag nanoparticles for ocular cell proliferation and antimicrobial application. Materials Science and Engineering: C 2020, 111, 110767. [CrossRef]
- Liu, X.; Guo, C.; Zhuang, K.; Chen, W.; Zhang, M.; Dai, Y.; Tan, L.; Ran, Y. A recyclable and light-triggered nanofibrous membrane against the emerging fungal pathogen Candida auris. PLoS Pathog 2022, 18, e1010534. [CrossRef]
- Machado, R.; da Costa, A.; Silva, D.M.; Gomes, A.C.; Casal, M.; Sencadas, V. Antibacterial and Antifungal Activity of Poly(Lactic Acid)–Bovine Lactoferrin Nanofiber Membranes. Macromolecular Bioscience 2018, 18, 1700324. [CrossRef]
- Jalvo, B.; Mathew, A.P.; Rosal, R. Coaxial poly(lactic acid) electrospun composite membranes incorporating cellulose and chitin nanocrystals. Journal of Membrane Science 2017, 544, 261-271. [CrossRef]
- Richter, A.R.; Carneiro, M.J.; de Sousa, N.A.; Pinto, V.P.T.; Freire, R.S.; de Sousa, J.S.; Mendes, J.F.S.; Fontenelle, R.O.S.; Feitosa, J.P.A.; Paula, H.C.B.; et al. Self-assembling cashew gum-graft-polylactide copolymer nanoparticles as a potential amphotericin B delivery matrix. International Journal of Biological Macromolecules 2020, 152, 492-502. [CrossRef]
- Alshehri, S.; Imam, S.S. Formulation and evaluation of butenafine loaded PLGA-nanoparticulate laden chitosan nano gel. Drug Delivery 2021, 28, 2348-2360. [CrossRef]
- Yang, M.; Du, K.; Hou, Y.; Xie, S.; Dong, Y.; Li, D.; Du, Y. Synergistic Antifungal Effect of Amphotericin B-Loaded Poly(Lactic-Co-Glycolic Acid) Nanoparticles and Ultrasound against Candida albicans Biofilms. Antimicrobial Agents and Chemotherapy 2019, 63, e02022-02018, doi:doi:10.1128/AAC.02022-18.
- Radwan, M.A.; AlQuadeib, B.T.; Šiller, L.; Wright, M.C.; Horrocks, B. Oral administration of amphotericin B nanoparticles: Antifungal activity, bioavailability and toxicity in rats. Drug Deliv 2017, 24, 40-50. [CrossRef]
- Hauser, M.; Nowack, B. Probabilistic modelling of nanobiomaterial release from medical applications into the environment. Environment International 2021, 146, 106184. [CrossRef]
- Greatti, V.R.; Oda, F.; Sorrechia, R.; Kapp, B.R.; Seraphim, C.M.; Weckwerth, A.C.V.B.; Chorilli, M.; Silva, P.B.D.; Eloy, J.O.; Kogan, M.J.; et al. Poly-ε-caprolactone Nanoparticles Loaded with 4-Nerolidylcatechol (4-NC) for Growth Inhibition of Microsporum canis. Antibiotics 2020, 9, 894.
- Abdel-Rashid, R.S.; Helal, D.A.; Alaa-Eldin, A.A.; Abdel-Monem, R. Polymeric versus lipid nanocapsules for miconazole nitrate enhanced topical delivery: In vitro and ex vivo evaluation. Drug Deliv 2022, 29, 294-304. [CrossRef]
- Abdelbar, M.F.; Shams, R.S.; Morsy, O.M.; Hady, M.A.; Shoueir, K.; Abdelmonem, R. Highly ordered functionalized mesoporous silicate nanoparticles reinforced poly (lactic acid) gatekeeper surface for infection treatment. International Journal of Biological Macromolecules 2020, 156, 858-868. [CrossRef]
- AbouSamra, M.M.; Basha, M.; Awad, G.E.A.; Mansy, S.S. A promising nystatin nanocapsular hydrogel as an antifungal polymeric carrier for the treatment of topical candidiasis. Journal of Drug Delivery Science and Technology 2019, 49, 365-374. [CrossRef]
- El-Naggar, M.E.; Abdelgawad, A.M.; Abdel-Sattar, R.; Gibriel, A.A.; Hemdan, B.A. Potential antimicrobial and antibiofilm efficacy of essential oil nanoemulsion loaded polycaprolactone nanofibrous dermal patches. European Polymer Journal 2023, 184, 111782. [CrossRef]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm inhibition by biocompatible poly(ε-caprolactone) nanocapsules loaded with essential oils and their cyto/genotoxicity to human keratinocyte cell line. International Journal of Pharmaceutics 2021, 606, 120846. [CrossRef]
- Abdel-Rashid, R.S.; Helal, D.A.; Alaa-Eldin, A.A.; Abdel-Monem, R. Polymeric versus lipid nanocapsules for miconazole nitrate enhanced topical delivery: In vitro and ex vivo evaluation. Drug Delivery 2022, 29, 294-304. [CrossRef]
- Zimmermann, E.S.; Ferreira, L.M.; Denardi, L.B.; Sari, M.H.M.; Cervi, V.F.; Nogueira, C.W.; Alves, S.H.; Cruz, L. Mucoadhesive gellan gum hydrogel containing diphenyl diselenide-loaded nanocapsules presents improved anti-candida action in a mouse model of vulvovaginal candidiasis. European Journal of Pharmaceutical Sciences 2021, 167, 106011. [CrossRef]
- Saqib, M.; Ali Bhatti, A.S.; Ahmad, N.M.; Ahmed, N.; Shahnaz, G.; Lebaz, N.; Elaissari, A. Amphotericin B Loaded Polymeric Nanoparticles for Treatment of Leishmania Infections. Nanomaterials 2020, 10, 1152.
- Kalita, S.; Kandimalla, R.; Devi, B.; Kalita, B.; Kalita, K.; Deka, M.; Kataki, A.; Sharma, A.; Kotoky, J. Dual delivery of chloramphenicol and essential oil by poly-ε-caprolactone–Pluronic nanocapsules to treat MRSA-Candida co-infected chronic burn wounds. RSC Advances 2017, 3, 1749-1758. [CrossRef]
- Arias, E.R.; Angarita-Villamizar, V.; Baena, Y.; Parra-Giraldo, C.; Perez, L.D. Phospholipid-Conjugated PEG-b-PCL Copolymers as Precursors of Micellar Vehicles for Amphotericin B. Polymers 2021, 13, 1747.
| Loaded drugs | Other components | Fungal | Zeta potential (mV) | Diameters | Loading content (LC) | Encapsulation efficiency (EE) | Drug release | PDI | Antifungal efficacy | In vivo study | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| O. syriacum essential oil (OSEO) and imidazolium-Zn(II)Salen | / | Candida albicans | +58.39 | 120.15 ±62.65 nm | 0.2241 | 35.17 %, | 80%(50h) | 0.31-0.39 | ZOI: 29.48 ± 1.26 mm; MIC; 3.25–45.25 μg/mL | N | [37] |
| Iron oxide nanoparticles/ chlorhexidine (CHX) | / | Candida albicans /Aspergillus flavus | +18.10 ± 0.82 | 33.6 ± 10.7 nm | / | / | / | 1.25 ±0.06 | MIC: 400 μg/mL | N | [51] |
| Cinnamic acid grafted CS | cinnamic acid grafted chitosan | M. canis | -69.74 | 263.0±81.4nm | / | 0.8493 | / | / | inhibition: 53.96%, MIC: 200 μg/mL | N | [35] |
| Metronidazol | / | C. albicans | +10.6 ± 1.3 | 188.7nm | / | 12μg/mg | 63%(8h) | / | MIC: 18 to 36 μg/mL | N | [52] |
| Fluconazole (Flu) | / | C. albicans | +3.36 | 82 nm | 0.602 | 0.787 | 8.12%(94h) | / | MIC: 1.25mg/mL,ZOI: 22.3 ± 1.6 mm | N | [36] |
| Ceftriaxone | / | / | +32 ±2.4 | 56 nm | 0.5437 | 0.7943 | 8.12%(94h) | / | ZOI: 19.5 ± 0.6 mm | N | [53] |
| Secondary Metabolites from P. fluorescens | / | Candida albicans /Aspergillus niger /Trichophyton tonsurans /Aspergillus flavis | +27.9 | 83.74±1.33nm | 90.29±1.73% | / | 7%(12h) | 0.34 | ZOI: 23.5±1.1 mm | Y | [54] |
| AmB | / | C. albicans | +15.84 ± 1.41 | 174.47±5.12 nm | 3.05% ± 0.13% | / | 80.6%(25h) | 0.17 | MIC: 1 μg/mL | N | [55] |
| Loaded drugs | Other components | Fungal | Zeta potential(mV) | Diameters | Loading content (LC) | Encapsulation efficiency (EE) | Drug release | PDI | Antifungal efficacy | In vivo study | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nystatin (Nys) | / | C. albicans | -37.42±1.07(pH 7.5); -35.22 ± 1.40(pH 5.5) | 24.41 μm | Surface 7.63±1.81% /Inside 17.45 ± 2.34% |
Surface 07.63±1.81 /Inside 17.45±2.34 | About 62%(18h) | / | exhibited a marked fungicidal activity | Y | [56] |
| Voriconazole | Chitosan | / | -24 ± 0.9 | 185 ±1nm | 10.38 ±0.87% | 91.31 ± 1.05% | About 68%(50h) | / | / | N | [58] |
| Miltefosine | / |
C.albicans /C.gattii. |
-39.7±5.2 | 279.1±56.7 nm | / | 81.70±6.64% | 55.24%(181h) | / | MIC: 0.03 to 2 µg/mL | Y | [60] |
| Sodium selenate | / | Fusarium oxysporum Schltdl | -7.25 | 80 nm | / | / | About 60%(40h) | / | / | N | [61] |
| Miltefosine | / | Galleria mellonella caterpillars | -39.7±5.2 | 279.1±56.7 nm | About 80% | 81.70%±6.64 | 55.24%(181h) | / | MIC: 0.03 µg/mL | Y | [62] |
| Ketoconazole | poloxamer 407, carbopol 940 | Candida albicans | +82.2 ± 64.94 | 34.8 ± 73.34 nm | / | 97.5 ± 41.95% | 43.75±5.38%(6h) | / | / | Y | [63] |
| Ethionamide | Chitosan | Mycobacterial | -24 ± 9 | 324 ± 62 nm | 0.59 | About100%(80h) | 0.35 ± 0.09 | MIC: 0.43 µg/mL | N | [64] |
| Loaded drugs | Other components | Fungal | Zeta potential (mV) | Diameters | Loading content (LC) | Encapsulation efficiency (EE) | Drug release | PDI | Antifungal efficacy | In vivo study | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spectinomycin | / | / | / | 250.9 nm | 0.1-0.5g/100mL | / | / | / | ZOI: 22mm | N | [68] |
| Fluconazole/ Cinnamaldehyde | Poly(Vinyl Alcohol) | Candida albicans | / | 334 ± 56 nm | 0.2 + 2.6 wt% | 73.84%(CA) and 68.58%(FLU) | CA87%(8h) /FLU61%(12h) | / | ZOI: 36 ± 1mm | N | [70] |
| Amp B | carboxymethyl ɩ-carrageenan | Candida glabrata | -25 ± 5.3 | 343 ± 12 nm | 2wt % | 78 ± 0.68% | 99% (40days) | < 0.3 | No viable C. glabrata was detected in Macrophage cells. | N | [69] |
| Amp B | polyethylene oxide | Candida tropicalis/ Candida krusei/ Candida parapsilosis/Candida glabrata/ Candida dubliniensis/ Aspergillus flavus | / | 351 ± 73 nm | 0-9% | / | 78% (11h) | / | ZOI: 19 ± 0.5mm | N | [71] |
| Mmethylene blue | / | Candida albicans | +30.8 | 100 nm | 3.13% to 6.75% | 84.0% ± 1.3 | 48% (180h) | 0.107 | / | N | [72] |
| Daptomycin/ Polymyxin B/ Tobramycin/ Vancomycin/ Caspofungin/Amp B | polydopamine | Candida albicans | / | 998 ± 250 nm | 0.005 | / | 80% (24h) | / | ZOI: 31mm | Y | [67] |
| Loaded drugs | Other components | Fungal | Zeta potential(mV) | Diameters | Loading content (LC) | Encapsulation efficiency (EE) | Drug release | PDI | Antifungal efficacy | In vivo study | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tobramycin/AgNPs | / | Pseudomonas aeruginosa(PA) | -39.2±1.5 | 167.2 ± 3.56 nm | >75% | Ag>95%/Tob78±2.5% | / | 0.241±0.008 | MIC: 2 μg/ mL | Y | [88] |
| Ciprofloxacin (CIP) /mucolytic enzyme papain (PAP) | / | PA | -51.0 ± 1.9 | 223 ± 99 nm | / | 0.88 | 100%(40min) | 0.51 ± 0.05 | / | N | [89] |
| Curcumin (CUR) | poly-lactic acid | / | +35(±7.23) | 248 (±86.39) | / | 0.7381 | 50%(16h) | 0.21 (± 0.09) | / | Y | [90] |
| Amp B | poly-lactic acid | / | 37 | 644±52nm | / | 0.56 | 100%(5min) | 0.27 | / | Y | [91] |
| Itraconazole (ITZ) | / | / | (-) 47 ± 0.8 | 400 ± 120nm | 65 ± 6 | 93 ± 2 | / | / | / | N | [92] |
| Loaded drugs | Other components | Fungal | Zeta potential(mV) | Diameters | Loading content (LC) | Encapsulation efficiency (EE) | Drug release | PDI | Antifungal efficacy | In vivo study | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [2-(methacryloyloxy)ethyl] trimethylammonium chloride solution | / | Candida albicans | / | / | 0.4 | / | / | / | inhibition > 99.9% | N | [110] |
| Fluconazole | tetraethyl orthosilicate | Candida albicans | RNF-25.4±1.13 /WNF-24.4±1.15 | RNF for 441.7 nm /WNF for 407.7 nm | 1%w/v | / | 30% (24h) | 0.735 for RNF /0.655 for WNF | ZOI: 39mm | Y | [107] |
| ciclopirox olamine and Boswellia serrata | / | Candida albicans, Candida parapsilosis | / | / | 10.1 ± 3.1 % | 10.0 ± 2.2 % | 79.1 ± 17.7 % (48 h) | / | ZOI: 20mm | N | [111] |
| gold nanoparticles | / | C.albicans,A.terreus,A. niger, andA. fumigatus | -3.16 | 54.49 nm | / | / | pH5.5, >45%. pH 7 <5% pH 9 <1%. | / | MIC: 20 μg/ml | N | [108] |
| hydroxyapatite | lysine | Candida albicans | / | 600 nm | 50-70% | / | / | / | ZOI: 28 mm | N | [112] |
| Griseofulvin | diatom | / | -13 ± 2 mV | 2–3 ± 0.5 μm | / | / | / | 0.675 | / | N | [109] |
| Amp | / | Candida albicans | -16.10 ± 2.6 mV | 150 ± 9.23 nm | 5 μg/mL | 60 ± 2% | 18 ± 2.1 % (12h) | 0.258±0.005 | MIC: 0.145 ± 0.01µg/mL | Y | [113] |
| Lliconazole | Polyvinyl alcohol | Candida albicans, Aspergillus niger | -14.6–32.3mV | 300-600 nm | 0.01 | 70–80% | up to 8 h | 0.108-0.497 | strong antifungal activity | N | [114] |
| Citin’ nanocrystals | / | Aspergillus | / | 60 nm | 0-10 % | / | / | / | inhibition: 98.87% | N | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




