Submitted:
14 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study participants
2.2. Dietary intake pattern
2.3. Other study variables
2.4. Statistical analysis
3. Results
3.1. Baseline characteristics
| Sociodemographic characteristics | |
| Age | 59.2 ± 9.9 |
| < 55 years | 190 (30.4) |
| 55 - < 60 years | 129 (20.7) |
| 60 - < 65 years | 140 (22.4) |
| ≥ 65 years | 165 (26.4) |
| Male | 362 (58.0) |
| Live with spouse/ or partner | 530 (84.9) |
| Monthly household income | |
| < 2 million Won | 89 (14.3) |
| 2 - < 4 million Won | 134 (21.5) |
| ≥ 4 million Won | 217 (34.8) |
| Unknown | 184 (29.5) |
| Education achievement | |
| ≤ Middle school | 70 (11.2) |
| High school | 211 (33.8) |
| ≥ College | 189 (30.3) |
| Unknown | 154 (24.7) |
| Smoking status | |
| Never smoking |
352 (56.4) |
| Ex-smoking | 230 (36.9) |
| Current smoking | 30 (4.8) |
| Unknown | 12 (1.9) |
| Alcohol consumption | |
| Non-current drinking | 442 (70.8) |
| Current drinking | 182 (29.2) |
| Clinical characteristics | |
| Age at cancer diagnosis | 52.5 ± 10.2 |
| < 45 years | 138 (22.1) |
| 45 - < 55 years | 231 (37.0) |
| ≥ 55 years | 255 (40.9) |
| Lapse after diagnosis | 6.7 ± 3.0 |
| < 1 years | 14 (2.2) |
| 1 - < 5 years | 152 (24.4) |
| 5 - < 10 years | 395 (63.3) |
| ≥ 10 years | 63 (10.1) |
| Stage of Cancer | |
| Stage 0 | 7 (1.1) |
| Stage 1 | 423 (67.8) |
| Stage 2 | 102 (16.3) |
| Stage 3 | 60 (9.6) |
| Stage 4 | 9 (1.4) |
| Unknown | 23 (3.7) |
| Type of surgery received | |
| Total gastrectomy 14 |
142 (22.8) |
| Subtotal gastrectomy | 470 (75.3) |
| Biloth-1 subtotal gastrectomy | 328 (52.8) |
| Biloth-2 subtotal gastrectomy | 75 (12.0) |
| Pylorus preserving surgery | 63 (10.1) |
| Not specifically stated | 4 (0.6) |
| Wedge resection | 6 (1.0) |
| Endoscopic submucosal dissection | 3 (0.5) |
| Unknown | 3 (0.5) |
| Type of cancer treatment received | |
| Chemotherapy | 179 (28.7) |
| Radiotherapy | 76 (12.2) |
| Preoperative, body mass index | 23.8 ± 3.1 |
| < 18.5 kg/m2 | 13 (2.1) |
| 18.5 - 22.9 kg/m2 | 252 (40.4) |
| 23 - 24.9 kg/m2 | 140 (22.4) |
| ≥ 25 kg/m2 | 198 (31.7) |
| Unknown | 21 (3.4) |
| Psychological characteristics | |
| High fear of cancer recurrence (FCRI ≥ 13) | 206 (33.0) |
| Depression (HADS-D ≥ 8) | 270 (43.3) |
| Anxiety (HADS-A ≥ 8) | 91 (14.6) |
3.2. Distribution of Dietary habit change
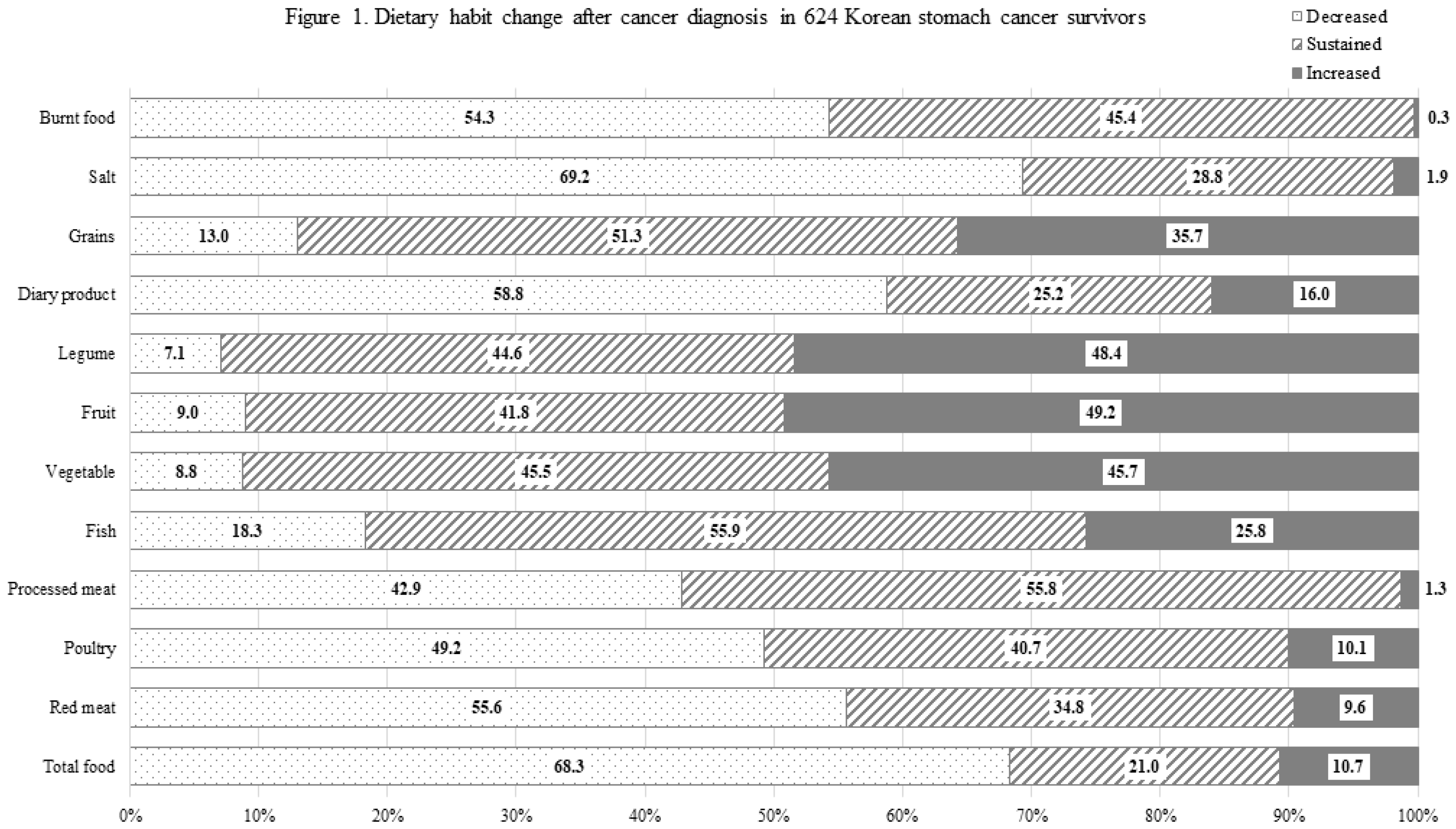
3.3. Dietary habit change stratified by age and time lapse after cancer diagnosis
3.4. Factors associated with dietary habit changes toward healthier directions
| Variable | Decreased Red meat |
Increased Poultry |
Decreased Processed meat |
Increased Fish |
Increased Vegetable |
Increased Fruit |
Increased Legume |
Increased Dairy product |
Decreased Grains |
Decreased Salt |
Decreased Burnt food |
| Sociodemographic characteristics | |||||||||||
| Age at the time of cancer diagnosis | |||||||||||
| < 45 years | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 45 - < 55 years | 1.37 (0.85, 2.19) |
0.77 (0.38, 1.54) |
0.68 (0.41, 1.11) |
1.77 (1.05, 2.98) |
0.68 (0.41, 1.11) |
0.87 (0.53, 1.44) |
0.69 (0.41, 1.13) |
1.44 (0.78, 2.66) |
0.78 (0.38, 1.59) |
1.16 (0.68, 1.98) |
0.96 (0.57, 1.62) |
| ≥ 55 years | 1.56 (0.92, 2.63) |
0.43 (0.19, 1.01) |
0.41 (0.24, 0.71) |
0.82 (0.45, 1.50) |
0.43 (0.24, 0.74) |
0.46 (0.26, 0.80) |
0.47 (0.27, 0.82) |
1.23 (0.61, 2.48) |
0.90 (0.42, 1.95) |
0.94 (0.53, 1.67) |
0.54 (0.31, 0.96) |
| Continuous, increase by 1 year | 1.01 (0.99, 1.04) |
0.96 (0.93, 0.99) |
0.97 (0.95, 0.99) |
0.99 (0.96, 1.01) |
0.97 (0.94, 0.99) |
0.96 (0.94, 0.98) |
0.96 (0.94, 0.98) |
0.99 (0.97, 1.02) |
0.99 (0.96, 1.02) |
0.99 (0.97, 1.01) |
0.97 (0.95, 0.99) |
| Female (vs. Male) | 1.01 (0.61, 1.66) |
0.94 (0.41, 2.12) |
1.01 (0.59, 1.73) |
1.22 (0.66, 2.26) |
0.86 (0.50, 1.49) |
0.76 (0.44, 1.30) |
1.15 (0.67, 1.97) |
0.91 (0.46, 1.82) |
1.14 (0.53, 2.45) |
1.35 (0.79, 2.29) |
0.54 (0.31, 0.93) |
| Live with spouse (vs. without spouse) | 1.05 (0.61, 1.80) |
0.77 (0.34, 1.70) |
1.51 (0.83, 2.74) |
1.03 (0.53, 1.98) |
1.16 (0.65, 2.09) |
1.07 (0.60, 1.92) |
1.01 (0.56, 1.83) |
0.61 (0.31, 1.19) |
0.44 (0.21, 0.91) |
1.02 (0.55, 1.86) |
0.90 (0.50, 1.63) |
| Education achievement | |||||||||||
| ≤ Middle school | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| High school | 1.08 (0.59, 1.99) |
0.91 (0.34, 2.42) |
1.07 (0.58, 1.99) |
1.28 (0.63, 2.59) |
1.99 (1.07, 3.73) |
1.55 (0.84, 2.86) |
1.55 (0.83, 2.88) |
1.57 (0.68, 3.64) |
0.34 (0.16, 0.73) |
1.25 (0.65, 2.39) |
1.92 (1.03, 3.56) |
| ≥ College | 1.01 (0.53, 1.94) |
1.33 (0.48, 3.74) |
2.49 (1.28, 4.84) |
2.15 (1.02, 4.53) |
2.64 (1.35, 5.18) |
2.16 (1.11, 4.20) |
1.07 (0.58, 1.99) |
1.56 (0.64, 3.79) |
0.30 (0.13, 0.70) |
1.29 (0.64, 2.62) |
1.90 (0.97, 3.71) |
| Monthly Household Income | |||||||||||
| < 2millions, Won | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 - < 4millions, Won | 1.34 (0.74, 2.44) |
1.56 (0.59, 4.12) |
1.79 (0.95, 3.36) |
1.70 (0.85, 3.38) |
2.17 (1.16, 4.04) |
2.18 (1.17, 4.07) |
1.55 (0.83, 2.88) |
1.71 (0.79, 3.70) |
1.44 (0.61, 3.42) |
2.07 (1.09, 3.96) |
1.39 (0.74, 2.62) |
| ≥ 4millions, Won | 1.35 (0.75, 2.45) |
0.89 (0.33, 2.42) |
1.76 (0.95, 3.29) |
1.12 (0.57, 2.23) |
1.20 (0.65, 2.20) |
1.11 (0.54, 2.29) |
1.07 (0.58, 1.99) |
1.20 (0.55, 2.61) |
1.79 (0.76, 4.22) |
2.03 (1.08, 3.81) |
1.28 (0.68, 2.40) |
| Smoking status | |||||||||||
| Never smoking | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ex-smoking | 1.28 (0.78, 2.11) |
0.94 (0.40, 2.20) |
1.05 (0.62, 1.80) |
1.79 (0.99, 3.25) |
1.49 (0.87, 2.55) |
1.28 (0.75, 2.19) |
1.33 (0.78, 2.27) |
0.94 (0.47, 1.87) |
1.05 (0.49, 2.25) |
1.19 (0.70, 2.02) |
1.26 (0.73, 2.18) |
| Current smoking | 0.88 (0.37, 2.06) |
1.02 (0.20, 5.22) |
0.83 (0.33, 2.12) |
1.24 (0.45, 3.41) |
0.79 (0.14, 4.41) |
0.58 (0.23, 1.47) |
1.05 (0.42, 2.61) |
1.15 (0.36, 3.65) |
0.68 (0.17, 2.75) |
0.57 (0.24, 1.38) |
0.73 (0.28, 1.90) |
| Alcohol consumption | |||||||||||
| Non-current drinking | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Current drinking | 1.13 (0.75, 1.70) |
0.28 (0.12, 0.67) |
1.14 (0.74, 1.75) |
1.00 (0.63, 1.60) |
0.96 (0.62, 1.48) |
0.94 (0.61, 1.45) |
0.71 (0.46, 1.10) |
0.61 (0.34, 1.09) |
1.69 (0.92, 3.11) |
1.11 (0.71, 1.73) |
1.54 (0.98, 2.42) |
| Clinical characteristics | |||||||||||
| Lapse after diagnosis | |||||||||||
| < 5 years | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 5 - < 10 years | 1.24 (0.39, 3.98) |
0.52 (0.10, 2.85) |
0.93 (0.26, 3.42) |
0.26 (0.07, 0.95) |
2.06 (0.50, 8.56) |
1.10 (0.30, 4.08) |
0.87 (0.25, 3.07) |
0.42 (0.11, 1.60) |
0.30 (0.08, 1.14) |
1.41 (0.38, 5.25) |
2.30 (0.57, 9.29) |
| ≥ 10 years | 1.16 (0.37, 3.62) |
0.45 (0.09, 2.34) |
0.82 (0.23, 2.94) |
0.24 (0.07, 0.84) |
1.99 (0.49, 8.09) |
0.85 (0.23, 3.07) |
0.74 (0.22, 2.56) |
0.32 (0.09, 1.19) |
0.20 (0.05, 0.76) |
0.59 (0.17, 2.09) |
2.34 (0.59, 9.25) |
| Continuous, increase by 1 year | 1.01 (0.96, 1.08) |
0.93 (0.83, 1.04) |
0.98 (0.92, 1.05) |
0.95 (0.89, 1.02) |
1.03 (0.96, 1.10) |
0.96 (0.90, 1.02) |
1.03 (0.96, 1.10) |
0.96 (0.88, 1.04) |
0.85 (0.75, 0.95) |
0.94 (0.88, 1.00) |
0.99 (0.92, 1.05) |
| Cancer stage | |||||||||||
| 0-1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.23 (0.64, 2.34) |
0.49 (0.16, 1.45) |
1.60 (0.81, 3.16) |
0.65 (0.30, 1.43) |
1.25 (0.62, 2.55) |
1.26 (0.63, 2.53) |
1.29 (0.65, 2.58) |
1.23 (0.50, 3.04) |
2.80 (1.05, 7.47) |
1.64 (0.82, 3.27) |
1.15 (0.56, 2.35) |
| 3-4 | 0.67 (0.32, 1.38) |
0.51 (0.16, 1.69) |
0.86 (0.39, 1.86) |
0.78 (0.33, 1.83) |
0.75 (0.33, 1.69) |
0.96 (0.43, 2.14) |
1.56 (0.71, 3.42) |
1.13 (0.42, 3.08) |
2.92 (0.95, 8.99) |
2.07 (0.91, 4.70) |
1.01 (0.44, 2.33) |
| Surgery type* | |||||||||||
| Total gastrectomy | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Biloth-1 Subtotal gastrectomy | 0.87 (0.55, 1.36) |
0.76 (0.38, 1.55) |
1.17 (0.72, 1.88) |
0.67 (0.40, 1.10) |
1.69 (1.04, 2.74) |
1.17 (0.73, 1.89) |
1.46 (0.91, 2.36) |
0.90 (0.50, 1.62) |
0.71 (0.37, 1.36) |
1.02 (0.63, 1.65) |
0.97 (0.59, 1.58) |
| Biloth-2 Subtotal gastrectomy | 0.87 (0.47, 1.62) |
0.80 (0.29, 2.20) |
0.73 (0.38, 1.42) |
0.84 (0.42, 1.70) |
1.55 (0.80, 3.01) |
1.26 (0.65, 2.43) |
1.34 (0.69, 2.58) |
0.69 (0.28, 1.68) |
0.34 (0.11, 1.02) |
1.37 (0.68, 2.73) |
0.76 (0.39, 1.48) |
| Pylorus preserving surgery | 0.68 (0.35, 1.32) |
0.85 (0.28, 2.56) |
0.64 (0.31, 1.33) |
0.75 (0.34, 1.63) |
1.23 (0.59, 2.56) |
1.01 (0.49, 2.08) |
0.98 (0.48, 2.02) |
0.83 (0.34, 2.05) |
1.06 (0.41, 2.73) |
1.13 (0.54, 2.37) |
1.11 (0.53, 2.31) |
| Chemotherapy recipient (vs. non-chemotherapy recipient) |
1.17 (0.62, 2.19) |
3.06 (1.13, 8.29) |
0.89 (0.45, 1.73) |
1.38 (0.66, 2.91) |
1.45 (0.72, 2.93) |
1.49 (0.74, 2.99) |
0.87 (0.44, 1.72) |
0.65 (0.27, 1.57) |
0.44 (0.16, 1.21) |
0.63 (0.32, 1.22) |
0.88 (0.43, 1.77) |
| Radiotherapy recipient (vs. non-radiotherapy recipient) |
1.03 (0.55, 1.94) |
0.27 (0.09, 0.80) |
0.88 (0.45, 1.70) |
0.59 (0.28, 1.23) |
0.68 (0.35, 1.33) |
0.53 (0.27, 1.05) |
0.59 (0.30, 1.15) |
0.79 (0.32, 1.92) |
0.86 (0.33, 2.23) |
1.51 (0.74, 3.12) |
1.37 (0.68, 2.77) |
| Preoperative body mass index | |||||||||||
| < 23 kg/m2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 23 - 24.9 kg/m2 |
1.78 (1.13, 2.80) |
0.75 (0.34, 1.63) |
1.05 (0.64, 1.72) |
0.91 (0.53, 1.57) |
1.43 (0.87, 2.36) |
1.16 (0.71, 1.90) |
0.70 (0.43, 1.15) |
0.69 (0.37, 1.29) |
0.94 (0.45, 1.94) |
0.86 (0.53, 1.40) |
0.73 (0.44, 1.20) |
| ≥ 25 kg/m2 |
2.54 (1.66, 3.89) |
0.71 (0.34, 1.47) |
1.69 (1.08, 2.65) |
1.14 (0.70, 1.87) |
1.35 (0.85, 2.13) |
1.18 (0.75, 1.86) |
0.76 (0.49, 1.19) |
0.61 (0.34, 1.09) |
1.72 (0.94, 3.17) |
1.07 (0.68, 1.69) |
1.06 (0.66, 1.70) |
| Continuous, increase by 1 kg/m2 |
1.08 (1.01, 1.14) |
0.94 (0.85, 1.04) |
1.04 (0.98, 1.11) |
1.05 (0.97, 1.12) |
1.03 (0.97, 1.10) |
1.04 (0.98, 1.11) |
0.96 (0.90, 1.03) |
0.97 (0.90, 1.05) |
1.15 (1.05, 1.26) |
1.00 (0.94, 1.07) |
1.03 (0.96, 1.10) |
| Psychological characteristics | |||||||||||
| High fear of cancer recurrence, FCRI ≥13 (vs. FCRI <13) |
1.07 (0.71, 1.61) |
1.05 (0.55, 2.01) |
1.16 (0.76, 1.76) |
1.58 (1.01, 2.46) |
1.37 (0.90, 2.09) |
1.76 (1.15, 2.70) |
1.49 (0.98, 2.27) |
1.16 (0.96, 2.71) |
0.65 (0.35, 1.22) |
1.30 (0.82, 2.04) |
1.66 (1.06, 2.58) |
| Depression, HADS-D≥8 (vs. HADS-D <8) |
1.55 (1.07, 2.24) |
0.96 (0.53, 1.76) |
1.74 (1.19, 2.56) |
0.92 (0.60, 1.41) |
1.17 (0.79, 1.72) |
1.24 (0.84, 1.82) |
0.95 (0.65, 1.40) |
0.84 (0.51, 1.38) |
1.31 (0.763, 2.26) |
1.08 (0.72, 1.62) |
1.70 (1.14, 2.54) |
| Anxiety, HADS-A≥8 (vs. HADS-A <8) |
1.03 (0.60, 1.76) |
1.12 (0.50, 2.50) |
1.11 (0.64, 1.95) |
1.71 (0.98, 3.01) |
1.43 (0.81, 2.53) |
0.94 (0.53, 1.65) |
0.97 (0.56, 1.70) |
0.87 (0.44, 1.72) |
2.67 (1.32, 5.42) |
1.36 (0.72, 2.54) |
1.01 (0.56, 1.85) |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-49. [CrossRef]
- Jung KW, Won YJ, Hong S, Kong HJ, Im JS, Seo HG. Prediction of Cancer Incidence and Mortality in Korea, 2021. Cancer Res Treat. 2021;53:316-22. [CrossRef]
- Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2019. Cancer Res Treat. 2022;54:330-44. [CrossRef]
- Carrillo GM, Santamaría NP. Life after a gastrectomy: Experience of patients with gastric cancer. Enferm Clin (Engl Ed). 2019;29:27-33. [CrossRef]
- Rha SY, Lee HJ, Lee J. Unmet needs in the physical and daily living domain mediates the influence of symptom experience on the quality of life of gastric cancer patients. Support Care Cancer. 2020;28:1419-31. [CrossRef]
- Jeong SM, Shin DW, Lee JE, Jin SM, Kim S. Increased Risk of Osteoporosis in Gastric Cancer Survivors Compared to General Population Control: A Study with Representative Korean Population. Cancer Res Treat. 2019;51:530-7. [CrossRef]
- Seo GH, Kang HY, Choe EK. Osteoporosis and fracture after gastrectomy for stomach cancer: A nationwide claims study. Medicine (Baltimore). 2018;97:e0532.
- Jones LW, Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. The Lancet Oncology. 2006;7:1017-26. [CrossRef]
- Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50:167-78. [CrossRef]
- Rock CL, Thomson CA, Sullivan KR, Howe CL, Kushi LH, Caan BJ, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72:230-62. [CrossRef]
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243-74. [CrossRef]
- Ghelfi F, Tieri M, Gori S, Nicolis F, Petrella MC, Filiberti A, et al. Do cancer patients change their diet in the e-health information era? A review of the literature and a survey as a proposal for the Italian population. Food Res Int. 2018;104:59-68. [CrossRef]
- Helft PR, Hlubocky F, Daugherty CK. American oncologists’ views of internet use by cancer patients: a mail survey of American Society of Clinical Oncology members. J Clin Oncol. 2003;21:942-7. [CrossRef]
- Ryu SW, Son YG, Lee MK. Motivators and barriers to adoption of a healthy diet by survivors of stomach cancer: A cross-sectional study. Eur J Oncol Nurs. 2020;44:101703. [CrossRef]
- Tollosa DN, Tavener M, Hure A, James EL. Adherence to multiple health behaviours in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2019;13:327-43. [CrossRef]
- Moazzen S, Cortés-Ibañez FO, van Leeuwen BL, Alizadeh BZ, de Bock GH. Assessment of Diet Quality and Adherence to Dietary Guidelines in Gastrointestinal Cancer Survivors: A Cross-Sectional Study. Nutrients. 2020;12. [CrossRef]
- Hoang T, Lee J, Kim J, Park B. Food Intake Behavior in Cancer Survivors in Comparison With Healthy General Population; From the Health Examination Center-based Cohort. J Cancer Prev. 2019;24:208-16. [CrossRef]
- Continuous Update Project Expert Report 2018. Recommendations and Public Health and Policy Implications. World Cancer Research Fund/American Institute for Cancer Research. 2018.
- Song S, Lee JE, Song WO, Paik HY, Song Y. Carbohydrate intake and refined-grain consumption are associated with metabolic syndrome in the Korean adult population. J Acad Nutr Diet. 2014;114:54-62. [CrossRef]
- Park S, Ahn J, Kim NS, Lee BK. High carbohydrate diets are positively associated with the risk of metabolic syndrome irrespective to fatty acid composition in women: the KNHANES 2007-2014. Int J Food Sci Nutr. 2017;68:479-87. [CrossRef]
- Kang Y, Lee K, Lee J, Kim J. Grain Subtype and the Combination of Grains Consumed Are Associated with the Risk of Metabolic Syndrome: Analysis of a Community-Based Prospective Cohort. J Nutr. 2020;150:118-27. [CrossRef]
- Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr. 2008;17:370-4.
- Beekman E, Verhagen A. Clinimetrics: Hospital Anxiety and Depression Scale. J Physiother. 2018;64:198. [CrossRef]
- Shin J, Goo A, Ko H, Kim JH, Lim SU, Lee HK, et al. Validation Study for the Korean Version of Fear of Cancer Recurrence Inventory. J Korean Med Sci. 2017;32:1792-9. [CrossRef]
- Simard S, Savard J. Screening and comorbidity of clinical levels of fear of cancer recurrence. J Cancer Surviv. 2015;9:481-91. [CrossRef]
- Park B, Lee J, Kim J. Imbalanced Nutrient Intake in Cancer Survivors from the Examination from the Nationwide Health Examination Center-Based Cohort. Nutrients. 2018;10. [CrossRef]
- Tan SY, Wong HY, Vardy JL. Do cancer survivors change their diet after cancer diagnosis? Support Care Cancer. 2021;29:6921-7.
- Continuous Updated Project Diet, Nutrition Physical Activity and Stomach Cancer. World Cancer Research Fund/American Institute for Cancer Research. Revised 2018.
- Charnley G, Tannenbaum SR. Flow cytometric analysis of the effect of sodium chloride on gastric cancer risk in the rat. Cancer Res. 1985;45:5608-16.
- Fang X, Wei J, He X, An P, Wang H, Jiang L, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer. 2015;51:2820-32. [CrossRef]
- Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59:4823-8.
- Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM, Jr., et al. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81:2258-67. [CrossRef]
- Costa BVL, Menezes MC, Oliveira CDL, Mingoti SA, Jaime PC, Caiaffa WT, et al. Does access to healthy food vary according to socioeconomic status and to food store type? an ecologic study. BMC Public Health. 2019;19:775. [CrossRef]
- Walker MS, Vidrine DJ, Gritz ER, Larsen RJ, Yan Y, Govindan R, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2370-7. [CrossRef]
- Food, Nutrition, Physical Activity, and Prevention of Cancer: a Global perspective. World Cancer Research Fund/American Institute for Cancer Research. 2007.
- Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323-53. [CrossRef]
- Iqbal A. Effect of Food on Causation and Prevention of Gastric Cancer. Journal of Cancer Prevention & Current Research. 2017;8. [CrossRef]
- Fruits and vegetables, IARC Handbooks of Cancer Prevention Volume 8. Lyon, France: International Agency for Research on Cancer, World Health Organization; 2003:384.
- Wang Q, Chen Y, Wang X, Gong G, Li G, Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer. 2014;50:1498-509. [CrossRef]
- Zheng G, Sundquist K, Sundquist J, Chen T, Försti A, Hemminki A, et al. Second Primary Cancers After Gastric Cancer, and Gastric Cancer as Second Primary Cancer. Clin Epidemiol. 2021;13:515-25. [CrossRef]
- Nicklett EJ, Kadell AR. Fruit and vegetable intake among older adults: a scoping review. Maturitas. 2013;75:305-12. [CrossRef]
- Nakazono M, Aoyama T, Komori K, Watanabe H, Kano K, Nagasawa S, et al. The Comparison of the Dietary Intake Loss Between Elderly and Non-Elderly Patients After Gastrectomy for Gastric Cancer. J Gastrointest Cancer. 2023;54:35-43. [CrossRef]
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18:67. [CrossRef]
- McMaughan DJ, Oloruntoba O, Smith ML. Socioeconomic Status and Access to Healthcare: Interrelated Drivers for Healthy Aging. Front Public Health. 2020;8:231. [CrossRef]
- Zajacova A, Lawrence EM. The Relationship Between Education and Health: Reducing Disparities Through a Contextual Approach. Annu Rev Public Health. 2018;39:273-89. [CrossRef]
- Ruiz-Tovar J, Bozhychko M, Del-Campo JM, Boix E, Zubiaga L, Muñoz JL, et al. Changes in Frequency Intake of Foods in Patients Undergoing Sleeve Gastrectomy and Following a Strict Dietary Control. Obes Surg. 2018;28:1659-64. [CrossRef]
- Brown J, Byers T, Thompson K, Eldridge B, Doyle C, Williams AM. Nutrition during and after cancer treatment: a guide for informed choices by cancer survivors. CA Cancer J Clin. 2001;51:153-87; quiz 89-92. [CrossRef]
- Séguin Leclair C, Lebel S, Westmaas JL. Can Physical Activity and Healthy Diet Help Long-Term Cancer Survivors Manage Their Fear of Recurrence? Front Psychol. 2021;12:647432.
- Ramp D, Mols F, Ezendam N, Beijer S, Bours M, Winkels R, et al. Psychological distress and lower health-related quality of life are associated with need for dietary support among colorectal cancer survivors with overweight or obesity. Support Care Cancer. 2021;29:7659-68. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





