Submitted:
14 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. VCO emulsion preparation
2.3. Film preparation
2.4. Nanocomposite film characterization
2.4.1. Film thickness
2.4.2. Film color
2.4.3. Film solubility in water
2.4.4. Water vapor transmission rate of the film
2.4.5. Mechanical properties of the film
2.4.6. Fourier transform infrared spectroscopy (FTIR)
2.4.7. Scanning electron microscope (SEM)
3. Results and Discussion
3.1. Film thickness
| Treatment | Thickness (mm) | L* | a* | b* |
|---|---|---|---|---|
| VCO 0 wt% | 0.214 ± 0.02 | 98.55 ± 0.34 | 0.11 ± 0.04 | 1.63 ± 0.15 |
| VCO 3 wt% | 0.219 ± 0.02 | 98.77 ± 0.26 | 0.23 ± 0.05 | 1.60 ± 0.05 |
| VCO 5 wt% | 0.237 ± 0.01 | 98.98 ± 0.15 | 0.30 ± 0.04 | 1.90 ± 0.02 |
3.2. Film color
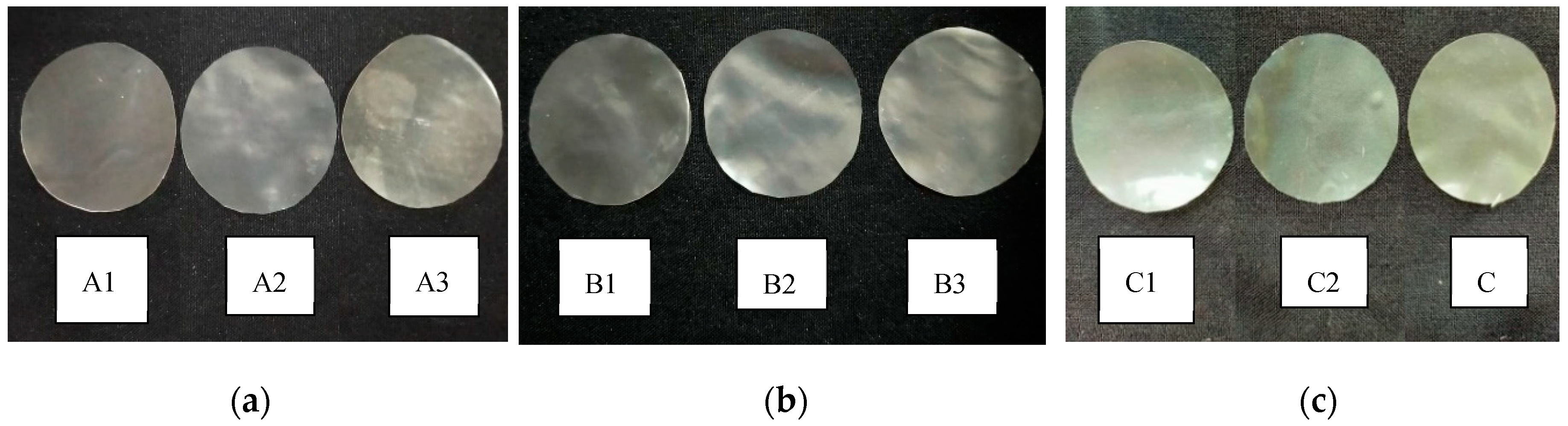
3.3. Film solubility in water
| Treatment | Film solubility in water(%) | WVTR (g/m2.h) |
|---|---|---|
| VCO 0 wt% | 40.71 ± 2.39 | 4.982 ± 0.10 |
| VCO 3 wt% | 40.51 ± 0.73 | 4.721 ± 0.53 |
| VCO 5 wt% | 40.02 ± 0.84 | 5.324 ± 0.59 |
3.4. Water vapor transmission rate of the film
3.5. Mechanical properties of the film
| Treatment | Tensile Strength MPa |
Elongation (%) |
Modulus Elasticity MPa |
|---|---|---|---|
| VCO 0 wt% | 4.800 ± 0.73 | 79.84 ± 2.07 | 0.060 ± 0.01 |
| VCO 3 wt% | 4.243 ± 0.31 | 68.58 ± 1.54 | 0.062 ± 0.01 |
| VCO 5 wt% | 4.237 ± 0.36 | 83.87 ± 5.85 | 0.051 ± 0.01 |
3.6. Scanning Electron Microscopy (SEM)
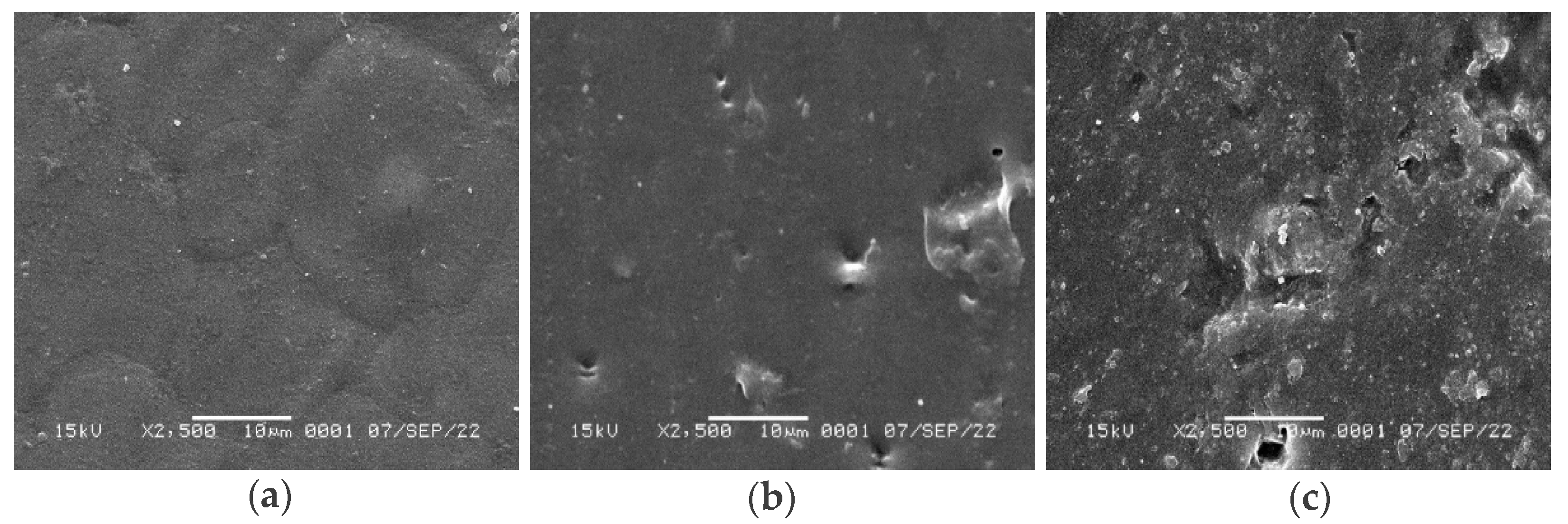
3.7. Fourier transform infrared spectroscopy (FTIR)
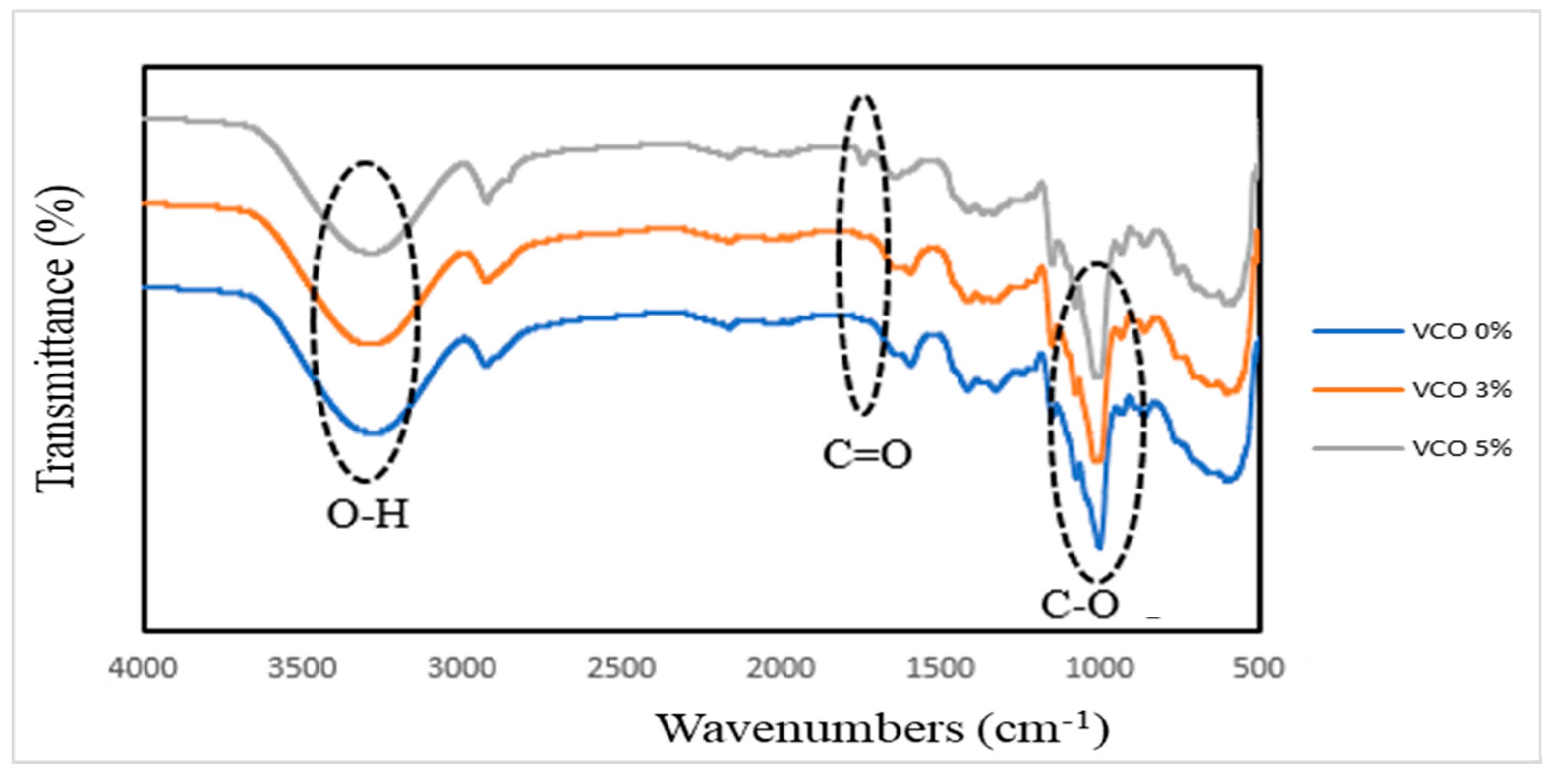
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zibaei, R. Applications of emerging botanical hydrocolloids for edible films: A review. Carbohydrate Polymers 2021, 256, 117554. [Google Scholar] [CrossRef] [PubMed]
- Wilfer, P.B.; Giridaran, G.; Jeevahan, J.J.; Joseph, G.B.; Kumar, G.S.; Thykattuserry, N.J. Effect of starch type on the film properties of native starch based edible films. Mater. Today Proc. 2021, 44, 3903–3907. [Google Scholar] [CrossRef]
- Bayram, B.; Ozkan, G.; Kostka, T.; Capanoglu, E. Valorization and Application of Fruit and Vegetable Wastes and By-Products for Food Packaging Materials. Molecules 2021, 26, 4031. [Google Scholar] [CrossRef] [PubMed]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.R.M. Antimicrobial activity, physical, mechanical and barrier properties of sugar palm based nanocellulose/starch biocomposite films incorporated with cinnamon essential oil. J. Mater. Res. Technol. 2021, 11, 144–157. [Google Scholar] [CrossRef]
- Basumatary, I.B.; Mukherjee, A.; Katiyar, V.; Kumar, S. Biopolymer-based nanocomposite films and coatings: recent advances in shelf-life improvement of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2020, 1–24. [Google Scholar] [CrossRef]
- Tavares, K.M.; De Campos, A.; Mitsuyuki, M.C.; Luchesi, B.R.; Marconcini, J.M. Corn and Cassava Starch with Carboxymethyl Cellulose Films and its Mechanical and Hydrophobic Properties. Carbohydr. Polym. 2019, 223. [Google Scholar] [CrossRef]
- Kusumawati, D.H.; Dwi, W.; Putri, R.; Koresponsdensi, P. Karakteristik fisik dan kimia edible film pati jagung yang diinkorporasi dengan perasan temu hitam. J. Pangan dan Agroindustri 2013, 1, 90–100. [Google Scholar]
- Farajpour, R.; Djomeh, Z.E.; Moeini, S.; Tavahkolipour, H.; Safayan, S. Structural and physico-mechanical properties of potato starch - olive oil edible films reinforced with zein nanoparticles. Int. J. Biol. Macromol. 2020, 149, 941–950. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M.R. Effect of Surface Energy on Dispersion and Mechanical Properties of Polymer/Nanocrystalline Cellulose Nanocomposites. Biomacromolecules 2013, 14, 3155–3163. [Google Scholar] [CrossRef]
- Utomo, P.P.; Salahudin, F. Pengaruh Inkorporasi Lipid dan Antioksidan terhadap Sifat Mekanik dan Permeabilitas Edible Film Pati Jagung. Biopropal Ind. 2015, 6, 37–42. [Google Scholar]
- Marina, A.M.; Man, Y.B.C.; Nazimah, S.A.H.; Amin, I. Chemical Properties of Virgin Coconut Oil. J. Am. Oil Chem. Soc. 2009, 86, 301–307. [Google Scholar] [CrossRef]
- Jer, Y.; et al. A comprehensive review on the techniques for coconut oil extraction and its application. Bioprocess Biosyst. Eng. 2021. [CrossRef]
- Fernandez, L.; de Apodaca, E.D.; Cebrian, M.; Villaran, C.; Mate, J.I. Effect of the unsaturation degree and concentration of fatty acids on the properties of WPI-based edible films. Eur. Food Res. Technol. 2007, 224, 415–420. [Google Scholar] [CrossRef]
- Wang, R.; Liu, P.; Cui, B.; Kang, X.; Yu, B. Effects of different treatment methods on properties of potato starch-lauric acid complex and potato starch-based fi lms. Int. J. Biol. Macromol. 2019, 124, 34–40. [Google Scholar] [CrossRef]
- Liu, P.; Wang, R.; Kang, X.; Cui, B.; Yu, B. Effects of ultrasonic treatment on amylose-lipid complex formation and properties of sweet potato starch-based films. Ultrason. Sonochem. 2018, 44, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, R.; Ghanbarzadeh, J.; Dehghannya; Hosseini, M.; Falcone, P.M. The effect of Macro and Nano-emulsions of cinnamon essential oil on the properties of edible active films. Food Sci. Nutr. 2020, 8, 6568–6579. [Google Scholar] [CrossRef] [PubMed]
- Bhasney, S.M.; Patwa, R.; Kumar, A.; Katiyar, V. Plasticizing effect of coconut oil on morphological, mechanical, thermal, rheological, barrier, and optical properties of poly (lactic acid): A promising candidate for food packaging. J. Appl. Polym. Sci. 2017, 134, 45390. [Google Scholar] [CrossRef]
- Xiao, M.; Luo, L.; Tang, B.; Qin, J.; Wu, K.; Jiang, F. Physical, structural, and water barrier properties of emulsified blend film based on konjac glucomannan/agar/gum Arabic incorporating virgin coconut oil. LWT-Food Sci. Technol. 2022, 154, 112683. [Google Scholar] [CrossRef]
- Carpiné, D.; Luiz, J.; Dagostin, A.; Bertan, L.C.; Mafra, M.R. Development and Characterization of Soy Protein Isolate Emulsion-Based Edible Films with Added Coconut Oil for Olive Oil Packaging: Barrier, Mechanical, and Thermal Properties. Food Bioprocess Technol 2015, 8, 1811–1823. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishekar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT-Food Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Sogut, E. Active whey protein isolate fi lms including bergamot oil emulsion stabilized by nanocellulose. Food Packag. Shelf Life 2020, 23. [Google Scholar] [CrossRef]
- Syafiq, R.; Sapuan, S.M.; Zuhri, M.R.M. Antimicrobial activity, physical, mechanical and barrier properties of sugar palm based nanocellulose/starch biocomposite films incorporated with cinnamon essential oil. J. Mater. Res. Technol. 2021, 11, 144–157. [Google Scholar] [CrossRef]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte films based on chitosan / olive oil and reinforced with cellulose nanocrystals. Carbohydr. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Fangfang, Z.; Xinpeng, B.; Wei, G.; Wang, G.; Shi, Z.; Jun, C. Effects of virgin coconut oil on the physicochemical, morphological and antibacterial properties of potato starch-based biodegradable films. Int. J. Food Sci. Technol. 2020, 55, 192–200. [Google Scholar] [CrossRef]
- Binsi, P.K.; Ravishankar, C.N.; Gopal, T.K.S. Development and Characterization of an Edible Composite Film Based on Chitosan and Virgin Coconut Oil with Improved Moisture Sorption Properties. J. Food Sci. 2013, 78, E526–E534. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Ziaee, E.; Eskandari, M.H.; Mohammad, S.; Hosseini, H. Characterization of basil seed gum-based edible films incorporated with Zataria multiflora essential oil nanoemulsion. Carbohydr. Polym. 2017. [CrossRef]
- Arifin, H.R.; Djali, M.; Nurhadi, B.; Hasim, S.A.; Hilmi, A.; Puspitasari, A.V. Improved properties of corn starch-based bio-nanocomposite film with different types of plasticizers reinforced by nanocrystalline cellulose. Int. J. Food Prop. 2022, 25, 509–521. [Google Scholar] [CrossRef]
- Warkoyo; Rahardjo, B.; Marseno, D.W.; Karyadi, J.N.W. Sifat fisik, mekanik dan barrier edible film berbasis pati umbi kimpul (Xanthosoma sagittifolium) yang diinkorporasi dengan kalium sorbat. Agritech 2014, 34, 72–81. [Google Scholar]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S. Physicochemical and antifungal properties of bio-nanocomposite film based on gelatin-chitin nanoparticles. Int. J. Biol. Macromol. 2016. [CrossRef]
- ASTM. Standard test methods for water transmission of material-ASTM E96-80; American Society for Testing and Material: Philadelphia, 1981. [Google Scholar]
- ASTM. Standard test methods for tensile properties of thin plastic sheeting (D882) (Annual Book of ASTM Standards; American Society for Testing and Material: Philadelpia, 2010. [Google Scholar]
- Setiani, W.; Sudiarti, T.; Rahmidar, L. Preparasi Dan Karakterisasi Edible Film Dari Poliblend Pati. Valensi 2013, 3, 100–109. [Google Scholar] [CrossRef]
- Febriati, N.L. Optimasi sifat fisik edible film berbasis karagenan murni dengan metode permukaan respon (response surface methodology). Eksakta 2018, 1, 71–90. [Google Scholar]
- Arham, R.; Mulyati, M.; Metusalach, M.; Salengke, S. Physical and mechanical properties of agar based edible film with glycerol plasticizer. Int. Food Res. J. 2016, 23, 1669–1675. [Google Scholar]
- JIS. Japanese International Standart Z 1707; Japanese Standards Association: Tokyo, 1975. [Google Scholar]
- Seifari, F.K.; Yousefi, S.; Ahari, H.; Hosseini, S.H. Corn Starch-Chitosan Nanocomposite Film Containing Nettle Essential Oil Nanoemulsions and Starch Nanocrystals: Optimization and Characterization. Polymers 2021, 13, 2113. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Cran, B.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Chiralt, A.; Santamarina, P.; Rosello, J.; González-Martínez, C.; Cháfer, M. Antifungal films based on starch-gelatin blend, containing essential oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Yam, K.L.; Papadakis, S.E. A simple digital imaging method for measuring and analyzing color of food surfaces. J. Food Eng. 2004, 61, 137–142. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Julianto, G.E.; Ustadi; Husni, A. Characterization of gelatin edible film from skin of red nile by adding of sorbitol and palmitic acid as plasticiser. J. Perikan. 2011, 13, 27–34. [Google Scholar]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Nafchi, M. The synergistic effects of cinnamon essential oil and nano TiO 2 on antimicrobial and functional properties of sago starch films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- Raphaelides, S.N.; Dimitreli, G.; Exarhopoulos, S.; Mintzas, D.; Lykidou, A. Effect of processing conditions on the physicochemical and structural characteristics of pregelatinised starch–fatty acid–glycerol extrudates. Carbohydr. Polym. 2012, 88, 282–289. [Google Scholar] [CrossRef]
- Liu, P.; Sun, S.; Hou, H.; Wang, W.; Dong, H. Effect of Five Saturated Fatty Acids on the Properties of Sweet-Potato-Starch-Based Films. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Anandito, R.B.K.; Nurhartadi, E.; Bukhori, A. Pengaruh gliserol terhadap karakteristik edible film berbahan dasar tepung jali. J. Teknol. Has. Pertan. 2012, V, 17–23. [Google Scholar]
- Natania, K.; Setiawan, F. Characterization of Antimicrobial Edible Films with Single and Double Emulsions from Clove (Syzygium aromaticum) Oil. Reaktor 2020, 20, 38–46. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovichs, N.E. Caseinate films modified with tung oil. Food Hydrocoll. 2010, 24, 800–808. [Google Scholar] [CrossRef]
- Zhang, Y.P.; et al. Preparation and characterization of bio-nanocomposites film of chitosan and montmorillonite incorporated with ginger essential oil and its application in chilled beef preservation. Antibiotics 2021, 10, 796. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Del Carmen Nuñez-Santiago, M.; Romero-Bastida, C.A.; Martinez-Bustos, F. Effects of coconut oil concentration as a plasticizer and Yucca schidigera extract as a surfactant in the preparation of extruded corn starch films. Starch/Staerke 2014, 66, 1079–1088. [Google Scholar] [CrossRef]
- Lian, H.; Peng, Y.; Shi, J.; Wang, Q. Effect of emulsifier hydrophilic-lipophilic balance (HLB) on the release of thyme essential oil from chitosan films. Food Hydrocoll. 2019, 97, 105213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





