1. INTRODUCTION
P. aeruginosa is a gram-negative, rod-shaped bacterium frequently encountered in hospital settings as an opportunistic pathogen in nosocomial infections with high mortality rates1. P. aeruginosa can grow in several environments including but not limited to hospital water systems, humidifiers, and other types of medical equipment such as pacemakers, catheters, and ventilators2. P. aeruginosa has the ability to cause both acute and chronic infections through different courses of infection. Acute infections, which are generally more manageable, typically involve P. aeruginosa in the planktonic form. In this form, the infection is treatable using antibiotics. The harder-to-treat chronic infections are usually the result of P. aeruginosa forming antibiotic-resistant biofilms. When this bacterium forms biofilm communities, it exhibits a high degree of multidrug resistance (MDR), thereby complicating the treatment of biofilm-based bacterial infections such as Chronic Obstructive Pulmonary Disease diseases (COPD)3. P. aeruginosa imposes a financial burden on hospitals and healthcare systems because of limited prevention and treatment strategies due to a lack of detection methods and the narrow availability of effective antibiotics3. Its antibiotic resistance can be categorized into three main mechanisms: intrinsic, acquired, and adaptive resistance. Intrinsic resistance is associated with factors such as reduced outer membrane permeability, the presence of efflux pumps, and the production of enzymes that deactivate antibiotics. Acquired resistance occurs through the transfer of resistance genes via horizontal gene transfer or genetic mutations. In contrast, adaptive resistance is characterized by the formation of biofilms or the development of multidrug-tolerant cells within biofilms. The active efflux pumps play a crucial role in establishing multidrug resistance, highlighting the potential of developing efflux pump inhibitors as a promising adjunctive therapy4. Efflux pumps are membrane-associated proteins responsible for expelling substances from inside bacterial cells, including antibiotics, waste products, and toxins.
Several studies have shown efflux pumps may play a significant role in the multidrug-resistant phenotype and bacterial virulence5. The P. aeruginosa genome has been correlated to encoding multiple RNA efflux pumps, with emphasis on the following four: MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY-OprM. MexAB-OprM and MexXY-OprM6. The RND-type efflux pumps account for the major cause of intrinsic resistance to most antimicrobial agents in P. aeruginosa7. MexXY-OprM and MexAB-OprM are known to be the largest and only intrinsic multidrug-resistant efflux pumps within the resistance nodulation division (RND) family in P. aeruginosa8.
The pumps extrude antimicrobials across the outer membrane, conferring resistance to beta-lactams targeting cell wall synthesis. MexAB-OprM consists of three different peptides: a MexB translocase solute/proton RND antiporter, an outer membrane porin-OprM, and a membrane fusion protein MexA, which docks MexB to OprM. Studies suggest MexAB-OprM system is constitutively expressed and contributes to the efflux of a broad range of antibiotics, while the MexXY system is inducible and primarily involved in the efflux of aminoglycosides 8. The hyperexpression of these major efflux pump systems contributes to the intrinsic resistance of P. aeruginosa to a number of antimicrobials including fluoroquinolones, tetracyclines, and β-lactams, such as carbapenem9,10. The resistance exhibited by biofilms to antimicrobials appears to align with the broad specificity of multidrug resistance (MDR) mechanisms. Biofilms demonstrate significantly elevated levels of resistance, and it remains uncertain whether the mechanisms that confer lower resistance in planktonic cells also play a significant role in biofilms. By deleting individual efflux pumps or combinations of pumps, this study examines this effect on the susceptibility of Pseudomonas aeruginosa to antibiotic treatment. Investigation of any observed changes in activity or mutations, if present, in efflux pumpsgene through reverse transcription polymerase chain reaction (RT-PCR) analysis was conducted. The objectives of this study encompass determining the minimum bactericidal concentration (MBC) and minimum inhibitory concentration (MIC) of P. aeruginosa PA01 biofilms. Furthermore, this review aims to illuminate the involvement of the only broad-spectrum intrinsic MexAB-OprM efflux pump system in antibiotic resistance within biofilms by comparing the expression levels of MexB genes in P. aeruginosa planktonic cells and biofilms, pre- and post-antibiotic exposure.
2. MATERIALS & METHODS
2.1. Literature Search
A literature review between August 2020 and December 2020, gathering studies related to current treatment approaches for P. aeruginosa and different efflux transporters. Promising antibiotics identified for treating P. aeruginosa include Tobramycin (TOB), Ofloxacin (OFX), and Ceftazidime (CAZ). The Basic Local Alignment Search Tool (BLAST) was utilized to identify regions of similarity in the genetic sequences of MexA, MexB, MexX, and MexY genes from the PA01 strain. These sequences were compared to well-studied genes from various strains, which are extensively documented in the literature regarding their functionality under different stress conditions. BLAST is an indispensable tool for biologists as it efficiently and sensitively compares nucleotide and protein sequences with both individual sequences and large databases, enabling the design of primers.
2.2. Planktonic cell growth
Modifications to the planktonic cell growth measurement assay based on the method described by Qu et al. in 2016 were done. Initially, PA01 cells that were grown overnight were diluted 1:100 in LB broth. These diluted samples were then incubated at 37°C with continuous agitation at 160 rpm. To monitor the turbidity of the planktonic culture, we used an Eppendorf spectrophotometer to measure the absorbance at 550nm at hourly intervals.
2.3. Determination of Minimal Bacterial Concentration (MBC) and Minimal Inhibition Concentration (MIC)
The minimal bacteriostatic concentration of an antimicrobial agent was determined using a 96-well plate. Each well contained 12 columns with 3 rows of antibiotics and the P. aeruginosa bacteria. To achieve minimal inhibition, 25 µL of antibiotic and 100 µL of diluted bacteria were simultaneously added to each well. The antibiotics (TOB, OFX, and CAZ) were diluted to concentrations of 256 µg/mL, 128 µg/mL, 64 µg/mL, 32 µg/mL, 16 µg/mL, 8 µg/mL, 4 µg/mL, 2 µg/mL, 1 µg/mL, 0.5 µg/mL, 0.25 µg/mL, and a control. The final volume in each well was 125 µL. The samples were then incubated for 24 hours at 37°C.
For the minimum bactericidal concentration (MBC), 100 µL of PA01 was added to each well and incubated for 24 hours at 37°C. Then, 25 µL of antibiotic was added to each well, totaling 125 µL, followed by a subsequent 24-hour incubation at 37°C. After incubation was complete for both plates, the plates were washed three times with deionized water and allowed to dry for 4-6 hours. Subsequently, to test the sensitivity to specific antibiotic concentrations, 135 µL of a 0.1% solution of crystal violet was added to each well and incubated at room temperature for 10-15 minutes. Another three sets of rinses were performed, and the plate was left to dry overnight. To quantify the biofilm, 125 µL of a 30% acetic acid solution in water was added to each well to dissolve the crystal violet, followed by a 10-15 minute incubation. The 96-well plate was then placed in a spectrophotometer to measure absorbance (OD at 550nm).
2.4. Viability assay using spectrofluorometric analysis
To investigate the antibacterial efficacy of the three different antibiotics (TOB, OFX, and CAZ) on the PAO1 stand of Pseudomonas Aeruginosa, we also examined the reduction in cell viability by employing Propidium Iodide (PI) fluorescence staining. PI is a fluorescent dye that cannot penetrate intact bacterial cell membranes. However, when the membrane integrity is compromised, PI can enter the cells and bind to their nucleic acids.
The PA01 cells were cultivated and exposed to various concentrations of the antibiotics as previously described. Subsequently, the cells were treated with 10 µM of Propidium iodide (PI). After incubating the cells with the stain for 10 minutes, the average fluorescence intensities resulting from the binding of PI to the cells were measured using a Molecular Devices SpectraMax Gemini® microplate spectrofluorometer, following the instructions provided in the BacLight™ LiveDead Kit (Molecular Probes).
2.4. Gene expression analysis
After determining the minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC), concentrations of 8 µg/ml and 32 µg/ml of TOB, OFX, and CAZ were selected for MIC and MBC evaluations, respectively. These concentrations were chosen based on optimal observation of inhibition (at 8 µg/ml) and eradication (at 32 µg/ml).
To analyze the expression of the efflux transporter genes, total RNA was extracted from the cells and treated with the selected concentrations using the RNeasy Mini kit (Qiagen), following the kit instructions. Additionally, RNAprotect® Bacteria Reagent was added at the appropriate step, as indicated in the RNeasy kit instructions, to ensure RNA stability. The purity and concentration of the extracted RNA were determined by measuring the absorbance at 260 nm and 280 nm (260/280 nm ratio). For cDNA synthesis, 1 μL of RNA was used with the TransScript All-in-One First-Strand cDNA Synthesis SuperMix (Transgene, China).
The expression of the efflux transporter genes was assessed by quantitative real-time PCR (qRT-PCR) amplification and quantification using the synthesized cDNA. The qRT-PCR was conducted using the TransStart™ Green qPCR SuperMix UDG kit (Transgene, China). The qRT-PCR conditions consisted of an initial denaturation step at 94°C for 10 minutes, followed by 40 cycles of amplification with 5 seconds at 94°C and 30 seconds at 60°C. The obtained data were normalized to the endogenous reference gene RPSL of the P. aeruginosa PA01 strain. Changes in gene expression in each sample relative to the control were analyzed using the threshold cycle method (2^(-ΔΔCT)). The qRT-PCR experiments were performed in triplicate using the ProtoScript® First Strand cDNA Synthesis Kit, and the entire experiment was repeated twice using RNA samples extracted from independent cultures.
3. RESULTS & DISCUSSION
3.1. Selection of primers for gene expression studies
The BLAST tool available on the National Library of Medicine website was utilized to align and compare the DNA sequences of the genes mexA, mexB, mexX and mexY (Figure 1), which were selected for further studies on the effect of antibiotics on planktonic cells and biofilms formed by the P. aeruginosa PA01 strain based on literature search in order to design the primers needed to facilitate the amplification and quantification of gene expression levels by quantitative real-time polymerase chain reaction (qRTPCR).
The BLAST alignment tool was used to locate similar nucleotides between the biological sequences of the four efflux transporter genes mexA, mexB, mexX and mexY, from P. aeruginosa PA01, with simultaneous comparison to other strains and recorded genes.
We utilized qRT-PCR-based amplification and quantification of cDNA obtained from transcribed RNA isolated from the tested samples. Specific primers targeting the four genes associated with efflux transporter pumps were sequenced, as each of these genes plays a vital role in actively expelling antibiotics from bacterial cells. Our analysis focused on determining the relative expression levels of these genes and investigating their potential involvement in antibiotic extrusion and resistance in P. aeruginosa PA018,11,12.
Table 1.
Summary of the Functions of Efflux Transporters MexA, MexB, MexX, and MexY in Bacteria.
Table 1.
Summary of the Functions of Efflux Transporters MexA, MexB, MexX, and MexY in Bacteria.
| Protein |
Function |
Role in Antibiotic Resistance |
Primer Sequence |
| Multidrug Resistant Efflux Pump MexA |
Resistance-
Nodulation-
Cell
Division (RND) multidrug efflux
Periplasmic membrane fusion protein precursor |
Transports structurally varied molecules, including antibiotics,
out of the bacterial cell |
F: 5’-acctacgaggccgactaccaga-3’
R: 5’-gttggtcaccagggcgcctc-3’ |
| Multidrug Resistant Efflux Pump MexB |
Resistance-
Nodulation-
Cell
Division (RND) Inner membrane multidrug efflux
Transporter protein |
Transports structurally varied molecules, including antibiotics,
out of the bacterial cell |
F: 5’-gtgttcggctcgcagtactc-3’
R: 5’-aaccgtcgggattgaccttg-3’ |
| Outer Membrane Protein OprM |
Major intrinsic multiple antibiotic
resistance efflux outer membrane
protein
OprM
precursor |
Channel-forming outer membrane protein |
F: 5’- ccatgagccgccaactgtc-3’
R: 5’-cctggaacgccgtctggat-3’ |
Multidrug Resistant
Efflux Pump MexX |
Resistance-
Nodulation-
Cell
Division (RND) multidrug efflux
membrane fusion protein MexX
precursor |
Transports structurally varied molecules, including antibiotics,
out of the bacterial cell |
F: 5’-tgtacgcgtattcggaacaaggcgtctgc-3’
R: 5’-ttctgctagcgatgtgcatgggtgtccctc-3’ |
| Multidrug Resistant Efflux Pump MexY |
Resistance-
Nodulation-
Cell
Division (RND) multidrug efflux
transporter MexY |
Transports structurally varied molecules, including antibiotics,
out of the bacterial cell |
F: 5’-tgtactagttgatgcccctagcgaaactctc-3’
R: 5’-tttaagcttgacctacaggacgctgctg-3’ |
| Ribosomal Subunit RpsL |
Ribosomal subunit binding rRNA and tRNA, expressed constitutively |
Structural constituent of ribosome which serves as Internal control |
F: 5’-gctgcaaaactgcccgcaacg-3’
R: 5’-acccgaggtggtccagcgaacc-3’ |
The four potent RND-type multidrug resistance efflux pumps- MexA, MexB, MexX and MexY- remove intracellular harmful chemicals in
P. aeruginosa, including antibiotics. By diminishing the intracellular antibiotic concentration, leading to decreased susceptibility and increased resistance, the efflux pump systems confer plays a direct role in the development of multidrug-resistant
P. aeruginosa. (
Table 1). The ribosomal subunit (RpsL) responsible for rRNA and tRNA binding is constitutively expressed and utilized as the internal control for monitoring changes in the efflux transporters’ expression levels in response to biofilm formation and the addition of varied antibiotics
12,13.
3.2. Effectiveness of different antibiotics for inhibition and eradication of Pseudomonas aeruginosa biofilm.
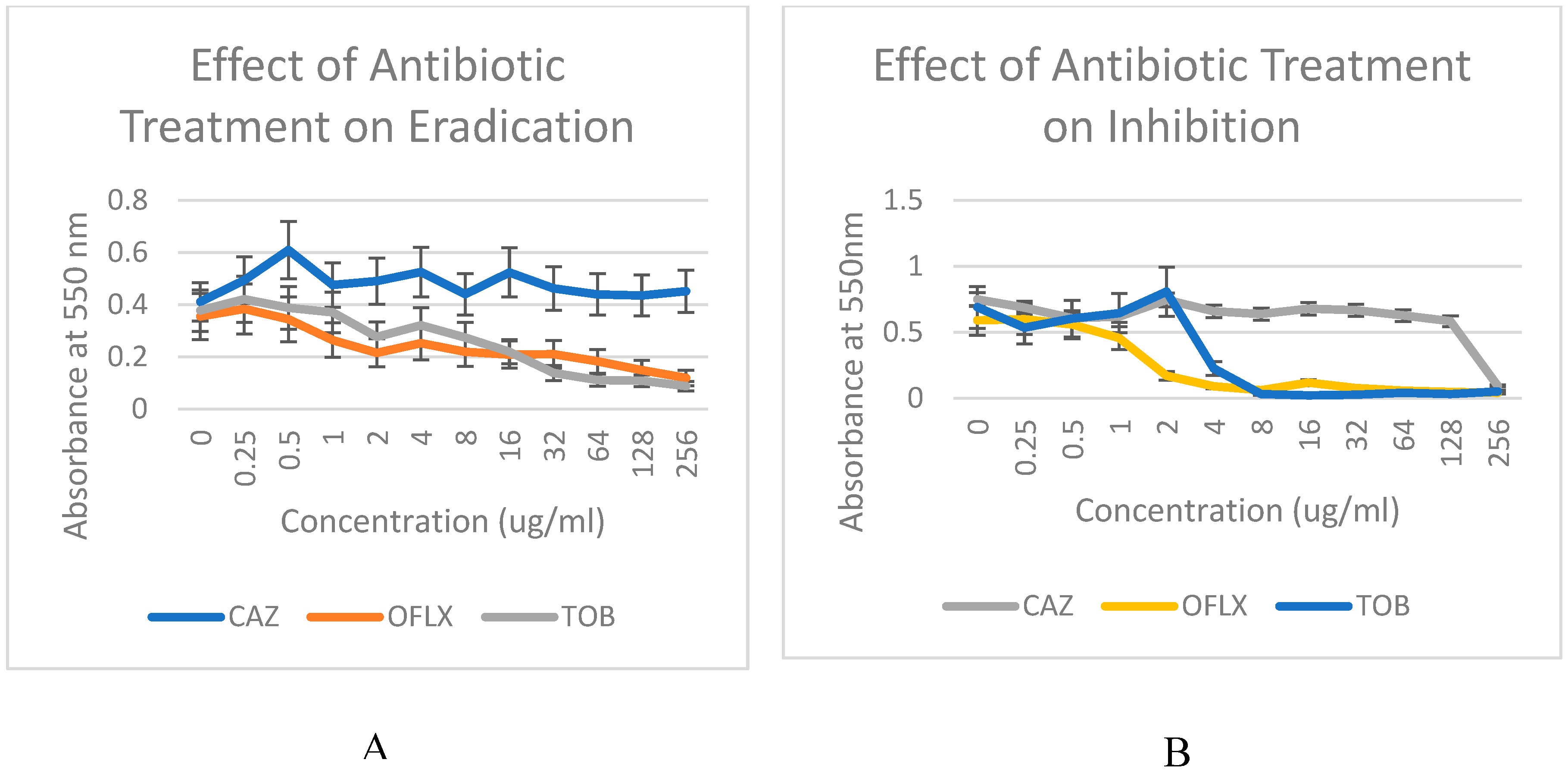
Figure 2A and 2B compare the effectiveness of three antibiotics, ceftazidime (CAZ), ofloxacin (OFX), and tobramycin (TOB), in inhibiting biofilm formation and eradicating mature biofilms in the
P. aeruginosa PA01. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values of these antibiotics assess the antibiotic capacity to inhibit and eradicate biofilms.
Figure 2B, the eradication data, evaluate the effectiveness of these antibiotics on mature biofilms, which mimic clinical biofilm infections. Comparatively, the inhibition data in
Figure 2A investigates the effects of the mentioned antibiotics on biofilm formation originating from free-living or planktonic cells.
Figure 2.
Effect of increasing concentration of the ceftazidime [CAZ], ofloxacin [OFLX] and tobramycin [TOB] on (A) inhibition and (B) eradication of Pseudomonas aeruginosa biofilm.
Figure 2.
Effect of increasing concentration of the ceftazidime [CAZ], ofloxacin [OFLX] and tobramycin [TOB] on (A) inhibition and (B) eradication of Pseudomonas aeruginosa biofilm.
The impact of antibiotics on biofilm inhibition (n = 6), involving the application of the antibiotic during inoculation (a), as well as biofilm eradication (n = 6), where the antibiotic is applied after the formation of mature biofilms (b), was evaluated at different concentrations. The x-axis illustrates the different concentrations of the three antibiotics, while the y-axis represents the degree of inhibition or eradication of P. aeruginosa biofilms resulting from treatment with the antibiotics. The measurement of residual biofilms was conducted using spectrophotometric absorbance at 550 nm, providing a quantitative assessment of biofilm levels.
In order to compare the effectiveness of the three antibiotics in inhibiting P. aeruginosa biofilms, the impact of different concentrations of each antibiotic on biofilm formation capacity was evaluated using the microbroth format. This assessment aimed to determine the Minimum Inhibitory Concentration (MIC) values. The results indicated a dose-dependent sensitivity of PA01 biofilms to both ofloxacin and tobramycin at concentrations of 8µg/ml, while exhibiting high resistance to ceftazidime during the inhibition phase. In contrast, the efficacy of the three antibiotics in eradicating P. aeruginosa biofilms was assessed by evaluating the remaining biofilm following incubation with different concentrations of each antibiotic to determine the Minimum Bactericidal Concentration (MBC) values.
The results demonstrated notable resistance to all antibiotics, except for ofloxacin, when targeting mature biofilms. During the eradication phase, biofilms exhibited sensitivity to ofloxacin, but at significantly higher concentrations (32 µg/ml) compared to the inhibition phase. Additionally, biofilms showed sensitivity to tobramycin only at the highest concentration tested (256 µg/ml). Ceftazidime displayed the highest level of resistance, demonstrating insensitivity even at the highest concentration, and also exhibited considerable resistance to tobramycin at lower concentrations during the eradication phase. For the inhibition phase (
Figure 2A), antibiotics were administered at the time of inoculation in a 96-well microtiter plate. In the eradication phase (
Figure 2B), antibiotics were applied 24 hours after the 37°C inoculation. At 550 nm, the optical density (OD) of each sample was measured following incubation with increasing antibiotic dosing Overall, ofloxacin exhibited the greatest inhibitory and eradication effects, while ceftazidime demonstrated the least effectiveness (Figures 2A and 2B) in both conditions. These observations support studies that suggest
P. aeruginosa biofilms impart resistance to beta-lactams (Ceftazidime), aminoglycosides (Tobramycin), and fluoroquinones (Ofloxacin) through the upregulation of efflux pumps
14. In particular, the MexAB-OprM efflux pump system was reported to contribute to conferring inherent resistance to β-lactams and quinolones
15. Biofilms will not be eradicated with low-dose tobramycin
16, but ofloxacin is supported as the choice of drug for
P. aeruginosa infection
11.
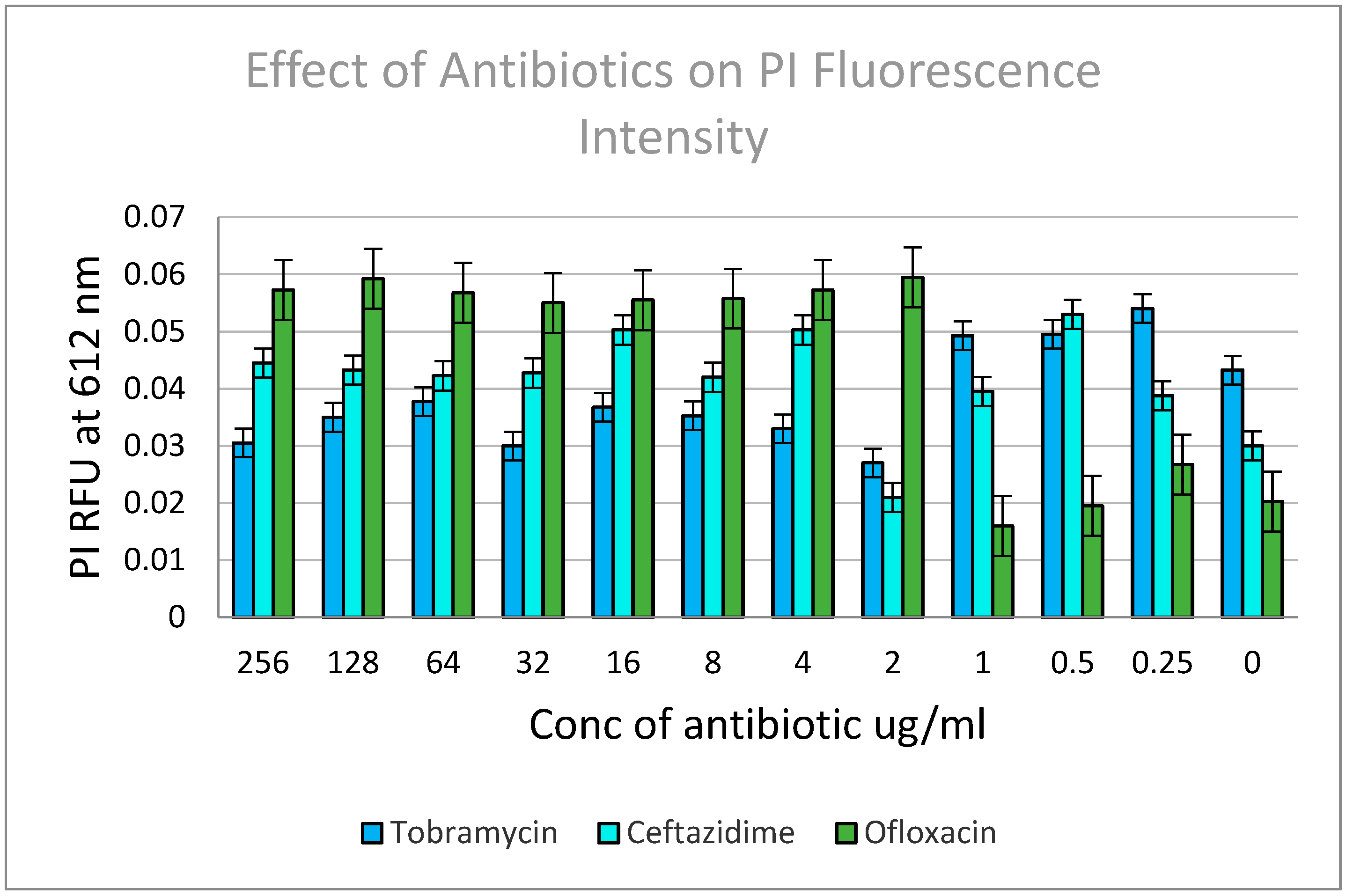
Figure 3.
Effect of various antibiotics on the viability of PA01 cells within a biofilm.
Figure 3.
Effect of various antibiotics on the viability of PA01 cells within a biofilm.
Cell viability was assessed using the fluorescence intensity of the PI stain, as it serves as a reliable indicator of membrane damage and loss of integrity. To evaluate membrane permeability and leakage, the cells were stained with the membrane-impermeable PI dye. The relative viability of the cell suspensions was analyzed using a fluorescence microplate reader, and the mean fluorescence intensity was determined through spectrofluorometric evaluation, with each data point representing the average of ten measurements. The samples were excited at 480 ± 20 nm, and the integrated intensities of the emission from the suspensions were recorded in the green range (530 ± 12.5 nm) and red range (620 ± 20 nm). Treatment with ofloxacin exhibited the highest antibacterial activity per the increase in PI fluorescence, suggesting greater membrane damage.
3.3. Changes in expression of efflux transporter genes in P. aeruginosa during biofilm formation
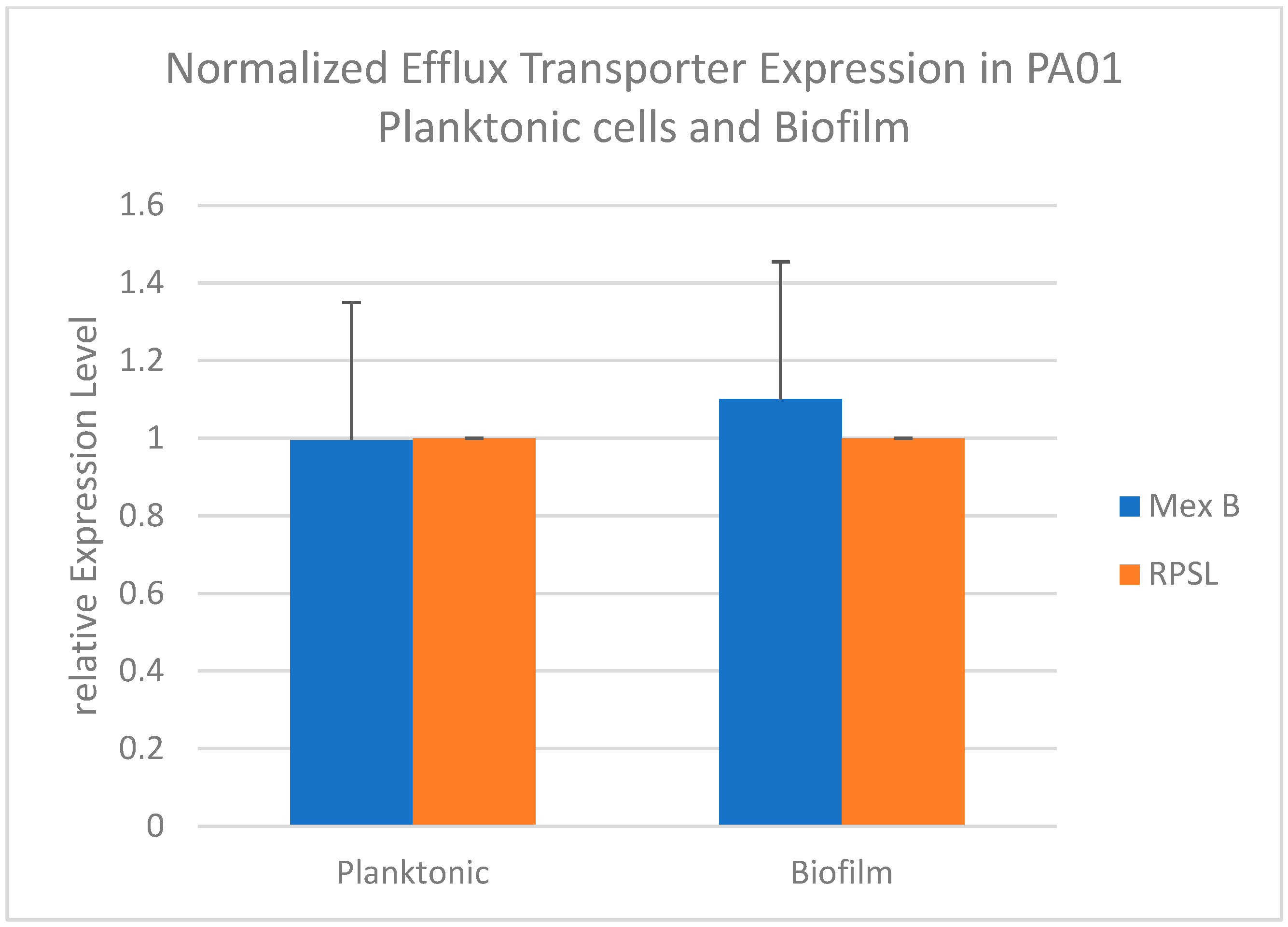
To investigate the impact of biofilm formation triggers on the expression levels of efflux transporter system MexAB-OprM, a comparison was made between their expression in PA01 planktonic cells and biofilms. Biofilms are well-known for their higher resistance to antibiotic treatment compared to planktonic cells17. The objective was to understand any alterations in the expression of this efflux transporter gene mex B in response to factors that induce biofilm formation, conferring increased resistance to both antibiotic inhibition and eradication.
The fold change values (mean of triplicate samples) were determined by comparing the transcription level of the
mexB efflux transporter gene with that of the internal control RPSL. The expression of the
mexB gene was assessed using qRTPCR to investigate its differential expression in planktonic and biofilm states. The results showed upregulation of
mexB gene expression in the biofilm stage compared to the planktonic stage (
Figure 4). This finding aligns with recent publications indicating that a significant proportion of antibiotic-resistant clinical isolates of
P. aeruginosa harbor the
mexA and
mexB genes, suggesting the crucial role of these active efflux pump systems in multi-drug resistance
11. These studies also highlight the contribution of the
mexA and
mexB genes to the elevated antibiotic resistance observed in biofilms formed by clinical isolates of
P. aeruginosa 9. In contrast, the expression levels of the
rpsL control gene remained consistent between the planktonic and biofilm stages.
Figure 4.
Normalized Expression of Efflux Transporter Mex B in PA01 Planktonic Cells and Biofilm.
Figure 4.
Normalized Expression of Efflux Transporter Mex B in PA01 Planktonic Cells and Biofilm.
3.4. Effect of various antibiotics on expression of efflux transporter genes in inhibition and eradication phases
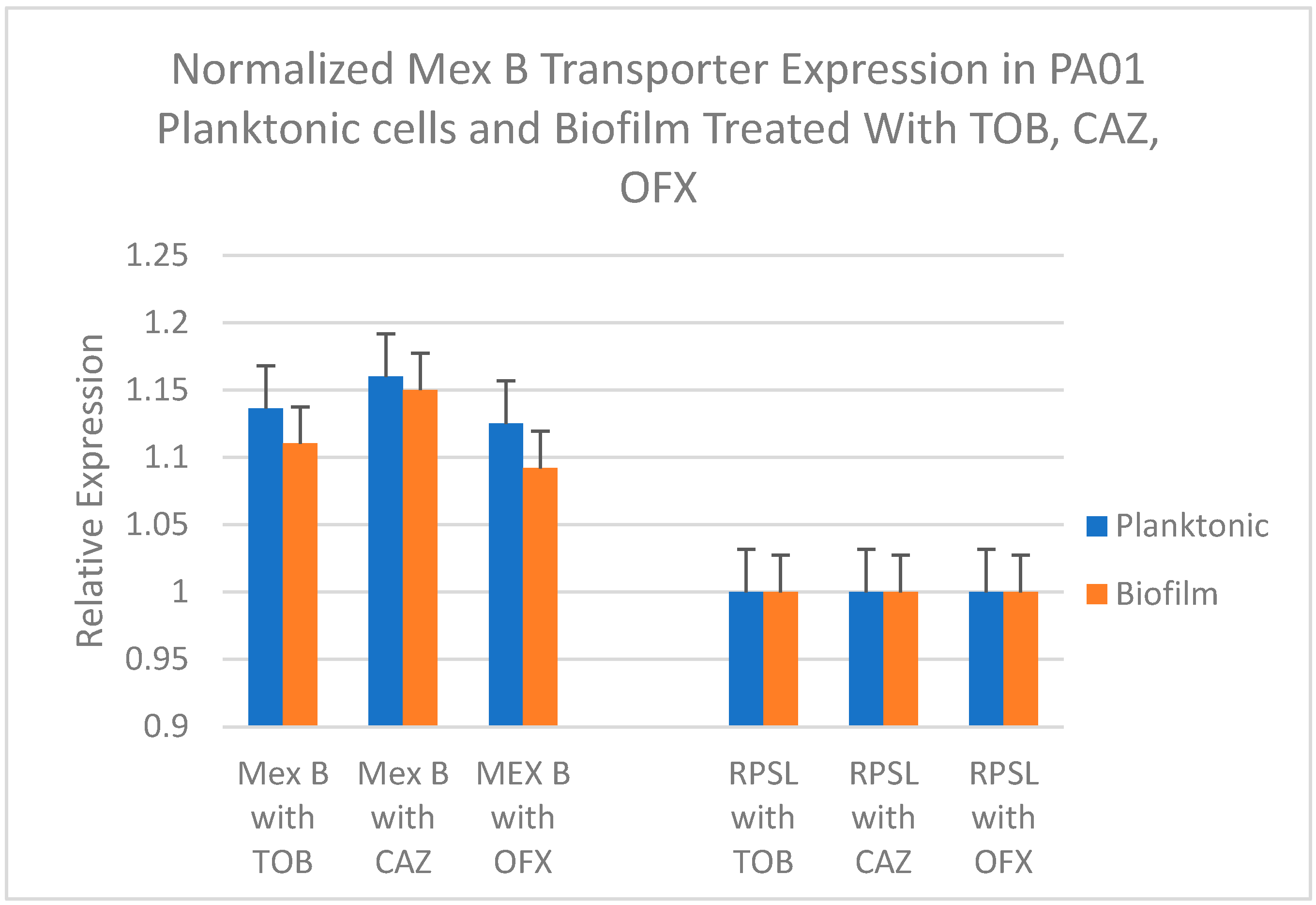
The study aimed to investigate the impact of ceftazidime, ofloxacin, and tobramycin on the expression levels of the MexAB-OprM transporter systems in newly formed and mature PA01 biofilms. The relative expression levels of MexB were compared after exposure to the antibiotics at their corresponding minimum inhibitory concentration (MIC) values for newly formed biofilms and minimum bactericidal concentration (MBC) values for mature biofilms. The objective was to assess whether these efflux pumps contribute to the reduced sensitivity to the tested antibiotics observed during and after biofilm formation.
Figure 5 represents the relative gene expression levels of mexB in PA01 planktonic cells versus biofilms after treatment with the antibiotics at their determined MIC. Values represent fold change (mean of triplicate samples) of the efflux transporter gene
mexB in comparison with the transcription level of the internal control
rpsL. Statistical analysis using paired t-test was done to test if the mean
mexB gene expression difference between planktonic and biofilm cultures in presence of various antibiotics was significant.
Figure 5.
Relative Expression of Efflux Pump Gene mexB in PA01 during (Planktonic) Inhibition of new biofilm formation and during (Biofilm) Eradication of mature biofilms after Treatments with three antibiotics, Tobramycin (TOB), Ceftazidime (CAZ) and Ofloxacin (OFX), at their Minimum Inhibitory Concentrations (MIC) for planktonic cultures and Minimum Bactericidal Concentrations (MBC) for biofilm cultures respectively as determined previously. Statistically highly significant as P < 0.001.
Figure 5.
Relative Expression of Efflux Pump Gene mexB in PA01 during (Planktonic) Inhibition of new biofilm formation and during (Biofilm) Eradication of mature biofilms after Treatments with three antibiotics, Tobramycin (TOB), Ceftazidime (CAZ) and Ofloxacin (OFX), at their Minimum Inhibitory Concentrations (MIC) for planktonic cultures and Minimum Bactericidal Concentrations (MBC) for biofilm cultures respectively as determined previously. Statistically highly significant as P < 0.001.
When comparing the impact of the tested antibiotics on the expression of
mexB genes in newly forming and mature PA01 biofilms, it was observed that ceftazidime (CAZ) treatment led to a significant increase in the expression of the efflux transporter
mexB gene relative to the internal control during both the inhibition (planktonic) and eradication (biofilm) phases. Previous studies have also reported the hyperexpression of MexAB-OprM genes, which are associated with increased resistance to cephalosporins
18,19, in
P. aeruginosa biofilms, as evident from
Figure 5. In contrast, treatment with ofloxacin and tobramycin resulted in much lesser increases in the expression of
mexB compared to the expression of
rpsL during both inhibition and eradication phases (
Figure 5). A paired t-test to determine the level of significance of the difference in
mexB expression between planktonic and biofilm cultures showed a highly significant reduction of
mexB expression in PA01 biofilm compared to planktonic cultures in the presence of Ofloxacin and Tobramycin (P<0.001) demonstrating their effectiveness in eradicating biofilm growth, while ceftazidime does not seem to produce a significant difference in mexB expression level showing that CAZ is not as effective as TOB and OFX. These findings support the results obtained from studying the effectiveness of these antibiotics in inhibiting and eradicating
P. aeruginosa biofilms, where ceftazidime exhibited the least efficacy with the highest MIC and MBC values (
Figure 2A and 2B). This could be attributed to the higher expression of the MexAB-OprM efflux pump system, which leads to increased extrusion of ceftazidime, thereby reducing its effectiveness. On the other hand, both ofloxacin and tobramycin demonstrated greater effectiveness compared to ceftazidime, with significantly lower MexB expression levels. The MIC and MBC values also supported ofloxacin as the superior treatment option (
Figure 2A and 2B). These findings are further supported by the lower expression levels of the efflux pump
mexB gene observed after treating the PA01 biofilms with ofloxacin under both inhibition and eradication conditions (
Figure 5).
In cases of suspected P. aeruginosa infection, empirical antibiotic therapy typically involves the use of monotherapy or combination therapy. Monotherapy options include β-lactam antibiotics or aminoglycosides. Combination therapy may involve the use of a β-lactam antibiotic (penicillin or cephalosporin) in combination with an aminoglycoside, or the use of a carbapenem (imipenem or meropenem) in combination with antipseudomonal quinolones and an aminoglycoside2,11.
The findings presented in this study also demonstrate a time-dependent efficacy of antibiotics against biofilm formation. The results, as depicted in
Figure 2, indicate that a lower concentration of antibiotics is required to treat an early
P. aeruginosa infection compared to a mature biofilm that has formed after 24 hours. The study reveals that PA01 biofilms are more susceptible to antibiotic treatment during the inhibition phase, as evidenced by their sensitivity to lower antibiotic concentrations during the early stages of formation. In contrast, during the eradication phase when biofilms have matured, they only exhibit sensitivity to much higher antibiotic concentrations. These findings provide support for the use of monotherapy in empirical antibiotic therapy rather than combination treatments once the infection is confirmed. Furthermore, considering a transition to a more contemporary combination antibiotic therapy to address resistant strains of P. aeruginosa has the potential to enhance treatment outcomes. Given the increasing prominence of
P. aeruginosa multi-drug resistance and prevalence, the identification of alternative antibiotics is vital.
To gain insights into the resistance mechanism of PA01 biofilms against the selected antibiotics, additional genomic analysis was performed to compare the expression levels of genes responsible for efflux pump proteins. This analysis aimed to identify any overexpressed or suppressed genes during antibiotic treatment. The results revealed that the
mexB genes exhibited higher expression levels during the biofilm phase compared to the planktonic phase (
Figure 4). Notably, when PA01 biofilms were treated with ceftazidime, a significant upregulation of the
mexB efflux gene was observed, in contrast to the treatment with ofloxacin and tobramycin, as shown in
Figure 5. The observed upregulation of MexB may contribute to reduced antibiotic sensitivity by actively pumping the antibiotics out of the bacterial cells within the biofilm phase. These findings suggest that MexB efflux pump could serve as a potential target for combating antibiotic resistance in the future.
5. CONCLUSIONS
Ofloxacin, Tobramycin, and Ceftazidime have been widely used antibiotics that demonstrate effectiveness in treating P. aeruginosa infections. Among the three antibiotics tested during both the inhibition and eradication phases, Ofloxacin (OFX) exhibited the highest efficacy in inhibiting the growth of P. aeruginosa strain PA01. Conversely, Ceftazidime (CAZ) displayed comparatively lower effectiveness in inhibiting P. aeruginosa growth. Although Tobramycin (TOB) exhibited slightly lower effectiveness compared to OFX, it still demonstrated greater efficacy than CAZ. Additionally, both tobramycin and ofloxacin were effective in eradicating biofilm growth. Based on all these results, ofloxacin was the most effective antibiotic for both inhibition and eradication of P. aeruginosa biofilms at lower concentrations as opposed to ceftazidime, which was seen to be less effective since the PAO1 strain showed resistance in both MIC and MBC phases.
Moreover, a comparative analysis of gene expression levels was performed to investigate the potential involvement of the MexB efflux transporter in the observed antibiotic resistance during biofilm formation. The analysis revealed higher expression of this gene in PA01 biofilms compared to planktonic cells. Significantly elevated expression levels of the selected efflux pump gene were detected in Pseudomonas aeruginosa biofilms treated with ceftazidime, which was found to be the least effective compared to ofloxacin and tobramycin in both the inhibition and eradication phases. These findings support a contribution of this efflux transporter in the mechanism of antibiotic resistance associated with these PA01 biofilms. To assess the impact of MexB efflux pump on the susceptibility of PAO1 biofilms to antibiotics, it is necessary to perform deletion studies. These studies would help determine the extent of its involvement in reducing the susceptibility of PAO1 biofilms to antibiotics. By neutralizing the MexB efflux pump, intrinsic resistance could be reduced, rendering P. aeruginosa biofilms more susceptible to antibiotics. This approach holds promise for developing new treatment strategies, as it could potentially address the high levels of antibiotic resistance displayed by P. aeruginosa biofilm infections. Although the efflux pump seems to play an important role in increasing the resistance towards different antibiotics, the role of other agents and mechanisms in the evolution of resistance should not be ignored21,22. Quorum sensing utilized by P. aeruginosa to coordinate the expression of virulence factors and biofilm formation also holds the potential for further evaluation and manipulation in relation to efflux transporter gene expression22,23.
The development of new antibiotics that can overcome the impact of efflux pumps remains a formidable challenge. Therefore, it is crucial to conduct additional studies to unravel the underlying mechanisms and establish the structure-function relationship of bacterial efflux systems24,25. Additionally, investigating the interactions between these pumps and other resistance mechanisms is highly recommended. Such studies will provide valuable insights for the development of novel strategies to tackle antibiotic resistance effectively.
Acknowledgments
The author is thankful to the Landers College of Men, Touro University, New York, USA for offering laboratory facilities to carry out this study.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Sousa, A. M., & Pereira, M. O. (2014). Pseudomonas aeruginosa diversification during infection development in cystic fibrosis Lungs—A review. Pathogens (Basel), 3(3), 680-703. [CrossRef]
- Walker, J., & Moore, G. (2014). Pseudomonas aeruginosa in hospital water systems: Biofilms, guidelines, and practicalities. The Journal of Hospital Infection, 89(4), 324-327. doi:10.1016/j.jhin.2014.11.019. [CrossRef]
- Lund-Palau, H., Turnbull, A. R., Bush, A., Bardin, E., Cameron, L., Soren, O., . . . Davies, J. C. (2016). Pseudomonas aeruginosa infection in cystic fibrosis: Pathophysiological mechanisms and therapeutic approaches Informa UK Limited. doi:10.1080/17476348.2016.1177460. [CrossRef]
- Li, X., Plésiat, P., & Nikaido, H. (2015). The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clinical Microbiology Reviews, 28(2), 337-418. doi:10.1128/CMR.00117-14. [CrossRef]
- Alav, I., Sutton, J. M., & Rahman, K. M. (2018). Role of bacterial efflux pumps in biofilm formation. Journal of Antimicrobial Chemotherapy, 73(8), 2003-2020. doi:10.1093/jac/dky042. [CrossRef]
- Marquez, B. (2005). Bacterial efflux systems and efflux pumps inhibitors. Biochimie, 87(12), 1137-1147. doi:10.1016/j.biochi.2005.04.012. [CrossRef]
- Goli, H. R., Nahaei, M. R., Rezaee, M. A., Hasani, A., Kafil, H. S., Aghazadeh, M., . . . Khalili, Y. (2018). Role of MexAB-OprM and MexXY-OprM efflux pumps and class 1 integrons in resistance to antibiotics in burn and intensive care unit isolates of pseudomonas aeruginosa. Journal of Infection and Public Health, 11(3), 364-372. doi:10.1016/j.jiph.2017.09.016. [CrossRef]
- Morita, Y., Tomida, J., & Kawamura, Y. (2012). MexXY multidrug efflux system of Pseudomonas aeruginosa. Frontiers in Microbiology, 3, 408. doi:10.3389/fmicb.2012.00408. [CrossRef]
- Hassuna, N. A., Darwish, M. K., Sayed, M., & Ibrahem, R. A. (2020). Molecular epidemiology and mechanisms of high-level resistance to meropenem and imipenem in Pseudomonas aeruginosa. Infection and Drug Resistance, 13, 285-293. doi:10.2147/IDR.S233808. [CrossRef]
- Poole, K. (2004). Efflux-mediated antimicrobial resistance. Journal of Antimicrobial Chemotherapy, 53(5), 1-5. doi: 10.1093/jac/dkh050. [CrossRef]
- Bhandari, S., Adhikari, S., Karki, D., Chand, A. B., Sapkota, S., Dhungel, B., Banjara, M. R., Joshi, P., Lekhak, B., & Rijal, K. R. (2022). Antibiotic Resistance, Biofilm Formation and Detection of mexA/mexB Efflux-Pump Genes Among Clinical Isolates of Pseudomonas aeruginosa in a Tertiary Care Hospital, Nepal. Front. Trop. Dis, 17(2), 2021. https://doi.org/10.3389/fitd.2021.810863. [CrossRef]
- Lorusso, A.B., Carrara, J.A., Barroso, C.D.N., Tuon, F.F., & Faoro, H. (2022). Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 15779. https://doi.org/. [CrossRef]
- Kishk, R.M., Abdalla, M.O., Hashish, A.A., Nemr, N.A., El Nahhas, N., Alkahtani, S., Abdel-Daim, M.M., & Kishk, S.M. (2020). Efflux MexAB-Mediated Resistance in P. aeruginosa Isolated from Patients with Healthcare Associated Infections. Pathogens 9(6), 471. https://doi.org/10.3390/pathogens906047110.3390/ijms232415779. [CrossRef]
- Masuda, N., Sakagawa, E., Ohya, S., Gotoh, N., Tsujimoto, H., & Nishino, T. (2000). Contribution of the MexX-MexY-OprM efflux system to intrinsic resistance in Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy, 44(9), 2242-2246. doi:10.1128/AAC.44.9.2242-2246.2000. [CrossRef]
- Köhler, T., Michea-Hamzehpour, M., Henze, U., Gotoh, N., Curty, L. K., & Pechère, J. C. (1997). Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Molecular Microbiology, 23(2), 345-354. doi: 10.1046/j.1365-2958.1997.2291594.x. [CrossRef]
- Mangiaterra, G., Cedraro, N., Vaiasicca, S., Citterio, B., Galeazzi, R., Laudadio, E., Mobbili, G., Minnelli, C., Bizzaro, D., & Biavasco, F. (2020). Role of Tobramycin in the Induction and Maintenance of Viable but Non-Culturable Pseudomonas aeruginosa in an In Vitro Biofilm Model. Antibiotics, 9(7), 399. https://doi.org/10.3390/antibiotics9070399. [CrossRef]
- Patel, D., Sen, P., Hlaing, Y., Boadu, M., Saadeh, B, & Basu, P. (2021). Antimicrobial Resistance in Pseudomonas aeruginosa Biofilms. Journal of Pure and Applied Microbiology, 15(4):2520-2528. https://doi.org/10.22207/JPAM.15.4.79. [CrossRef]
- Horna G, López M, Guerra H, Saénz Y, Ruiz J. Interplay Between MexAB-OprM and MexEF-OprN in Clinical Isolates of Pseudomonas Aeruginosa. Sci Rep (2018) 8:1–11. doi: 10.1038/s41598-018-34694-z. [CrossRef]
- Llanes C, et al. Role of the MexEF-OprN efflux system in low-level resistance of Pseudomonas aeruginosa to ciprofloxacin. Antimicrob. Agents Chemother. 2011;55:5676–5684. doi: 10.1128/AAC.00101-11. [CrossRef]
- Pourakbari, B., Yaslianifard, S., Yaslianifard, S., Mahmoudi, S., Keshavarz-Valian, S., & Mamishi, S. (2016). Evaluation of efflux pumps gene expression in resistant Pseudomonas aeruginosa isolates in an Iranian referral hospital. Iranian journal of microbiology, 8(4), 249–256.
- Lee, J., & Zhang, L. (2014). The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein & Cell, 6(1), 26-41. doi:10.1007/s13238-014-0100-x. [CrossRef]
- Qu, L., She, P., Wang, Y., Liu, F., Zhang, D., Chen, L., . . . Wu, Y. (2016). Effects of norspermidine on pseudomonas aeruginosa biofilm formation and eradication. MicrobiologyOpen (Weinheim), 5(3), 402-412. [CrossRef]
- Rutherford, S. T., & Bassler, B. L. (2012). Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine, 2(11), a012427. [CrossRef]
- Smith, R. S., & Iglewski, B. H. (2003). Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. The Journal of Clinical Investigation, 112(10), 1460-1465. doi:10.1172/JCI200320364. [CrossRef]
- Zakhour, J., Sharara, S.L., Hindy, J.-R., Haddad, S.F., Kanj, S.S. (2022). Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis. Antibiotics, 11(10), 1432. [CrossRef]
- Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3322-3327.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).