Submitted:
14 June 2023
Posted:
14 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Methodology
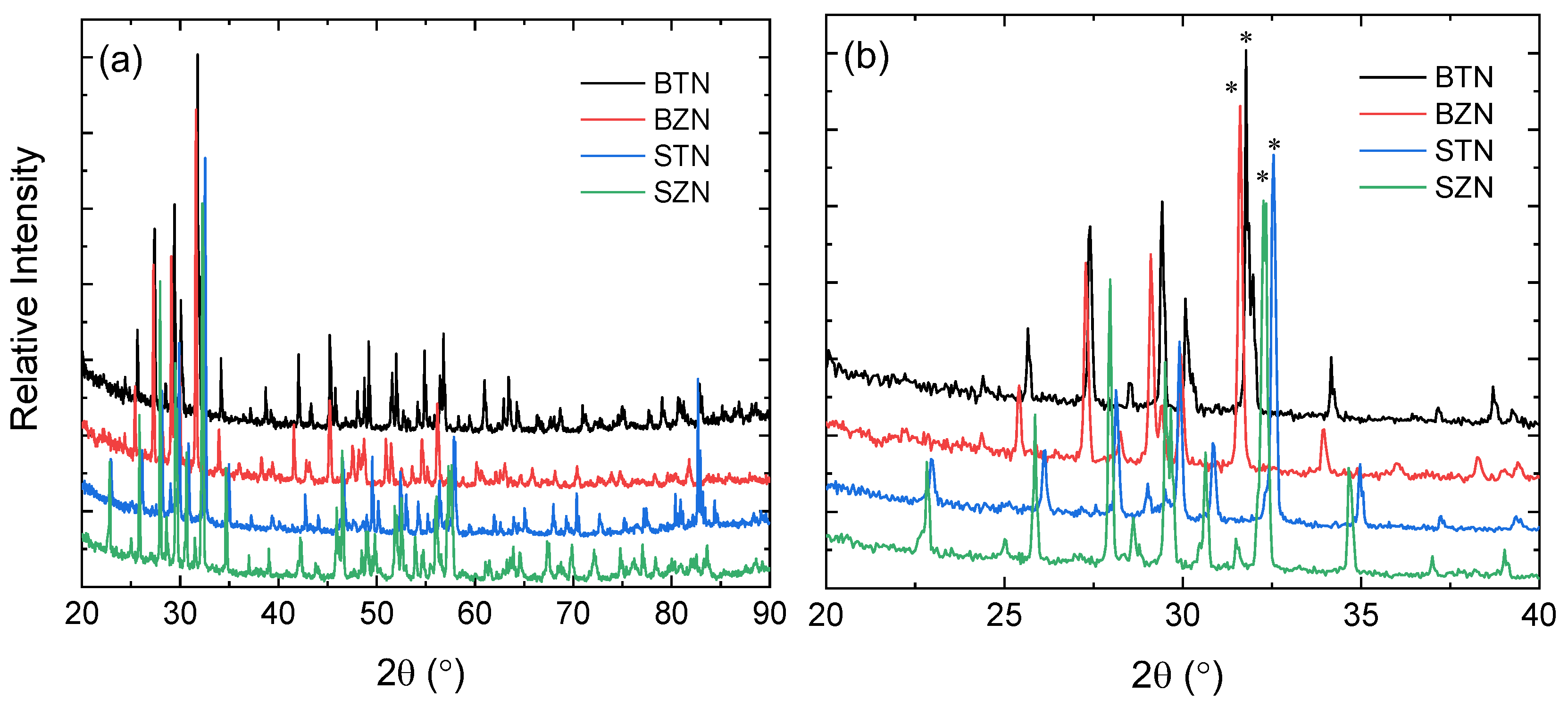
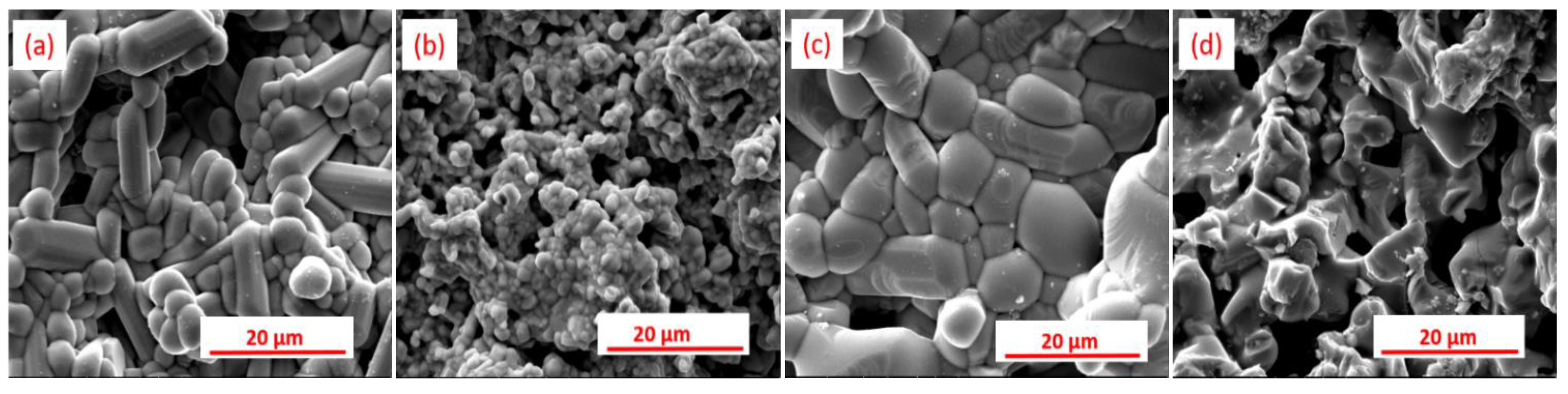
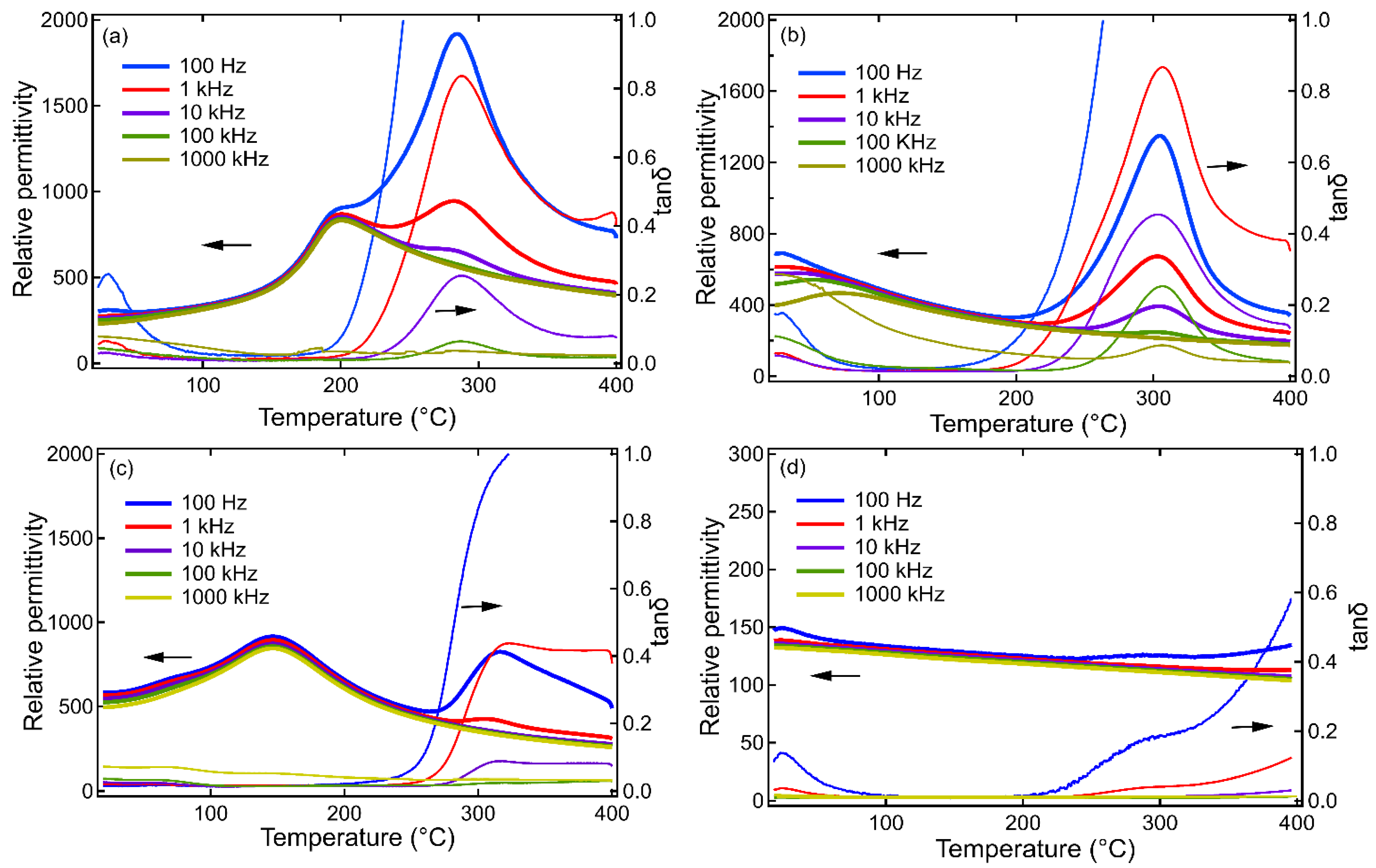
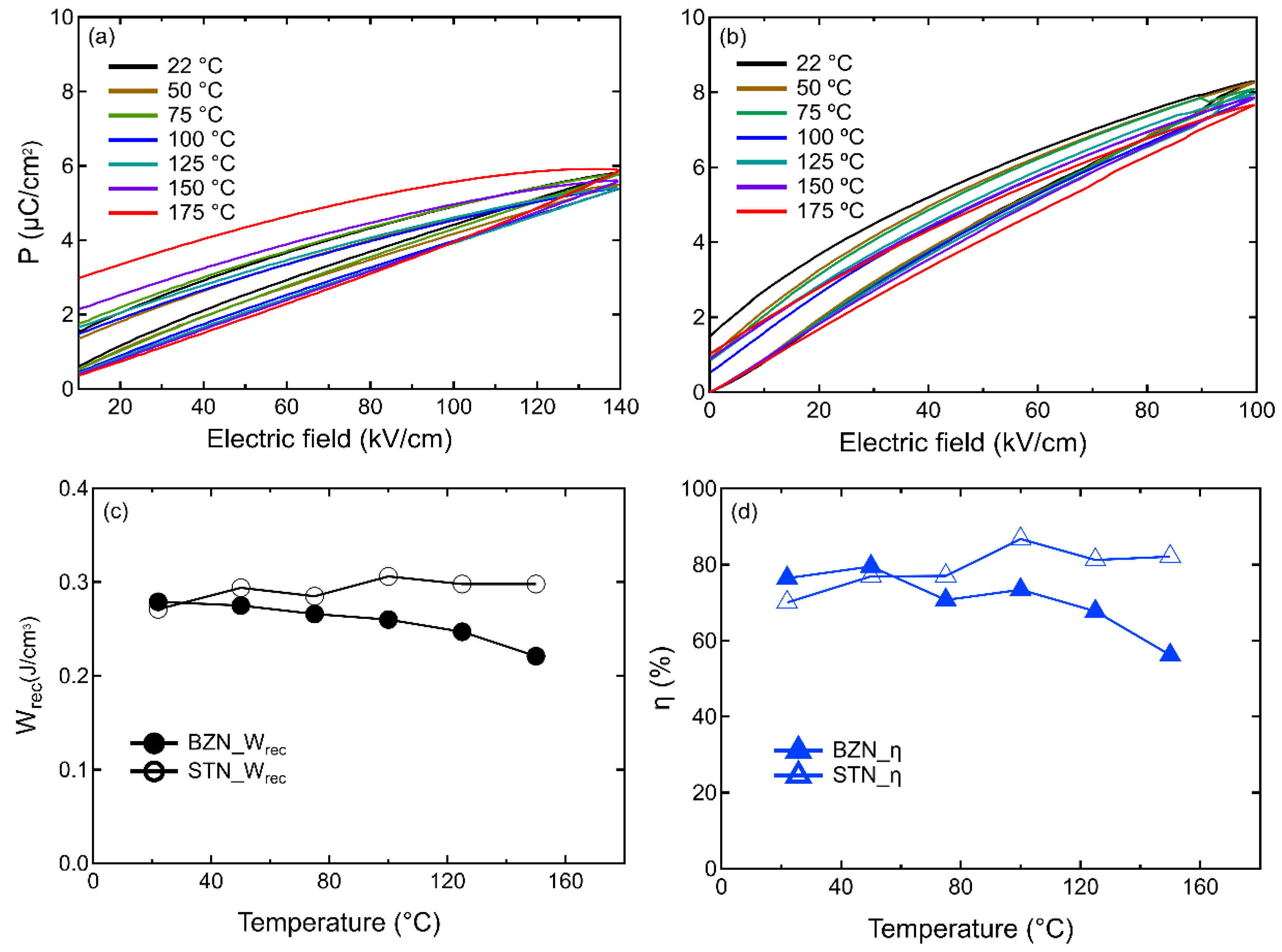
Results and Discussion
Conclusions
Declaration of Competing Interest
Acknowledgments
References
- X. Zhang, W. Ye, X. Bu, P. Zheng, L. Li, F. Wen, W. Bai, L. Zheng, Y. Zhang, Remarkable capacitive performance in novel tungsten bronze ceramics, J Dalton Transactions 50(1) (2021) 124-130. [CrossRef]
- Q. Li, C. Zhou, J. Xu, L. Yang, X. Zhang, W. Zeng, C. Yuan, G. Chen, G. Rao, Tailoring antiferroelectricity with high energy-storage properties in Bi 0.5 Na 0.5 TiO 3–BaTiO 3 ceramics by modulating Bi/Na ratio, J Journal of Materials Science: Materials in Electronics 27 (2016) 10810-10815.
- H. Ogihara, C.A. Randall, S. Trolier-McKinstry, High-energy density capacitors utilizing 0.7 BaTiO3–0.3 BiScO3 ceramics, J Journal of the American Ceramic Society 92(8) (2009) 1719-1724.
- Z. Yang, H. Du, S. Qu, Y. Hou, H. Ma, J. Wang, J. Wang, X. Wei, Z. Xu, Significantly enhanced recoverable energy storage density in potassium–sodium niobate-based lead free ceramics, J Journal of materials chemistry A 4(36) (2016) 13778-13785. [CrossRef]
- P. Jamieson, S. Abrahams, J.J.T.J.o.C.P. Bernstein, Ferroelectric tungsten bronze-type crystal structures. I. Barium Strontium niobate Ba0. 27Sr0. 75Nb2O5. 78, 48(11) (1968) 5048-5057.
- L. Cao, Y. Yuan, Z. Yang, E. Li, S. Zhang, Crystal structure, relaxor behaviors and energy storage performance of (Sr0. 7Ba0. 3) 5LaNb7Ti3O30 tungsten bronze ceramics, J Ceramics International 46(5) (2020) 6108-6114. [CrossRef]
- M.-S. Kim, P. Wang, J.-H. Lee, J.-J. Kim, H.Y. Lee, S.-H. Cho, Site Occupancy and Dielectric Characteristics of Strontium Barium Niobate Ceramics: Sr/Ba Ratio Dependence, Japanese Journal of Applied Physics 41(Part 1, No. 11B) (2002) 7042-7047. [CrossRef]
- S. Jindal, A. Vasishth, S. Devi, G.J.I.F. Anand, A review on tungsten bronze ferroelectric ceramics as electrically tunable devices, 186(1) (2018) 1-9. [CrossRef]
- Simon, J. Ravez, Solid-state chemistry and non-linear properties of tetragonal tungsten bronzes materials, J Comptes Rendus Chimie 9(10) (2006) 1268-1276. [CrossRef]
- B.-X. Wang, M. Krogstad, H. Zheng, R. Osborn, S. Rosenkranz, D.J.J.o.P.C.M. Phelan, Active and passive defects in tetragonal tungsten bronze relaxor ferroelectrics, 34(40) (2022) 405401. [CrossRef]
- T.A. Whittle, T. Lu, P. Blanchard, J.R. Hester, Q. Gu, Y. Liu, S. Schmid, Synthesis, structure and dielectric properties of the Sr 3 Ti 1− x Zr x Nb 4 O 15,(0≤ x≤ 1), series of tungsten bronze type compounds, J CrystEngComm 22(30) (2020) 4994-5001.
- G.-h. Chen, B.J.J.o.a. Qi, compounds, Effect of CASP glass doping on sintering and dielectric properties of SBN ceramics, 473(1-2) (2009) 414-417. [CrossRef]
- S.T. Zhang, G. Yuan, J. Chen, Z.B. Gu, B. Yang, J. Yin, W. Cao, Structural evolving sequence and porous Ba 6 Zr 2 Nb 8 O 30 ferroelectric ceramics with ultrahigh breakdown field and zero strain, J Journal of the American Ceramic Society 96(2) (2013) 555-560.
- H. Bai, J. Li, Y. Wu, Y. Hong, K. Shi, Z. Zhou, Exploring determinants of lattice structure and high energy storage properties of Fe-doped SBN ceramics, J Ceramics International 45(8) (2019) 11109-11113. [CrossRef]
- N. Stephenson, The crystal structure of the tetragonal bronze, Ba6Ti2Nb8O30, J Acta Crystallographica 18(3) (1965) 496-501. [CrossRef]
- D. Jiang, D. Ekren, F. Azough, S.J. Day, K. Chen, A. Mahajan, D.M. Kepaptsoglou, Q.M. Ramasse, M.J. Reece, R. Freer, The structure and thermoelectric properties of tungsten bronze Ba6Ti2Nb8O30, J Journal of Applied Physics 126(12) (2019) 125115. [CrossRef]
- G. Roberts, R. Cava, W. Peck, J. Krajewski, Dielectric properties of barium titanium niobates, J Journal of materials research 12(2) (1997) 526-530.
- Y. Itoh, H. Iwasaki, Ferroelectric and optical properties of Ba6Ti2Nb8O30 single crystals, J Journal of Physics 34(10) (1973) 1639-1645. [CrossRef]
- V. Massarotti, D. Capsoni, M. Bini, C. Azzoni, M. Mozzati, P. Galinetto, G. Chiodelli, Structural and spectroscopic properties of pure and doped Ba6Ti2Nb8O30 tungsten bronze, J The Journal of Physical Chemistry B 110(36) (2006) 17798-17805.
- Q. He, S. Schmid, X. Chen, B. Peng, C. Li, C. Hu, L. Liu, M.J.J.o.A.P. Hinterstein, Structure and relaxor ferroelectric behavior of the novel tungsten bronze type ceramic Sr5BiTi3Nb7O30, 131(16) (2022) 164102. [CrossRef]
- E.O. Chi, A. Gandini, K.M. Ok, L. Zhang, P.S.J.C.o.m. Halasyamani, Syntheses, structures, second-harmonic generating, and ferroelectric properties of tungsten bronzes: A6M2M ‘8O30 (A= Sr2+, Ba2+, or Pb2+; M= Ti4+, Zr4+, or Hf4+; M ‘= Nb5+ or Ta5+), 16(19) (2004) 3616-3622.
- R. Neurgaonkar, J. Nelson, J. Oliver, Ferroelectric properties of the tungsten bronze M2+ 6M4+ 2Nb8O30 solid solution systems, J Materials research bulletin 27(6) (1992) 677-684. [CrossRef]
- D. Jiang, Tungsten bronze structured compounds for thermoelectric applications, The University of Manchester (United Kingdom)2019.
- K.S. Rao, A. Subrahmanyam, P. Viswarupachary, Microstructural and anomalous resistivity behavior of modified ferroelectric Ba6Ti2Nb8O30, J Ferroelectrics 215(1) (1998) 95-102.
- Y. Yuan, X. Chen, Y.J.J.o.a.p. Wu, Diffused ferroelectrics of Ba 6 Ti 2 Nb 8 O 30 and Sr 6 Ti 2 Nb 8 O 30 with filled tungsten-bronze structure, 98(8) (2005) 084110. [CrossRef]
- R.-J. Xie, Y. Akimune, K. Matsuo, T. Sugiyama, N. Hirosaki, T.J.A.P.L. Sekiya, Dielectric and ferroelectric properties of tetragonal tungsten bronze Sr 2− x Ca x NaNb 5 O 15 (x= 0.05–0.35) ceramics, 80(5) (2002) 835-837.
- Bendahhou, P. Marchet, A. El-Houssaine, S. El Barkany, M. Abou-Salama, Relationship between structural and dielectric properties of Zn-substituted Ba5CaTi2−xZnxNb8O30 tetragonal tungsten bronze, CrystEngComm 23(1) (2021) 163-173.
- Parida, P.R. Das, R. Padhee, R.J.J.o.a. Choudhary, compounds, Phase transition and conduction mechanism of rare earth based tungsten-bronze compounds, 540 (2012) 267-274. [CrossRef]
- P.R. Das, L. Biswal, B. Behera, R.J.M.R.B. Choudhary, Structural and electrical properties of Na2Pb2Eu2W2Ti4X4O30 (X= Nb, Ta) ferroelectric ceramics, 44(6) (2009) 1214-1218.
- X. Zhang, H. Wang, X. Bu, P. Zheng, L. Li, F. Wen, W. Bai, J. Zhang, L. Zheng, J. Zhai, Simultaneously Realizing Superior Energy Storage Properties and Outstanding Charge–Discharge Performances in Tungsten Bronze-Based Ceramic for Capacitor Applications, J Inorganic Chemistry 60(9) (2021) 6559-6568. [CrossRef]
- S. Xu, R. Hao, Z. Peng, F. Zhang, D. Wu, P. Liang, X. Chao, L. Wei, Z. Yang, Relaxor nature and superior energy storage performance of Sr2Ag0. 2Na0. 8Nb5O15-based tungsten bronze ceramics through B-site substitution, J Chemical Engineering Journal 433 (2022) 133812. [CrossRef]
- B. Yang, J. Zhang, X. Lou, Y. Gao, P. Shi, Y. Yang, M. Yang, J. Cui, L. Wei, S. Sun, Interfaces, Enhancing Comprehensive Energy Storage Properties in Tungsten Bronze Sr0. 53Ba0. 47Nb2O6-Based Lead-free Ceramics by B-Site Doping and Relaxor Tuning, J ACS Applied Materials 14(30) (2022) 34855-34866.
- S. Xu, R. Hao, Z. Yan, S. Hou, Z. Peng, D. Wu, P. Liang, X. Chao, L. Wei, Z. Yang, Enhanced energy storage properties and superior thermal stability in SNN-based tungsten bronze ceramics through substitution strategy, J Journal of the European Ceramic Society 42(6) (2022) 2781-2788. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




