1. Introduction
Traditionally, tuberculosis (TB) has been the leading occupational health problem in healthcare workers (HCW) who provide medical care under poor infection control conditions [
1]. However, during 2020 and 2021, the disease caused by SARS-CoV-2 (COVID-19) has become the primary occupational respiratory infection in unvaccinated HCWs [
2]. The World Health Organization (WHO) recommends the use of filtering-facepiece respirators (FFR) type N95 (NIOSH 42 CFR84 standard) or FFP2 (EN149:2001 standard) for the care of patients with TB and COVID-19, especially when they involve aerosol-generating procedures [
3,
4,
5]. Type N95 and FFP2 FFRs provide 94-95% filtration efficiency of aerosol particles with a mean diameter of 0.3 µm [
6]. However, the protective capacity of FFRs is not only based on filtration efficiency but also the quality of the facial fit [
7]. A non-optimal facial fit can account for one-sixth of the airflow entering the FFRs [
8], one of the main routes of contamination [
9].
The evaluation of facial fit is named the fit test. It is the main parameter of the Respiratory Protection Program proposed by the National Institute for Occupational Safety and Health (NIOSH) [
10]. According to the US Occupational Safety and Health Administration (OSHA), the fit test must be applied to each worker to select the design and size of FFR that provides an appropriate and acceptable fit and protection when appropriately [
11]. This activity will allow the worker to know which FFR fits his/her face and look for large stocks of FFR for its permanent replacement. The quantitative fit test (QNFT) is considered the gold standard and is based on the count of environmental particles outside and inside the FFR through air sampling lines, from which a ratio, called the fit factor, is calculated [
12,
13]. This value measures the degree of particles entering through the edges of the FFR and the face due to a poor seal. A fit factor equal to or greater than 100 (pass level) for type N95 and FFP2 FFRs is necessary to ensures optimal tightness of the design and size of the FFR evaluated whenever it is used in the same way [
14].
The fit test is an essential procedure in the field of occupational health, specifically aimed at workers exposed to biological risks such as tuberculosis in endemic countries[
15]. In this regard, Peru is the country with the second highest burden of TB in Latin America and the Caribbean, [
16] and one of the 30 countries with the highest burden of Multidrug-Resistant TB (MDR-TB) in the world [
17]. Peruvian legislation regulates the application of administrative, environmental, and respiratory protection control measures for the prevention and control of TB [
18]. However, fit testing in Peruvian HCWs of primary healthcare centers is almost nonexistent due to the absence of a respiratory protection program [
19]. Additionally, due to the global shortage of FFRs generated by the COVID-19 pandemic, new FFRs with poorly technical specifications or suspected of being counterfeit were provided to HCWs [
20]. In this sense, we evaluated the respiratory protection measures focused on applying QNFT in FFR by HCWs who care for TB patients under our PROFIT (PROmoting the FIT) study 2020.
2. Materials and Methods
2.1. Study design
The PROFIT study 2020 represents an initiative of the National Center for Occupational Health and Environmental Health (CENSOPAS in Spanish) of the Peruvian National Institute of Health to evaluate respiratory protection measures focused on FFR facial fit in Peruvian HCW developed within the framework of Institutional Operational Plan. During November and December 2020, we visited 37 primary healthcare centers (PHC) to enroll HCWs in work conditions to apply QNFT in FFRs worn by them. The PHC were selected based on the highest burden of care for TB cases in the metropolitan area of Lima and Callao in Peru through data from the SIGTB platform (tuberculosis management information system) of the Tuberculosis Prevention and Control Directorate of the Ministry of Health of Peru [
21]. The selection of the participants was for convenience according to the time availability of the HCWs, prioritizing those who work in the TB Control Program, the unit responsible for detecting, diagnosing, and treating TB cases in PHC [
22]. We asked enrolled participants about their sex, occupation, age, work area, and the number of hours of FFR use through a questionnaire. The FFRs evaluated by QNFT were N95, FFP2, KN95, and other equivalent types. They were characterized according to their brand, model, type, size, design, presence of batch number on their surface, and country of origin of the manufacturer. We excluded from the analysis workers with facial hair [
23], workers with 3M 1860 FFRs suspected of counterfeiting [
24], or workers with expired FFRs as long as we had access to the boxes where the expiration date is indicated
.
2.2. Quantitative fit testing
We used the PortaCount® model 8048 equipment (TSI Inc., St. Paul, MN, USA) with which we determined two fit factors: real-time fit factor (rt-FF) and overall fit factor (overall-FF) [
25,
26]. Initially, we determine the rt-FF to train on properly donning the FFR and making adjustments in real-time [
25]. The rt-FF is updated with intervals per second on the PortaCount® screen when using FitCheck® mode and allows us to identify non-tight adjustment areas to adapt the FFR to face manually. Subsequently, we determine the overall-FF by applying the OSHA 29 CFR 1910.134 Appendix A standard, which consists of 08 movements that simulate work conditions: normal breathing, deep breathing, left-right head turns, up-down head turns, speaking loudly, gestures and grimaces, leaning forward and normal breathing [
14]. Each movement lasted 60 seconds except for gestures and faces, which lasted 15 seconds. A fit factor (rt-FF or overall-FF) equal to or greater than 100 was optimal (passing result), while a fit factor (rt-FF or overall-FF) less than 100 was non-optimal (failed result).
2.3. Procedures
We ask the participant to give us their FFR to connect it to the PortaCount® through an air sampling line, and then ask them to put on the FFR as they usually do at work without our support. Subsequently, we recorded the pre-instruction rt-FF 10 seconds after the participant was put on the FFR. The post-instruction rt-FF was recorded 10 seconds after providing instruction focused on improving the position of the elastic band, adjusting the nose clip, and fit check based on the recommendations of the Centers for Disease Control and Prevention [
27]. The results of rt-FF pre- and post-instruction were determined only for the last 184 participants because this process was incorporated during the study. Regardless of the result of the rt-FF, we perform a first QNFT to determine the overall-FF. We gave the participant the option of requesting from the employer a new FFR of any brand or model to carry out a second QNFT in case they obtained a non-optimal overall-FF in the first QNFT. Each PortaCount® sample line was cleaned with isopropyl alcohol at the end of each QNFT, according to the manufacturer's recommendations in the context of the COVID-19 pandemic [
28].
2.4. Data analysis
The rt-FF and overall-FF were expressed as geometric means and were also categorized into ≥100 and <100, which were arbitrarily subcategorized into 50 -99 and <50 for a better understanding of the level of fit. We compared the pre- and post-instruction geometric means of rt-FF categories using a paired T-test comparing two population proportions with a significance of 95%. We performed the analyzes using Stata version 16 software (Stata Corporation, College Station, TX, USA) and the graphs using Graph Pad Prism software v5.
3. Results
3.1. Description
We interviewed 279 participants, of whom 263 (94.6%) were enrolled according to inclusion criteria. Of the 263 participants enrolled
(Table S1), we obtained rt-FF pre- and post-instruction results in 184 (70.3%) participants and overall-FF results participants in all the 263 participants (first QNFT)
(Table S2). Furthermore, only 10 participants who previously had a non-optimal overall-FF with the FFR in use had another QNFT with a new FFR (second QNFT). The excluded sixteen participants from the analysis were due the following reasons: 11 with facial hair, 3 with counterfeit FFR, and 2 with timeout FFR. However, due to the characteristics of the PROFIT study 2020, the excluded participants were also evaluated by QNFT to provide evidence to health authorities
(Table 3).
We identified 12 types of FFR models, all one size fits all (standard, regular, or adult) and predominantly of Asian origin: Chinese (91/263) and Taiwanese (55/263)
(Table 1 and Figure 1). All the FFRs were provided by the PHC except 03 FFR, which the workers acquired on their account: 02 3M 1860 FFR and 01 Lucca Light FFR. The main FFRs identified were 3M model 1860 (33.1%), Xiantao Zhong Yi model ZYB-11 (24.7%), and Makrite model 9500 (20.5%), mainly. All the Xiantao Zhong Yi FFRs identified in the 37 PHCs belonged to only 3 batch numbers (200701, 200601, and 200901), while the 3M 1860 FFR had 18 different lot numbers. The Makrite, Grande, Y&Z, PGT Care, Lucca Light, and GIKO FFRs evaluated in our study do not display their batch numbers on their surface.
3.2. Instruction and real-time fit factor assessment
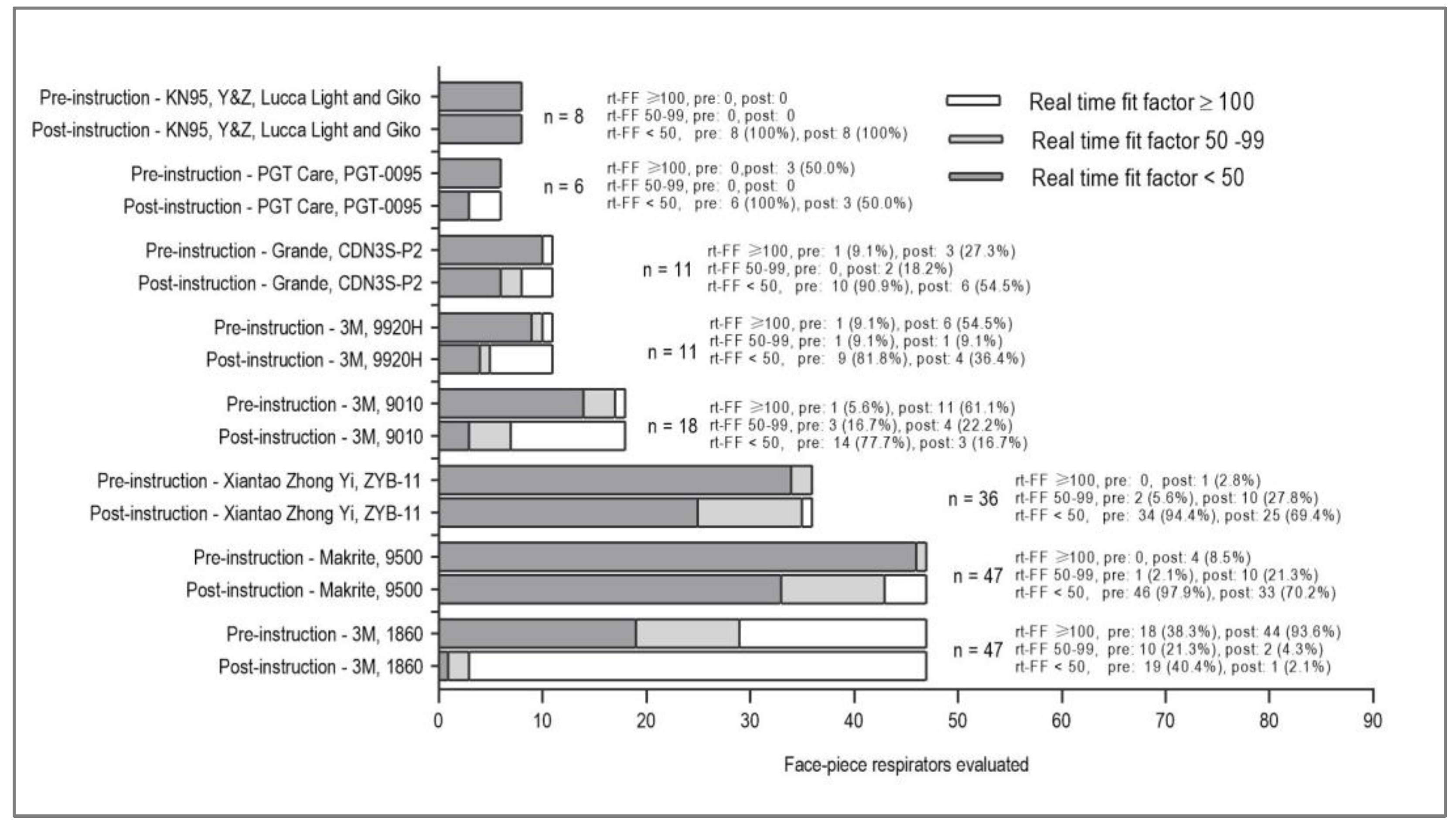
The proportion of optimal rt-FF increased significantly from 21 (11.4%) to 72 (39.1%) (p<0.01) because of instruction, while the frequency of non-optimal fit factor less than 50 decreased significantly from 146 (79.4%) to 83 (45.1%) (p<0.01)
(Table 2). 3M 1860 FFRs had an optimal pre-instructional rt-FF of 38.3% (18/47) and a significantly higher optimal post-instructional rt-FF of 93.6% (44/47) (p<0.01). 3M 9010 FFRs had an optimal pre-instruction rt-FF of 5.6% (1/18) that increased significantly post-instruction to 61.1% (11/18) (p<0.01). Similarly, 3M 9920H FFRs had an optimal pre-instruction rt-FF of 9.1% (1/11), increasing to 54.5% (6/11) (p=0.01). Neither Xiantao Zhong Yi ZYB-11 nor Makrite 9500 FFRs achieved optimal pre-instruction rt-FF, but after instruction, five of them achieved an optimal rt-FF. Grande and PGT FFRs showed minimal increase in optimal rt-FF ratio, while KN95, Y&Z, Lucca Light, and Giko FFRs did not show optimal post-instruction rt-FF
(Figure 2).
3.3. Overall fit factor assessment
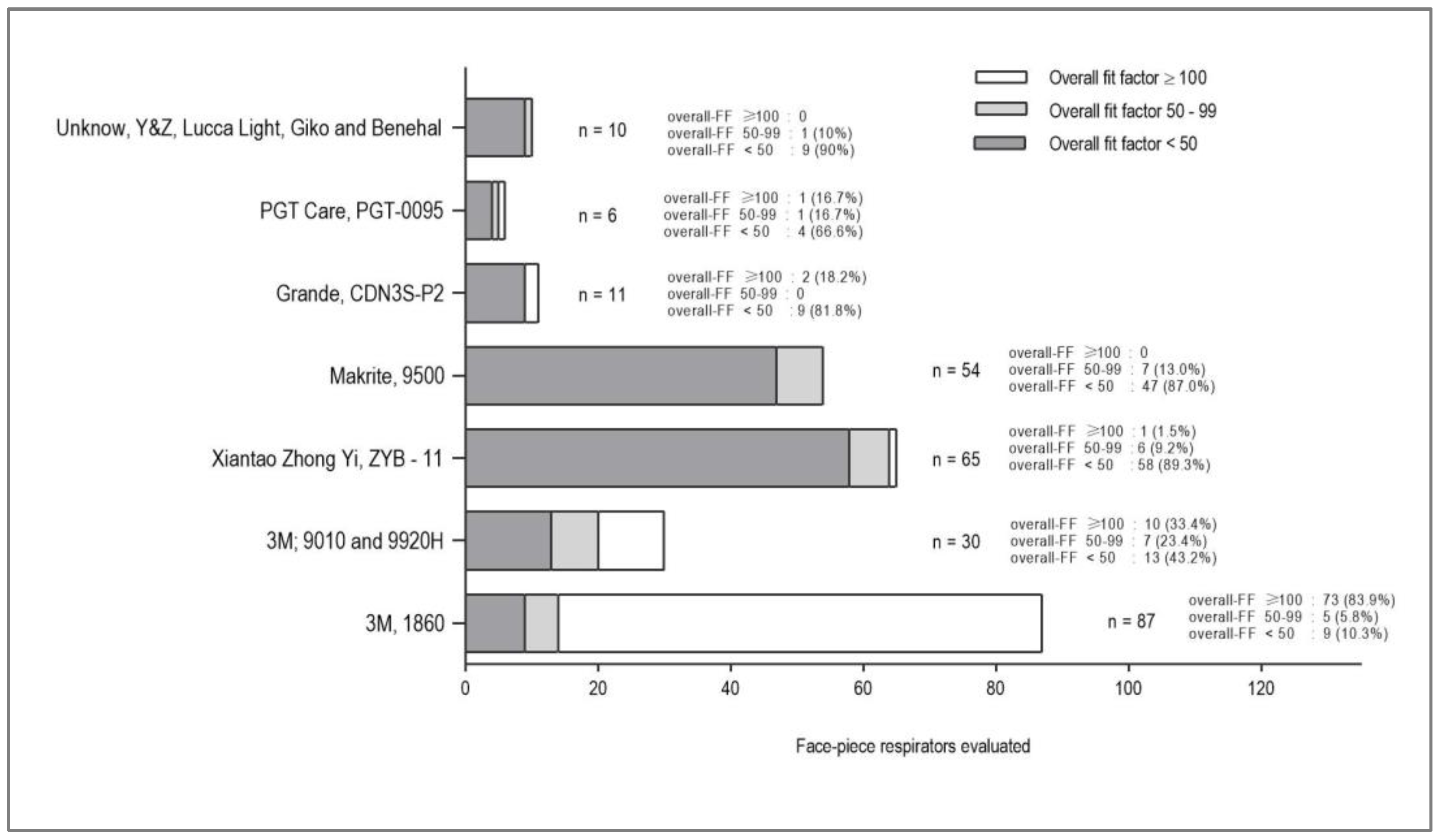
The 3M 1860 showed an optimal overall-FF at 73 (83.9%) FFR. The 3M 9010 and 3M 9920H FFRs had an optimal overall-FF of 33.4% (10/30). The Xiantao Zhong Yi ZYB-11 has a single FFR with optimal overall-FF (1.5%), while the Makrite 9500 had a non-optimal result, as well as the FFRs of the brands Lucca Light, Giko, and Benehal. The percentage of HCWs using FFRs with an overall-FF of less than 50 were high (56.7%) were represented mainly by two of the most widely used FFRs: Xiantao Zhong Yi ZYB-11 and Makrite 9500
(Figure 3). The overall-FF did not show a statistical association with age, sex, FFR design (conical or foldable), or elastic band arrangement (earloop or head loop) of the FFR.
Of the 263 evaluations carried out, 87 (33.1%) participants had an optimal overall-FF, 27 (10.3%) had an overall-FF between 50-99, and 149 (56.7%) had an overall-FF less than 50
(Table 3). Of the 87 participants with optimal overall-FF, 73 (83.9%) were 3M model 1860 FFR. The 3M model 1860 FFR had a geometric mean of 126.3 (95% CI: 109.4–146.6), the only one that exceeds the threshold of 100. Likewise, of the 27 participants with overall-FF between 50-99, 7 (25.9%) were FFR Makrite model 9500, while of the 149 with overall-FF less than 50, 58 (38.9%) and 47 (31.5%) were Xiantao Zhong Yi model ZYB-11 and Makrite model 9500, respectively. The FFRs evaluated in the first QNFT had a median time of use of 12 hours (interquartile range: 18.0), which were also not associated with the overall-FF (p>0.05).
Table 3.
Overall fit factors in evaluated FFR in use by HCWs in the PROFIT study 2020 (N = 263).
Table 3.
Overall fit factors in evaluated FFR in use by HCWs in the PROFIT study 2020 (N = 263).
| Brand, model, and type of FFR |
Hours of FFR use a
|
overall-FF, n = 263 |
| < 50 |
50 - 99 |
≥ 100 |
Geometric mean (IC 95%) |
| n (%) |
n (%) |
n (%) |
| 3M, 1860, N95 |
12 (19) |
9 (6.0) |
5 (18.5) |
73 (83.9) |
126.0 (109.4-146.6) |
| Xiantao Zhong Yi, ZYB-11, N95 |
12 (26) |
58 (38.9) |
6 (22.2) |
1 (1.2) |
15.1 (11.7-19.4) |
| Makrite, 9500, N95 |
6 (14) |
47 (31.5) |
7 (25.9) |
- |
12.4 (9.1-17.0) |
| 3M, 9010, N95 |
5.5 (16) |
7 (4.7) |
5 (18.5) |
7 (8.0) |
44.9 (22.7-88.9) |
| 3M, 9920H, PFF |
18 (20) |
6 (4.0) |
2 (7.4) |
3 (3.4) |
34.1 (11.2-103.5) |
| Grande, CDN3S-P2, FFP2 |
12 (20) |
9 (6.0) |
- |
2 (2.3) |
13.8 (5.0-37.7) |
| Brand and model unknown, KN95 b
|
6 (9) |
6 (4.0) |
- |
- |
2.8 (1.1-7.4) |
| PGT Care, PGT-0095, FFP2 |
3 (15) |
4 (2.7) |
1 (3.7) |
1 (1.2) |
21.0 (4.6-96.0) |
| GIKO, 1200H, N95 |
6 |
1 (0.7) |
- |
- |
- |
| Benehal, MS6115L, N95 |
3 |
- |
1 (3.7) |
- |
- |
| Y&Z, Safety Work F720, N95 |
3 |
1 (0.7) |
- |
- |
- |
| Lucca Light, Lucca Care, KN95 / FFP2 |
- |
1 (0.7) |
- |
- |
- |
| Total |
12 (18) |
149 (56.7) |
27 (10.3) |
87 (33.0) |
31.5 (26.3-37.8) |
3.4. Second QNFT
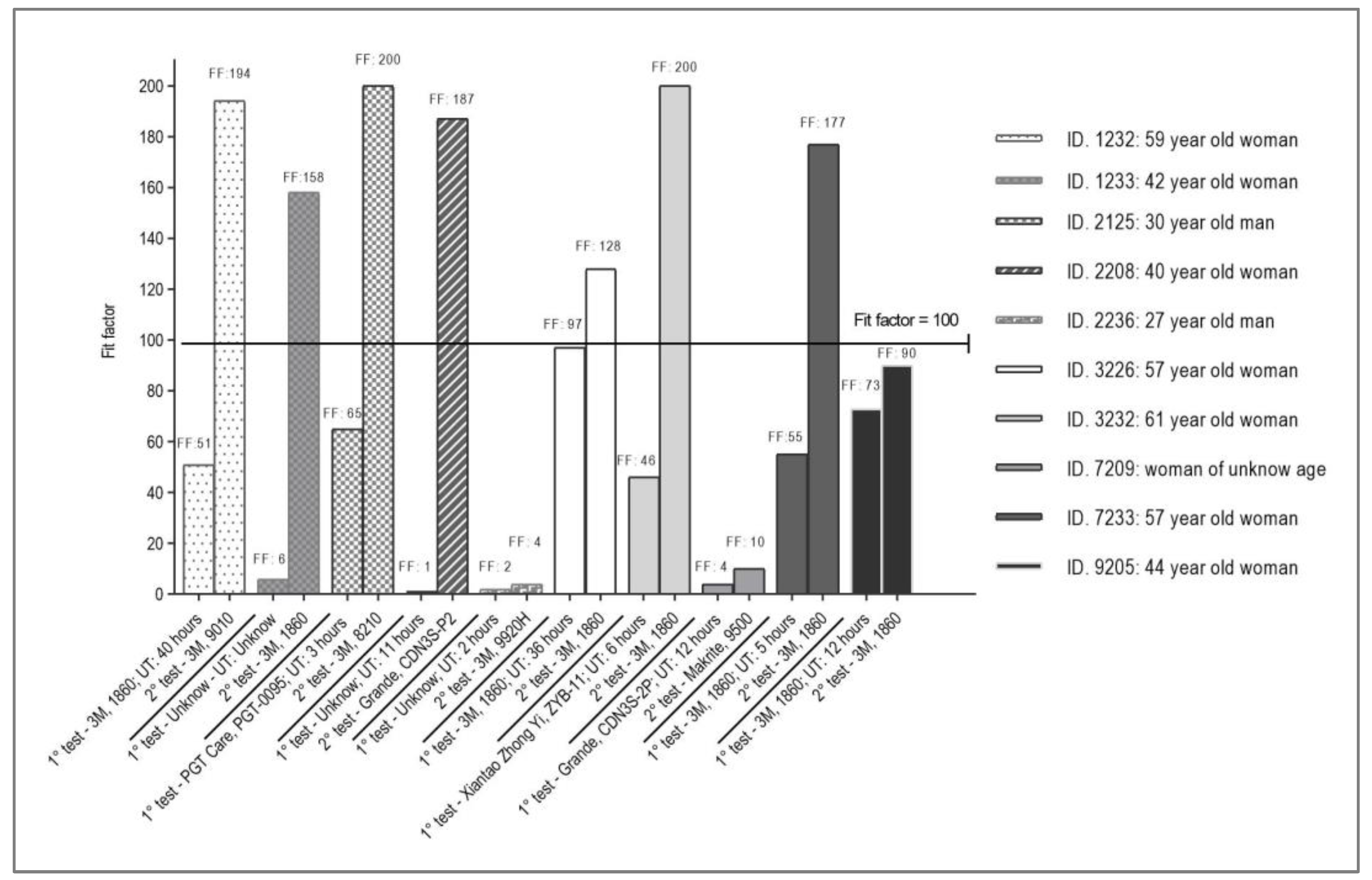
Finally, we evaluated the results of the second QNFT in FFR of 10 participants expressed in overall-FF and its comparation with the results of the first QNFT
(Figure 4). Of them, 7 opted for a different brand FFR, and 3 opted for a new FFR of the same brand and model (3M 1860). Of the ten new evaluations, all had optimal results, except for 3 participants with 3M 9920H (ID. 2236), Makrite 9500 (ID 7209), and 3M 1860 FFR (ID 9205). Three participants used 3M 1860 FFR in the first and second QNFTs (ID 3226, ID 7233, ID 9205), of which 02 had optimal overall-FF in the second QNFT. Of the total participants evaluated in the second QNFT, 07 had an optimal overall-FF: 06 were 3M FFR (1860, 9010, and 8210 models), and 1 was Grande FFR.
4. Discussion
We found a non-optimal overall-FF in the first QNFT of 67.0% (176/263) of the evaluated participants, of which 84.6% (149/176) present an overall-FF of less than 50. The 3M FFRs had a significantly higher adaptability capacity for facial adjustment than the Xiantao Zhong Yi and Makrite FFRs. The non-optimal overall-FF in the first QNFT was not related to the gender, age, design of the FFR (foldable or conical) or time of use. Indeed, the Xiantao Zhong Yi and Makrite FFRs showed poor adaptability to the facial dimensions, mainly in the nasal and chin areas.
The nose clip of most of the Makrite and Xiantao Zhong Yi FFR did not adapt correctly to the shape of the nose of the participants, despite the attempts. This limitation generated noticeable openings in the nasal area that would explain the lower overall-FF. Some participants reported that the nose clip of Makrite FFR, initially in the form of an arch, has limited malleability and yields to its original shape after adjustment. We also observed a loss of fit in the chin region, partly evidenced by the excess length of the elastic bands. Given this, some participants reported that the elastic bands tend to remain stretched due to the loss of their elasticity, and therefore, they must tie them to increase the fit. The nose clip and the elastic bands of the FFR can show serious flaws and affect the facial fit, being necessary to be evaluated more precisely with appropriate instruments [
29]
(Figure S1).
On the other hand, we identified some FFRs that show insufficient technical characteristics. Six FFRs only display the KN95 GB2626-2006 inscription on their surface, with no known brand, model, or manufacturer. The Y&Z FFR identified in our study was produced by the Fido Mask Company and had a NIOSH TC-841-4227 voluntary certification revocation since August 2014 [
30]. It cannot be manufactured, assembled, sold, or distributed as a NIOSH-approved product, so it is probably a timeout. We also identified a Lucca Light FFR of Chinese origin and an unknown manufacturer that was banned in the European Union for having a filtration efficiency of less than 57.2% [
31]. The Y&Z FFR was worn by a nursing staff and provided by the PHC, while the Lucca Light FFR was worn by a security staff and purchased at their own expense.
The lack of adaptability of the FFR in our study may be mainly due to 03 factors: (i) incorrect size, (ii) damage to its support structure (elastic bands, nose clip) due to excessive reuse, and/or (iii) deficiencies in its filtration efficiency. Regarding the first factor, the overall-FF of 10 achieved by a new Makrite FFR in the second QNFT in ID 7209 would demonstrate its limited adaptability to the participant's facial dimensions. The second factor would support those two participants (ID 3226 and ID 7233) with 3M 1860 FFR and usage time of 36 hours, had a non-optimal result in the first QNFT changing to optimal in the second QNFT using the same FFR model (
figure 3). Likewise, we must consider that all the FFRs evaluated in the analysis were standard sizes. Regarding the third factor, it is crucial to indicate that the overall-FF does not discriminate between particles that have entered through the edges of the FFR or the filter [
7]. This situation leads us to consider if the high proportion of non-optimal overall-FF of less than 50 would involve filter penetration.
Except for 3M FFRs, there is no information in the literature on most of the FFRs evaluated in our study. A recent study evaluated the overall-FF of 03 FFRs: Xiantao Zhong Yi ZYB-11, Makrite 9500, and KN95 (manufactured by Zhong Jian Le of Chengde Technology Co. Ltd.) in 07 participants [
32]. Of the 07 participants with the Xiantao Zhong Yi FFR, none had an optimal overall-FF; however, all had values less than 60. On the other hand, of the 07 participants with the Makrite FFR, one had an optimal overall-FF; however, the rest had an overall-FF lower than 20. Regarding the KN95 FFRs, the overall-FF results were all lower than 3, having the same performance as a cloth mask. The study suggests that the fit loss was due to the inadequate sealing of the FFR in the chin area.
One publication evaluating the tightness of FFRs available at a PHC in the United States found KN95 FFRs that did not display brand, model, or lot number data; and had non-optimal overall-FF results [
33]. These FFRs did not show elastic band support on the head and had perforations on their surface with the text "KN95" and "GB2626-2006" in low-relief that gave rise to a thin non-protective layer of the filter. Likewise, a study applied a qualitative fit test to a panel of 7 individuals using 12 types of FFR between KN95 and N95 FFR (3M 1860) and their results showed an optimal fit percentage of 3% (1/36) for KN95 FFR, while the 3M 1860 FFR had a 100% (12/12) best fit [
34].
Regarding limitations, our study was making a single measurement of QNFT per FFR in use, unlike other studies that perform up to three measurements, considering that the overall-FF presents a level of error [
37,
38]. Also, we did not check the expiration date of all the FFRs because that depended on access to the boxes. On the other hand, we identified 3M 1860 counterfeit FFRs as we are familiar with them since their use was predominant before the pandemic. However, we could not identify counterfeit FFRs from other brands, such as the Makrite 9500. This point is essential given the continual reporting of fake alerts by Makrite displayed on their website [
39]. Therefore, our results should be taken with caution.
The PROFIT study allowed us to evaluate respiratory protection measures focused on FFR facial fit in Peruvian HCW in the context of the COVID-19 pandemic. The main strength of the study was the application of QNFT, which does not depend on the sensitivity or attitude of the person, unlike qualitative fit tests [
40]. We believe that our findings are important to consider for tuberculosis-endemic countries that have not yet implemented respiratory protection measures. Finally, it is essential that the PHC, through the Occupational Health and Safety services, comply with the programs for the prevention and control of occupational risks and diseases, including those transmitted by air, to strengthen the primary prevention activities in workplaces.
5. Conclusions
We found a non-optimal fit provided by in-use FFRs in Peruvian healthcare workers (HCWs) during the COVID-19 pandemic. Most of these FFRs were new brands not previously used by HCWs, which appeared due to shortages of FFRs. This finding helps us to understand the exposure to TB transmission during a pandemic scenario where there was a limited access to diagnosis, follow-up and treatment that could increase the TB cases in settings with precarious health system.
We recommend implementing measures that prioritize educational interventions with a practical approach to recognizing the FFR, correct handling, fit check, and good storage practices. Compliance with these activities must be monitored, supervised, and evaluated by the Respiratory Infection Control Plan established in the Technical Health Standard for TB Care in Peru.
Supplementary Materials
Table S1. Characteristics of 263 workers enrolled in the PROFIT study 2020. Table S2. Real-time and overall fit factor obtained in FFR included of analysis (N = 263); Table S3. Real-time and overall fit factor obtained in FFR excluded of analysis (N = 16). Figure S1. Visible openings in the nasal area in participants using the Makrite 9500 FFR (top photographs) and loss of FFR adjustment mainly in users with elastic bands that are too extended and therefore must be tied to increase the FFR adjustment (lower photographs).
Author Contributions
Conceptualization: J.I. and J.R.; methodology: J.I., M.C. and R.A.; resources: K.M. and R.A.; formal analysis: J.I. and J.A.F.; writing—original draft preparation: J.I., K.M.; writing—review and editing: J.R. and J.A.F.; project administration: K.M. All authors have read and agreed to the published version of the manuscript.
Funding
The PROFIT study 2020 is part of an occupational surveillance program for respiratory protection focused on HCWs developed within the framework of the National Center for Occupational Health and Environmental Health (CENSOPAS in Spanish) Institutional Operational Plan entitled "Tuberculosis care services with infection control and biosafety measures"
[44]. “The APC was funded by Universidad Privada San Juan Bautista”.
Institutional Review Board Statement
This study was approved by the Institutional Ethics Committee of the Universidad Privada San Juan Bautista with code number 445-2023-CIEI-UPSJB.
Informed Consent Statement
We apply an informed consent to all subjects involved in the study which describes the purpose of the study, the procedures, benefits, and rights of the participants. The participation of the HCWs was voluntary, and the management of the participant's data was encrypted to guarantee their anonymity.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We posthumously thank Luis Santa María Juárez, former director of CENSOPAS, whose management allowed us to start the activities considered in this publication. We also thank Julia Ríos Vidal, director of Tuberculosis Prevention and Control Directorate of the Ministry of Health of Peru; and César Ugarte-Gil for their support in the development and analysis of study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azeredo ACV, Holler SR, de Almeida EGC, Cionek O, Loureiro MM, Freitas AA, et al. Tuberculosis in Health Care Workers and the Impact of Implementation of Hospital Infection-Control Measures. Workplace health & safety. 2020;68(11):519-25. Epub 2020/06/06. [CrossRef] [PubMed]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. Jama. 2020;323(11):1061-9. [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care. Geneva: World Health Organization; 2014.
- Infection prevention and control during health care when coronavirus disease (COVID-19) is suspected or confirmed. Overview. Geneva: World Health Organization; 2021. p. 23.
- WHO Guidelines Approved by the Guidelines Review Committee. WHO guidelines on tuberculosis infection prevention and control: 2019 update. Geneva: World Health Organization © 2019.; 2019.
- Coia JE, Ritchie L, Adisesh A, Makison Booth C, Bradley C, Bunyan D, et al. Guidance on the use of respiratory and facial protection equipment. J Hosp Infect. 2013;85(3):170-82. Epub 2013/09/21. [CrossRef] [PubMed] [PubMed Central]
- Or P, Chung J, Wong T. Does training in performing a fit check enhance N95 respirator efficacy? Workplace health & safety. 2012;60(12):511-5. Epub 2012/12/06. [CrossRef] [PubMed]
- Cooper DW, Hinds WC, Price JM, Weker R, Yee HS. Common materials for emergency respiratory protection: leakage tests with a manikin. American Industrial Hygiene Association journal. 1983;44(10):720-6. Epub 1983/10/01. [CrossRef] [PubMed]
- Grinshpun SA, Haruta H, Eninger RM, Reponen T, McKay RT, Lee SA. Performance of an N95 filtering facepiece particulate respirator and a surgical mask during human breathing: two pathways for particle penetration. Journal of occupational and environmental hygiene. 2009;6(10):593-603. Epub 2009/07/15. [CrossRef] [PubMed] [PubMed Central]
- Healthcare Respiratory Protection Resources. US: Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health; 2020 [updated February 5, 2021; cited June 1, 2022]. Available from: https://www.cdc.gov/niosh/npptl/hospresptoolkit/default.html.
- Standard 1910.134: Personal Protective Equipment. US: Department of Labour, Occupational Safety and Health Administration; 2020 [cited 2022 01/06/2022]. Available from: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134.
- Lam SC, Lee JKL, Yau SY, Charm CYC. Sensitivity and specificity of the user-seal-check in determining the fit of N95 respirators. J Hosp Infect. 2011;77(3):252-6. Epub 2011/01/13. [CrossRef] [PubMed]
- Regli A, Sommerfield A, von Ungern-Sternberg BS. The role of fit testing N95/FFP2/FFP3 masks: a narrative review. Anaesthesia. 2021;76(1):91-100. [CrossRef] [PubMed]
- Appendix A to § 1910.134 - Fit Testing Procedures. US Department of Labour, Occupational Safety and Health Administration; 2004 [updated August 4, 2004; cited June 1, 2022]. Available from: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA.
- Niu S. ILO list of occupational diseases and health care workers. African Newsletter on occupational health and safety. 2010;20(1):4-9.
- Tuberculosis en las Américas. Informe regional 2019. Washington, D.C.: Organización Panamericana de la Salud; 2019.
- Global Tuberculosis Report 2020. Geneva: World Health Organization; 2020.
- Ley N° 30287 de prevención y control de la tuberculosis en el Perú Perú: Ministerio de Salud; 2014 [updated 2014; cited June 1, 2022]. Available from: https://www.gob.pe/institucion/minsa/normas-legales/296991-30287.
- Huaroto L, Espinoza MM. Recomendaciones para el control de la transmisión de la tuberculosis en los hospitales. Revista Peruana de Medicina Experimental y Salud Publica. 2009;26:364-9.
- Livingston E, Desai A, Berkwits M. Sourcing Personal Protective Equipment During the COVID-19 Pandemic. Jama. 2020;323(19):1912-4. [CrossRef]
- Sistema de Información Gerencial de Tuberculosis Perú: Ministerio de Salud; 2021 [cited 2022 01/06/2022]. Available from: https://appsalud.minsa.gob.pe/sigtbdata/WFLogin.aspx.
- Norma técnica de salud para la atención integral de las personas afectadas por tuberculosis. Perú: Ministerio de Salud; 2013 [cited 2022| 01/06/2022]. Available from: http://www.tuberculosis.minsa.gob.pe/portaldpctb/recursos/20180308083418.pdf.
- To Beard or not to Beard? That’s a good Question! US: Centers for Disease Control and Prevention; 2020 [cited 2022 01/06/2022]. Available from: https://blogs.cdc.gov/niosh-science-blog/2017/11/02/noshave/.
- Fighting Respirator Fraud, Counterfeiting, and Price Gouging. US: 3M; 2022 [cited 2022]. Available from: https://www.3m.com/3M/en_US/worker-health-safety-us/covid19/covid-fraud/.
- PortaCount Respirator Fit Tester 8048 US: TSI Incorporated; 2020 [cited 2022 01/06/2022]. Available from: https://www.tsi.com/products/respirator-fit-testers/portacount-respirator-fit-tester-8048/.
- Welker RW. Chapter 4 - Size Analysis and Identification of Particles. In: Kohli R, Mittal KL, editors. Developments in Surface Contamination and Cleaning. Oxford: William Andrew Publishing; 2012. p. 179-213.
- Proper N95 Respirator Use for Respiratory Protection Preparedness. US: Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health; 2020 [updated March 16, 2020; cited June 1, 2022]. Available from: https://blogs.cdc.gov/niosh-science-blog/2020/03/16/n95-preparedness/.
- Official response to questions related to use of the Portacount Respirator Fit Tester during a pathogenic outbreak. RFT-032. US: TSI Incorporated; 2020.
- Brun D, Curti C, Mekideche T, Benech A, Hounliasso I, Lamy E, et al. Stockpiled N95 respirator/surgical mask release beyond manufacturer-designated shelf-life: a French experience. J Hosp Infect. 2020;106(2):258-63. Epub 2020/08/01. [CrossRef] [PubMed]
- Voluntary Rescission of Fido Mask Company Certificates of Approvals. US: Centers for Disease Control and Prevention, National Institute of Occupational Safety and Health; 2014 [updated August 3, 2014; cited June 3, 2022]. Available from: https://www.cdc.gov/niosh/npptl/usernotices/notices/notice08032014.html.
- Non-compliant respiratory protection Bavikhove - Belgium: European Safety Federation; 2020 [cited 2022 01/06/2022]. Available from: https://www.eu-esf.org/personal-protective-equipment/non-compliant/respiratory-protection/5442-rapex-2020-49-particle-filter-mask-lucca-light-kn95-ffp2.
- O’Kelly E, Arora A, Pirog S, Ward J, Clarkson PJ. Comparing the fit of N95, KN95, surgical, and cloth face masks and assessing the accuracy of fit checking. PLOS ONE. 2021;16(1):e0245688. [CrossRef]
- Plana D, Tian E, Cramer AK, Yang H, Carmack MM, Sinha MS, et al. Assessing the filtration efficiency and regulatory status of N95s and nontraditional filtering face-piece respirators available during the COVID-19 pandemic. BMC infectious diseases. 2021;21(1):712-. [CrossRef] [PubMed]
- Mottay L, Le Roux J, Perumal R, Esmail A, Timm L, Sivarasu S, et al. KN95 filtering facepiece respirators distributed in South Africa fail safety testing protocols. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2020;0(0):13162. Epub 2020/12/19. [CrossRef] [PubMed]
- Zhuang Z, Landsittel D, Benson S, Roberge R, Shaffer R. Facial Anthropometric Differences among Gender, Ethnicity, and Age Groups. The Annals of Occupational Hygiene. 2010;54(4):391-402. [CrossRef]
- Zhuang Z, Bradtmiller B, Shaffer RE. New respirator fit test panels representing the current U.S. civilian work force. Journal of occupational and environmental hygiene. 2007;4(9):647-59. Epub 2007/07/07. [CrossRef] [PubMed]
- Coffey CC, Lawrence RB, Zhuang Z, Duling MG, Campbell DL. Errors associated with three methods of assessing respirator fit. Journal of occupational and environmental hygiene. 2006;3(1):44-52. Epub 2006/02/21. [CrossRef] [PubMed]
- Bergman M, Zhuang Z, Brochu E, Palmiero A. Fit Assessment of N95 Filtering-Facepiece Respirators in the U.S. Centers for Disease Control and Prevention Strategic National Stockpile. J Int Soc Respir Prot. 2015;32(2):50-64. Epub 2016/02/16. [PubMed] [PubMed Central]
- Warning Notice. Taiwan Makrite Industries Inc.; 2021 [cited 2021 01/06/2022]. Available from: http://www.makrite.com/sellers-on-amazon/.
- Hon CY, Danyluk Q, Bryce E, Janssen B, Neudorf M, Yassi A, et al. Comparison of qualitative and quantitative fit-testing results for three commonly used respirators in the healthcare sector. Journal of occupational and environmental hygiene. 2017;14(3):175-9. Epub 2016/10/09. [CrossRef] [PubMed]
- Reyes-Vega MF, Soto-Cabezas MG, Cárdenas F, Martel KS, Valle A, Valverde J, et al. SARS-CoV-2 prevalence associated to low socioeconomic status and overcrowding in an LMIC megacity: A population-based seroepidemiological survey in Lima, Peru. EClinicalMedicine. 2021;34:100801. Epub 2021/04/06. [CrossRef] [PubMed] [PubMed Central]
- Saunders MJ, Evans CA. COVID-19, tuberculosis and poverty: preventing a perfect storm. Eur Respir J. 2020;56(1):2001348. [CrossRef] [PubMed]
- Ley N°29783 de Seguridad y Salud en el Trabajo. Perú: Congreso de la República; 2011 [cited 2022]. Available from: https://diariooficial.elperuano.pe/pdf/0052/ley-seguridad-salud-en-el-trabajo.pdf.
- Resolución Jefatural N° 271-2019-J-OPE/INS. Perú: Ministerio de Salud, Instituto Nacional de Salud; 2019 [cited 2022 01/06/2022]. Available from: https://www.gob.pe/institucion/ins/normas-legales/991092-271-2019-j-ope-ins.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).