1. Introduction
Mosquito-borne diseases are among the leading causes of mortality and morbidity in humans [
1]. In the absence of effective vaccines, the vector control is one of the few available strategies for limiting pathogen transmission. Although the use of chemical products remains the most common method, alternatives have emerged aiming at reducing the environmental impact of vector control. Among the methods under development, the control of vector populations through the mass release of sterilizing males is appealing as it is highly specific and not polluting, and does not require the introduction of any new species into the environment [
2,
3]. This approach requires a robust sex separation system, especially in mosquitoes, because the accidental release of females creates biting nuisance and may have detrimental epidemiological consequences.
In mosquitoes, a variety of sex separation methods have been established and are based on size and developmental dimorphisms, morphological traits, behavioral differences or a sex specific genetic selectable marker [
4]. Among all these methods, the genetic approach seems as an ideal system because it can potentially separate quickly and in a large quantities males and females at an early development stage, reducing the time and cost of production [
5]. For example, a Genetic Sexing Strain (GSS) has been developed for the mosquito
Anopheles stephensi based on the expression of a green fluorescent protein at the 3rd instar larvae stage in males only [
6,
7]. Recently, an
Aedes albopictus transgenic line has been constructed that expresses ectopically
Nix, inducing masculinization in combination with a fluorescence marker [
8]. Other more classical genetic approaches have been developed aiming at randomly translocating (through low-dose irradiation) a conditionally lethal or any selectable marker near the male-determining factor. The majority of GSS developed through chromosomal translocation use an insecticide resistance gene as a selectable marker, as originally, including GSS developed for
Culex tarsalis [
9] and
Anopheles arabiensis [
10]. Similarly, our group recently developed an
Aedes albopictus GSS line based on the Y linkage of the
rdl gene conferring resistance to dieldrin and allowing the production of 98% of males following selection at third-instar larvae [
11].
However, it has been shown that the dieldrin ingested by the larvae of an
An.
Arabiensis GSS (ANO IPCL1) during sex selection can be detected in adults, raising concerns about the use of this insecticide at industrial scales [
10]. In this context we optimized the protocol developed by Lebon et al. [
11] in order to reduce larval exposure to dieldrin. We demonstrated that the selection protocol can be easily modulated by modifying the dose and/or exposure time to recover nearly all resistant males while maintaining a female contamination rate averaging 1%. We also showed that in certain conditions, dieldrin induced mortality on resistant male larvae and that this toxic effect is positively correlated with the dieldrin quantity found in adults. Finally, and interestingly, we showed that using insecticide exposure conditions allowing recovering all resistant males after selection, the dieldrin detected in adults was below the sensitivity threshold of the detection method. All these results showed that dieldrin selection can be adjusted so that sexing remains efficient while removing the toxicity on resistant males and maintaining the levels of dieldrin residues at hardly detectable levels.
2. Materials and Methods
2.1. Mosquito strain and rearing conditions
All experiments were performed using the
Aedes albopictus GSS referred as Tikok, obtained by sex linkage of the
rdl gene conferring dieldrin resistance [
11]. The Tikok line was maintained in conditions described in Lebon et al. [
11] from its original construction up to the F34 (generation used for the presented experiments). Briefly, at each generation, eggs were allowed to hatch for 24 hours in a 250 mL jar to obtain approximately 1,000 first instar larvae per jar. Larvae were transferred in a tray (34x23x4 cm) and fed with tetraMin (©TETRA) until L3 stage. Larvae were then exposed to a dieldrin concentration of 0.1 ppm for 24h. All survivors were rinsed, transferred in a tray and fed until pupation. All pupae were manually sex sorted under a microscope to determine the female contamination rate at each generation. Females were discarded and male pupae were placed in an adult cage (30x30x30 cm, Bugdorm 4S3030, Taichung, Taiwan), crossed with females from the susceptible line (S-RUN line, see [
11]) and adults were maintained under classic climatic conditions for reproduction (31±1 °C, 70±5% RH, 12:12 hour light:dark photoperiod).
2.2. Dieldrin selection at the L1 stage
With the aim to improve the dieldrin selection protocol, we tested sexing efficiency using L1 rather than L3 larvae, with the same dieldrin concentration and exposure time. This protocol was used from the F34 to the F42 (generation used for the following experiments) and the female contamination rate was measured at each generation.
2.3. Effect of dieldrin concentration on sexing
We optimized the dieldrin selection protocol performed on L1 by testing different dieldrin concentrations in order to decrease the quantity of dieldrin used. Six different concentrations were tested: 0.01, 0.02, 0.04, 0.06, 0.08 and 0.1 ppm. For each concentration, 1,000 L1 were manually counted and exposed for 24 hours, then rinsed with clean water and reared until the pupae stage. The experiment was duplicated for each dose. All pupae were manually sex sorted under a microscope to determine the female contamination rate.
2.4. Effect of dieldrin exposure time on sex sorting
We tested different times of exposure at a dieldrin concentration of 0.08 ppm (the lowest concentration giving a female contamination rate < 1% for 1,000 L1 exposed) to reduce L1 exposure to dieldrin. For this, 1,000 L1 were manually counted and exposed to dieldrin for different times: 1h, 2h, 4h, 6h, 12h, 18h and 24h, then rinsed with clean water and reared until pupal stage. All pupae were manually sex sorted under a microscope to determine female contamination rate.
2.5. Measurement of dieldrin toxicity effect on resistant males
Although males are genetically resistant to dieldrin, the insecticide may still cause some aberrations to the nervous system by interfering with the ion exchange [
12]. To quantify this potential toxic effect, we exposed 1,000 manually counted L1 to a dieldrin concentration of 0.08 ppm at three different times: 0, 6 and 24h. For each selection time, four repetitions were performed. All pupae were manually sex sorted under a microscope to determine the female contamination rate. The male recovery was used as a proxy of dieldrin toxicity and calculated as follows: male recovery (%) = [mean (number of male pupae recovered after dieldrin selection) / mean (number of male pupae recovered without selection) x 100].
2.6. Quantification of dieldrin residues in resistant male adults
2.6.1. Quantification at a small production scale
We quantified dieldrin in adult mosquitoes after larvae selection inducing a low or a high toxic effect on resistant male larvae. For this, we used the same protocol as describe above, i.e. 1,000 manually counted L1 were exposed to a dieldrin concentration of 0.08 ppm for three different times: 0, 6 and 24h. Six and twenty repetitions of 1,000 L1 exposed for 6h and 24h were performed respectively in order to have at least 2,000 surviving male pupae for each condition. For each exposure time, all surviving larvae were pooled and reared until pupation. All pupae were manually sex sorted under a microscope to determine female contamination rate, and male recovery was calculated as described above. All male pupae were allowed to emerge in an adult cage (30x30x30 cm, Bugdorm 4S3030) and fed with a 5% sucrose solution. 2-4 day old adult males were frozen at -20°C for 1 hour and for each selection time 3 vials of 50 mL containing 500 adult males were used for dieldrin quantification.
2.6.2. Quantification of dieldrin on a mass production context
After quantifying the dieldrin residues in adults exposed at different times, we quantified dieldrin residues in a mass production context exposing L1 to different dieldrin concentrations. A hundred and sixty milligrams of brushed eggs were allowed to hatch for 8 hours in a pot of 200 mL containing 30 mL of hatching solution. First instar larvae were then exposed for 16h to five different dieldrin concentrations: 0.1, 0.2, 0.4, 0.6 and 1 ppm, then rinsed with clean water and reared until pupation. All trays were fed with the same quantity of food (tetraMin (©TETRA), day 1: 0.3 g, day 2: 0.6 g, day 3: 20 g, day 4: 25, day 5: 20 g, day 6: 15 g, day 7: 10 g), pupae were collected and sex separated using a pupae sex sorter (Wolbaki, WBK-P0001-V1 model). All pupae were then counted using a mosquito pupae counter (Radiation General Ltd, Budapest, Hungary) [
13]. For each dieldrin concentration, 3 repetitions were performed, and the female contamination rate as well as male recovery were measured for each repetition. All male pupae were allowed to emerge in adult cages (30x30x30 cm, Bugdorm 4S3030) and fed with a 5% sucrose solution. 2-4 day-old males were frozen at -20°C for 1 hour and 3 vials of 50 mL containing 2,000 adult males were prepared for each dieldrin concentration. A female contamination rate and a male recovery rate were associated to each dieldrin concentration for each vial (corresponding to a specific tray).
2.7. Dieldrin extraction and quantification
The quantification was performed by an independent private laboratory (Berkem groupe, Blanquefort, France). All samples were weighted before extraction. All adult males were then transferred in a vial containing 20 mL of acetonitrile and placed in an ultrasonic bath at 45°C for 90 minutes for agitation. The content was then filtered and transferred into a new vial until a total evaporation of acetonitrile. Two milliliters of isohexane were added and the quantification was performed through GS/MS/MS with a sensitivity threshold of 2 µg/L.
2.8. Statistical analysis
Fisher exact tests were used to compare the percentage of female contamination rate (%) between protocols expositing L1 or L3 stages, the female contamination rate (%) obtained after different dieldrin concentrations or exposure times, and the male recovery (%) after different dieldrin exposure times.
3. Results
3.1. Stability of Tikok line and efficiency of sex sorting at L1 stage
Female contamination rate was stable across generations, from the F27 to the F42, with a percentage comprised between 1.29% and 0.40% (
Table 1). We didn’t detect differences in female contamination between protocols exposing L3 (from F27 to F34) or L1 larvae (from F35 to F42), with a female contamination rate averaging 0.84 (±0.26) and 0.75 (±0.17), respectively (Fisher exact test, P=0.161).
3.2. Effect of dieldrin concentration on the female elimination efficiency
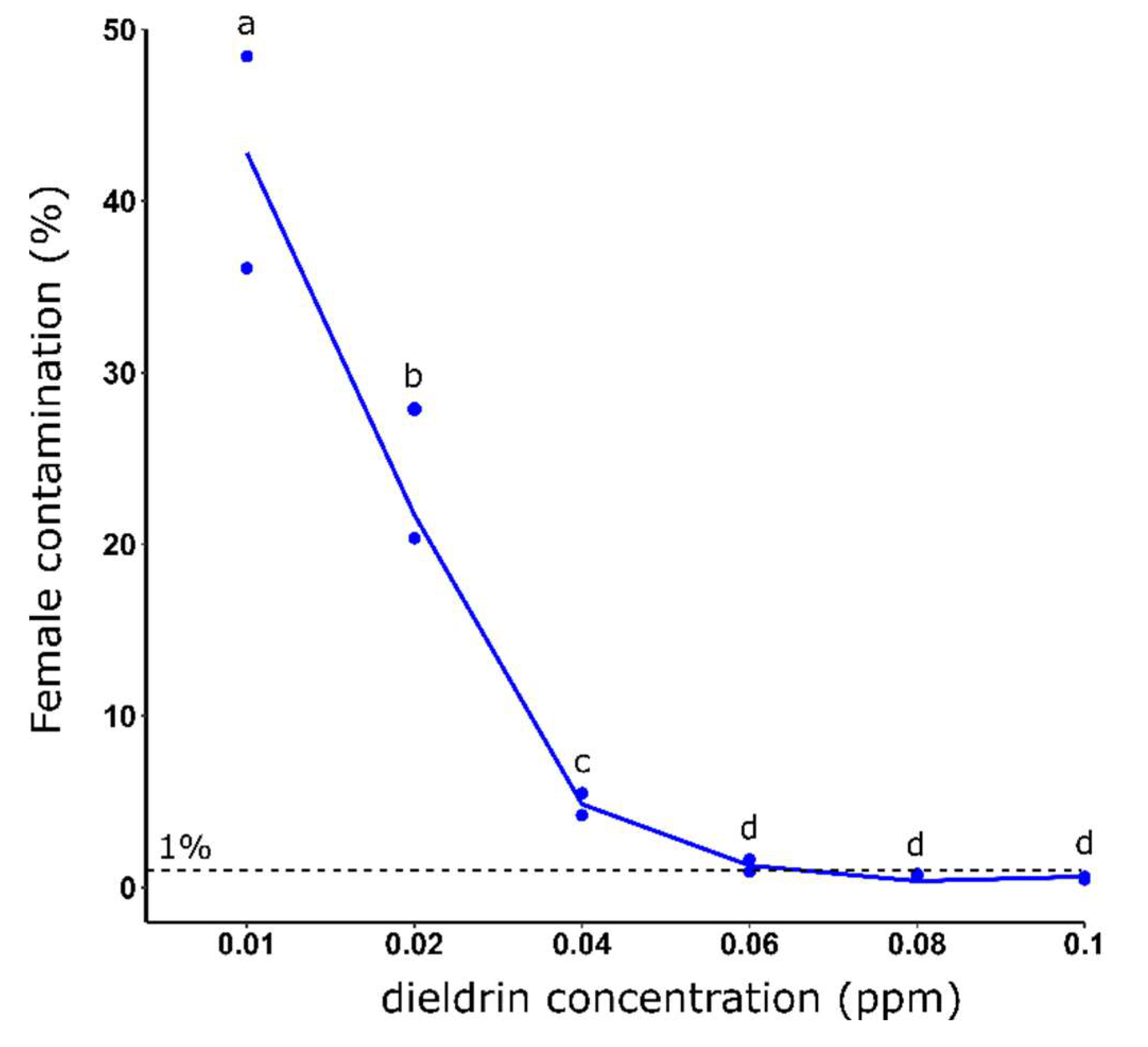
We showed that for 1,000 exposed L1, increasing dieldrin concentration improved female elimination efficiency with a female contamination rate averaging 45.26% and 0.54% for dieldrin concentrations of 0.01 and 0.1 ppm, respectively (
Figure 1). Female contamination rate decreased significantly for concentrations of 0.01 to 0.06 ppm, and then decreased slightly until 0.1 ppm of dieldrin selective pressure (
Figure 1).
3.3. Effect of dieldrin exposure time on sex sorting
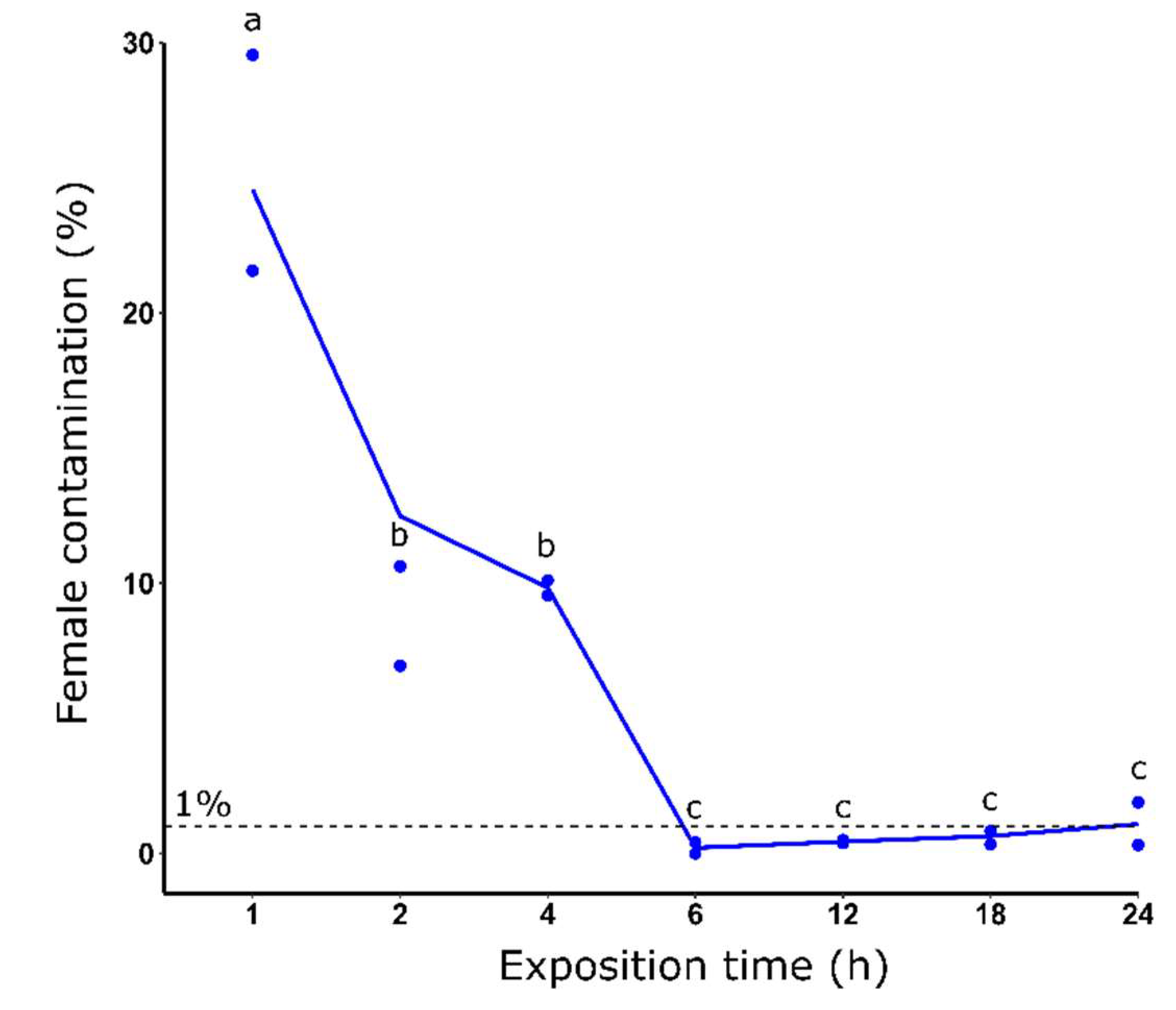
At a dieldrin concentration of 0.08 ppm, increasing the exposure time increased the female elimination efficiency from 25.55% to 1.10% of average female contamination rate, for 1h and 24h of exposure, respectively (
Figure 2). More precisely, the female contamination rate decreased significantly from 1 to 6 hours of selection. Interestingly, after 6 h and until 24 h of selection, we found a slight increase of female mean contamination rate that increased from 0.21% to 1.10%.
3.3. Measurement of dieldrin toxicity effect on resistant males
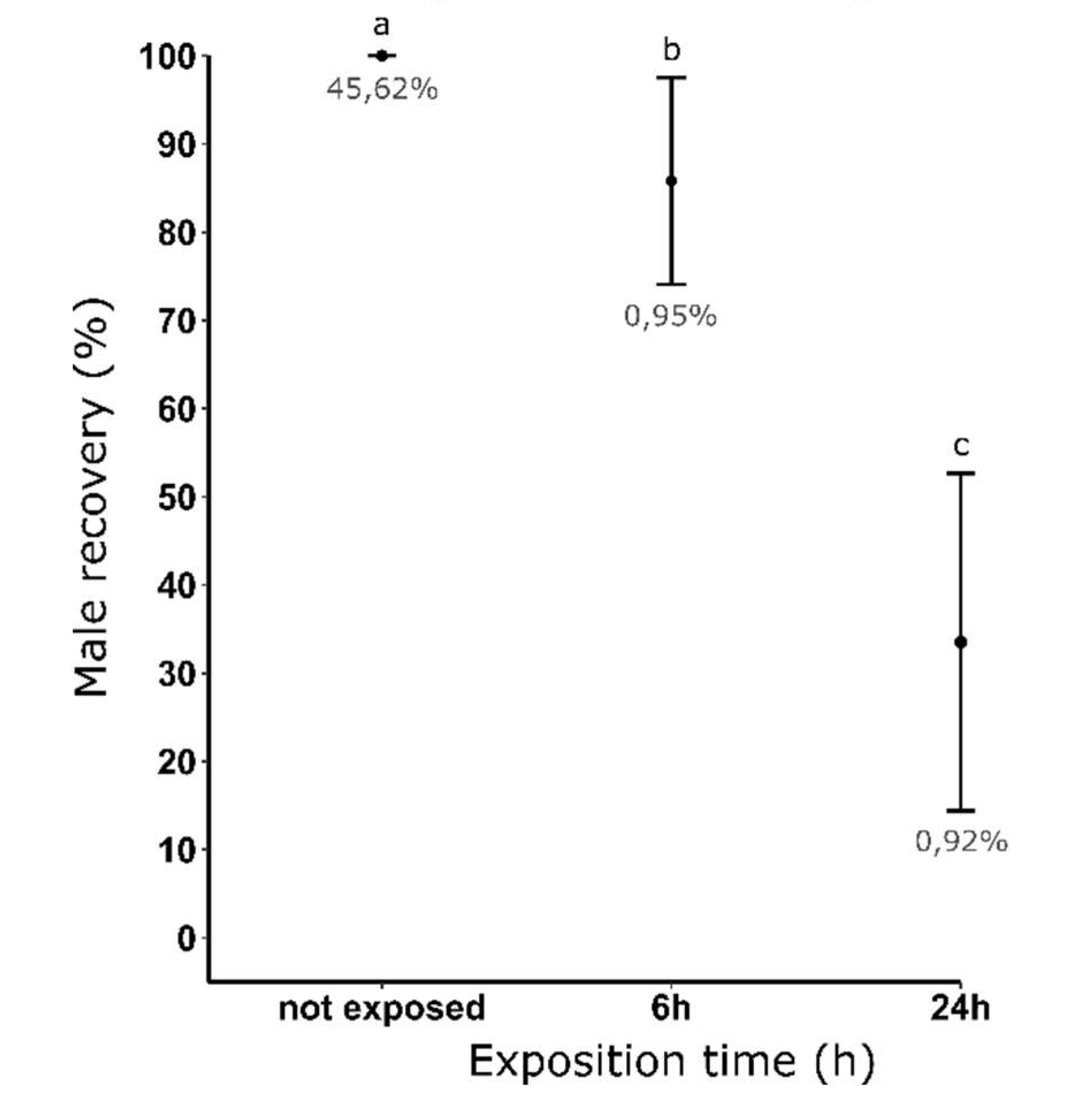
We showed that increasing the exposure time from 6 to 24 hours while maintaining a constant dieldrin concentration decreased the percentage of male recovery from 85.80 % to 33.52%, respectively (
Figure 3). In addition, we showed that this toxic effect on resistant male larvae did not affect female contamination rate as this rate averaged 0.9% for both exposure times.
3.4. Quantification of dieldrin residues in resistant male adults
3.4.1. Quantification at a small production scale
Dieldrin was detected in all mosquito samples for which L1 larvae had been exposed at 0.08 ppm for 6 or 24h. For 6h exposure, one of three quantification replicates was below the sensitivity threshold of quantification (2 µg/L), one was at the threshold, and the last replicate revealed a 2.1 µg/L dieldrin in males, corresponding to a mean of 8.32±0.3 pg dieldrin per adult male. We showed that dieldrin quantity found in resistant male adults was positively correlated with the exposure time as mosquitoes exposed for 24h showed approximately a thousand times more dieldrin (1,94±0.6 ng per mosquito, see
Table 2) than those exposed for 6h. We showed that the female contamination rate remained low (< 1%) for both exposure times. Lastly, we confirmed that extended dieldrin exposure induced mortality on resistant larvae with 33.88% of male recovery for an exposure of 24h, compared with 90.70% for 6h exposure.
3.4.2. Quantification of dieldrin in males obtained in a mass production context
Dieldrin was detected in all samples containing mosquitoes for which L1 larvae had been exposed for 16h at concentrations ranging from 0.1 to 1 ppm (
Table 3). For 0.1, 0.2, and 0.4 ppm, dieldrin detection was below the quantification threshold (2 µg/L), corresponding to ≤ 2 pg of dieldrin per adult male. Two out of the three replicates using 0.6 ppm dieldrin were also below the quantification threshold, corresponding to ≤ 2.33 pg of dieldrin per adult male. For the highest tested concentration (1 ppm), we found 6.66 pg dieldrin per adult male.
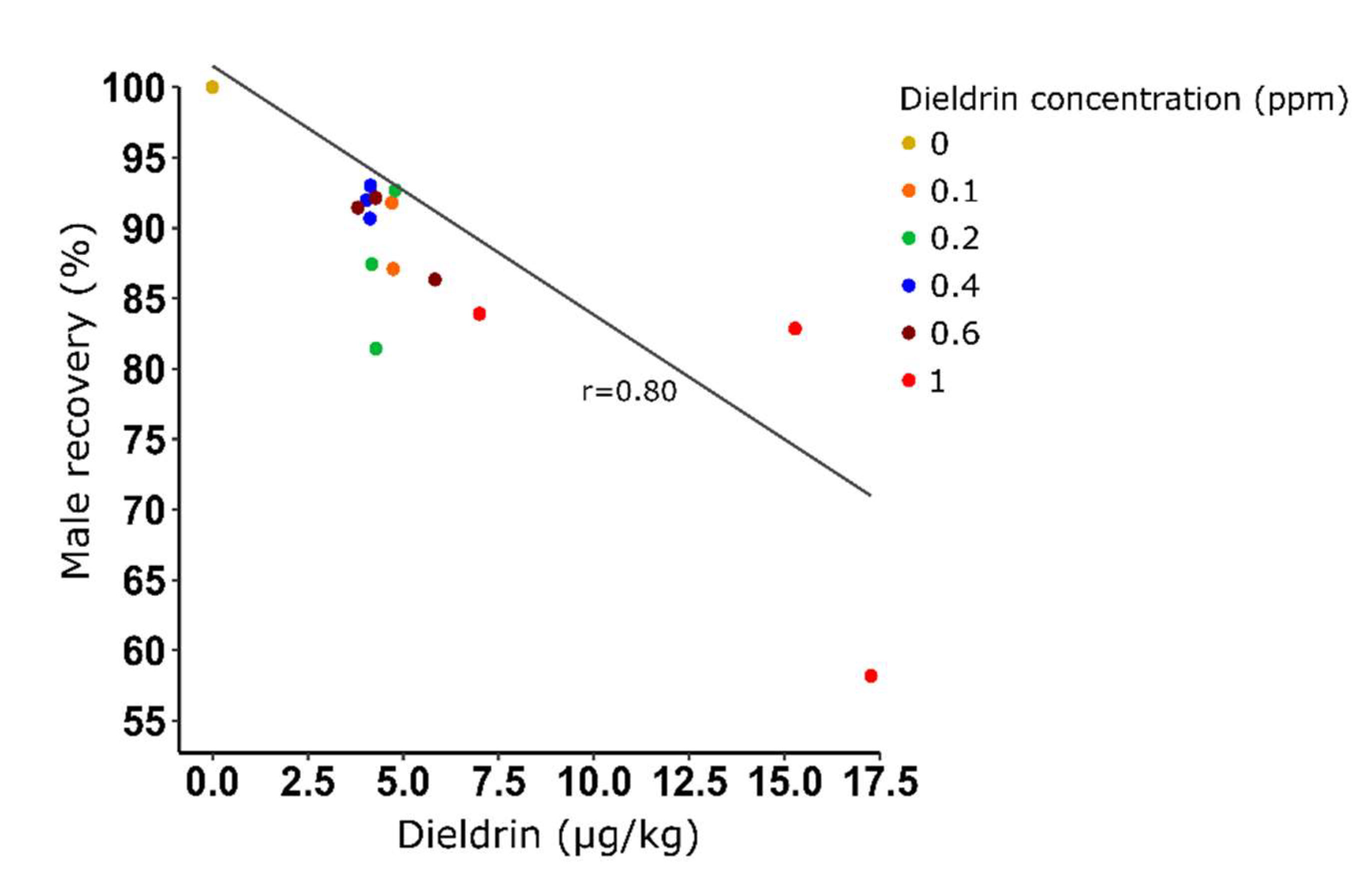
We also confirmed that an exposure to a high dieldrin concentration induced mortality on resistant larvae with a male recovery of 74.97% (±14.56) for 1 ppm to be compared to lower concentrations for which the male recovery averaged 90%. Lastly, we demonstrated that the dieldrin found in adults was positively correlated with male recovery (Pearson’s product-moment correlation, df=14, P<0.05) (
Figure 4).
4. Discussion
The Ae. Albopictus GSS line used in this study has been genetically stable since its construction and until the F42, corresponding to the last generation used in this study, with a female contamination rate ranging between 0.4% and 1.7%. Interestingly, the selection protocol modification that we tested herein in which L1 instead L3 larvae were exposed to dieldrin did not affect this stability.
We showed that the increase of dieldrin concentration and/or exposure time increased the female elimination up to a certain threshold. Beyond, a toxic effect of dieldrin was observed on resistant male larvae, reducing male recovery to 50%. Although males are homozygous resistant to dieldrin, the insecticide may still cause some aberrations to the nervous system by interfering with ion exchange [
12]. In keeping with this hypothesis, we demonstrated that the toxic effect on resistant male larvae was positively correlated with the dieldrin quantity found in adults. Most interestingly, we showed that when the toxic effect is low, with a male recovery averaging 90%, the dieldrin is hardly detectable (using a limit of detection of 2 µg/L corresponding to the quantification method used herein). Therefore, in a mass production context (growing ~ 3,000 larvae per tray following selection), we detected dieldrin at a concentration of 2 pg/mosquito (always below the detection threshold) and recorded a male recovery of 92% with a female contamination rate of 4% (L1 larvae exposed at 0.4 ppm during 16h). By comparison, it has been quantified in the GSS of
An.
Arabiensis ANO IPCL 1, 9 ng/mosquito with a comparable level of female elimination [
10]. We hence detected a thousand times less dieldrin per mosquito.
Although < 2 pg per mosquito seems low, it is important to evaluate the amount of dieldrin potentially dispersing in the field with the deployment of a sterile-male program. The abundance of
Ae.
albopictus populations on Reunion Island peaks at 6,000 wild males per hectare during the rainy season [
14]. To reach a ratio of 5:1 (sterile male: wild male), approximately 30,000 sterile males have to be released in the field per hectare and week, corresponding to a maximum of 0.06 µg of dieldrin per hectare and week. Thus, a six months treatment corresponds to less than 144 µg of dieldrin per km
2. The Food and Agriculture Organization/World Health Organization’s acceptable daily intake for the combined total of aldrin and dieldrin for humans is 0.1 µg/kg of body weight, corresponding to 8 µg per day for an 80 kg person [
15]. In the European legislation, a default value of 10 µg/kg has been adopted to facilitate the control of residues of pesticides for which no maximum residue levels have been established [
16]. In humans, the lowest reported lethal dose has been estimated at 5000 µg/kg of body weight [
17]. The total dose without negative effects observed is 25 µg/kg and 40µg/kg of body weight in rats and dogs, respectively [
17]. Compared to these data, a level of less than 144 µg of dieldrin per km
2 for 6 months of releasing sterile male seems negligible. However, the persistence of dieldrin in the field and its bioaccumulation in the food chain were not evaluated herein [
10]. In addition, the liquid wastes generated by the mosquito production can be challenging to recycle. Hence the use of such GSS could be powerful for sterile-male programs implemented at a small scale and on a short period of time but not relevant for industrial scale-up.
Author Contributions
Conceptualization, J.C; methodology, S.S., B.G., J.E., D.M. and J.C.; software, J.C.; validation, S.S., B.G., J.E., Q.L., M.D., D.M. and J.C.; formal analysis, J.C.; investigation, S.S., B.G., J.E., Q.J., M.D., D.M. and J.C.; resources, J.C.; data curation, J.C.; writing—original draft preparation, P.T., and J.C.; writing—review and editing, P.M., P.T. and J.C.; visualization, J.C.; supervision, J.C.; project administration, B.G. and J.C.; funding acquisition, P.M., P.T. and J.C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Organization, W.H. Global Vector Control Response 2017-2030. Glob. vector Control response 2017-2030. 2017.
- Bourtzis, K.; Dobson, S.L.; Xi, Z.; Rasgon, J.L.; Calvitti, M.; Moreira, L.A.; Bossin, H.C.; Moretti, R.; Baton, L.A.; Hughes, G.L.; et al. Harnessing Mosquito-Wolbachia Symbiosis for Vector and Disease Control. Acta Trop. 2014, 132, S150–S163. [Google Scholar] [CrossRef] [PubMed]
- Lees, R.S.; Gilles, J.R.; Hendrichs, J.; Vreysen, M.J.; Bourtzis, K. Back to the Future: The Sterile Insect Technique against Mosquito Disease Vectors. Curr. Opin. Insect Sci. 2015, 10, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Papathanos, P.A.; Bourtzis, K.; Tripet, F.; Bossin, H.; Virginio, J.F.; Capurro, M.L.; Pedrosa, M.C.; Guindo, A.; Sylla, L.; Coulibaly, M.B.; et al. A Perspective on the Need and Current Status of Efficient Sex Separation Methods for Mosquito Genetic Control. Parasites and Vectors 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Lutrat, C.; Giesbrecht, D.; Marois, E.; Whyard, S.; Baldet, T.; Bouyer, J. Sex Sorting for Pest Control: It’s Raining Men! Trends Parasitol. 2019, 35, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, F.; Galizi, R.; Menichelli, M.; Papathanos, P.A.; Dritsou, V.; Marois, E.; Crisanti, A.; Windbichler, N. Site-Specific Genetic Engineering of the Anopheles Gambiae y Chromosome. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 7600–7605. [Google Scholar] [CrossRef] [PubMed]
- Catteruccia, F.; Benton, J.P.; Crisanti, A. An Anopheles Transgenic Sexing Strain for Vector Control. Nat. Biotechnol. 2005, 23, 1414–1417. [Google Scholar] [CrossRef] [PubMed]
- Lutrat, C.; Olmo, R.P.; Baldet, T.; Bouyer, J.; Marois, E. Transgenic Expression of Nix Converts Genetic Females into Males and Allows Automated Sex Sorting in Aedes Albopictus. Commun. Biol. 2022, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.T.; Asman, S.M. ; others A Genetic-Sexing Strain Based on Malathion Resistance for Culex Tarsalis. Mosq. News 1982, 42, 531–536. [Google Scholar]
- Yamada, H.; Jandric, Z.; Chhem-Kieth, S.; Vreysen, M.J.B.; Rathor, M.N.; Gilles, J.R.L.; Cannavan, A. Anopheles Arabiensis Egg Treatment with Dieldrin for Sex Separation Leaves Residues in Male Adult Mosquitoes That Can Bioaccumulate in Goldfish (Carassius Auratus Auratus). Environ. Toxicol. Chem. 2013, 32, 2786–2791. [Google Scholar] [CrossRef] [PubMed]
- Lebon, C.; Benlali, A.; Atyame, C.; Mavingui, P.; Tortosa, P. Construction of a Genetic Sexing Strain for Aedes Albopictus: A Promising Tool for the Development of Sterilizing Insect Control Strategies Targeting the Tiger Mosquito. Parasites and Vectors 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Vreysen, M.J.B.; Bourtzis, K.; Tschirk, W.; Chadee, D.D.; Gilles, J.R.L. The Anopheles Arabiensis Genetic Sexing Strain ANO IPCL1 and Its Application Potential for the Sterile Insect Technique in Integrated Vector Management Programmes. Acta Trop. 2015, 142, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Esnault, J.; Scussel, S.; Gaudillat, B.; Lejarre, Q.; Toussaint, M.; Duployer, M.; Gárdos, M.; Bán, P.; Mavingui, P.; Tortosa, P.; et al. Development of an Automated Mosquito Pupae Counter. J. Med. Entomol. 2023, 1–5. [Google Scholar] [CrossRef]
- Le Goff, G.; Damiens, D.; Ruttee, A.H.; Payet, L.; Lebon, C.; Dehecq, J.S.; Gouagna, L.C. Field Evaluation of Seasonal Trends in Relative Population Sizes and Dispersal Pattern of Aedes Albopictus Males in Support of the Design of a Sterile Male Release Strategy. Parasites and Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) Aldrin and Dieldrin in Drinking-Water. 2003, 14.
- European Commission Regulation (EC) No 396/2005, Maximum Residue Levels of Pesticides in/on Food and Feed of Plant and Animal. Off. J. Eur. Union 2005, 1–16.
- INERIS Dieldrine. Données Tech. sur les Subst. Chim. en Fr. 2007, 1–13.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).