Submitted:
14 June 2023
Posted:
15 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Follow-up assessment
2.3. Primary and secondary endpoint
2.4. Statistical analysis
3. Results
3.1. Comparison of bilateral and unilateral multifocal PTC
3.2. Univariate and multivariate analyses of the risk factors of recurrence before PSM
3.3. Comparison of patients with bilateral and unilateral multifocal PTC who underwent TT
3.4. Univariate and multivariate analyses of recurrence risk factors after PSM
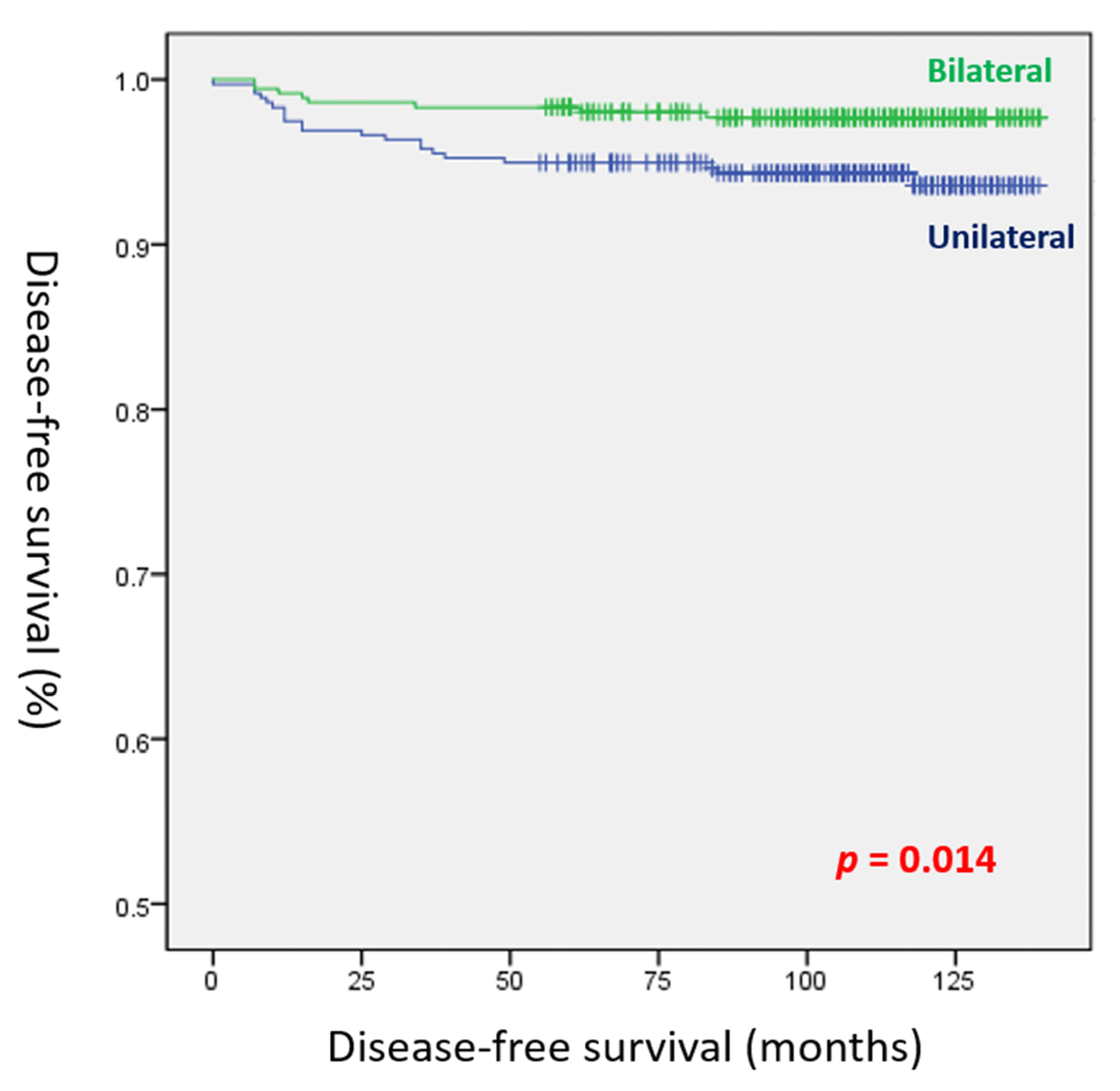
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, S.; Han, Y. The overdiagnosis of thyroid micropapillary carcinoma: the rising incidence, inert biological behavior, and countermeasures. J. Oncol. 2021, 2021, 5544232. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Welch, H. Increasing incidence of thyroid cancer in United States. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [PubMed]
- Megwalu, U.; Moon, P. Thyroid cancer incidence and mortality trends in the United States: 2000-2018. Thyroid 2022, 32, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Iida, F.; Yonekura, M.; Miyakawa, M. Study of intraglandular dissemination of thyroid cancer. Cancer 1969, 24, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Katoh, R.; Sasaki, J.; Kurihara, H.; Suzuki, K.; Iida, Y.; Kawaoi, A. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma: a clinicopathologic study of 105 consecutive patients. Cancer 1992, 70, 1585–1590. [Google Scholar] [CrossRef]
- Tam, A.A.; Ozdemir, D.; Cuhaci, N.; Baser, H.; Aydin, C.; Yazgan, A.K.; Ersoy, R.; Cakir, B. Association of multifocality, tumor number, and total tumor diameter with clnicopathological features in papillary thyroid cancer. Endocrine 2016, 53, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, T.M.; Westra, W.H.; Ladenson, P.W.; Arnold, A. Independent clonal origins of distinct tumor foci in multifocal papillary thyroid carcinoma. N. Engl. J. Med. 2005, 352, 2406–2412. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, I.; Woo, J.-W.; Lee, J.H.; Choe, J.-H.; Kim, J.-H.; Kim, J.S. Predictive factors for lymph node metastasis in papillary thyroid microcarcinoma. Ann. Surg. Oncol. 2016, 23, 2866–2873. [Google Scholar] [CrossRef]
- Genpeng, L.; Jianyong, L.; Jiaying, Y.; Ke, J.; Zhihui, L.; Rixiang, G.; Lihan, Z.; Jingqiang, Z. Independent predictors and lymph node metastasis characteristics of multifocal papillary thyroid cancer. Medicine 2018, 97, e9619. [Google Scholar] [CrossRef]
- Haddad, R.I.; Bischoff, L.; Ball, D.; Bernet, V.; Blomain, E.; Busaidy, N.L.; Campbell, M.; Dickson, P.; Duh, Q.-Y.; Ehya, H.; Goldner, W.S.; Guo, T.; Haymart, M.; Holt, S.; Hunt, J.P.; Iagaru, A.; Kandeel, F.; Lamonica, D.M.; Mandel, S.; Markovina, S.; McIver, B.; Raeburn, C.D.; Rezaee, R.; Ridge, J.A.; Roth, M.Y.; Scheri, R.P.; Shah, J.P.; Sipos, J.A.; Sippel, R.; Sturgeon, C.; Wang, T.N.; Wirth, L.J.; Wong, R.J.; Yeh, M.; Cassara, C.J.; Darlow, S. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 925–951. [Google Scholar] [CrossRef]
- Kim, H.J.; Sohn, S.Y.; Jang, H.W.; Kim, S.W.; Chung, J.H. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J. Surg. 2013, 37, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Polat, S.B.; Cakir, B.; Evranos, B.; Baser, H.; Cuhaci, N.; Aydin, C.; Ersoy, R. Preoperative predictors and prognosis of bilateral multifocal papillary thyroid carcinomas. Surg. Oncol. 2019, 28, 145–149. [Google Scholar] [CrossRef]
- Jeon, Y.W.; Gwak, H.G.; Lim, S.T.; Schneider, J.; Suh, Y.J. Long-term prognosis of unilateral and multifocal papillary thyroid microcarcinoma after unilateral lobectomy versus total thyroidectomy. Ann. Surg. Oncol. 2019, 26, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Duh, Q.Y.; Sugino, K.; Iwasaki, H.; Kameyama, K.; Mimura, T.; Ito, K.; Takami, H.; Takanashi, Y. Lymph node metastasis from 259 papillary thyroid carcinomas: frequency, pattern of occurrence and recurrence and optimal strategy for neck dissection. Ann. Surg. 2003, 237, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Fu, P.; Harth, K.C.; Margevicius, S.; Wilhelm, S.M. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery 2010, 148, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; Schuff, K.G.; Sherman, S.I.; Sosa, J.A.; Steward, D.L.; Tuttle, R.M.; Wartofsky, L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Karatzas, T.; Vasileiadis, I.; Charitoudis, G.; Karakostas, E.; Tseleni-Balafouta, S.; Kouraklis, G. Bilateral versus unilateral papillary thyroid microcarcinoma: predictive factors and associated histopathological findings following total thyroidectomy. Hormones (Athens) 2013, 12, 529–536. [Google Scholar] [CrossRef]
- Kim, K.; Zheng, X.; Kim, J.K.; Lee, C.R.; Kang, S.-W.; Lee, J.; Jeong, J.J.; Nam, K.-H.; Chung, W.Y. The contributing factors for lateral neck lymph node metastasis in papillary thyroid microcarcinoma (PTMC). Endocrine 2020, 69, 149–156. [Google Scholar] [CrossRef]
- Kim, S.K.; Park, I.; Woo, J.-W.; Lee, J.H.; Choe, J.-H.; Kim, J.-H.; Kim, J.S. Predicting factors for bilaterality in papillary thyroid carcinoma with tumor size <4cm. Thyroid 2017, 27, 207–214. [Google Scholar] [CrossRef]
- Wang, N.; Qian, L.-X. Predictive factors for occult bilateral papillary thyroid carcinoma. Acad. Rad. 2021, 28, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-L.; Gao, E.-L.; Zhang, W.; Yang, H.; Guo, G.-L.; Zhang, X.-H.; Wang, O.-C. Factors predictive of papillary thyroid microcarcinoma with bilateral involvement and central lymph node metastasis: a retrospective study. World J. Surg. Oncol. 2012, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Zhang, L.; Wu, W.-L.; Ji, Q.-H.; Lu, Z.-W.; Zhu, Y.-X.; Lin, D.-Z. Bilaterality weighs more than unilateral multifocallity in predicting prognosis in papillary thyroid cancer. Tumour Biol. 2016, 37, 8783–8789. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-W.; Qu, Z.; Qin, A.-C.; Pan, H.; Ye, J.; Jiang, Y. Significance of multifocality in papillary thyroid carcinoma. Eur. J. Surg. Oncol. 2020, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Qiu, W.; Song, J.; Ying, T.; Fan, Y.; Yang, Z. Bilateral multifocality, a marker for aggressive disease, is not an independent prognostic factor for papillary thyroid microcarcinoma: A propensity score matching analysis. Clin. Endocrinol. 2021, 95, 209–216. [Google Scholar] [CrossRef]
- Cai, J.; Fang, F.; Chen, J.; Xiang, D. Unilateral multifocality and bilaterality could be two different multifocal entities in patients with papillary thyroid microcarcinoma. BioMed Res. Int. 2020, 9854964. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, T.; Xie, C.; Di, Z. BRAF V600E gene mutation is associated with bilateral malignancy of papillary thyroid cancer. Am. J. Med. Sci. 2018, 356, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, W.; Wang, H.; Teng, X.; Wang, H.; Chen, X.; Li, Z.; Yu, X.; Fahey, T.J.; Teng, L. Poorer prognosis and higher prevalence of BRAFV600E mutation in synchronous bilateral papillary thyroid carcinoma. Ann. Surg. Oncol. 2012, 19, 31–36. [Google Scholar] [CrossRef]
- Reilly, J.; Faridmoayer, E.; Lapkus, M.; Pastewski, J.; Sun, F.; Elassar, H.; Studzinski, D.M.; Callahan, R.E.; Czako, P.; Nagar, S. Vascular invasion predicts advanced tumor characteristics in papillary thyroid carcinoma. Am. J. Surg. 2022, 223, 487–491. [Google Scholar] [CrossRef]
- Kim, J.W.; Roh, J.-L.; Gong, G.; Cho, K.-J.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Recurrence in patients with clinically early-stage papillary thyroid carcinoma according to tumor size and surgical extent. Am. J. Surg. 2016, 212, 419–425. [Google Scholar] [CrossRef]
- Shobab, L.; Gomes-Lima, C.; Zeymo, A.; Feldman, R.; Jonklaas, J.; Wartofsky, L.; Burman, K.D. Clinical, pathological, and molecular profiling for radioactive iodine refractory differentiated thyroid cancer. Thyroid 2019, 29, 1262–1268. [Google Scholar] [CrossRef]
- Tang, J.; Kong, D.; Cui, Q.; Wang, K.; Zhang, D.; Liao, X.; Gong, Y.; Wu, G. The role of radioactive iodine therapy in papillary thyroid cancer: an observation study based on SEER. Onco Targets Ther. 2018, 11, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
| Bilateral (n=1002) | Unilateral-multifocal (n=743) | p-value | |
|---|---|---|---|
| Age (years) | 48.9 ± 12.1 | ||
| (range, 15 - 83) | 47.5 ± 11.7 | ||
| (range, 16 - 82) | 0.018 | ||
| Male gender | 199 (19.9%) | 168 (22.6%) | 0.172 |
| Extent of surgery | <0.001 | ||
| Less than TT | 0 | 372 (50.1%) | |
| TT and/or mRND | 1002 (100%) | 371 (49.9%) | |
| Subtype of PTC | 0.111 | ||
| Non-aggressive | 848 (84.6%) | 649 (87.3%) | |
| Aggressive | 154 (15.4%) | 94 (12.7%) | |
| Tumor size (cm) | 1.2 ± 0.9 | ||
| (range, 0.2 – 6.7) | 0.9 ± 0.6 | ||
| (range, 0.2 – 6.5) | <0.001 | ||
| gross ETE | 95 (9.5%) | 29 (3.9%) | <0.001 |
| Lymphatic invasion | 367 (36.6%) | 194 (26.1%) | <0.001 |
| Vascular invasion | 34 (3.4%) | 24 (3.2%) | 0.893 |
| Perineural invasion | 39 (3.9%) | 10 (1.3%) | 0.001 |
| BRAFV600E positivity | 712/853 (83.5%) | 489/596 (82.0%) | 0.479 |
| Harvested LNs | 19.1 ± 21.4 | 13.2 ± 16.0 | <0.001 |
| Positive LNs | 3.9 ± 6.3 | 2.4 ± 4.5 | <0.001 |
| T stage | <0.001 | ||
| T1 | 826 (82.4%) | 689 (92.7%) | |
| T2 | 68 (6.8%) | 23 (3.1%) | |
| T3a | 13 (1.3%) | 2 (0.3%) | |
| T3b | 93 (9.3%) | 27 (3.6%) | |
| T4a | 2 (0.2%) | 2 (0.3%) | |
| N stage | <0.001 | ||
| N0 | 387 (38.6%) | 359 (48.3%) | |
| N1a | 455 (45.4%) | 310 (41.7%) | |
| N1b | 160 (16.0%) | 74 (10.0%) | |
| M stage | 0.402 | ||
| M1 | 4 (0.4%) | 1 (0.1%) | |
| TNM stage | 0.002 | ||
| Stage I | 802 (80.0%) | 643 (86.5%) | |
| Stage II | 196 (19.6%) | 100 (13.5%) |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Gender | ||||
| Female | ref. | |||
| Male | 1.770 (1.091-2.870) | 0.021 | ||
| Extent of surgery | ||||
| Less than TT | ref. | |||
| TT and/or mRND | 1.408 (0.760-2.610) | 0.277 | ||
| Subtype of PTC | ||||
| Non-aggressive | ref. | |||
| Aggressive | 1.523 (0.866-2.681) | 0.144 | ||
| Tumor size | ||||
| ≤ 1cm | ref. | |||
| > 1cm | 2.877 (1.821-4.547) | <0.001 | ||
| gross ETE | 3.376 (1.919-5.940) | <0.001 | 12.674 (1.6119-99.713) | 0.016 |
| Multifocality | ||||
| Unilateral | ref. | |||
| Bilateral | 0.953 (0.605-1.500) | 0.835 | ||
| Lymphatic invasion | 3.738 (2.345-5.957) | <0.001 | ||
| Vascular invasion | 3.581 (1.721-7.451) | 0.001 | ||
| Perineural invasion | 5.011 (2.499-10.050) | <0.001 | 2.273 (1.005-5.139) | 0.049 |
| Harvested LNs | 1.017 (1.010-1.024) | <0.001 | ||
| Positive LNs | 1.078 (1.060-1.096) | <0.001 | 1.102 (1.053-1.154) | <0.001 |
| T stage | ||||
| T1 | ref. | |||
| T2 | 2.741 (1.301-5.776) | 0.008 | ||
| T3a | 4.257 (1.036-17.488) | 0.044 | ||
| T3b | 3.635 (2.012-6.568) | <0.001 | ||
| T4b | 9.142 (1.261-66.257) | 0.029 | ||
| N stage | ||||
| N0 | ref. | ref. | ||
| N1a | 7.535 (3.215-17.662) | <0.001 | 2.303 (0.912-5.819) | 0.078 |
| N1b | 14.065 (5.770-34.286) | <0.001 | 3.565 (1.086-11.710) | 0.036 |
| M stage | 10.883 (2.668-44.389) | 0.001 | ||
| RAI therapy | 18.685 (5.880-59.289) | <0.001 | 7.760 (2.275-26.470) | 0.001 |
| Before matching | After matching | ||||||
|---|---|---|---|---|---|---|---|
| Bilateral (n=1002) | Unilateral-multifocal (n=371) | p-value | Bilateral (n=357) | Unilateral-multifocal (n=357) | p-value | ||
| Age (years) | 48.9 ± 12.1 (range, 15 - 83) |
48.1 ± 12.2 (range, 20 - 82) |
0.305 | 46.5 ± 12.4 (range, 14 - 79) |
46.2 ± 11.8 (range, 11 - 81) |
||
| Male gender | 199 (19.9%) | 89 (24.0%) | 0.101 | 83 (23.2%) | 83 (23.2%) | 1.000 | |
| Subtype of PTC | 0.933 | 0.336 | |||||
| Non-aggressive | 848 (84.6%) | 315 (84.9%) | 311 (87.1%) | 301 (84.3%) | |||
| Aggressive | 154 (15.4%) | 56 (15.1%) | 46 (12.9%) | 56 (15.7%) | |||
| Tumor size (cm) | 1.2 ± 0.9 (range, 0.2 – 6.7) |
1.0 ± 0.7 (range, 0.2 – 6.5) |
<0.001 | 1.0 ± 0.6 (range, 0.2 – 4.5) |
1.0 ± 0.7 (range, 0.2 – 6.5) |
0.739 | |
| gross ETE | 95 (9.5%) | 21 (5.7%) | 0.028 | 22 (6.2%) | 21 (5.9%) | 1.000 | |
| Lymphatic invasion | 367 (36.6%) | 135 (36.4%) | 0.950 | 128 (35.9%) | 126 (35.3%) | 0.938 | |
| Vascular invasion | 34 (3.4%) | 11 (3.0%) | 0.865 | 10 (2.8%) | 11 (3.1%) | 1.000 | |
| Perineural invasion | 39 (3.9%) | 8 (2.2%) | 0.134 | 14 (3.9%) | 8 (2.2%) | 0.279 | |
| BRAFV600E positivity | 712/853 (83.5%) | 254/307 (82.7%) | 0.789 | 255/298 (85.6%) | 248/298 (83.2%) | 0.498 | |
| Harvested LNs | 19.1 ± 21.4 | 19.2 ± 20.5 | 0.986 | 19.1 ± 21.4 | 19.2 ± 20.5 | ||
| Positive LNs | 3.9 ± 6.3 | 3.9 ± 5.7 | 0.824 | 3.9 ± 6.3 | 3.9 ± 5.7 | ||
| T stage | 0.015 | 0.987 | |||||
| T1 | 826 (82.4%) | 333 (89.7%) | 318 (89.1%) | 319 (89.4%) | |||
| T2 | 68 (6.8%) | 16 (4.3%) | 16 (4.5%) | 16 (4.5%) | |||
| T3a | 13 (1.3%) | 1 (0.3%) | 1 (0.3%) | 1 (0.3%) | |||
| T3b | 93 (9.3%) | 20 (5.4%) | 20 (5.6%) | 20 (5.6%) | |||
| T4a | 2 (0.2%) | 1 (0.3%) | 2 (0.6%) | 1 (0.3%) | |||
| N stage | 0.189 | 0.842 | |||||
| N0 | 387 (38.6%) | 131 (35.3%) | 128 (35.9%) | 129 (36.1%) | |||
| N1a | 455 (45.4%) | 166 (44.8%) | 157 (44.0%) | 162 (45.4%) | |||
| N1b | 160 (16.0%) | 74 (19.9%) | 72 (20.2%) | 66 (18.5%) | |||
| M stage | 0.579 | N/A | |||||
| M1 | 4 (0.4%) | 0 | 0 | 0 | |||
| TNM stage | 0.391 | 0.303 | |||||
| Stage I | 802 (80.0%) | 309 (83.3%) | 288 (80.7%) | 296 (82.9%) | |||
| Stage II | 196 (19.6%) | 62 (16.7%) | 67 (18.8%) | 61 (17.1%) | |||
| Stage III | 2 (0.2%) | 0 | 2 (0.6%) | 0 | |||
| Stage IVb | 2 (0.2%) | 0 | 0 | 0 | |||
| RAI therapy | 726 (72.5%) | 251 (67.7%) | 0.093 | 246 (68.9%) | 242 (67.8%) | 0.809 | |
| Recurrence | 43 (4.3%) | 21 (5.7%) | 0.313 | 8 (2.2%) | 21 (5.9%) | 0.021 | |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Tumor size | ||||
| ≤ 1cm | ref. | |||
| > 1cm | 2.102 (1.014-4.358) | 0.046 | ||
| gross ETE | 3.458 (1.318-9.070) | 0.012 | ||
| Multifocality | ||||
| Bilateral | ref. | ref. | ||
| Unilateral | 2.660 (1.178-6.004) | 0.019 | 2.664 (1.180-6.017) | 0.018 |
| Lymphatic invasion | 3.586 (1.667-7.712) | 0.001 | ||
| Vascular invasion | 5.768 (2.006-16.582) | 0.001 | 3.839 (1.331-11.073) | 0.013 |
| Perineural invasion | 3.882 (1.175-12.833) | 0.026 | ||
| Positive LNs | 1.057 (1.023-1.093) | 0.001 | ||
| T stage | ||||
| T1 | ref. | |||
| T3b | 3.041 (1.047-8.830) | 0.041 | ||
| T4b | 12.909 (1.727-96.474) | 0.013 | ||
| N stage | ||||
| N0 | ref. | |||
| N1a | 4.437 (1.293-15.228) | 0.018 | ||
| N1b | 6.678 (1.836-24.288) | 0.004 | ||
| RAI therapy | 13.334 (1.814-98.005) | 0.011 | 12.124 (1.640-89.630) | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





