1. Introduction
The use of zeolite-based materials as adsorbents and photocatalysts for the extraction of heavy metals and degradation of wide variety of dyes, pharmaceutical compounds like antibiotics and other organic pollutants was broadly discussed [
1,
2,
3,
4], highlighting the advantage of their use in the treatment of contaminated water. Zeolites offer high surface area, nanoporous structure, strong acidity, high ion exchange ability and hydrothermal stability for synthesis of highly efficient photocatalysts. Unfortunately, the micropores from zeolites and active sites from the surface are less accessible to organic pollutants due to the limitations of mass transport. Furthermore, materials with a mesoporous structure are widely used as a support in the synthesis of photocatalysts because of their highly ordered mesoporous channels and large surface area [
5,
6,
7]. The solution to keep the properties of zeolites, along with the advantage of mass transfer through mesopores, seems to be hierarchical zeolites. These materials have significant advantages in reactions involving large molecules by coupling the native micropores with intracrystalline mesopores network. Higher adsorption capacity and degradation efficiency of amoxicillin were obtained for Au-Ti [
7] and Fe–Ti [
8] bimetallic photocatalysts supported on hierarchical zeolites Y photocatalysts. Whereas all the zeolite supports are not photoactive they were activated by incorporation of semiconductors and metal nanoparticles. The preparation of nanocomposite photocatalysts by immobilization of nanosized titanium dioxide into zeolite is a very promising solution to removal of organic and inorganic pollutants from water [
4,
9,
10,
11]. The utilization of zeolite as a carrier for titanium dioxide led to smaller particle sizes of this oxide. Also, the photocatalytic activity of the TiO
2-zeolite system increased comparatively to that of TiO
2. However, TiO
2 is a wide band gap semiconductor (~3.2 eV) and high recombination rate for electron-hole pairs with limited practical utility [
12,
13]. Recently, there has been increased the interest in the synthesis of nanocomposite materials as photocatalysts more efficient and active under visible light irradiation by doping of TiO
2 with noble metals [
7,
14,
15] and transition metals semiconductors [
8,
16,
17,
18,
19]. The increase of catalytic activity with transition metals is alternative to highly expensive noble metals. The transition metal cations have been found to be dopants and cocatalysts in the photocatalytic reactions. Among different transition metal ions, Ni and Co attracted attention for the development of visible light active TiO
2 materials used in photocatalytic degradation of organic pollutants [
20,
21,
22,
23,
24,
25,
26,
27]. At the interfacial contact between n-type TiO
2 semiconductor and a p semiconductor as nickel and cobalt oxides is established an p-n heterojunction which represents an effective way to hinder electron hole pair recombination after light absorption and prolong the charge carrier life time [
28,
29,
30]. Theoretically, the p–n junction generates an internal electric field at the interface, which causes, at equilibrium, a negative charge in p-type semiconductor region while the TiO
2 region has a positive charge [
28]. Under the illumination, the photogenerated electron-hole pairs are separated by the internal electric field. Thus the electrons move in the positive field and the holes in the negative field. Their separation leads to a significant increase in photocatalytic activity.
Cobalt oxide was reported as good photocatalyst for pollutants degradation [
5]. Bahnemann et al. [
22] highlighted the effect of the synthesis method on the photocatalytic activity of TiO
2 doped with cobalt. It was also shown that the photocatalytic activity under visible light irradiation depends largely on the valence state of the Co ions in the dopant and its concentration rather than the specific surface area of the support and the crystallinity of the anatase [
5,
21]. Also, in the case of TiO
2 doping with NiO, it was evidenced [
29] that the synergistic interactions of heterojunctions, design, synthesis method, and structure influenced the photocatalytic performances. PL spectra [
31] showed that the small amount of Ni on TiO
2 enhances the recombination of photogenerated electrons and holes and Ni excess decrease reaction rate, due to coverage effect of nickel species on the active site from titanium bioxide surface.
While, most of the works have been focused on modifying TiO2 based photocatalysts in order of their increasing efficiency in degradation of pollutants under sunlight or visible light irradiation a limited number had as their objective the activation of zeolite type supports and especially hierarchical zeolites. Also, a comparative study of the effect of Co and Ni doping on the activity of TiO2 immobilized on a zeolite support with a micro-mesoporous structure has not been carried out. The novelty also consists in evaluation with scavengers of the photocatalytic reactions mechanism.
In this work were studied zeolites Y containing Ti–Ni and Ti–Co mixed oxides as photocatalysts for degradation of amoxicillin from water. The zeolite Y supports, with microporous and hierarchical structure, were synthesized and impregnated, as active species, with different Ti amounts (5%, 10% TiO2) followed of Ni or Co species (5% NiO, CoO) loading. The effects of supports, TiO2 loading and type of the second immobilized metal (Ni or Co) on formation of reactive species, photocatalytic performances and mechanism were studied.
2. Materials and Methods
2.1. Materials
Sodium silicate solution (Na2O(SiO2)x·xH2O, reagent grade), sodium aluminate (NaAlO2), sodium hydroxide (NaOH, ≥98 %), 1-propanol (CH₃CH₂CH₂OH, ACS reagent, ≥99.5 %), tetradecyltrimethylammonium bromide—TTAB (CH3(CH2)13N(Br)(CH3)3, for synthesis), amoxicillin (C16H19N3O5S, 95.0–102.0% anhydrous basis) used for photocatalytic reactions, and terephthalic acid (98%) were purchased from Sigma Aldrich (Burlington, MA, USA), titanium (IV) n-butoxide (Ti(OCH2CH2CH2CH3)4, reagent grade, 97%) from ACROS Organics (Geel, Belgium), nickel (II) nitrate ((Ni(NO3)2 ·6H2O) and cobalt (II) nitrate ((Co(NO3)2 ·6H2O) from Merck (Darmstadt, Germany). Potassium iodide (KI) ethanol (C2H5OH), p-benzoquinone (C6H4O2) used as scavengers were purchased from Merck.
2.2. Photocatalysts Preparation
Hierarchical zeolite Y (noted hY) was obtained by a seed assisted method, previously reported [
16], in the presence of TTAB as mesopores structure directing agent. After 24 hours of aging, the synthesis mixture with the molar ratio 0.66Na
2O:0.21Al
2O
3:SiO
2:0.02TTAB:19.1H
2O was hydrotermally treated for 6 hours at 100 °C. Zeolite Y having only microporous structure (noted Y) was obtained in a similar way, but in the absence of surfactant. The precipitates obtained after the hydrothermal treatment were filtered, washed with deionized water until pH=9, dried at 60 °C and calcined at 600 °C, 6 hours.
Modification of zeolite Y and hierarchical zeolite Y with titanium dioxide was carried out by impregnation with an alcoholic solution of titanium butoxide. In the case of zeolite Y, the concentration of the immobilized TiO2 was 5%, while for the hierarchized zeolite hY, the percentage of TiO2 was varied (2, 5 and 10%). After impregnation, the samples were dried at room temperature for 24 h and then at 80 °C for 12 hours. Calcination was carried out in air for 6 hours at 600 °C with a heating rate of 2 °C/min. Further, these materials were impregnated with aqueous solution of Co(NO3)2 or Ni(NO3)2, prepared so that the mass percentage of metal oxide in the final powders to be 5%. After impregnation, the samples were dried at room temperature for 24 hours and then at 60 °C for 8 hours. Calcination was carried out in air for 6 hours at 450 °C with a heating rate of 2 °C/min. The obtained materials were noted hYT2C, hYT5C, YT5C, hYT10C in the case of Ti and Co oxides modification and hYT2N, hYT5N, YT5N, hYT10N for Ti and Ni oxides modified materials.
2.3. Photocatalysts Characterization
X-ray diffraction (XRD) patterns were recorded using a Rigaku Ultima IV diffractometer (Rigaku Corp., Tokyo, Japan) with Cu Kα, λ = 0.15406 nm. Phase analysis was performed using Rigaku PDXL software with Whole Powder Pattern Fitting (WPPF) module, connected to the database ICDD-PDF-2.
Textural properties were evaluated from TRISTAR 3000 sorptometer (Micromeritics, Merignac, France). Before measurements, the samples were outgassed under vacuum (pressure = 0.13 mBar) at 25 °C for 16 h.
The scanning electron microscopy (SEM) with EDX, FEI Quanta 3D FEG was used to analyze the morphology and composition of the samples.
UV-Vis diffuse reflectance spectra of the powders were recorded in the 250-900 nm wavelength range using a JASCO V570 spectrophotometer (Tokyo, Japan).
Hydrogen temperature-programmed reduction (H2-TPR) experiments were performed using a ChemBET 3000-Quantachrome instrument (USA) equipped with a thermal conductivity detectors (TCD). 50 mg of photocatalyst was used and a continuous flow of 5 vol% H2 in Ar (70 mL/min). The heating rate was 10 °C/min, up to 850°C. In order to remove the water vapor and ensure optimal stability of the TCD (thermal conductivity detector) a silica gel column was inserted.
The analysis of the surface of the samples was investigated by X-Ray Photoelectron Spectroscopy (XPS). XPS spectra were obtained in an AXIS Ultra DLD (Kratos Surface Analysis, Manchester, UK) setup, using Al Kα1 (1486.74 eV) radiation produced by a monochromatized X-Ray source at operating power of 192 W and the high resolution core level spectra were recorded using hybrid lens mode, 40 eV pass energy, slot aperture. The binding energy scale was calibrated to the C 1s standard value of 284.6 eV and the spectra of interest were deconvoluted using Voigt profiles, based on the methods described in ref. [
32].
Raman Scattering Spectra were collected on a Jobin-Yvon T64000 spectrometer equipped with an optical microscope in confocal mode (Horiba Jobin-Yvon, Palaiseau, France).
2.4. Photocatalystic Properties
The ability of the samples to generate, under irradiation, hydroxyl radicals in aqueous solution was evaluated with terephthalic acid. Thus, the fluorescence of the 2-hydroxyterephthalic acid (TAOH) resulting from the interaction of terephthalic acid (TA, 5×10–4 M TA prepared using an aqueous solution of NaOH 2×10–3 M) with hydroxyl radicals generated under irradiation by the samples (λexc=312 nm) was recorded using a spectrofluorometer FluoroMax 4P (Horiba Jobin Yvon, Northampton, UK).
The photocatalytic experiments were carried out in quartz microreactors, thermostated conditions (30 °C) and under stirring. Firstly, the amoxicillin aqueous solution (30 mg/L) and 20 mg of the photocatalyst were stirred for 30 minutes in darkness to allow AMX adsorption on the photocatalyst surface. Irradiation was then carried out for 5 hours, using 2 x 60 W halogen lamps. After 1, 3, and 5 hours of irradiation, 3 mL from reaction mixture was taken out, the photocatalyst was separated using Millipore syringe filter of 0.45 μm and the solution was spectrophotometrically measured using a JASCO V570 UV-Vis spectrophotometer (λ = 230 nm). AMX degradation was calculated using the solution phase concentration Ct (mg/L) at moment t and the initial concentration C0 (mg/L) at t = 0.
In the case of ROS-scavenging tests, the experimental procedure was similar as in a photocatalytic test. In each reaction system was added 0.1 mmol of scavenger.
3. Results
3.1. Characterization of the samples
3.1.1. X-Ray Diffraction
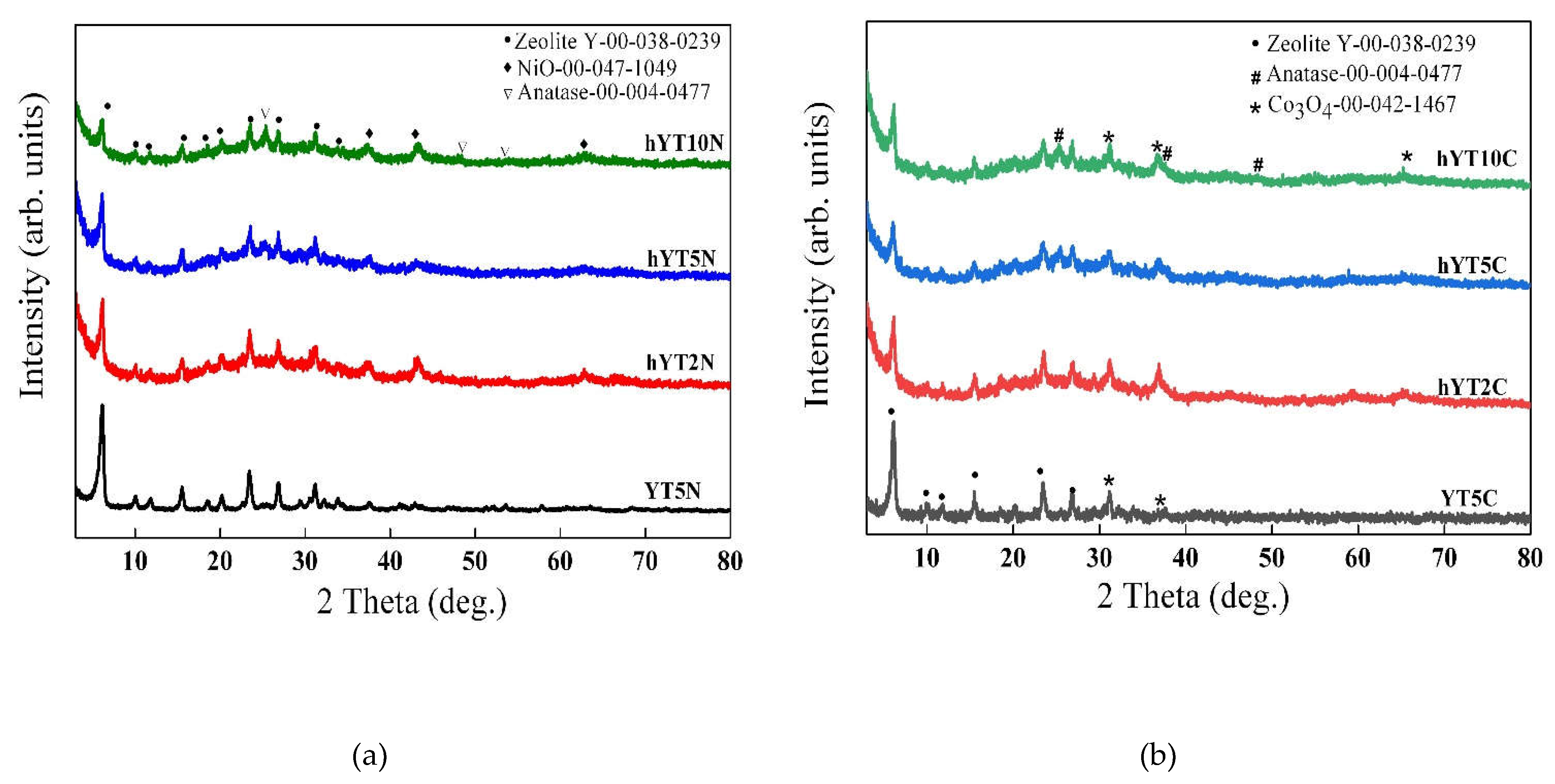
The wide angle XRD patterns of the synthesited samples are illustrated in
Figure 1 (a,b). For all the samples was evidenced the diffraction pattern of zeolite Y (ICDD 00-038-0239), used as support to obtain the photocatalysts. Furthermore, for all the prepared samples, was noticed a diffraction peak located at 25.2°, assigned to anatase [
33] whose intensity increases with TiO
2 concentration (from 2% to 10%), most likely as a result of the size variation of the supported TiO
2 nanoparticles. The X-ray diffractograms presented in
Figure 1a confirmed the presence of nickel as NiO species in the samples after inpregnation, by the appearance of characteristic diffraction lines at 37.3°, 43.3° and 62.9° [
34]. In the case of modification with cobalt, XRD results suggest the formation of Co
3O
4 (
Figure 1b), associated with the presence of some discrete diffraction peaks located at values of 2θ = 31.2°, 36.7° and 65.7° (ICDD 00-042-1467).
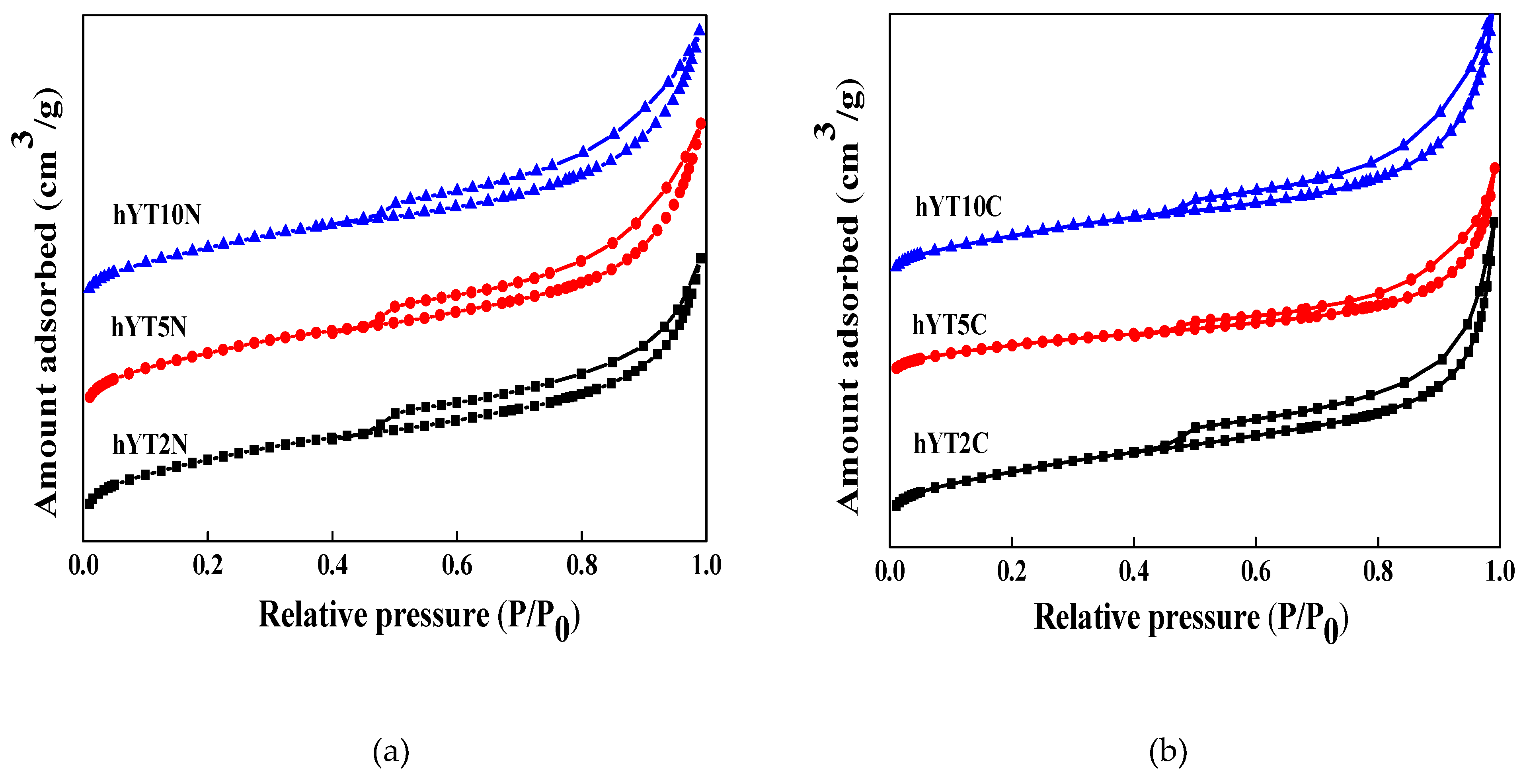
3.1.2. N2-sorption
The nitrogen adsorption-desorption isotherms recorded for the materials obtained by impregnation with titania and nickel or cobalt oxide are shown in
Figure 2.
No change in the isotherm type, which is characteristic of the hY support (type IV) was evidenced after double impregnation processes (
Figure 2). The specific surface area and pore volume values are shown in
Table 1.
A significant decrease of the specific surface after double impregnation with metal oxides was revealed, suggesting the blocking of the pores by the distribution of active species inside the micro- and mesopores.
3.1.3. Scanning Electron Microscopy (SEM)
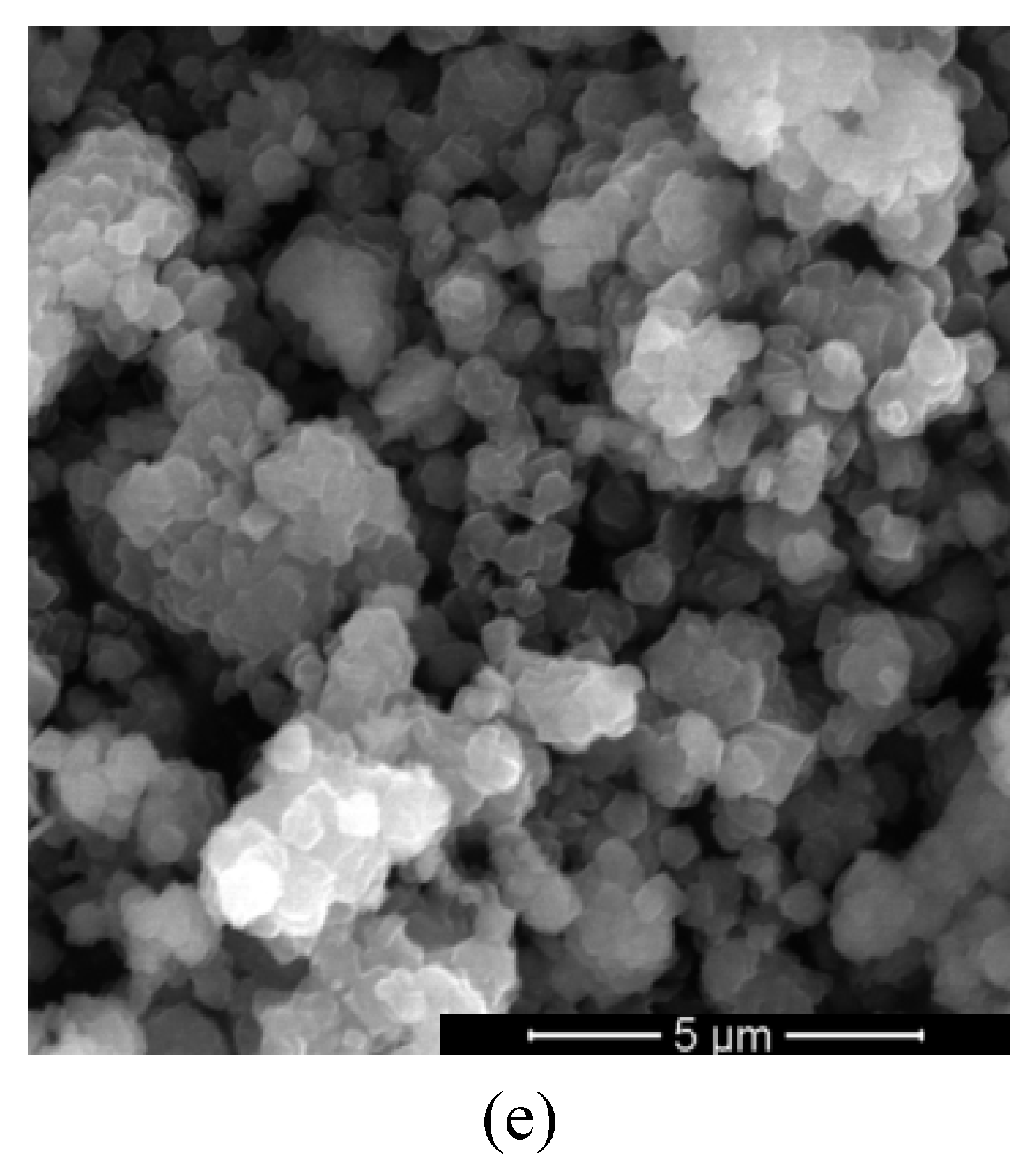
Morphology of the modified zeolite supports with Co-Ti or Ni-Ti was evaluated by scanning electron microscopy (
Figure 3). No change of morphology was observed after the impregnations of support. Thus, the preservation of octahedral morphology with smooth surface, specific to zeolite Y and no aggregates or any other observable defects in the structure of zeolite Y was evidenced.
The composition of the samples was evaluated by EDX analysis. The elemental analysis (
Figure 4) shows the presence of immobilized species Ti, Co or Ti, Ni (
Figure 4) and a higher content of elements (O, Si, Al, Na) from the composition of the zeolite supports.
3.1.4. UV-Vis absorption spectroscopy
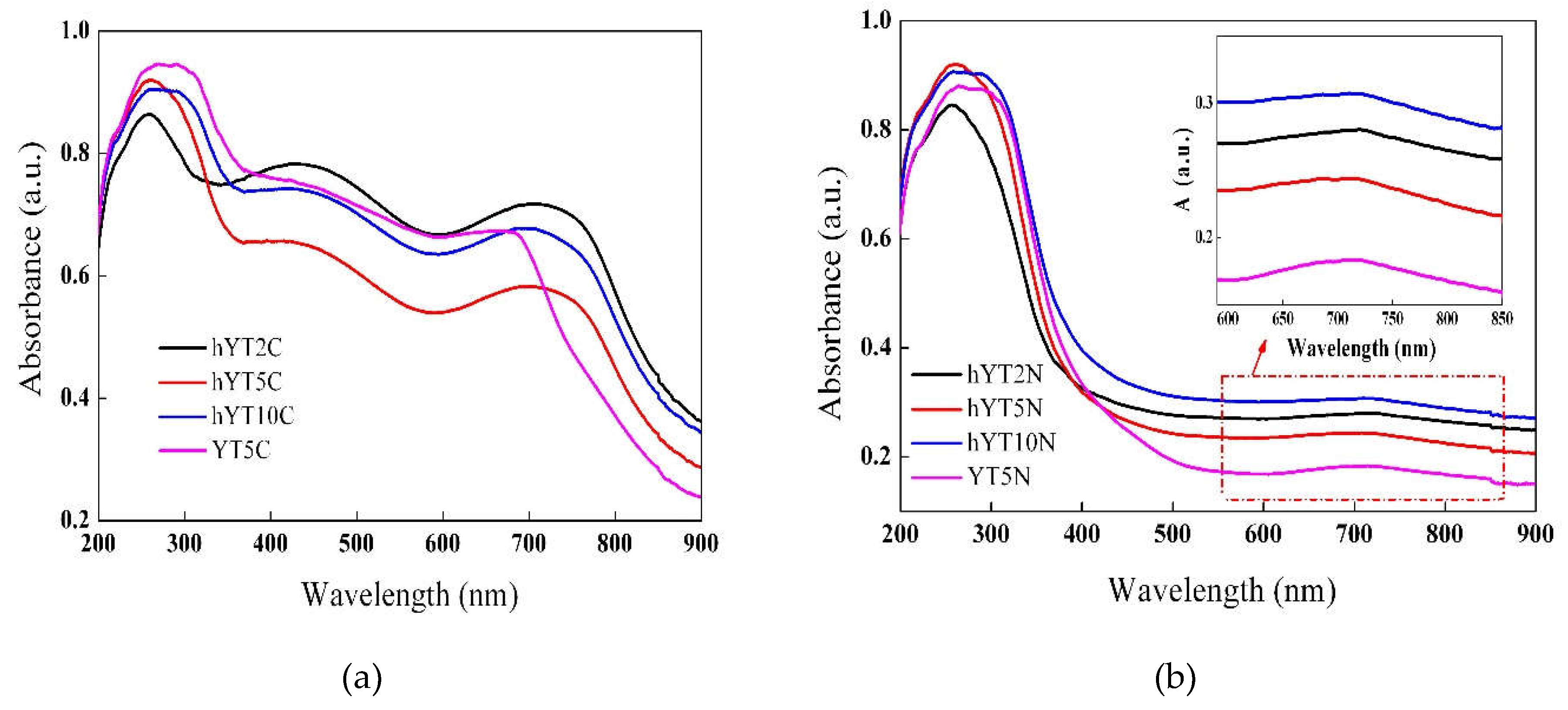
The optical properties of the samples were investigated by UV-Vis absorption spectroscopy, the results are shown in
Figure 5. It was evidenced the presence of a broad absorption band for all the samples in the UV range, with a maximum peak located at ~ 260 nm, assigned to ligand-to-metal charge transfer of the highly dispersed octahedrally coordinated titanium oxide species. By increasing the TiO
2 amount from 2% to 10%, it can be noticed a broadening of the UV absorption band towards wavelengths over 300 nm, most likely due to the growing of the anatase particles size [
35,
36].
Furthermore, in the case of Co-modified samples (
Figure 5a), it was noticed the presence of two absorption bands in the visible domain, specific to Co
3O
4 species, with maxima located at ~440 nm and ~710 nm [
37]. These are assigned to O
2-→Co
2+ and O
2-→Co
3+ charge transfer and also to 1A
1g→1T
2g electron transfer given by the octahedral Co(III) species. The hypsochromic shift of the characteristic absorption band of unsupported Co
3O
4 (located at 470 nm, as reported in the literature) indicates the obtaining of smaller sized Co
3O
4 species by dispersion on the Y zeolitic support [
38]. The same behavior was observed for the Ni-modified samples which exhibited a broad absorption band with the maximum located at ~715 nm, assigned to NiO species (Figura 5b) and not at 725 nm, as was reported for the unsupported NiO [
39].
The recorded UV-Vis absorption spectra indicate that the synthesized materials are active both in UV and visible light irradiation. Obtaining materials with visible light absorption capacity by modifying the zeolitic supports with Co or Ni oxide is an extremely important aspect in the current context where the maximization of efficiency is desired, but with minimal energy consumption [
40] and was also supported by the band gap values calculated using the Kubelka-Munk function (
Table 1).
3.1.5. H2-TPR measurements
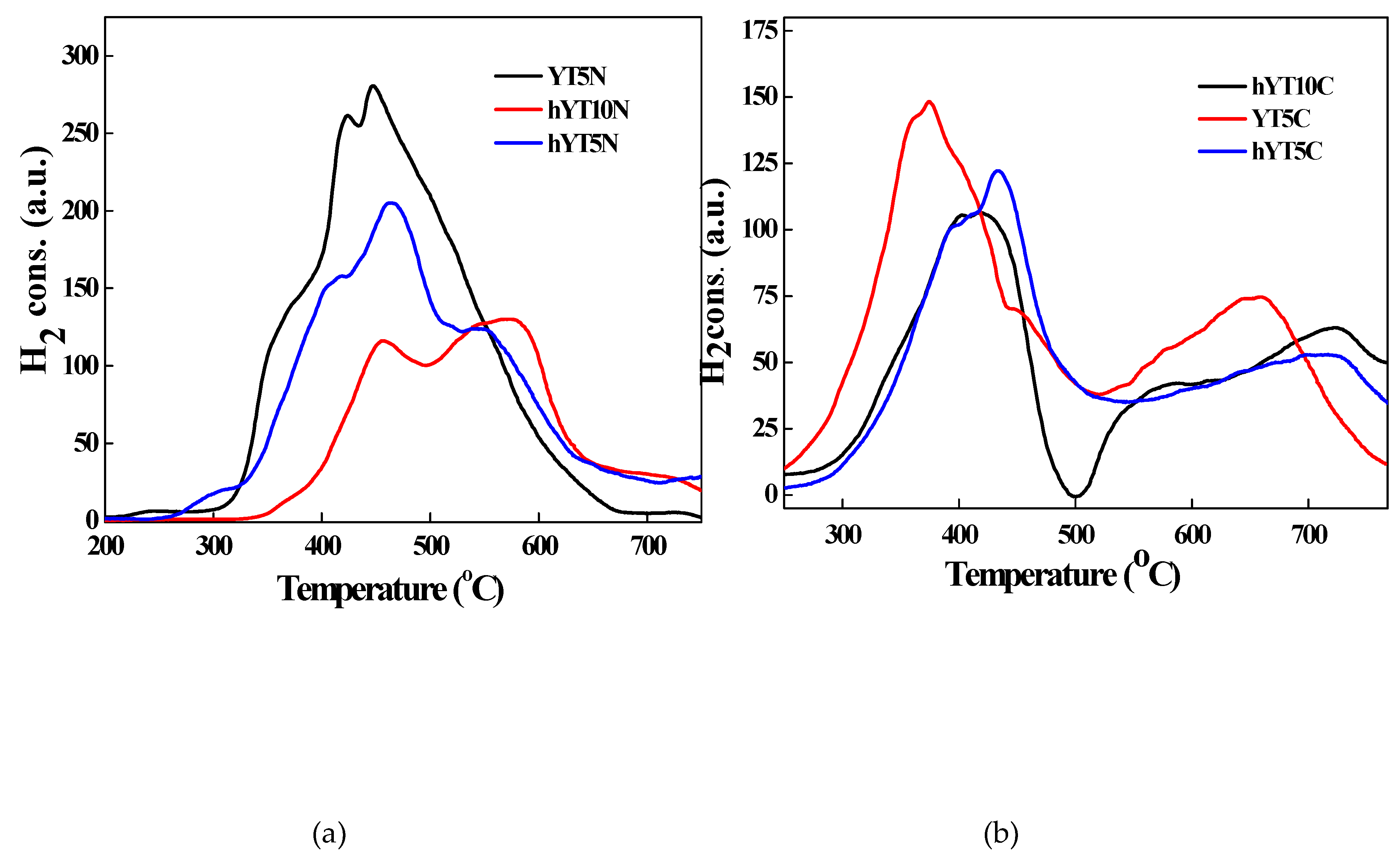
The reduction behavior of the synthesized photocatalysts was studied by thermoreduction measurements H
2-TPR to investigate the chemical state of the metal as well as to obtain information about the reducibility of the species on the photocatalyst surface and in the bulk. A strong interaction of the immobilized metal species with hierarchical zeolite Y can lead to changes in chemical status. A weak interaction with the support favors the reduction of metal oxides at lower temperatures, while the strong interaction with the support forms species that reduce at higher temperatures. The amount of H
2 (µmol) consumed per gram of photocatalyst is indicated in
Table 2.
The TPR profiles recorded for the samples are illustrated in
Figure 6 (a,b). In the case of materials modified with Ti and Ni oxides, the TPR profiles (
Figure 6a) show several signals which can be divided as follows: peaks occurring at temperatures below 500 ° C are attributed to various NiO species formed mainly on the outer surface of the zeolite, peaks occurring in the range of 500-650 °C are due to Ni ions present inside the zeolitic channels and peaks located at temperatures higher than 700 °C are attributed to Ni species incorporated in the zeolitic network, according to the literature [
41]. For YT5N sample, no reduction peak in temperature range of 500-650 °C was observed, which means that the microporous structure of the support did not allow the access of Ni ions inside the zeolitic channels with smaller dimensions than in the case of hierarchical zeolite. There is also a shift in the signals recorded for the hYT10N sample to higher values of reduction temperatures. This indicates stronger interactions between Ni oxide and supported titanium dioxide [
42], most likely due to the high concentration of TiO
2 (10%) which ensures a higher probability of interaction with nickel oxide. The high degree of interaction between NiO and TiO
2 in this sample is also supported by the absence of reduction peaks of bulk NiO which usually occurs in the temperature range between 280-300 °C. In the case of a moderate interaction of NiO with TiO
2, its reduction takes place at about 400 °C, while in the case of stronger interactions with titanium dioxide, the reduction temperature of NiO increases to 530 °C [
43,
44]. The presence of reduction peaks up to 500 °C for all the samples modified with Ti-Ni mixed oxides indicates the formation of NiO species, predominantly presented on the outer surface of zeolitic materials [
42] (both zeolite Y and hierarchical zeolite hY). The consumption of hydrogen from temperatures higher than 600
oC can be attributed to the reduction of titanium. The presence of metallic nickel particles on the zeolite subsurface favors the molecular dissociation of H
2 and, thus, the reduction of Ti
4+ to Ti
3+ or Ti species with a lower valence state. The amount of hydrogen needed to reduce nickel is greater than the stoichiometric amount. This confirms the hypothesis that the reduction of small amounts of titanium also takes place.
Figure 6b shows the TPR profiles for Ti-Co containing samples with three different domains of reduction temperatures. The first peaks observed at lower temperature (200
oC) correspond to the reduction to CoO on the surface of the support (I), second and third peaks located at 370 and 536
oC (II and III) can be attributed to the reduction Co
3+ →Co
2+→Co
0 and the fourth peak at 636
oC can be attributed to the reduction of Co
2+ which interacts strongly with the support [
45,
46,
47,
48,
49,
50]. TPR signals recorded at higher temperatures, between 600-700 °C are due to reduction of cobalt oxide species strongly bounded to the support (surface Co–O–Al species reduced to Co metal), as reported in the case of Co
3O
4 on alumina support [
50].
3.1.6. XPS analysis
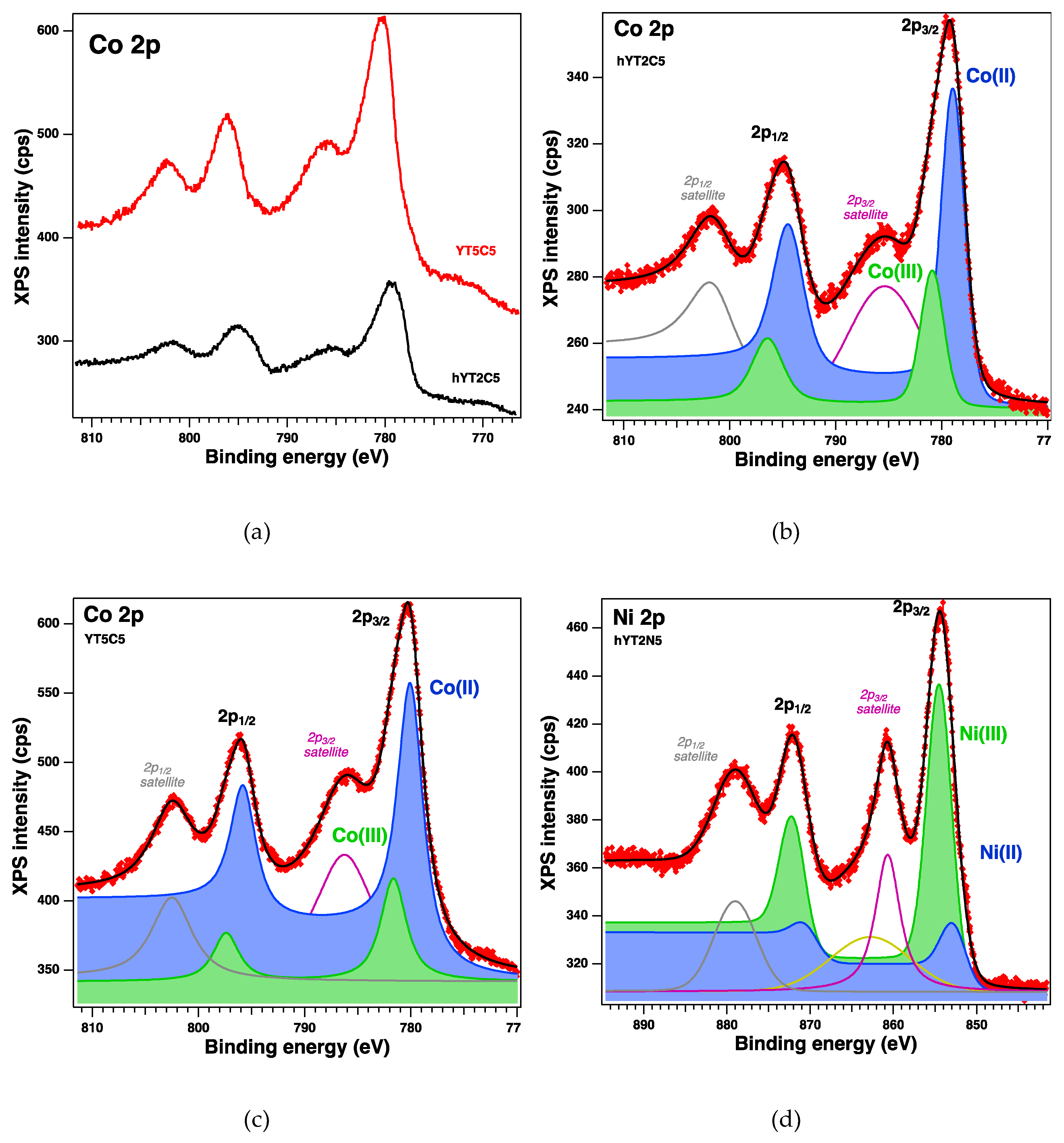
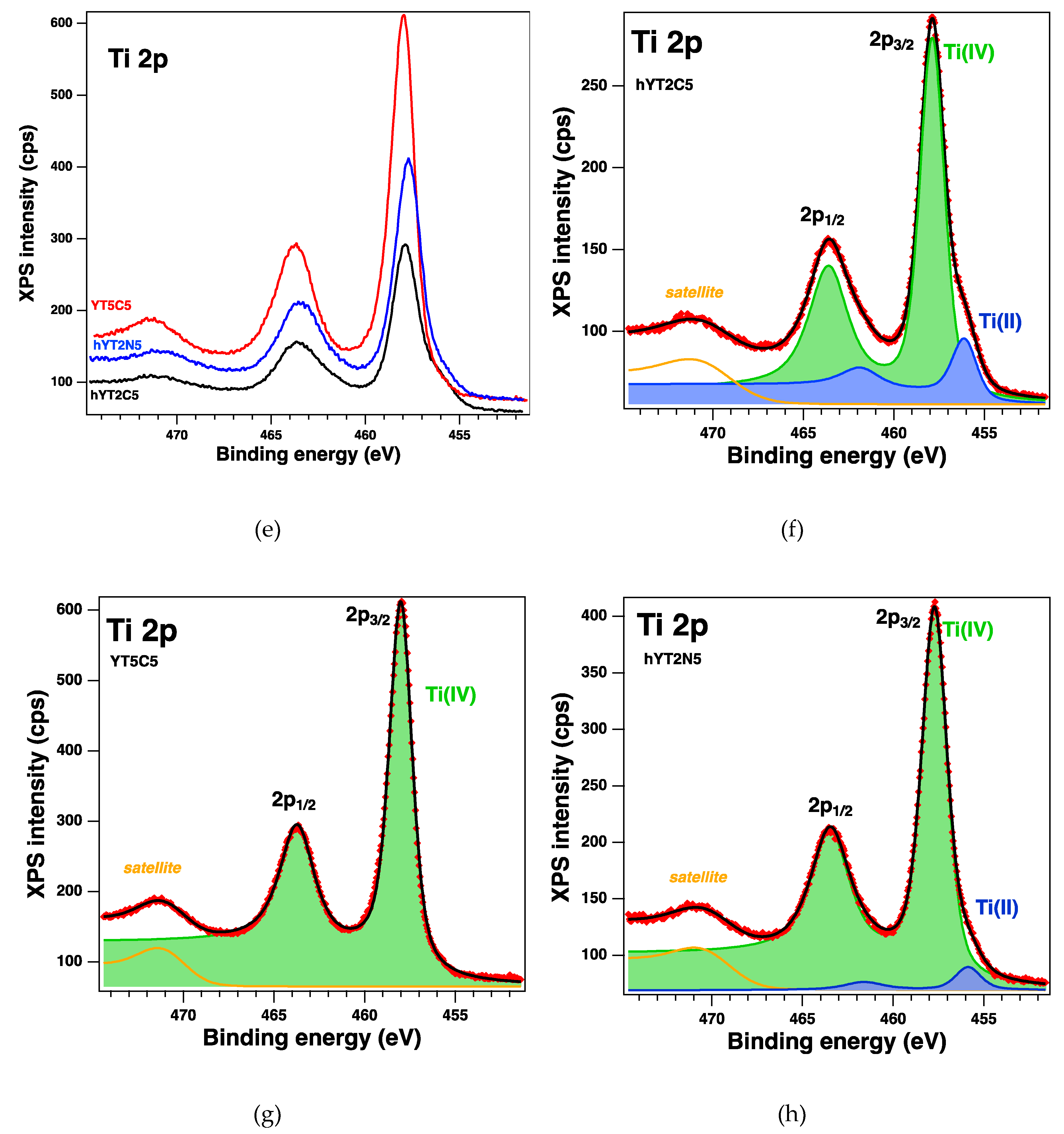
The surface of the samples and the oxidation states were investigated by XPS, which revealed the existence of the following core levels: O 1s, Si 2p, Al 2p, Na 1s, C 1s, Co 2p, Ti 2p and Ni 2p. The spectra of interest are illustrated in Fgure 7. The
Table 3 gives the relevant parameters obtained after the deconvolutions of the core levels of interest (Ti 2p, Co 2p and Ni 2p): binding energies, amplitudes and an assignment by consulting the existing database. The deconvolutions highlight the coexistence of different oxides states, the ratio varying depending on the synthesis method (the incorporated titanium concentration).
Thus, it can be observed that for sample YT5C, based on zeolite Y support, no titanium reduction occurs and the oxidized Co species are less (
Table 3). Instead, the formation of Ti(III) is observed in the case of samples supported on hierarchical Y zeolite. At the same time, several species of oxidized Co (III) are formed on this support, even if the percentage of TiO
2 is lower. We consider that it is the result of different interactions of titanium species with the surface of zeolite Y and that of aluminosilicate with mesopores. However, comparing hYT2C and hYT2N samples that have the same support and percentage of TiO
2 and Co or Ni oxide, a significant increase in the amount of oxidized Ni (III) was observed, although the amount of reduced Ti (III) is slightly higher.
Figure 7.
XPS spectra of the core levels Co 2p (a) for YT5C and hYT2C samples, and Ti 2p (e) for YT5C, hYT2C, and hYT2N doped samples, and (b–d,f–h) the fit and deconvolutions for the samples.
Figure 7.
XPS spectra of the core levels Co 2p (a) for YT5C and hYT2C samples, and Ti 2p (e) for YT5C, hYT2C, and hYT2N doped samples, and (b–d,f–h) the fit and deconvolutions for the samples.
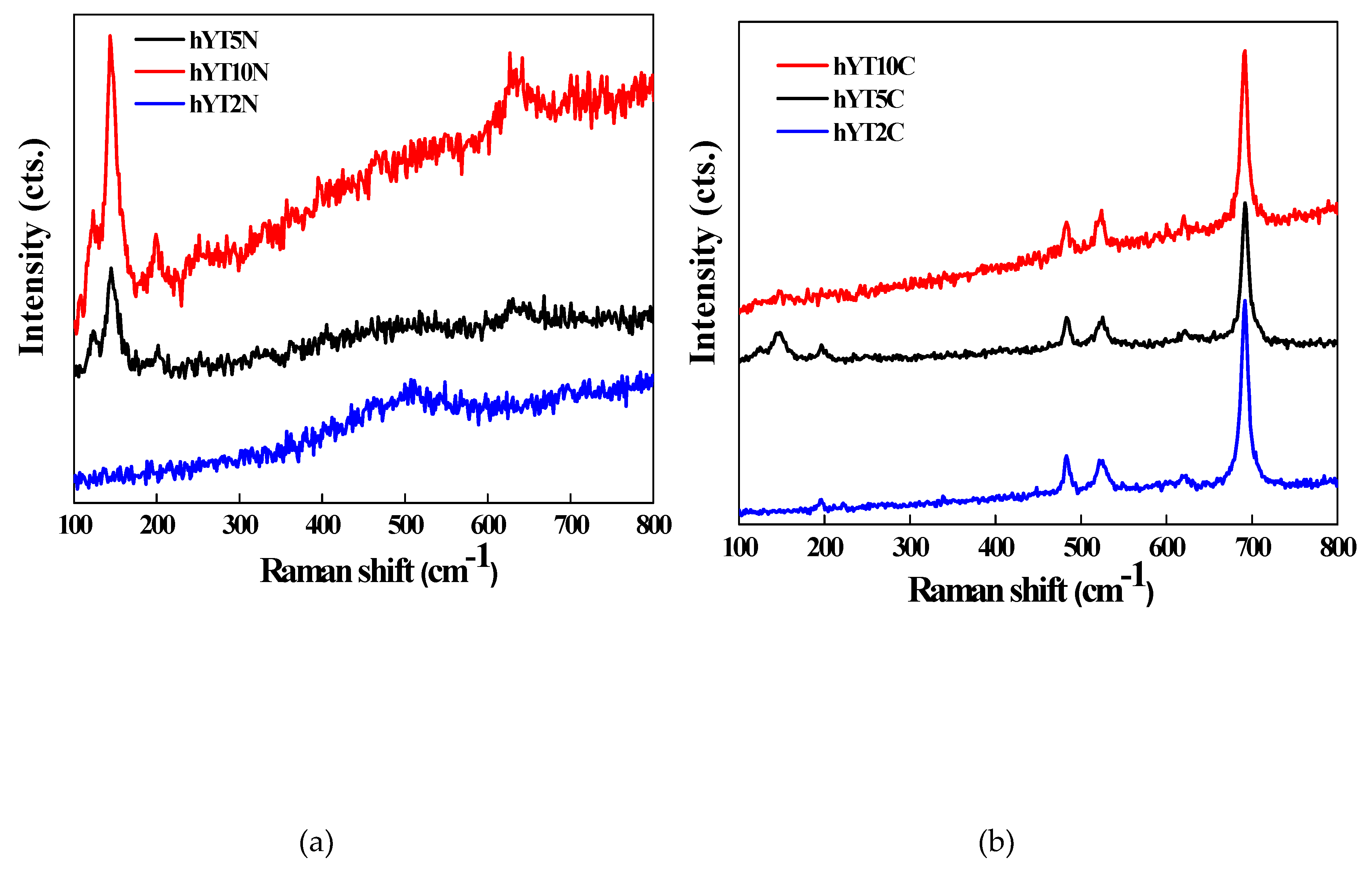
3.1.7. Raman spectroscopy
Raman spectra of the synthesized samples are illustrated in
Figure 8. For Ti-Ni containing samples (
Figure 8a), Raman modes of segregated anatase were depicted at 144 (E
g), 197 (E
g), 637 cm
-1 (E
g) while for the samples modified with Ti and Co oxides, more obvious are the peaks located at 192, 483, 527, 620 and 692 cm
−1 (
Figure 8b) which characterize the Raman active modes of Co
3O
4. The phonon symmetries of these Raman peaks are caused by lattice vibrations of the spinel structure, in which Co
2+ and Co
3+ cations are situated at tetrahedral and octahedral sites in the cubic lattice [
37].
3.2. Photocatalytic activity
3.2.1. Photocatalytic results
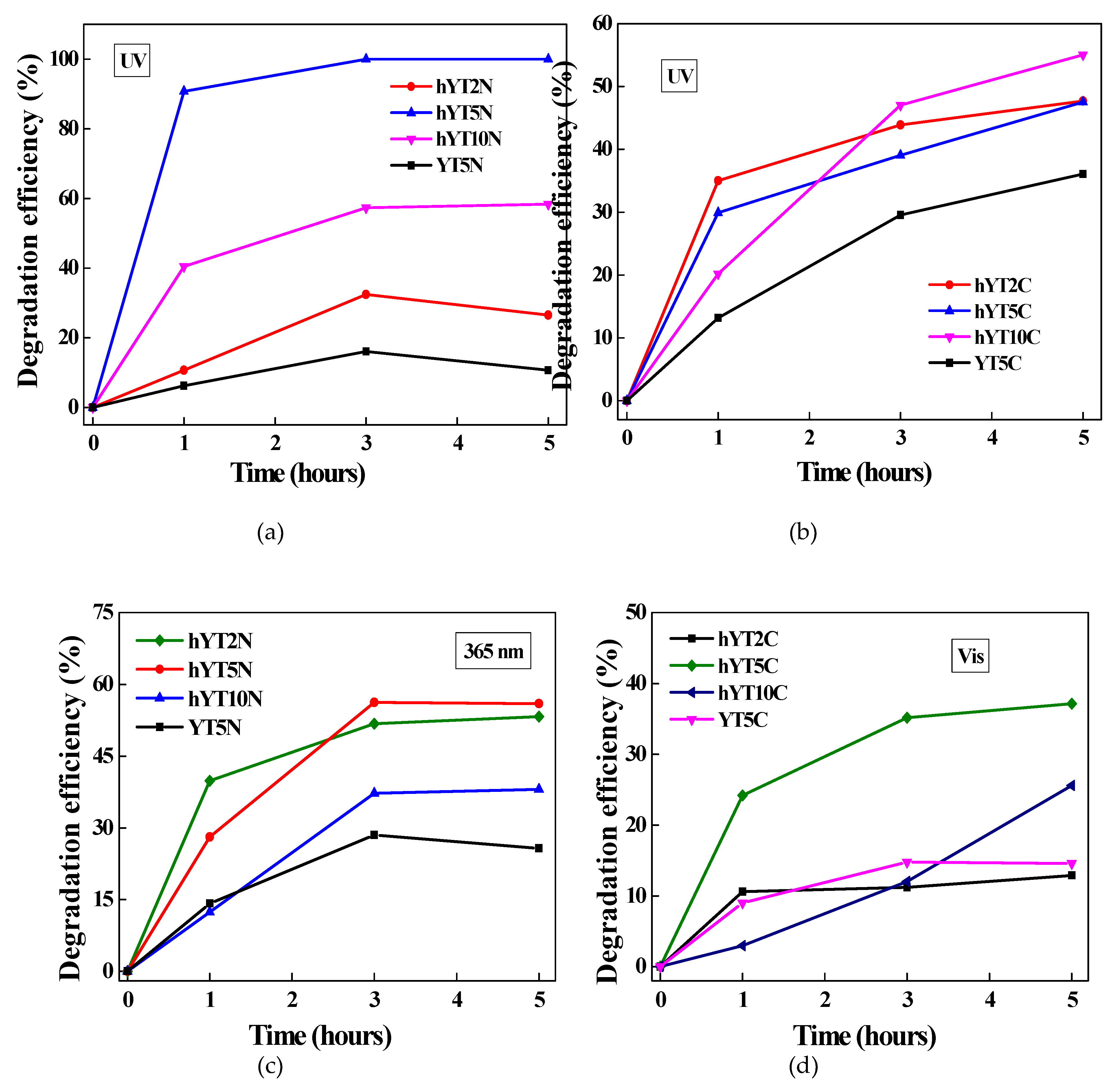
The synthesized materials were tested in the photocatalytic degradation of amoxicillin under UV and visible light irradiation. The results obtained are presented in
Figure 9 (a–d). In the case of UV light irradiation (
Figure 9a,b), it was observed a higher photocatalytic efficiency of materials based on hierarchical zeolite Y. This behavior is due to its characteristic properties which contribute to a high and uniform dispersion of metal species and also to improve the access of both light radiation and amoxicillin molecules to the metallic sites. Similar results were noticed under visible light irradiation (
Figure 9c,d), highlighting that the hierarchical zeolite Y used as a support improves the photocatalytic performances of the resulted materials compared to the non-hierarchical zeolite Y.
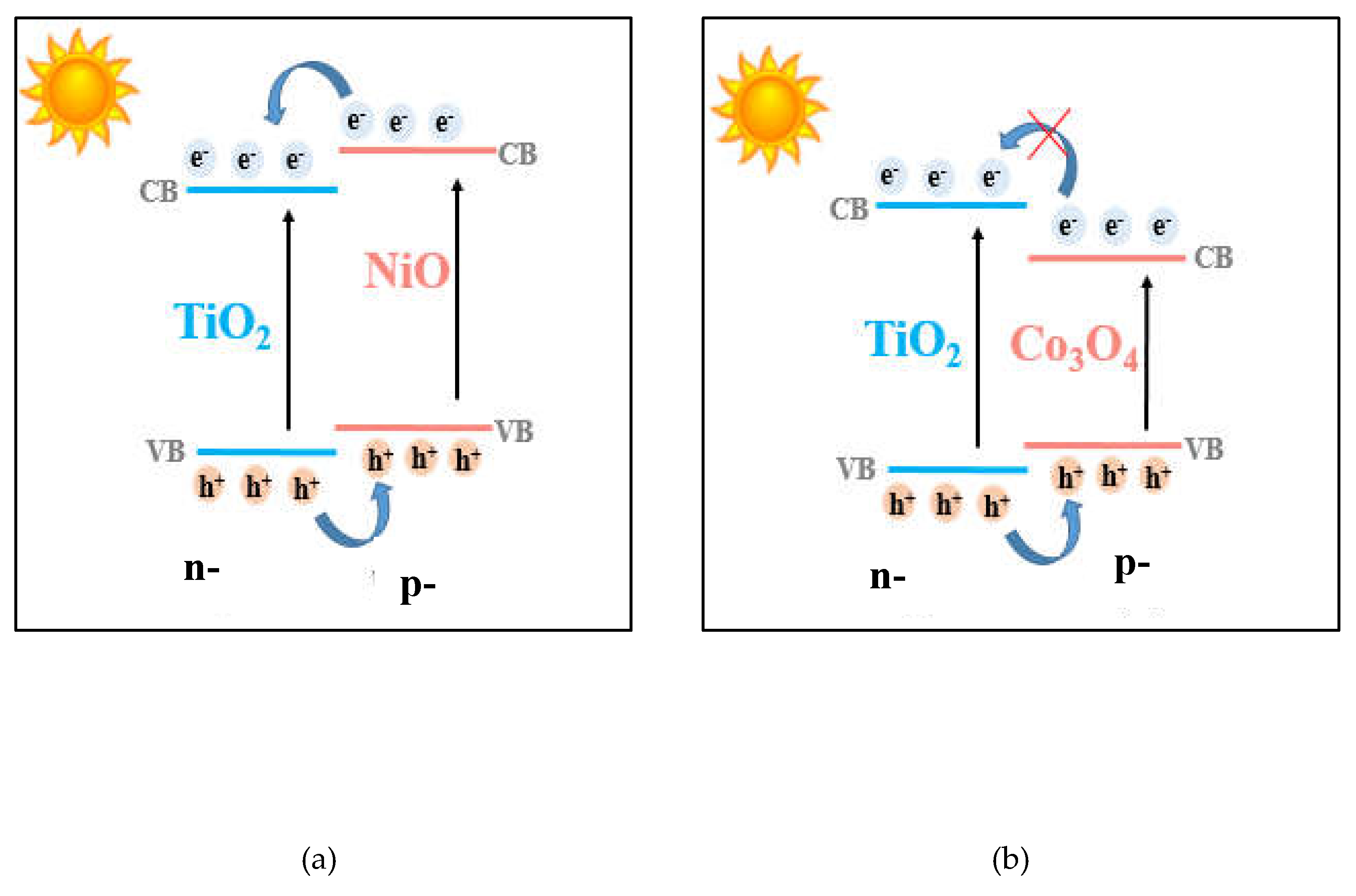
It was pointed out that Ti-Ni modified photocatalysts have better activity than those modified with Ti and Co oxides. It is explained by the efficient separation of photogenerated electron-hole pairs, as a synergistic effect of internal electric field resulted in a typical p-n heterojunction and band alignment of NiO and TiO
2 semiconductors, as can be seen in
Figure 10a. In contrast, for the photocatalytic systems containing TiO
2-Co
3O
4, the electrons transfer across the electric field is thermodynamically hindered, because the conduction band of Co
3O
4 semiconductor is lower than that of TiO
2 (
Figure 10b) [
51].
A better separation of charge careers in the case of Ti-Ni samples leads to a higher number of holes available to generate more hydroxyl radicals (·OH), species responsible for the degradation of amoxicillin in photocatalytic reactions [
16]. The ability of these materials to generate hydroxyl radicals was also supported by the fluorescence results obtained in the case of terephthalic acid reaction (
Figure 11). This method is based on the capacity of terephthalic acid (TA) to interact with ·OH radicals resulted in aqueous solution during the photocatalytic experiment, leading to formation of a photoluminescence compound, 2-hydroxyterephthalic acid (TAOH) which can be quantified.
3.2.2. Mechanism investigations
Generally, the main reactive species in photocatalytic degradation of organic pollutants are hydroxyl (·OH), superoxide (·O
2-) and holes (h
+) radicals [
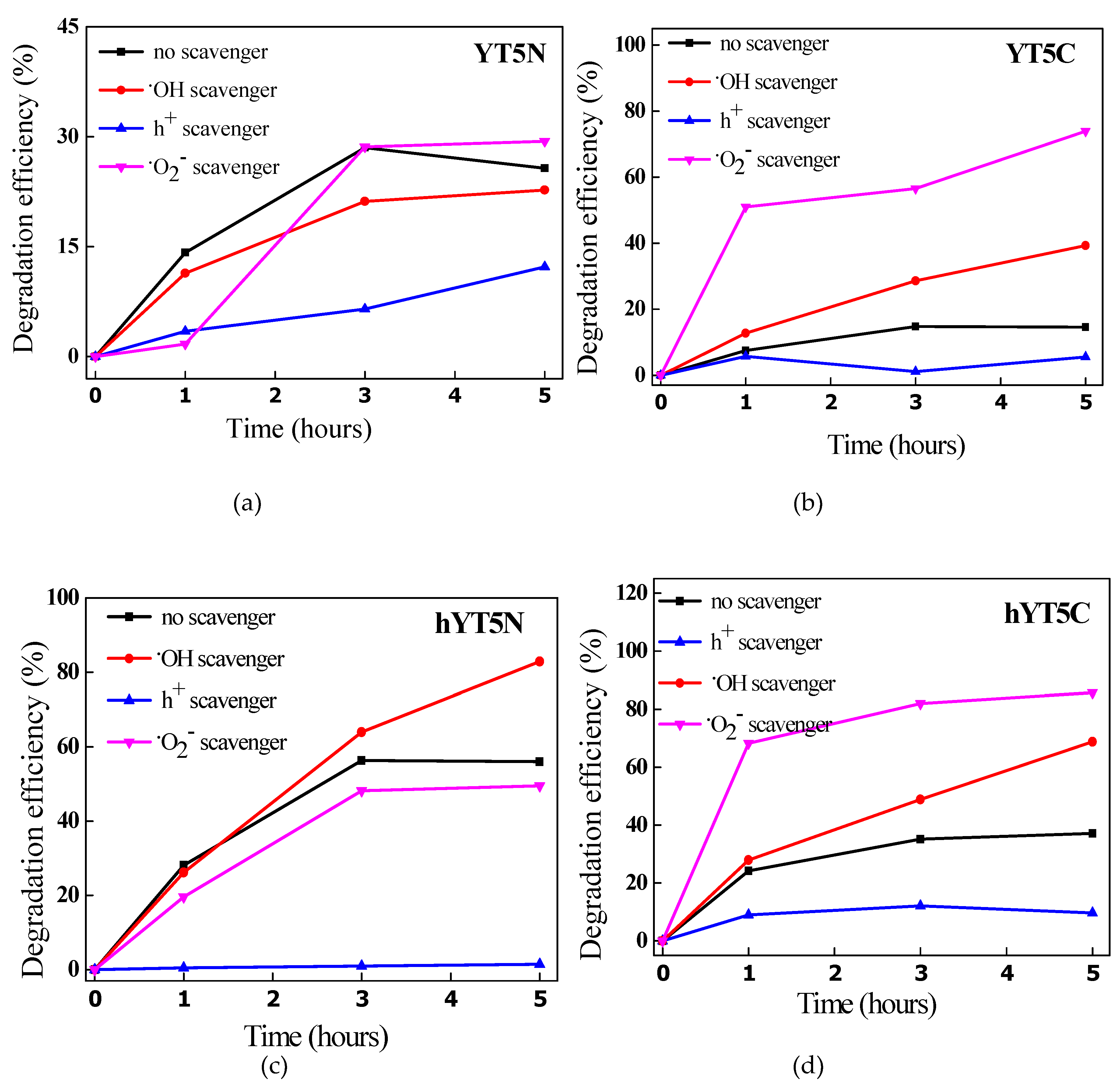
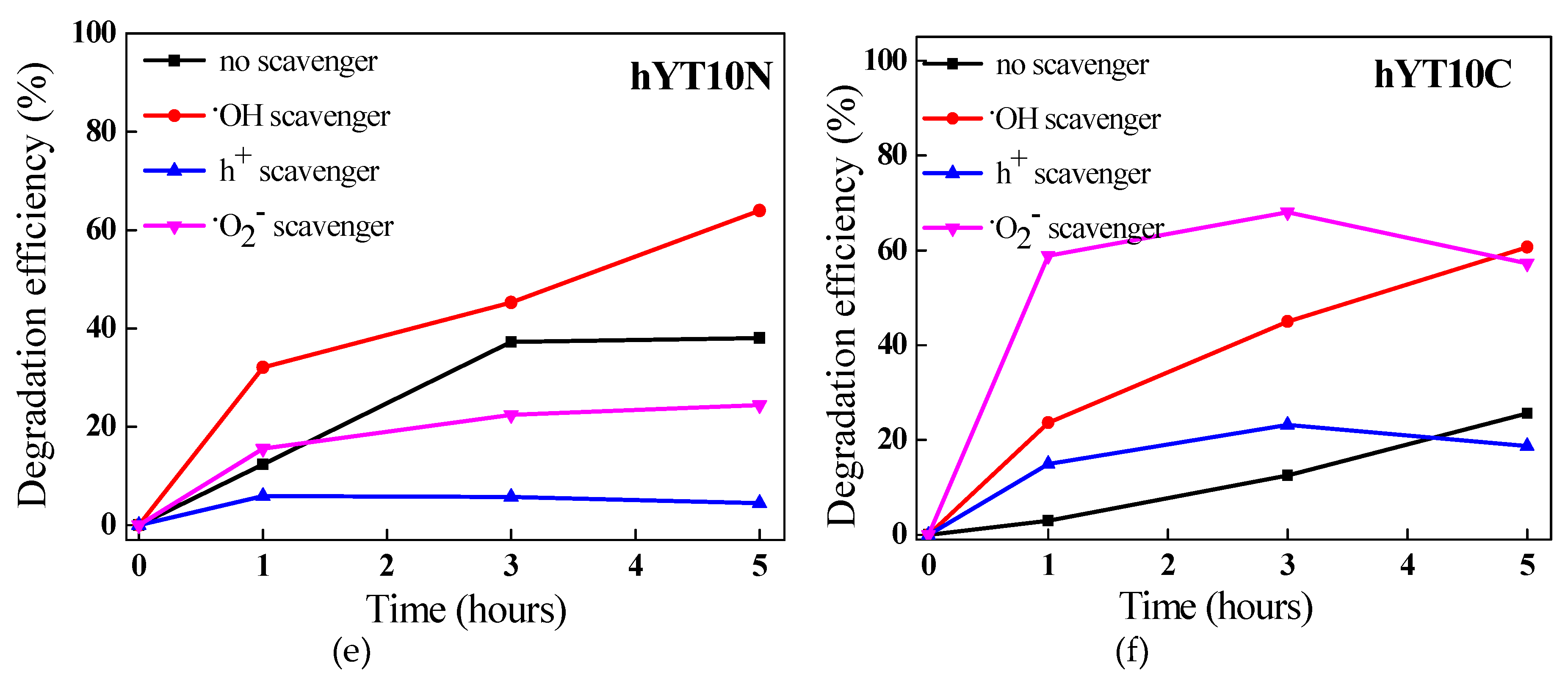
38]. To evaluate the mechanism of amoxicillin degradation using the synthesized materials, photocatalytic experiments were carried out in the presence of scavengers able to selectively interact with the highly reactive oxygen species (ROS) from the reaction system. The results obtained from these experiments are shown in
Figure 12.
It was noticed a different contribution of reactive oxygen species to AMX degradation depending on the metal species used to modify the zeolitic support. Thus, in the case of the samples containing Ti and Ni oxides, the following order was observed h+ >·O2−>·OH while for the samples modified with Ti and Co oxides, the order was h+ >·OH> ·O2−. The decrease in the degradation efficiency after KI addition suggests that holes are mainly responsible for AMX degradation. In fact, the holes have a dual role: they are directly involved in the degradation of amoxicillin molecules and in the formation of reactive hydroxyl species (·OH) through the oxidation of water.
Interesting is that by adding scavengers for ·O
2− and ·OH species, an increase of photocatytic efficiencies was recorded. It means that the lack of an active species in the system is compensated by the others with higher reactivity, depending on their oxidizing potential. For example, by capturing superoxide species, it is practically possible for amoxicillin molecules to be oxidized by hydroxyl radicals, known to be the most active of all reactive oxygen species [
52], hence the increase in catalytic performance in the case of adding p-benzoquinone (p-BQ) in the system, as was obtained for the samples modified with titanium and cobalt oxides (
Figure 12b,d,f).
4. Conclusions
New Co-Ti or Ni-Ti supported photocatalysts on zeolite Y with microporous and hierarchical structures were obtained and their high activity under UV and visible light was evidenced in amoxicillin photodegradation. A typical crystalline structure of zeolite Y and presence of mesopores were evidenced for all the obtained samples. A significant effect of the support was observed on Ti and Co/Ni species and their interaction. It was thus highlighted that the zeolite Y support stabilizes Ti in the 4+ oxidation state and reduces the oxidation of Co or Ni species. In the case of the hierarchical Y zeolite, reduced species of Ti (III) are formed on the surface, and the amount of the other oxidized metal species increases significantly in the case of nickel. The presence of Co and Ni species in 3+ and 2+ oxidation states was also confirmed by Raman spectroscopy and H2-TPR results. The mechanism of amoxicillin degradation under UV and visible light irradiation, investigated in the presence of scavengers, evidenced the effect of metal species interaction and zeolite support. The best results were obtained for the immobilized Ni-Ti species and hierarchical zeolite Y support.
Author Contributions
Conceptualization, J.-L. B. and V.P.; Data curation, G.P., I.A., F.P., S.P., A.B., N.G.A., S.P. and L.R.; Formal analysis, F.P., N.G.A., L.R.; Investigation, G.P., I.A., F.P., S.P., A.B., N.G.A. and L.R.; Methodology, G.P., V.P and J.-L.B; validation, J.-L.B. and V.P.; writing—original draft preparation, G.P., F.P., N.G.A., J.-L.B. and V.P.; writing—review and editing, J.-L.B., G.P. and V.P.; visualization, J.-L. B, G.P. and V.P.; supervision, J.-L. B and V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, Z.; Wang, N.; Zhang, H.; Shang, R.; Xing, J.; Zhang, D.; Li, J. Fabrication of ZSM-5 zeolite supported TiO2-NiO heterojunction photocatalyst and research on its photocatalytic performance, J. Solid State Chem. 2022, 309, 122895. [Google Scholar] [CrossRef]
- Umejuru, E. C.; Mashifana, T.; Kandjou, V.; Amani-Beni, M.; Sadeghifar, H.; Fayazi, M.; Karimi-Maleh, H.; Sithole, N.T. Application of zeolite based nanocomposites for wastewater remediation: Evaluating newer and environmentally benign approaches. Environ. Res. 2023, 231, 116073. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Benítez, J.E.; García-García, A.; Rivera-Manrique, S.I.; Ramírez-Aparicio, J. Multiwalled carbon nanotubes/zeolite composite for dye degradation under sunlight. Mater. Today Commun. 2023, 35, 106046. [Google Scholar] [CrossRef]
- Hu, G.; Yang, J.; Duan, X.; Farnood, R.; Yang, C.; Yang, J.; Liu, W.; Liu, Q. Recent developments and challenges in zeolite-based composite photocatalysts for environmental applications, Chem. Eng. J. 2021, 417, 129209. [Google Scholar] [CrossRef]
- Suraja, P.V.; Yaakob, Z.; Binitha, N.N.; Resmi, M.R.; Silija, P.P. Photocatalytic degradation of dye pollutant over Ti and Co doped SBA-15: Comparison of activities under visible light. Chem. Eng. J. 2011, 176–177, 265–271. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, K.P.; Ou, H.D.; Chiang, Y.C.; Chang, E.E.; Wang, C.F. Characterization and application of Ti-containing mesoporous silica for dye removal with synergistic effect of coupled adsorption and photocatalytic oxidation. J. Hazard. Mater. 2011, 186, 1174–1182. [Google Scholar] [CrossRef]
- Petcu, G.; Anghel, E.M.; Buixaderas, E.; Atkinson, I.; Somacescu, S.; Baran, A.; Culita, D.C.; Trica, B.; Bradu, C.; Ciobanu, M.; Parvulescu, V. Au/Ti Synergistically Modified Supports Based on SiO2 with Different Pore Geometries and Architectures. Catalysts 2022, 12, 1129. [Google Scholar] [CrossRef]
- Petcu, G.; Anghel, E.M.; Somacescu, S.; Preda, S.; Culita, D.C.; Mocanu, S.; Ciobanu, M.; Parvulescu, V. Hierarchical Zeolite Y Containing Ti and Fe Oxides as Photocatalysts for Degradation of Amoxicillin. J. Nanosci. Nanotechnol. 2020, 1158–1169. [Google Scholar] [CrossRef]
- Diban, N.; Pacuła, A.; Kumakiri, I.; Barquín, C.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. TiO2–Zeolite Metal Composites for Photocatalytic Degradation of Organic Pollutants in Water. Catalysts 2021, 11, 1367. [Google Scholar] [CrossRef]
- Joseph, C.G.; Sharain-Liew, Y.L.; Bono, A.; Teng, L.Y. Photodegradation of Indigo Dye Using TiO2 and TiO2/Zeolite System. Asian J. Chem. 2013, 25, 8402–8406. [Google Scholar] [CrossRef]
- Chatti, R.; Rayalu, S.S.; Dubey, N.; Labhsetwar, N.; Devotta, S. Solar-based photoreduction of methyl orange using zeolite supported photocatalytic materials. Sol. Energy Mater Sol. Cells 2007, 180–190. [Google Scholar] [CrossRef]
- Das, A.; Mrinal, K. Adak, M.K.; Mahata, N.; Biswas, B. Wastewater treatment with the advent of TiO2 endowed photocatalysts and their reaction kinetics with scavenger effect. J. Mol. Liq. 2021, 338, 116479. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Paul Chen, P.; Wang, Y.; Kun Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; Wang, L.; Liu, H.; Liu, Y.; Ruan, R. ; Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review, J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Sescu, A.M.; Favier, L.; Lutic, D.; Soto-Donoso, N.; Ciobanu, G.; Harja, M. TiO2 Doped with Noble Metals as an Efficient Solution for the Photodegradation of Hazardous Organic Water Pollutants at Ambient Conditions. Water 2021, 13, 19. [Google Scholar] [CrossRef]
- Goncearenco, E.; Morjan, I.P.; Dutu, E.; Scarisoreanu, M.; Fleaca, C.; Gavrila-Florescu, L.; Dumitrache, F.; Banici, A.M.; Teodorescu, V.S.; Anastasescu, C.; Sandulescu, A.; Balint, I. The effect of noble metal addition on the properties of oxide semiconductors nanoparticles. J. Solid State Chem. 2022, 307, 122817. [Google Scholar] [CrossRef]
- Krishnan, A.; Swarnalal, A.; Das, D.; Krishnan, M.; Saji, V.S.; Shibli, S.M.A. A review on transition metal oxides based photocatalysts for degradation of synthetic organic pollutants. J. Environ. Sci. [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Castillo-Deltell, A.; Lillo-Ródenas, M.A.; Román-Martínez, M. C. TiO2 Modification with Transition Metallic Species (Cr, Co, Ni, and Cu) for Photocatalytic Abatement of Acetic Acid in Liquid Phase and Propene in Gas Phase. Materials 2019, 12, 40. [Google Scholar] [CrossRef]
- Suligoj, A.; Arcon, I.; Mazaj, M.; Drazic, G.; Arcon, D.; Cool, P.; Stangar, U.L.; Tusar, N.N. Surface modified titanium dioxide using transition metals: nickel as a winning transition metal for solar light photocatalysis. J. Mater. Chem. A 2018, 6, 9882. [Google Scholar] [CrossRef]
- FeTi- SBA-15 magnetic nanocomposites with photocatalytic properties Mihaela Filip a, Petcu, G.; Anghel, E.M.; Petrescu, S.; Trica, B., Osiceanu, P.; Stanica, N.; Atkinson, I.; Munteanu, C.; Mureseanu, M.; Parvulescu, V. Catal. Today 2021, 366, 10–19. [CrossRef]
- Jo, W.-K.; Kumar, S.; Isaacs, A.A.; Lee, A. F.; Karthikeyan, S. Cobalt promoted TiO2/GO for the photocatalytic degradation ofoxytetracycline and Congo Red, Appl. Catal. B: Environ. 2017, 201 159–168. [CrossRef]
- Iwasaki, M.; Hara, M.; Kawada, H.; Taday, H.; Ito, S.; Cobalt Ion-Doped TiO2 Photocatalyst Response to Visible Light, J. Colloid Interface Sci. 2000, 224, 202–204. [CrossRef]
- Akel, S.; Boughaled, R.; Dillert, R.; El Azzouzi, M.; Bahnemann, D.W. UV/Vis Light Induced Degradation of Oxytetracycline Hydrochloride Mediated by Co-TiO2 Nanoparticles. Molecules 2020, 25, 249. [CrossRef]
- Li, J.; Liu, S.; He, Y.; Wang, J. Adsorption and degradation of the cationic dyes over Co doped amorphous mesoporous titania–silica catalyst under UV and visible light irradiation, Microporous Mesoporous Mater. 2008, 115, 416–425. [CrossRef]
- Najafabadi, A.T.; Taghipour, F. Cobalt precursor role in the photocatalytic activity of the zeolite-supported TiO2-based photocatalysts under visible light: A promising tool toward zeolite-based core–shell photocatalysis, J. Photochem. Photobiol. A: Chem. 2012, 248, 1– 7. [CrossRef]
- Song, Z.; Liu, J.; Hu, Y.; Li, S.; Zhang, X.; Ma,L.; Chen, L.; Zhang, Q. Solvent-controlled selective photocatalytic oxidation of benzyl alcohol over Ni@C/TiO2, Catal. Commun. 2023, 176, 106628. [CrossRef]
- Zhao, Z.; Wang, N.; Zhang, H.; Shang, R.; Xing, J.; Zhang, D.; Li, J. Fabrication of ZSM-5 zeolite supported TiO2-NiO heterojunction photocatalyst and research on its photocatalytic performance, J. Solid State Chem. 2022, 309, 122895. [Google Scholar] [CrossRef]
- Lu,X.; Liu, F.; Dang, Y.; Li, M.; Ruan, M.; Wu, M.; Zhu C.; Mani, T.; Suib, S.L.; Gao, P.-X. Transition-metal doped titanate nanowire photocatalysts boosted by selective ion-exchange induced defect engineering , Appl. Surf. Sci. 2022, 591, 153116. [CrossRef]
- Daia, G.; Liua, S.; Lianga, Y.; Luo, T. Synthesis and enhanced photoelectrocatalytic activity of p–n junction Co3O4/TiO2 nanotube arrays, Appl. Surf. Sci. 2013, 264, 157– 161. [CrossRef]
- Chen, J.; Wang, M.; Han, J.; Guo, R. TiO2 nanosheet/NiO nanorod hierarchical nanostructures: p–n heterojunctions towards efficient photocatalysis, J. Colloid Interface Sci. 2020, 562, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.; Nicolas, Y.; C. Oliver, C.; Jaegermann, W.; Rockstroh, N.; Junge H.; Toupance, T. Band alignment investigations of heterostructure NiO/TiO2 nanomaterials used as efficient heterojunction earth-abundant metal oxide photocatalysts for hydrogen production, Phys. Chem. Chem. Phys., 2017. [CrossRef]
- Tseng, Y.-H.; Huang, B.-K. Photocatalytic Degradation of NOx Using Ni-Containing TiO2, Int. J. Photoenergy. 2012. [CrossRef]
- Teodorescu, C.M.; Esteva, J.M.; Karnatak, R.C.; El Afif, A. An approximation of the Voigt I profile for the fitting of experimental x-ray absorption data. Nucl. Instrum. Meth. Phys. Res. A 1994, 345, 141–147. [Google Scholar] [CrossRef]
- Shindo,T.; Koizumi, N.; Hatekeyama, K.; Ikeuchi, T. Post-synthesis of TiO2 Dispersed Inside the Pore Channels of SBA-15 and its Photocatalytic Activity for the Degradation of Methylene Blue. Int. J. Soc. Mater. Eng. Resour. 2011, 18, 11-17. [CrossRef]
- Ziebro,J.; Łukasiewicz, I.; Borowiak-Palen, E.; Michalkiewicz, B. Low temperature growth of carbon nanotubes from methane catalytic decomposition over nickel supported on a zeolite, Nanotechnol. 2010, 21, 145308. [CrossRef]
- Sacaliuc, E.; Beale, A.M.; Weckhuysen, B.M.; Nijhuis, T.A. Propene epoxidation over Au/Ti-SBA-15 catalysts, J. Catal. 2007, 248, 235–248. [Google Scholar] [CrossRef]
- Yadav, R.; Amoli, V.; Singh, J.; Tripathi, M.K.; Bhanja, P.; Bhaumik, A.; Sinha, A.K. Plasmonic gold deposited on mesoporous TixSi1-xO2 with isolated silica in lattice: An excellent photocatalyst for photocatalytic conversion of CO2 into methanol under visible light irradiation. J. CO2 Util. 2018, 27, 11–21. [Google Scholar] [CrossRef]
- Ravindra, A.V.; Behera, B.C.; Padhan, P. Laser Induced Structural Phase Transformation of Cobalt Oxides Nanostructures, J. Nanosci. Nanotechnol. 2014, 14, 5591–5595. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Fan, Z.; Zhao, D.; Xiong, S.; Zhang, B.; Zhou, S.; Liu, G. Mechanisms for ·O−2 and ·OH Production on Flowerlike BiVO4 Photocatalysis Based on Electron Spin Resonance, Front. Chem. 2018, 6, 64. [Google Scholar] [CrossRef]
- Graca, I.; González, L.V.; Bacariza, M.C.; Fernandes, A.; Henriques, C.; Lopes, J.M.; Ribeiro, M.F. CO2 hydrogenation into CH4 on NiHNaUSY zeolites, Appl. Catal. B: Environ. 2014, 147, 101-110. [CrossRef]
- Iwasaki, M.; Hara, M.; Kawada, H.; Taday, H.; Ito, S. Cobalt Ion-Doped TiO2 Photocatalyst Response to Visible Light, J. Colloid Interface Sci. 2000, 224, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.J.; Meng, X.; Shi, L. Ni-exchanged Y-zeolite- An efficient heterogeneous catalyst for acetylene hydrocarboxylation, Appl. Catal. A 2014, 485, 163–171. [Google Scholar] [CrossRef]
- Mohan, V.; Raghavendra, C.; Pramod, C.V.; Raju, B.D.; Rao, K.S.R. Ni/H-ZSM-5 as a promising catalyst for vapour phase hydrogenation of levulinic acid at atmospheric pressure, RSC Adv. 2014, 4, 9660. [CrossRef]
- Deshmane, V.G.; Owen, S.L.; Abrokwah, R.Y.; Kuila, D. Mesoporous nanocrystalline TiO2 supported metal (Cu, Co, Ni, Pd, Zn,and Sn) catalysts: Effect of metal-support interactions on steamreforming of methanol, J. Mol. Catal A 2015, 408, 202–213. [Google Scholar] [CrossRef]
- Ivan, S.B.; Fechete, I.; Papa, F.; Marcu, I.C. Ethane oxydehydrogenation over TiP2O7-supported NiO catalysts, Catal. Today 2021, 366, 133–140. [Google Scholar] [CrossRef]
- Jongsomjit, B.; Panpranot, J.; Goodwin, J.G. Jr. Co-support compound formation in alumina-supported cobalt catalysts, J. Catal. 2001, 204, 98–109. [Google Scholar] [CrossRef]
- hang, Y.; Wei ,D.; Hammache, S.; Goodwin, J.G. Jr. Effect of Water Vapor on the Reduction of Ru-Promoted Co/Al2O3, J. Catal. 1999, 188, 281–290. [CrossRef]
- Srisawad, N.; Chaitree, W.; Mekasuwandumrong, O.; Shotipruk, A.; Jongsomjit, B.; Panpranot, J. CO2 hydrogenation over Co/Al2O3 catalysts prepared via a solid-state reaction of fine gibbsite and cobalt precursors, Reac Kinet Mech Cat 2012, 107, 179–188. [CrossRef]
- Ji, Y.; Zhao, Z.; Duan, A.; Jiang, G.; Liu, J. Comparative Study on the Formation and Reduction of Bulk and Al2O3-Supported Cobalt Oxides by H2-TPR Technique, J. Phys. Chem. C 2009, 113, 7186–7199. [Google Scholar] [CrossRef]
- Kruatim, J.; Jantasee, S.; Jongsomjit, B. Improvement of Cobalt Dispersion on Co/SBA-15 and Co/SBA-16 Catalysts by Ultrasound and Vacuum Treatments during Post-Impregnation Step, Eng. J. 2017, 21, 17–28. [Google Scholar] [CrossRef]
- Solsona, B.; Davies, T.E.; Garcia, T.; Vazquez, I.; Dejoz, A. Taylor, S.H. Total oxidation of propane using nanocrystalline cobalt oxide and supported cobalt oxide catalysts, Appl. Catal. B 2008, 84, 176–184. [CrossRef]
- Liu, L.; Li, Y. Understanding the Reaction Mechanism of Photocatalytic Reduction of CO2 with H2O on TiO2-Based Photocatalysts: A Review, Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases, Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
Figure 1.
X-ray diffractograms of (a) Ni-Ti/zeolite Y and (b) Co-Ti/zeolite Y samples (Y- zeolite Y with microporous structure, and hY-zeolite Y with hierarchical structure).
Figure 1.
X-ray diffractograms of (a) Ni-Ti/zeolite Y and (b) Co-Ti/zeolite Y samples (Y- zeolite Y with microporous structure, and hY-zeolite Y with hierarchical structure).
Figure 2.
The nitrogen adsorption–desorption isotherms of the zeolitic samples modified with (a) Ti-Ni oxides, and (b) Ti-Co oxides.
Figure 2.
The nitrogen adsorption–desorption isotherms of the zeolitic samples modified with (a) Ti-Ni oxides, and (b) Ti-Co oxides.
Figure 3.
SEM images of (a) hYT2N, (b) hYT10N, (c) hYT10C, (d) hYT5C, and (e) YT5C samples.
Figure 3.
SEM images of (a) hYT2N, (b) hYT10N, (c) hYT10C, (d) hYT5C, and (e) YT5C samples.
Figure 4.
EDX spectrum of Co-Ti and Ni-Ti supported samples.
Figure 4.
EDX spectrum of Co-Ti and Ni-Ti supported samples.
Figure 5.
UV-Vis absorption spectra of (a) Ti-Co modified samples and (b) Ti-Ni modified samples.
Figure 5.
UV-Vis absorption spectra of (a) Ti-Co modified samples and (b) Ti-Ni modified samples.
Figure 6.
H2-TPR profiles of the samples with (a) Ti-Ni oxides, and (b) Ti-Co oxides.
Figure 6.
H2-TPR profiles of the samples with (a) Ti-Ni oxides, and (b) Ti-Co oxides.
Figure 8.
Raman spectra of (a) hYT2N, hYT5N, hYT10N, and (b) hYT2C, hYT5C, hY110C samples.
Figure 8.
Raman spectra of (a) hYT2N, hYT5N, hYT10N, and (b) hYT2C, hYT5C, hY110C samples.
Figure 9.
Photocatalytic degradation of amoxicillin using the synthesized materials under UV (a,b) and visible light (c,d) irradiation.
Figure 9.
Photocatalytic degradation of amoxicillin using the synthesized materials under UV (a,b) and visible light (c,d) irradiation.
Figure 10.
Schematic representation of charge transfer in the p-n heterojunction of the synthesized materials.
Figure 10.
Schematic representation of charge transfer in the p-n heterojunction of the synthesized materials.
Figure 11.
Fluorescence emission spectra of 2-hydroxyterephthalic acid resulted after interaction between terephthalic acid and ·OH radicals.
Figure 11.
Fluorescence emission spectra of 2-hydroxyterephthalic acid resulted after interaction between terephthalic acid and ·OH radicals.
Figure 12.
The effect of scavengers on the photocatalytic degradation of amoxicillin under visible light irradiation for (a) YT5N, (b) YT5C, (c) hYT5N, (d) hYT5C, (e) hYT10N, and (f) hYT10C samples.
Figure 12.
The effect of scavengers on the photocatalytic degradation of amoxicillin under visible light irradiation for (a) YT5N, (b) YT5C, (c) hYT5N, (d) hYT5C, (e) hYT10N, and (f) hYT10C samples.
Table 1.
The specific BET surface areas (SBET), pore volume (Vpore), and band gap values of the synthesized photocatalysts.
Table 1.
The specific BET surface areas (SBET), pore volume (Vpore), and band gap values of the synthesized photocatalysts.
| Sample |
hY |
hYT2N |
hYT5N |
hYT 10N |
hYT2C |
hYT5C |
hYT 10C |
| SBET (m2/g) |
810 |
100 |
98 |
91 |
99 |
50 |
89 |
| Vpore (cm3/g) |
0.2 |
0.08 |
0.08 |
0.08 |
0.1 |
0.06 |
0.09 |
| Eg (eV) |
- |
2.18 |
2.93 |
2.88 |
1.55 |
2.51 |
2.05 |
Table 2.
The amount of hydrogen consumed, calculated from the TPR profiles of the samples.
Table 2.
The amount of hydrogen consumed, calculated from the TPR profiles of the samples.
| Sample |
The metallic species |
µmol H2/ g |
| YT5C |
Co2+, Co3+
|
1109 |
| YT5N |
Ni2+, Ni0
|
869 |
| hYT10C |
Co2+, Co3+, Ti4+, Ti3+
|
1151 |
| hYT10N |
Ni2+,Ni0
|
916 |
| hYT5N |
Ti4+,Ti3+
|
858 |
| hYT5C |
Co3+, Co2+
|
1090 |
Table 3.
Binding energies (BE), amplitudes, and assignment of the deconvolutions for the core levels of interest for the samples.
Table 3.
Binding energies (BE), amplitudes, and assignment of the deconvolutions for the core levels of interest for the samples.
| Sample |
Element |
BE (eV) |
Ampl. (cps) |
Assignment |
| hYT2C |
Ti2p |
C1 |
456.06 |
84.85 |
Ti(II) |
| C2 |
458.15 |
408.71 |
Ti(IV) |
| Ti(III)/ Ti(IV) |
0.21 |
| Co 2p |
C1 |
778.92 |
480.6 |
Co(II) |
| C2 |
780.88 |
214.7 |
Co(III) |
| Co(III)/ Co(II) |
0.45 |
| YT5C |
Ti 2p |
C1 |
458.07 |
242.65 |
Ti(IV) |
| Co 2p |
C1 |
780.04 |
1229.39 |
Co(II) |
| C2 |
781.65 |
471.77 |
Co(III) |
| Co(III)/ Co(II) |
0.38 |
| hYT2N |
Ti 2p |
C2 |
456.04 |
77.38 |
Ti(III) |
| C3 |
458.15 |
223.11 |
Ti(IV) |
| Ti(III)/ Ti(IV) |
0.35 |
| Ni 2p |
C1 |
852.75 |
124.61 |
Ni(II) |
| C2 |
854.5 |
673.48 |
Ni(III) |
| Ni(II)/ Ni(III) |
0.19 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).