1. Introduction
Extracellular electron transfer (EET) is a respiration process in which electroactive microorganisms (EAMs) produce energy for their development and maintenance by transporting electrons between cells and oxidized or reduced substances (Hernandez et al. 2001, Kato 2015a, Xiao et al. 2017, Zhao et al. 2017). Thus, EET enables EAMs to transport metabolically generated electrons to anodes for energy recovery or accept electrons from cathodes to synthesize high-value chemicals and fuels (Wang et al. 2021a). The use of EAMs for energy recovery and environmental remediation via their EET capabilities has sparked a lot of interest in the fields of environmental engineering and biotechnology. Since fungi, bacteria (both gram-negative and gram-positive), and archaea have all been found to be capable of acquiring and releasing electrons, EAMs are widely used to build various bioelectrochemical systems (BESs, also known as microbial electrochemical systems) for waste treatment, microbial degradation, seawater desalination, environmental remediation, biosensing, and energy and resource recovery (Chiranjeevi and Patil 2020, Tucci et al. 2021, Wang et al. 2021c, Zhao et al. 2021). The EET-based energy harvesting device known as a “microbial fuel cell” (MFC) is one of the most important BESs. MFCs’ ability to convert organic waste into power and their efficacy in wastewater treatment makes them a promising renewable energy source. MFCs can also be used as biosensors in water to detect harmful substances and monitor water quality (Deng et al. 2018).
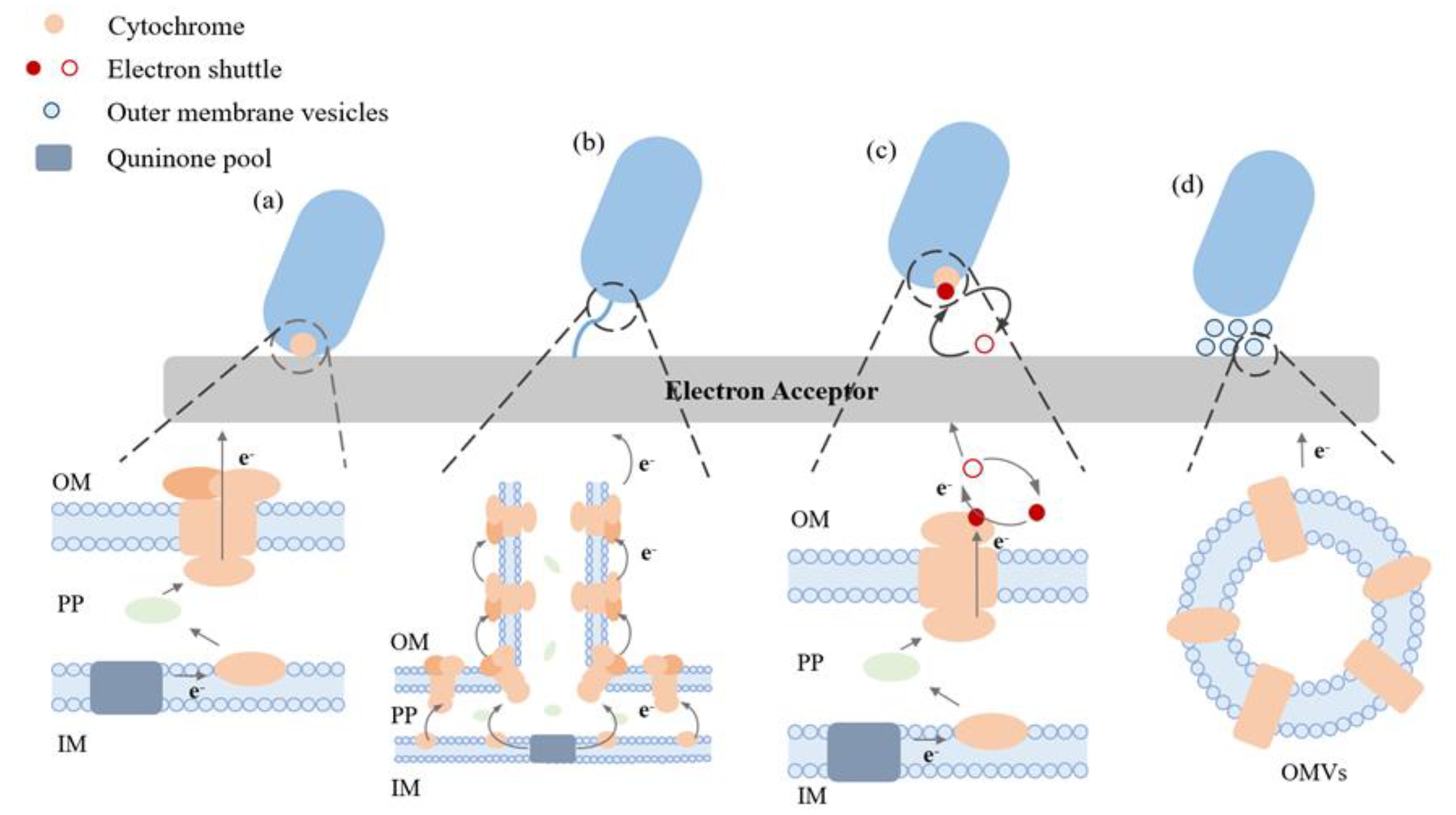
The EET process can be direct (DET) or indirect (IET) (
Figure 1). DET occurs through direct physical contact (via an outer membrane electron conduit) while IET occurs through electron shuttles (ESs, redox-active small molecules that can diffuse through the outer cellular envelope) (Kato 2015b, Light et al. 2018, Marsili et al. 2008).
Geobacter and
Shewanella have received the most attention in terms of EAM research, with extensive research into their physiology, ecology, and EET mechanisms (Wang et al. 2021a).
Shewanella oneidensis MR-1 has the capability of both DET and IET. In MR-1, a metal reduction (Mtr) pathway is essential for bi-directional electron transfer, during which OmcA, MtrC, MtrA, MtrB, CymA, STC, and Fcc3 are among the proteins participating in DET, while IET occurs via flavins which are used as ESs (Marsili et al. 2008). On the other hand,
Geobacter sulfurreducens can carry out DET with solid electron donors and acceptors, including metals, electrodes, and other cells, and it does this via conductive pili (e-pili) and
c-type cytochromes (Ueki 2021). There is no conflict between indirect and direct transport in EAMs, and the two pathways are usually used simultaneously to transport electrons.
Efficient EET is critical for BESs' long-term development, and EET's low efficiency is currently a major impediment to the development of BESs (Deng et al. 2018, Min et al. 2017, Yong et al. 2014a, Yong et al. 2014b). Engineering methods for improving the interaction between EAMs and electrodes can offer promising prospects for the improvement of EET efficiency of EAM. In light of recent research, this review discusses different strategies for enhancing EET through various approaches, such as through mediator materials, external electricity, photo, magnetism, and integrated strategies. Following that, we will examine the key directions, challenges, and opportunities in EET research.
2. Principles for enhancing EET
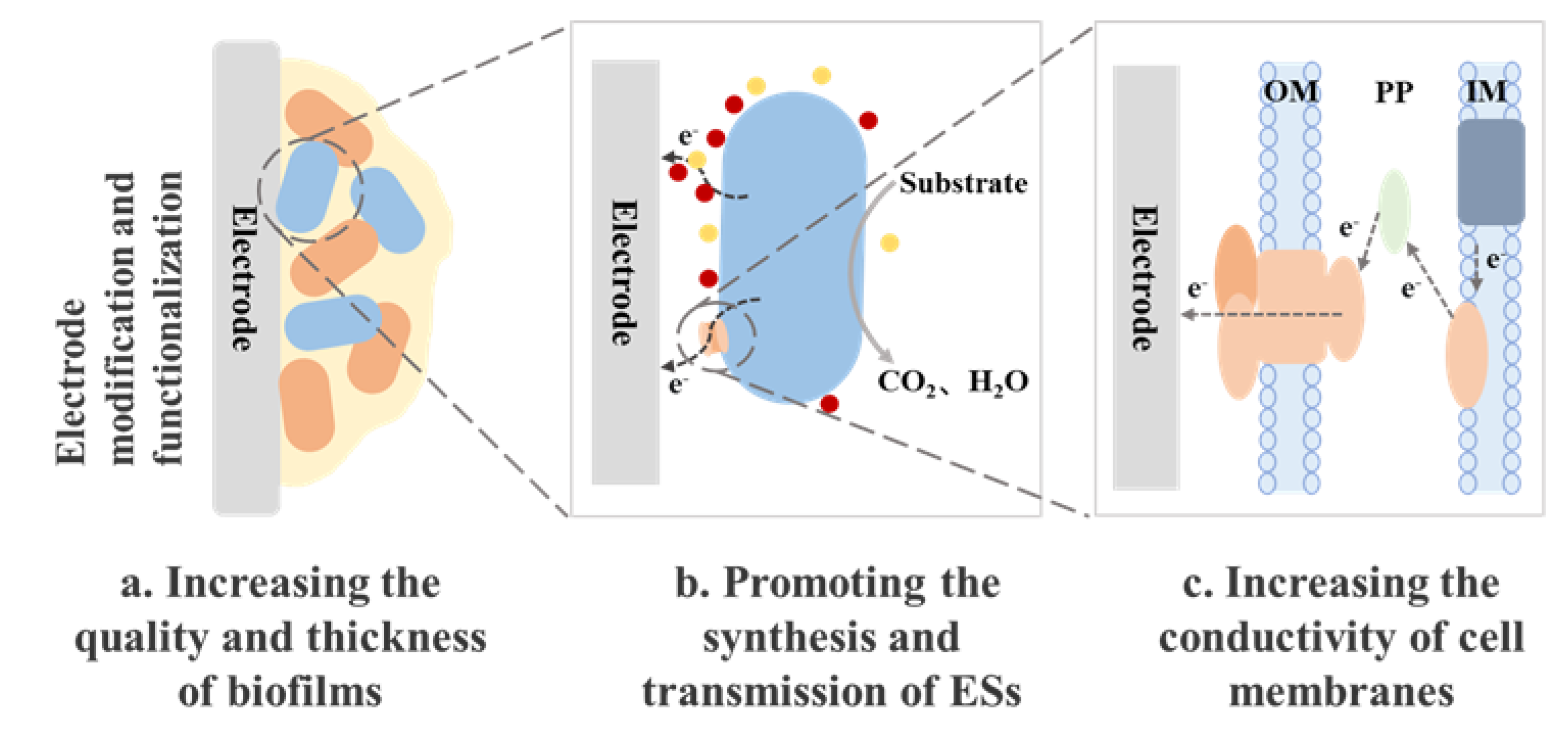
Microbial EET is highly related to transmembrane electron transport by cytochrome protein channels, synthesis and transmission of ESs, and formation of electrode biofilm (Shi et al. 2016). Engineering solutions for increasing EET capability, therefore, include improving transmembrane electron transport via cytochrome protein channels, accelerating electron transport via ESs, and enhancing the microbe-electrode interface interaction via biofilm development regulation (Surti et al. 2021, Xu et al. 2021, Yong et al. 2013).
Since some EAMs depend on cytochrome proteins for the passage of electrons outside the cell, increasing the expression of cytochrome proteins can break through the electrical barrier caused by the cell membrane's lack of electrical conductivity. For example, expressing the MtrCAB electron nanoconduit from S. oneidensis MR-1 with cymA (Vellingiri et al. 2019) or the addition of riboflavin could encourage cell proliferation and EET in Escherichia coli (Jensen et al. 2016).
While EET via cytochrome proteins usually requires direct contact between EAMs and electron acceptor/donor, ESs can diffuse into a solution to enable long-range electron transfer in biofilm, therefore promoting the synthesis and transmission of ESs can enhance the EET. Flavins are widely used mediators for microbial EET (Light et al. 2018, Marsili et al. 2008), and MFCs with higher flavin concentrations showed an increase in current and power output (Velasquez-Orta et al. 2010). EET can also be facilitated by using artificial redox mediators such as methyl viologen, anthraquinone-2,6-disulfonate, and neutral red (Rosenbaum et al. 2011, Wu et al. 2019). The transmission of ESs can be enhanced by porin proteins acting as hydrophilic channels, such as the porin protein OprF of P. aeruginosa (Yong et al. 2013), and the addition of certain substances perforating cells (Liu et al. 2012, White et al. 2016, Zhao et al. 2021).
Microbes can create a structure known as biofilm, and the electron exchange at the contact between a microbe and an electrode plays a crucial role in the production of the various biofilms, which impacts the overall performance of BES. Biofilms can be managed by increasing their thickness (
Figure 2) and conductivity using both synthetic biology and materials engineering. Biofilm mass can be increased through biological methods including the release of cyclic diguanosine monophosphate or signaling molecules from the quorum sensing system by bacteria. Flagella are also widely expressed in electroactive biofilms and their presence facilitated the development of thicker biofilms and acted as scaffolds for the biofilm matrix, allowing for the orderly arrangement of more extracellular cytochromes, which accelerated the rate of electron diffusion within the biofilm (Liu et al. 2019). Additionally, extracellular polymeric substances are essential to biofilm formation and long-range EET (Saunders et al. 2020, Wang et al. 2015, Xiao et al. 2017). Materials engineering is another strategy that can be used to improve the thickness and conductivity of biofilms via the use of conductive substances (Luckarift et al. 2010, Song et al. 2017) and electrode functionalization (Luo et al. 2020, Zou et al. 2019).
3. Enhancing EET by mediator materials
3.1. Electron mediators
ESs are a class of chemicals that can participate in redox reactions as electron carriers. According to the sources, it can be divided into endogenous electron mediator (produced by microorganisms themselves) and exogenous electron mediator (derived from the natural environment or synthesized). The use of exogenous and endogenous mediators are the two different ways through which mediated electron transfer might take place.
Endogenous ESs come directly from the microbial cell, for example, flavins from S. oneidensis MR-1 (Marsili et al. 2008), 2-amino-3-carboxy-1,4-naphthoquinone released by S. putrefaciens (Matsena and Chirwa 2022), and phenazines from Pseudomonas aeruginosa (Chukwubuikem et al. 2021). Genetic engineering is the main method used to manipulate the yields of endogenous ESs. For example, the expression of phzM in E. coli, a crucial gene in the synthesis of phenazine, increased the phenazine yield and sequentially the power density (Yong et al. 2011). Endogenous ESs usually are positively correlated with microbial respiration and cell growth (Wu et al. 2020), engineering method aiming to increase the production of ESs therefore can promote biofilm formation and sequentially multiply the EET efficiency.
Since the biosynthesis of endogenous ESs consumes a mass of substrate and energy, their concentration usually is at μM-level (Xiao et al. 2021) and exogenous ESs can be added into BESs to further boost the EET process by facilitating long-distance EET between microorganisms and electron acceptors such as minerals or electrodes (Wu et al. 2020). Various soluble exogenous ESs have been examined including quinine, phenoxazine, phenozine, thionine, and phenothiazine. Many MFCs using exogenous mediators, however, harbor a lot of bacteria that are not intrinsically electroactive since they can use the external mediator to transport electrons (Matsena and Chirwa 2022). Thus, the addition of exogenous ESs can markedly affect the microbial diversity in BESs.
3.2. Conductive materials
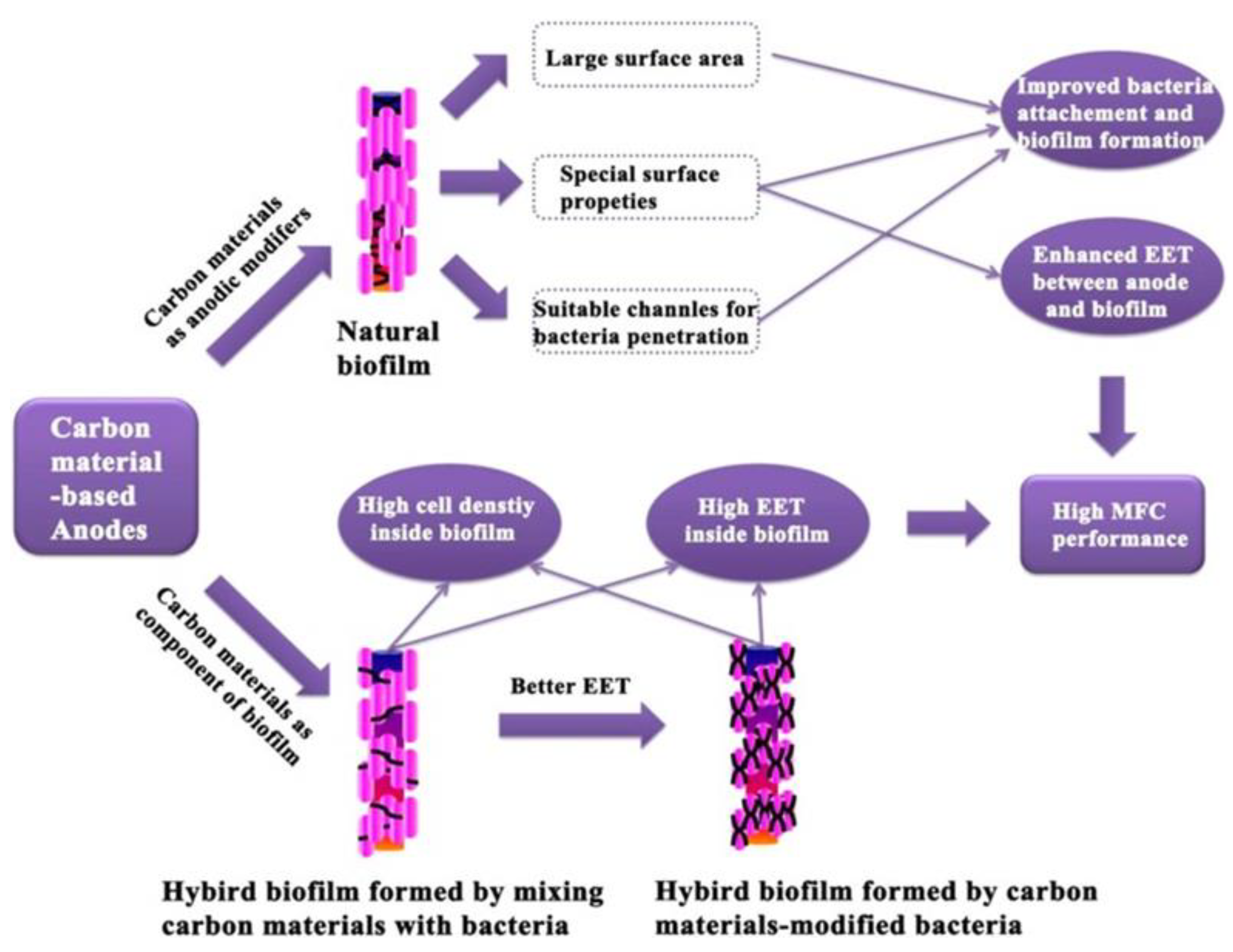
Solid mediators, including carbon and iron based-materials, can also be used to facilitate the EET process. Naturally, carbon-based materials have evolved into a fundamental component of MFC anode materials and they include commercially available materials such as carbon paper, carbon cloth, carbon felt, graphite rods, and flake graphite (Fan et al. 2021). Due to the poor biofilm formation on the electrode surface under natural settings, a series of electrode surface modifications are required to improve the interaction between bacteria and electrodes. This is most commonly accomplished by using carbon nanomaterials as modifiers (
Figure 3).
Graphene, carbon nanotubes, and their derivatives are examples of carbon nanomaterials (Wei et al. 2016). Graphene is one of the most significant examples of carbon materials that has a large specific surface area and excellent conductivity, stability, and biocompatibility, and is widely used for enhancing microbial EET (Chen et al. 2022, Huang et al. 2011, Zhang et al. 2022, Zhang et al. 2011). N-modified carbon nanomaterials were also directly used to promote the current output of BESs, and they can considerably lower the thermodynamic and kinetic resistances to increase both the DET and IET of
S. oneidensis MR-1 (Wang et al. 2020). Carbon nanotubes have a substantial specific surface area, high mechanical strength, exceptional conductivity, and distinctive fiber structure, and they are also another popular cluster as substrate materials for MFC anodes (Fan et al. 2021, Peng et al. 2010, Zhang et al. 2017). Although carbon nanomaterials have been demonstrated to be successful due to their enormous surface area (
Figure 3) and strong electrical conductivity, investigations have revealed that they have some biological toxicity, which may limit their application (Qiu et al. 2017, Wang et al. 2021b).
Also, some bacteria have been found to make use of iron-oxide minerals as (semi)conductive conduits for extracellular electron transfer (EET) to distant, insoluble electron acceptors. In a study that investigated how (semi)conductive iron-oxide minerals alter the EET pathway of Geobacter spp. (Kato et al. 2013), it was discovered that iron-oxide minerals increased current generation in Geobacter strains (Cheng et al. 2022) while having no effect on another strain. Their findings raise the notion that natural (semi)conductive iron oxide minerals provide Geobacter with energetic and ecological advantages, enabling their growth and survival in the natural environment. In another study, metal-organic framework-derived iron oxide-modified carbon fabric was made utilizing a simple hydrothermal roasting process to boost the extracellular electron transfer function of electricity-producing microbes in geological environments. The addition of MIL-Fe2O3 and MIL-Fe3O4 nanoparticles were seen to aid the improvement of the anode's biocompatibility, facilitated electroactive microorganism's adherence to the anode, and encouraged the secretion of additional EET-related proteins in addition to improving the anode's electrochemical performance (Wang et al. 2022a).
4. Enhancing EET by external electricity or electric field
The electric field can enhance the activity of biological enzymes, encourage microbial metabolism and growth, encourage the oxidation-reduction process of various microorganisms, and enhance the biodegradation process. By changing the electrochemical parameters in BES, such as voltage and current, the microbial community can be regulated, thereby enhancing the extracellular electron transport process of microorganisms.
Electrical energy or valuable compounds can be produced in BESs directly from waste biomass when electrons produced by their metabolisms are pumped via an artificial circuit by the redox potential gradient or external power, and EAMs can be selected by electric field intensity from a mixed inoculum (Du et al. 2018). Typically, BESs being controlled by a potentiostat can be used to ensure efficient EET process and system performance. The system consists of a working electrode and a counter electrode which aids the production of a gradient electric field in the same reactor. The working electrode's set potential can also maintain ideal cathodic potentials to, for instance, enable a bioelectrochemical electron-accepting reaction and promote the biofilm formation on the electrode surface (Goud and Mohan 2013, Liu et al. 2008, Torres et al. 2009). As a result, an external electricity-supported BES can be thought of as a specific example of a microbial electrolytic cell in which the working electrode is controlled under advantageous electrochemical circumstances while an external power input aids in driving the reaction (Rosenbaum et al. 2011).
It was discovered that the biomass produced by bacteria was the same for each electron respired by bacteria exposed to different redox potentials, indicating that the anodic potential did not affect the metabolic efficiency of Geobacter sulfurreducens (Bosch et al. 2014). However, some studies pointed out that setting different anode potentials of the BES will provide different thermodynamic energy to the microorganisms, which may respond to the anode potential and make adaptive metabolic adjustments (Busalmen et al. 2008, Torres et al. 2009). In a study, it was found that different anode potentials (0, -200 mV, and -400 mV) affect the maximum power density of the reactor, and in this experiment -200 mV was used as the optimal anode potential to maintain bacterial activity and growth with enhanced current and power generation (Aelterman et al. 2008). Therefore it may be necessary to find the appropriate redox potential for different culture systems.
The enrichment or development of electrotrophs at the cathode of BESs has also been accomplished using this electrochemical strategy, for example, an obligatory Fe(II)-oxidizing autotroph Mariprofundus ferrooxydans PV-1 at a poised cathode (Summers et al. 2013). Additionally, it provides a novel method for enriching electrotrophs that are challenging to cultivate (Jangir et al. 2019) and a precise means to look into, support, and track certain bioremediation and bioproduction processes (Nancharaiah et al. 2015). At fixed cathode potentials, the removal of other contaminants such as nitrates, uranium, and tetrachloroethene has been documented using several Geobacter species (Strycharz et al. 2008), and it has also been proved that H2 can be produced from waste and that CO2 can be converted into several other compounds (Aryal et al. 2017). With this strategy of using an external power source, the power supply, therefore, is not the main source of electrons, but rather it simply overcomes cathodic reaction overpotentials by raising the potential difference between the two electrodes.
5. Enhancing EET by photo
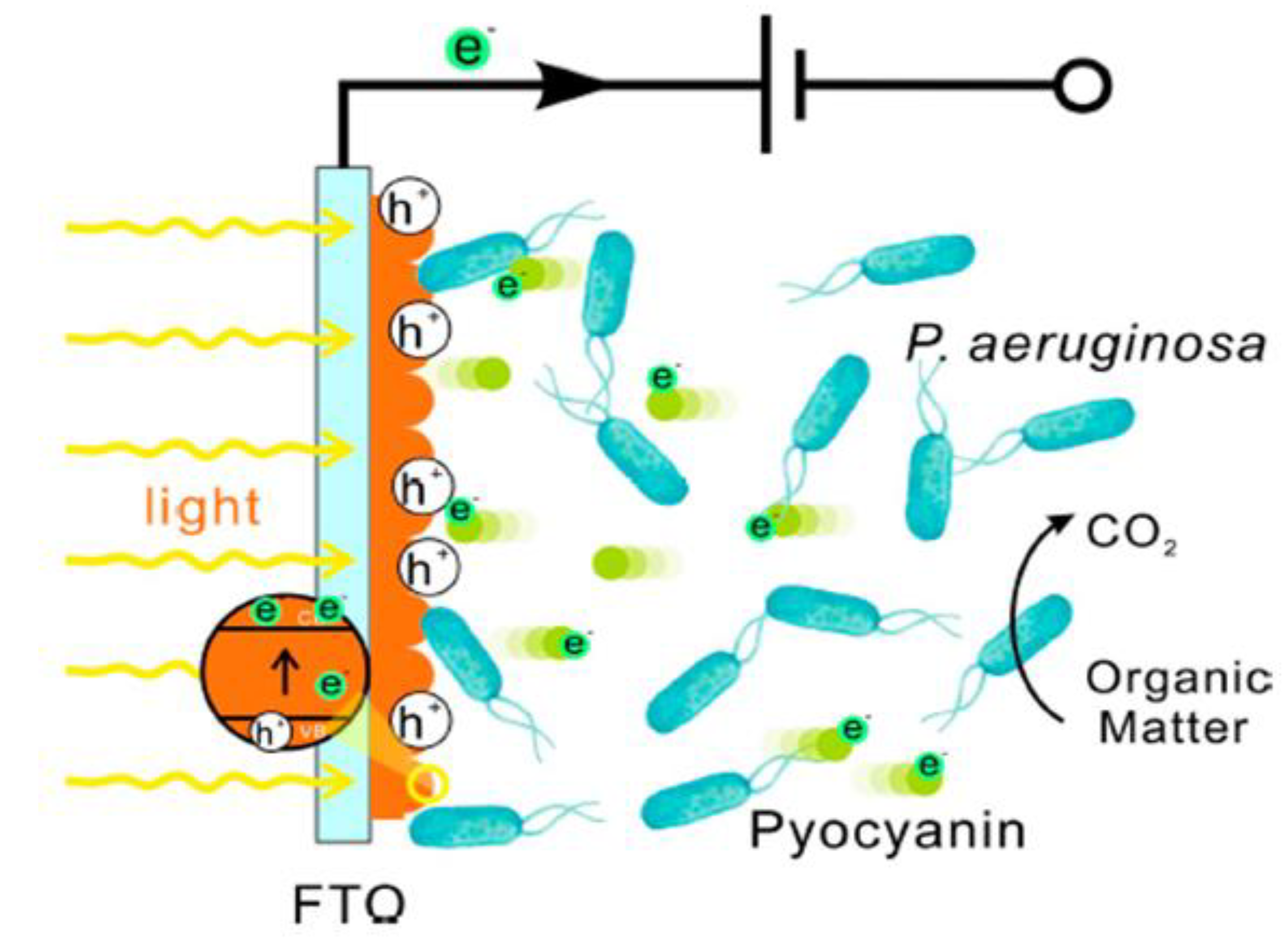
Recently, some progress has been made in the investigation of electron transfer between semiconducting minerals and microorganisms under solar illumination, as well as the development of a solar-assisted microbial photoelectrochemical system. Under the light, semiconductor minerals will generate photo-generated electron and hole pairs, which will impact the extracellular electron transfer mechanism of microorganisms. Light can excite crystalline Fe(III) oxide, a common electron acceptor for EAMs in nature, allowing photocatalysis and microbial culture-mediated photocurrent production in the absence of soluble ESs.
Non-photosynthetic microbes can utilise photogenerated electrons, which are produced when semiconductor materials absorb light energy, and this is in addition to obtaining electrons from reducing substances in an oxidizing environment. Photogenerated electrons are delivered to microorganism outer membrane proteins via the conduction band of semiconductor minerals, increasing microorganism metabolism and proliferation. Research has proved that photogenerated electrons produced by semiconductor minerals including metal oxides and metal sulfides activated by visible light accelerated the growth of both chemoautotrophic and heterotrophic microorganisms (Zhang et al. 2020). In addition, there can be a combination of electrons generated by microorganisms with photogenerated holes produced by semiconductor minerals, thus enhancing the separation of photogenerated electrons and holes in semiconductor minerals. Photo-generated electrons are also transmitted to external circuits, which improves electron transfer between semiconductors and microorganisms.
Figure 4 depicts the mechanism diagram of the electron transfer process. Ren et al. built a bioelectrochemical system with a photoanode composed of sodalite and Pseudomonas aeruginosa (PAO1). In their study, electrochemically prepared birnessite electrodes demonstrated a photoactive mineral response to visible light. When exposed to light, the hematite electrode submerged in live cell culture was able to generate 240% greater photocurrent density than that in the abiotic control of the medium, indicating that hematite and PAO1 are involved in a photo-enhanced extracellular electron transfer process. The findings added to the understanding of the relationship between microorganism EET and photocatalysis of semiconducting minerals in a natural environment and may open up new avenues for the future design of sunlight-powered bioelectronic devices. Photocatalysis and photoelectrocatalysis based on semiconductors are promising technologies for harvesting solar energy through pollutant degradation, hydrogen production, and CO
2 fixation, and thus serve the same purposes as MFCs (Du et al. 2017b).
There have been relatively few studies devoted to the interactions of the light-semiconducting minerals-microorganism system, and the numbered studies have primarily focused on the model bacteria Geobacter and Shewanella, with other microorganisms being overlooked. Until now, the mechanisms governing EET between bacteria and semiconducting minerals under solar irradiation had not been thoroughly studied. More research is needed to fully comprehend the EET process between microorganisms and mineral photocatalysis under solar illumination.
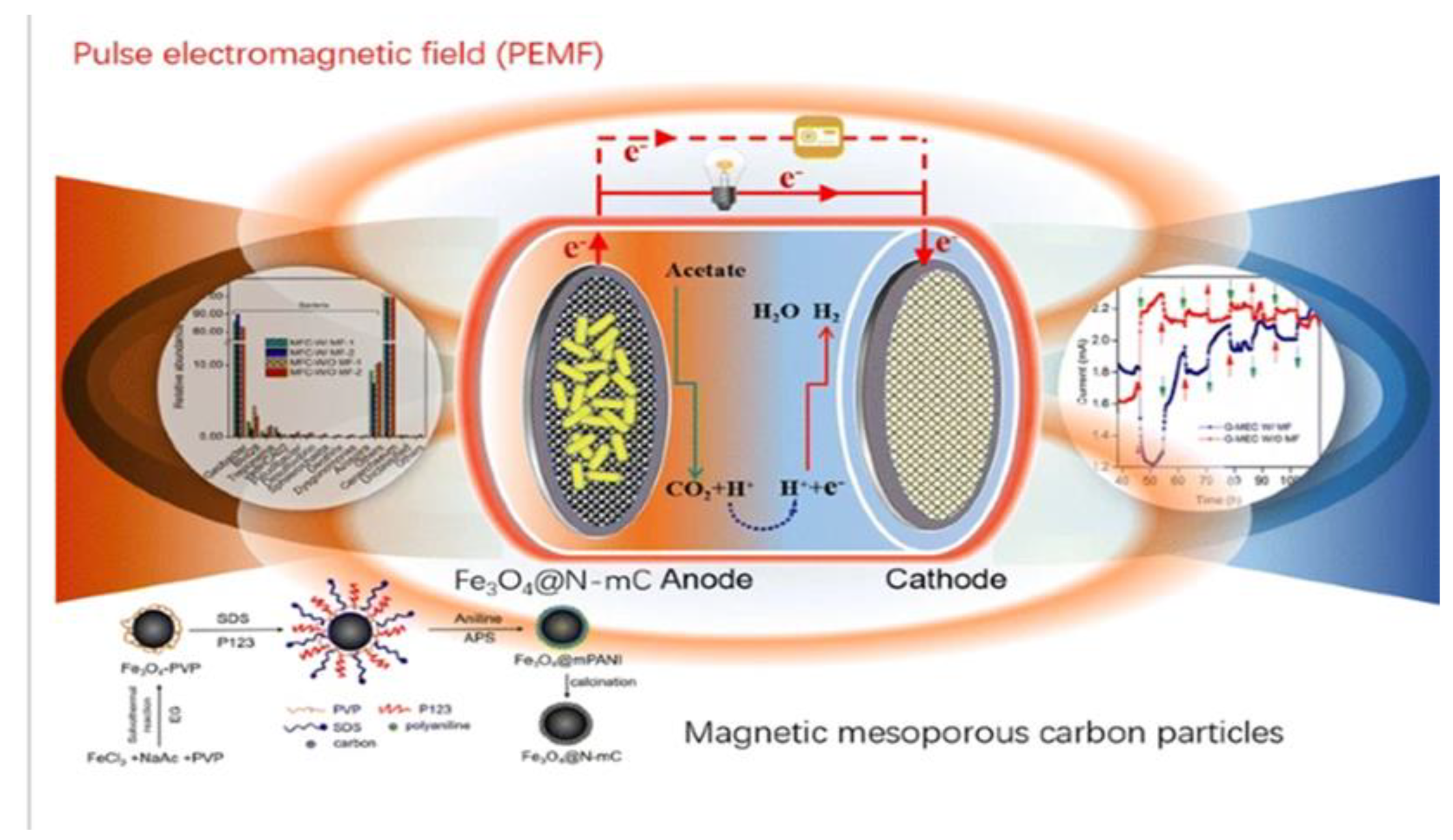
6. Enhancing EET by magnetism
The growth of microorganisms is affected by a magnetic field (Moore 1979). Magnetic fields are a low-cost and convenient method of changing microbial activity. Modulating the extracellular electron transfer process of microorganisms by adding a weak magnetic field is also an important strategy.
Static magnetic fields can promote the production of more extracellular polymers by microorganisms, facilitating the formation of anodic biofilms and thus their extracellular electron transfer processes (Zhao et al. 2016), and are most often used in microbial electrochemical systems. Static electromagnetic fields significantly increased the power production of MFC. Li et al. found that the application of a static magnetic field of 100 mT promoted the electrochemical activity of microorganisms, which in turn increased the maximum voltage of the MFC system by 20-27% (Li et al. 2011). The field strength of the static magnetic field is the most important parameter, which affects the electrochemical activity of microorganisms. A static magnetic field of appropriate strength promotes the extracellular electron transfer process of microorganisms and increases the output of the maximum voltage (Zhao et al. 2016) whereas, when the field strength is too high it can be harmful to microbial growth and have negative effects (Zhao et al. 2016). However, the appropriate range of magnetic field strengths that favor the enrichment of EAMs and thus enhance MFC power generation has not yet been determined and needs further study. Moreover, in the future, molecular regulation of EET-related genes and proteins will need to be researched utilizing a multi-omic technique to elucidate the mechanism of EET stimulation by the magnetic field (Zhou et al. 2019).
In addition, magnets can also play a role in facilitating extracellular electron transfer processes in microbial electrochemical systems with magnetic fields.
Due to the superparamagnetic properties of magnets, low mass transfer resistance, and selective separation of immobilized biomolecules and bacteria using magnetic fields, magnetite is now used for a wide range of applications. Magnetite (Fe
3O
4) is an electrically conductive magnetite mineral that can be used to facilitate the EET of Fe(III)-reducing microorganisms (
Figure 5). Its nanoparticle can facilitate interspecies electron transfer of EAMs and then allow them to establish a cooperative metabolism, for example,
G. sulfurreducens and
Thiobacillus denitrificans (Zhou et al. 2017). The combination of a magnetic field and Fe
3O
4 can change microbial metabolism and protein expression to enhance the EET process in BESs for electro-biosynthesis (Hou et al. 2021) and biodegradation (Zhou et al. 2019). The use of magnets as electrode materials appears to be a potential strategy for facilitating EET transmission. However, to build magnetic BESs with low internal resistance, more research into low-resistance magnetic materials with good conductivity is required.
7. Integrated strategies for enhancing EET
The EET process of microorganisms can be enhanced using mediator material, electricity, light, and a magnetic field. At present, research has also focused on the combination of multiple means to promote extracellular electron transport and improve the performance of microbial electrochemical systems.
A powerful enhancement technique is the simultaneous addition of various mediator materials to encourage EET in microbial electrochemical systems. According to studies, a constructed wetland-microbial fuel cell (CW-MFC) filled with manganese ore and activated carbon granules can boost the biochemistry of N-transformations under anoxic conditions. The paper also underlines how speeding up electron transmission may help with energy production and pollution reduction (Wang et al. 2022b). Furthermore, EET can be supported by the simultaneous coupling of mediator material strengthening and light enhancement. It has been shown that the nanomaterial carbon dots (CDs) can be internalized into Shewanella xiamenensis, and CDs not only enhance the electrical conductivity of cells but also promote the secretion of a large number of flavin-like electron shuttles to facilitate the EET process. Moreover, under light conditions, internalized CDs can further facilitate the EET process by coupling light enhancement means by allowing photogenerated electron transfer to cellular metabolism. (Liu et al. 2020).
Some studies couple the biological anode with the photoanode to improve the power output of the optocoupled system by constructing a hybrid photoelectrochemical and microbial electrochemical system (HPMFC) that uses both solar and biological energy (Du et al. 2017a). By combining visible light, the energy source for the biocathode reaction activation can successfully lower the cathode reaction barrier. Through the voltage difference between the anode and the cathode, the biocathode can facilitate the separation of photogenerated hole/electron pairs (Tong et al. 2022).
Synthetic biology is a research field that blends the investigative aspect of biology with the constructive nature of engineering, to promote extremely effective EET of electroactive cells. Broadening the spectrum of feedstocks, improving intracellular electron generation, improving conductive cytochrome systems, promoting the synthesis and secretion of electron shuttles, and generating conductive biofilms are some of the recent developments in synthetic biology techniques (Li et al. 2018). At present, a large number of studies have combined synthetic biology with mediator material enhancement, light enhancement, and other means to promote EET. In one study, a light-driven proton pump (proteorhodopsin) was expressed heterologously to increase the substrate (lactate) uptake rate of S. oneidensis. This increased the consumption of lactate at an accelerated rate upon illumination, resulting in an increase in current production by 250%, in comparison to the wild-type S. oneidensis MR-1 (Johnson et al. 2010). According to a research that modified E. coli to undertake EET by introducing a Pseudomonas phenazine-1-carboxylic acid biosynthetic route, findings show that the introduction of this self-excreted electron shuttle into E. coli increased the generation of phenazine-1-carboxylic acid, which improved the EET efficiency of microbial fuel cells (Feng et al. 2020). Additionally, Osmium-based polymers (OBP) have demonstrated efficacy in enabling direct EET in organisms that would not otherwise be able to do so by embellishing electrode surfaces and allowing electrons to pass along redox-active osmium units inside the polymer structure (Myers et al. 2022).To demonstrate this, the gram-positive bacterium Enterococcus faecalis was investigated to see if it could interact electrochemically with electrodes, and maximum current density was reached in experiments utilizing the redox polymer, but control experiments lacking the redox polymer showed no current response (Pankratova et al. 2017). Despite these advancements in synthetic biology, there are still huge knowledge gaps when it comes to creating novel gene editing tools and systematically comprehending the molecular pathways behind EET and this has limited the ability to further design exoelectrogens and apply BES in practical settings.
8. Conclusion
This paper summarises the various principles of strategies used to enhance the efficiency of the microbial extracellular electron transfer process from the perspective of enhancement by mediator materials, enhancement by external electricity or electric field, enhancement by photo, enhancement by magnetism, and the combination of various other strategies. For example, as regards enhancement by mediator materials, the most significant issue with the use of exogenous mediators is the requisite for continuous dosing, which raises treatment costs and elevates the risk of secondary contamination (Ren et al. 2022). As a result, many researches are still ongoing on how to ensure that exogenous redox mediators are improved with better performance. For enhancement via an electric field, determining the regulation of external resistance should be part of case-specific microbial fuel cell optimization (Koók et al. 2021).
As for EET enhancement by light, more study is needed to adapt it to practical sewage treatment and build a system for efficient pollutant removal and bioelectricity recovery (Zhang et al. 2020). Although the magnetic field has tremendous promise, there is a need to develop low-cost and powerful magnetic materials, future research should concentrate on understanding how the electrogenic activity of microbes and the magnetic field effect interact. (Al-Mayyahi et al. 2023). According to this paper, the current research focus in this field includes whether there is a synergistic effect of multiple enhancement methods, mechanisms, etc; key issues with the different enhancement strategies, and the use of these various enhancement strategies to solve engineering problems.
Author Contributions
Yong Xiao: Visualisation, Supervision, Writing – review and editing. Oluwadamilola Hazzan: Visualisation, Investigation, Writing - original draft. Biyi Zhao: Visualisaton, Investigation, Writing – review and editing. All authors have read and approved the submission of this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Fujian for Distinguished Young Scholars (2021J06036), National Natural Science Foundation of China (22276183, 51878640), and the Youth Innovation Foundation of Xiamen (3502Z20206089).
Data availability
Not applicable.
References
- Aelterman, P.; Freguia, S.; Keller, J.; Verstraete, W.; Rabaey, K. The anode potential regulates bacterial activity in microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayyahi, R.B.; Park, S.-G.; Jadhav, D.A.; Hussien, M.; Mohamed, H.O.; Castaño, P.; Al-Qaradawi, S.Y.; Chae, K.-J. Unraveling the influence of magnetic field on microbial and electrogenic activities in bioelectrochemical systems: A comprehensive review. Fuel 2023, 331. [Google Scholar] [CrossRef]
- Aryal, N.; Ammam, F.; Patil, S.A.; Pant, D. An overview of cathode materials for microbial electrosynthesis of chemicals from carbon dioxide. Green Chem. 2017, 19, 5748–5760. [Google Scholar] [CrossRef]
- Bosch, J.; Lee, K.-Y.; Hong, S.-F.; Harnisch, F.; Schröder, U.; Meckenstock, R.U. Metabolic Efficiency of Geobacter sulfurreducens Growing on Anodes with Different Redox Potentials. Curr. Microbiol. 2014, 68, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Busalmen, J.P.; Esteve-Nuñez, A.; Feliu, J.M. Whole Cell Electrochemistry of Electricity-Producing Microorganisms Evidence an Adaptation for Optimal Exocellular Electron Transport. Environ. Sci. Technol. 2008, 42, 2445–2450. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Yu, H.; Zhang, J.; Qin, H.Y. A short review of graphene in the microbial electrosynthesis of biochemicals from carbon dioxide. RSC Adv. 2022, 12, 22770–22782. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-L.; Xu, Q.; Yang, Q.-W.; Tian, R.-R.; Li, B.; Yan, S.; Zhang, X.-Y.; Zhou, J.; Yong, X.-Y. Enhancing extracellular electron transfer through selective enrichment of Geobacter with Fe@CN-modified carbon-based anode in microbial fuel cells. Environ. Sci. Pollut. Res. 2022, 30, 1–12. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Patil, S.A. Strategies for improving the electroactivity and specific metabolic functionality of microorganisms for various microbial electrochemical technologies. Biotechnol. Adv. 2019, 39, 107468. [Google Scholar] [CrossRef]
- Chukwubuikem, A.; Berger, C.; Mady, A.; Rosenbaum, M.A. Role of phenazine-enzyme physiology for current generation in a bioelectrochemical system. Microb. Biotechnol. 2021, 14, 1613–1626. [Google Scholar] [CrossRef]
- De Vrieze, J.; Gildemyn, S.; Arends, J.B.; Vanwonterghem, I.; Verbeken, K.; Boon, N.; Verstraete, W.; Tyson, G.W.; Hennebel, T.; Rabaey, K. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res. 2014, 54, 211–221. [Google Scholar] [CrossRef]
- Hsu, L. (.-H.; Deng, P.; Zhang, Y.; Nguyen, H.N.; Jiang, X. Nanostructured interfaces for probing and facilitating extracellular electron transfer. J. Mater. Chem. B 2018, 6, 7144–7158. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Mu, Q.; Cheng, T.; Li, N.; Wang, X. Real-Time Imaging Revealed That Exoelectrogens from Wastewater Are Selected at the Center of a Gradient Electric Field. Environ. Sci. Technol. 2018, 52, 8939–8946. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhou, X.; Qu, Y.; Liu, J.; Feng, Y.; Ren, N. Enhanced Electricity Generation and Pollutant Degradation by Hybrid Photoelectrochemical and Microbial Fuel Cells. Energy Technol. 2017a, 5, 402–405. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, X.; Qu, Y.; Liu, J.; Feng, Y.; Ren, N. Enhanced Electricity Generation and Pollutant Degradation by Hybrid Photoelectrochemical and Microbial Fuel Cells. Energy Technol. 2017b, 5, 402–405. [Google Scholar] [CrossRef]
- Fan, X. , Zhou, Y., Jin, X., Song, R.B., Li, Z. and Zhang, Q. (2021) Carbon material-based anodes in the microbial fuel cells. Carbon Energy 3(3), 449-472.
- Feng, J.; Lu, Q.; Li, K.; Xu, S.; Wang, X.; Chen, K.; Ouyang, P. Construction of an Electron Transfer Mediator Pathway for Bioelectrosynthesis by Escherichia coli. Front. Bioeng. Biotechnol. 2020, 8, 590667. [Google Scholar] [CrossRef]
- Goud, R.K.; Mohan, S.V. Prolonged applied potential to anode facilitate selective enrichment of bio-electrochemically active Proteobacteria for mediating electron transfer: Microbial dynamics and bio-catalytic analysis. Bioresour. Technol. 2013, 137, 160–170. [Google Scholar] [CrossRef]
- Hernandez, M. , Newman, D.J.C. and CMLS, M.L.S. (2001) Extracellular electron transfer. 58(11), 1562-1571.
- Hou, X.; Huang, L.; Zhou, P. Synergetic interaction of magnetic field and loaded magnetite for enhanced acetate production in biocathode of microbial electrosynthesis system. Int. J. Hydrogen Energy 2020, 46, 7183–7194. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Liu, X.-W.; Xie, J.-F.; Sheng, G.-P.; Wang, G.-Y.; Zhang, Y.-Y.; Xu, A.-W.; Yu, H.-Q. Graphene oxide nanoribbons greatly enhance extracellular electron transfer in bio-electrochemical systems. Chem. Commun. 2011, 47, 5795–5797. [Google Scholar] [CrossRef]
- Jangir, Y.; Karbelkar, A.A.; Beedle, N.M.; Zinke, L.A.; Wanger, G.; Anderson, C.M.; Reese, B.K.; Amend, J.P.; El-Naggar, M.Y. In situ Electrochemical Studies of the Terrestrial Deep Subsurface Biosphere at the Sanford Underground Research Facility, South Dakota, USA. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Jensen, H.M.; TerAvest, M.A.; Kokish, M.G.; Ajo-Franklin, C.M. CymA and Exogenous Flavins Improve Extracellular Electron Transfer and Couple It to Cell Growth in Mtr-Expressing Escherichia coli. ACS Synth. Biol. 2016, 5, 679–688. [Google Scholar] [CrossRef]
- Johnson, E.T.; Baron, D.B.; Naranjo, B.; Bond, D.R.; Schmidt-Dannert, C.; Gralnick, J.A. Enhancement of Survival and Electricity Production in an Engineered Bacterium by Light-Driven Proton Pumping. Appl. Environ. Microbiol. 2010, 76, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. Biotechnological Aspects of Microbial Extracellular Electron Transfer. Microbes Environ. 2015, 30, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. (2015b) Biotechnological aspects of microbial extracellular electron transfer. Microbes environments ME1 5028.
- Kato, S.; Hashimoto, K.; Watanabe, K. Iron-Oxide Minerals Affect Extracellular Electron-Transfer Paths of Geobacter spp. Microbes Environ. 2013, 28, 141–148. [Google Scholar] [CrossRef]
- Koók, L.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. The influential role of external electrical load in microbial fuel cells and related improvement strategies: A review. Bioelectrochemistry 2021, 140, 107749. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Liu, C.; Wu, D.; Song, H. Engineering exoelectrogens by synthetic biology strategies. Curr. Opin. Electrochem. 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Cai, P.-J.; Sun, M.; Xiao, X.; Wang, Y.-K.; Tong, Z.-H.; Dong, F.; Yu, H.-Q. Impact of a static magnetic field on the electricity production of Shewanella-inoculated microbial fuel cells. Biosens. Bioelectron. 2011, 26, 3987–3992. [Google Scholar] [CrossRef]
- Light, S.H. , Su, L., Rivera-Lugo, R., Cornejo, J.A., Louie, A., Iavarone, A.T., Ajo-Franklin, C.M. and Portnoy, D.A. (2018) A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 562, 140–144.
- Liu, J.; Qiao, Y.; Lu, Z.S.; Song, H.; Li, C.M. Enhance electron transfer and performance of microbial fuel cells by perforating the cell membrane. Electrochem. Commun. 2012, 15, 50–53. [Google Scholar] [CrossRef]
- Liu, S.; Yi, X.; Wu, X.; Li, Q.; Wang, Y. Internalized Carbon Dots for Enhanced Extracellular Electron Transfer in the Dark and Light. Small 2020, 16, e2004194. [Google Scholar] [CrossRef]
- Liu, X.; Zhuo, S.; Jing, X.; Yuan, Y.; Rensing, C.; Zhou, S. Flagella act as Geobacter biofilm scaffolds to stabilize biofilm and facilitate extracellular electron transfer. Biosens. Bioelectron. 2019, 146, 111748. [Google Scholar] [CrossRef]
- Liu, Y.; Harnisch, F.; Fricke, K.; Sietmann, R.; Schröder, U. Improvement of the anodic bioelectrocatalytic activity of mixed culture biofilms by a simple consecutive electrochemical selection procedure. Biosens. Bioelectron. 2008, 24, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Luckarift, H.R.; Sizemore, S.R.; Roy, J.; Lau, C.; Gupta, G.; Atanassov, P.; Johnson, G.R. Standardized microbial fuel cell anodes of silica-immobilized Shewanella oneidensis. Chem. Commun. 2010, 46, 6048–6050. [Google Scholar] [CrossRef]
- Luo, S.; Fu, B.; Liu, F.; He, K.; Yang, H.; Ma, J.; Wang, H.; Zhang, X.; Liang, P.; Huang, X. Construction of innovative 3D-weaved carbon mesh anode network to boost electron transfer and microbial activity in bioelectrochemical system. Water Res. 2020, 172, 115493. [Google Scholar] [CrossRef] [PubMed]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. 2008, 105, 3968–3973. [Google Scholar] [CrossRef]
- Matsena, M.T. and Chirwa, E.M.N. (2022) Biofuels and Bioenergy, pp. 321–358, Elsevier.
- Min, D.; Cheng, L.; Zhang, F.; Huang, X.-N.; Li, D.-B.; Liu, D.-F.; Lau, T.-C.; Mu, Y.; Yu, H.-Q. Enhancing Extracellular Electron Transfer of Shewanella oneidensis MR-1 through Coupling Improved Flavin Synthesis and Metal-Reducing Conduit for Pollutant Degradation. Environ. Sci. Technol. 2017, 51, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.L. Biological effects of magnetic fields: studies with microorganisms. Can. J. Microbiol. 1979, 25, 1145–1151. [Google Scholar] [CrossRef]
- Myers, B.; Hill, P.; Rawson, F.; Kovács, K. Enhancing Microbial Electron Transfer Through Synthetic Biology and Biohybrid Approaches: Part II: Combining approaches for clean energy. Johnson Matthey Technology Review 2022, 66, 455–465. [Google Scholar] [CrossRef]
- Nancharaiah, Y.; Mohan, S.V.; Lens, P. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef]
- Pankratova, G.; Hasan, K.; Leech, D.; Hederstedt, L.; Gorton, L. Electrochemical wiring of the Gram-positive bacterium Enterococcus faecalis with osmium redox polymer modified electrodes. Electrochem. Commun. 2017, 75, 56–59. [Google Scholar] [CrossRef]
- Peng, L.; You, S.-J.; Wang, J.-Y. Carbon nanotubes as electrode modifier promoting direct electron transfer from Shewanella oneidensis. Biosens. Bioelectron. 2010, 25, 1248–1251. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, D.; Geng, H.; Guo, J.; Qian, S.; Liu, X. How Oxygen-Containing Groups on Graphene Influence the Antibacterial Behaviors. Adv. Mater. Interfaces 2017, 4. [Google Scholar] [CrossRef]
- Ren, G.; Sun, Y.; Sun, M.; Li, Y.; Lu, A.; Ding, H. Visible Light Enhanced Extracellular Electron Transfer between a Hematite Photoanode and Pseudomonas aeruginosa. Minerals 2017, 7, 230. [Google Scholar] [CrossRef]
- Ren, Z.; Ma, P.; Lv, L.; Zhang, G.; Li, W.; Wang, P.; Liu, X.; Gao, W. Application of exogenous redox mediators in anaerobic biological wastewater treatment: A critical review. J. Clean. Prod. 2022, 372. [Google Scholar] [CrossRef]
- Rosenbaum, M. , Aulenta, F., Villano, M. and Angenent, L.T. (2011) Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresource technology 102(1), 324-333.
- Saunders, S.H.; Tse, E.C.; Yates, M.D.; Otero, F.J.; Trammell, S.A.; Stemp, E.D.; Barton, J.K.; Tender, L.M.; Newman, D.K. Extracellular DNA Promotes Efficient Extracellular Electron Transfer by Pyocyanin in Pseudomonas aeruginosa Biofilms. Cell 2020, 182, 919–932. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Genet. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Song, R.-B.; Wu, Y.; Lin, Z.-Q.; Xie, J.; Tan, C.H.; Loo, J.S.C.; Cao, B.; Zhang, J.-R.; Zhu, J.-J.; Zhang, Q. Living and Conducting: Coating Individual Bacterial Cells with In Situ Formed Polypyrrole. Angew. Chem. 2017, 129, 10652–10656. [Google Scholar] [CrossRef]
- Strycharz, S.M.; Woodard, T.L.; Johnson, J.P.; Nevin, K.P.; Sanford, R.A.; Löffler, F.E.; Lovley, D.R. Graphite Electrode as a Sole Electron Donor for Reductive Dechlorination of Tetrachlorethene by Geobacter lovleyi. Appl. Environ. Microbiol. 2008, 74, 5943–5947. [Google Scholar] [CrossRef]
- Summers, Z.M.; Gralnick, J.A.; Bond, D.R. Cultivation of an Obligate Fe(II)-Oxidizing Lithoautotrophic Bacterium Using Electrodes. Mbio 2013, 4, e00420–12. [Google Scholar] [CrossRef]
- Surti, P.; Kailasa, S.K.; Mungray, A.K. Genetic engineering strategies for performance enhancement of bioelectrochemical systems: A review. Sustain. Energy Technol. Assessments 2021, 47, 101332. [Google Scholar] [CrossRef]
- Tong, Y.; Wei, J.; Mo, R.; Ma, H.; Ai, F. Photocatalytic Microbial Fuel Cells and Performance Applications: A Review. Front. Chem. 2022, 10, 953434. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I. , Krajmalnik-Brown, R., Parameswaran, P., Marcus, A.K., Wanger, G., Gorby, Y.A. and Rittmann, B.E. (2009) Selecting anode-respiring bacteria based on anode potential: phylogenetic, electrochemical, and microscopic characterization. Environmental science technology 43(24), 9519-9524.
- Tucci, M.; Viggi, C.C.; Núñez, A.E.; Schievano, A.; Rabaey, K.; Aulenta, F. Empowering electroactive microorganisms for soil remediation: Challenges in the bioelectrochemical removal of petroleum hydrocarbons. Chem. Eng. J. 2021, 419, 130008. [Google Scholar] [CrossRef]
- Ueki, T. (2021) Cytochromes in extracellular electron transfer in Geobacter. Applied environmental microbiology 87(10), e03109-03120.
- Velasquez-Orta, S.B.; Head, I.M.; Curtis, T.P.; Scott, K.; Lloyd, J.R.; von Canstein, H. The effect of flavin electron shuttles in microbial fuel cells current production. Appl. Microbiol. Biotechnol. 2009, 85, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Vellingiri, A. , Song, Y.E., Munussami, G., Kim, C., Park, C., Jeon, B.H., Lee, S.G. and Kim, J.R. (2019) Overexpression of c-type cytochrome, CymA in Shewanella oneidensis MR-1 for enhanced bioelectricity generation and cell growth in a microbial fuel cell. Journal of Chemical Technology Biotechnology 94(7), 2115-2122.
- Wang, J.; Li, B.; Wang, S.; Liu, T.; Jia, B.; Liu, W.; Dong, P. Metal-organic framework-derived iron oxide modified carbon cloth as a high-power density microbial fuel cell anode. J. Clean. Prod. 2022, 341, 130725. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Sun, J.; Zhang, L.; Jiao, J.; Wang, Q.; Liu, S. Nanomaterials Facilitating Microbial Extracellular Electron Transfer at Interfaces. Adv. Mater. 2020, 33, e2004051. [Google Scholar] [CrossRef] [PubMed]
- Wang, R., Li, H., Sun, J., Zhang, L., Jiao, J., Wang, Q. and Liu, S. (2021b) Nanomaterials facilitating microbial extracellular electron transfer at interfaces. Advanced Materials 33(6), 2004051.
- Wang, S. , Liu, X., Liu, H., Zhang, L., Guo, Y., Yu, S., Wozniak, D.J. and Ma, L.Z. (2015) The exopolysaccharide Psl–eDNA interaction enables the formation of a biofilm skeleton in P seudomonas aeruginosa. Environmental microbiology reports 7(2), 330-340.
- Wang, Y.-X., Hou, N., Liu, X.-L. and Mu, Y. (2021c) Advances in interfacial engineering for enhanced microbial extracellular electron transfer. Bioresource Technology 126562.
- Wang, Y.-X.; Li, W.-Q.; He, C.-S.; Zhao, H.-Q.; Han, J.-C.; Liu, X.-C.; Mu, Y. Active N dopant states of electrodes regulate extracellular electron transfer of Shewanella oneidensis MR-1 for bioelectricity generation: Experimental and theoretical investigations. Biosens. Bioelectron. 2020, 160, 112231. [Google Scholar] [CrossRef]
- Wang, Y., Song, X., Cao, X., Xu, Z., Huang, W., Wang, Y. and Ge, X. (2022b) Integration of manganese ores with activated carbon granules into CW-MFC to trigger anoxic electron transfer and removal of ammonia nitrogen. Journal of Cleaner Production 334, 130202.
- Wei, H.; Wu, X.-S.; Zou, L.; Wen, G.-Y.; Liu, D.-Y.; Qiao, Y. Amine-terminated ionic liquid functionalized carbon nanotubes for enhanced interfacial electron transfer of Shewanella putrefaciens anode in microbial fuel cells. J. Power Sources 2016, 315, 192–198. [Google Scholar] [CrossRef]
- White, G.; Edwards, M.; Gomez-Perez, L.; Richardson, D.; Butt, J.; Clarke, T. Mechanisms of Bacterial Extracellular Electron Exchange. 2016, 68, 87–138. [CrossRef]
- Wu, Y.; Luo, X.; Qin, B.; Li, F.; Häggblom, M.M.; Liu, T. Enhanced Current Production by Exogenous Electron Mediators via Synergy of Promoting Biofilm Formation and the Electron Shuttling Process. Environ. Sci. Technol. 2020, 54, 7217–7225. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Zhang, X.; Bi, C. Engineering an electroactive Escherichia coli for the microbial electrosynthesis of succinate by increasing the intracellular FAD pool. Biochem. Eng. J. 2019, 146, 132–142. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, G.; Chen, Z.; Bai, R.; Zhao, B.; Tian, X.; Wu, Y.; Zhou, X.; Zhao, F. Interspecific competition by non-exoelectrogenic Citrobacter freundii An1 boosts bioelectricity generation of exoelectrogenic Shewanella oneidensis MR-1. Biosens. Bioelectron. 2021, 194, 113614. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, E.; Zhang, J.; Dai, Y.; Yang, Z.; Christensen, H.E.M.; Ulstrup, J.; Zhao, F. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci. Adv. 2017, 3, e1700623–e1700623. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z.; Jiang, Y.; Chen, M.; Chen, B.; Xin, F.; Dong, W.; Jiang, M. Recent advances in the improvement of bi-directional electron transfer between abiotic/biotic interfaces in electron-assisted biosynthesis system. Biotechnol. Adv. 2022, 54, 107810. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.-Y.; Shi, D.-Y.; Chen, Y.-L.; Jiao, F.; Lin, X.; Zhou, J.; Wang, S.-Y.; Yong, Y.-C.; Sun, Y.-M.; OuYang, P.-K.; et al. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour. Technol. 2013, 152, 220–224. [Google Scholar] [CrossRef]
- Yong, Y.-C.; Yu, Y.-Y.; Li, C.-M.; Zhong, J.-J.; Song, H. Bioelectricity enhancement via overexpression of quorum sensing system in Pseudomonas aeruginosa-inoculated microbial fuel cells. Biosens. Bioelectron. 2011, 30, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.-C.; Yu, Y.-Y.; Yang, Y.; Liu, J.; Wang, J.-Y.; Song, H. Enhancement of extracellular electron transfer and bioelectricity output by synthetic porin. Biotechnol. Bioeng. 2012, 110, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.C. , Yu, Y.Y., Zhang, X. and Song, H. (2014b) Highly active bidirectional electron transfer by a self-assembled electroactive reduced-graphene-oxide-hybridized biofilm. Angewandte Chemie International Edition 53(17), 4480-4483.
- Zhang, F. , Yang, K., Liu, G., Chen, Y., Wang, M., Li, S. and Li, R. (2022) Recent Advances on Graphene: Synthesis, Properties, and Applications. Composites Part A: Applied Science Manufacturing 107051.
- Zhang, P.; Liu, J.; Qu, Y.; Zhang, J.; Zhong, Y.; Feng, Y. Enhanced performance of microbial fuel cell with a bacteria/multi-walled carbon nanotube hybrid biofilm. J. Power Sources 2017, 361, 318–325. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, G.; Li, X.; Zhang, W.; Zhang, J.; Ye, J.; Huang, X.; Yu, C. A graphene modified anode to improve the performance of microbial fuel cells. J. Power Sources 2011, 196, 5402–5407. [Google Scholar] [CrossRef]
- Zhang, Z., Li, Z., Sun, M., Li, J., Wu, J., Song, Y. and Feng, Y. (2020) Enhancement mechanism of microbial extracellular electron transfer process and efficient transformation of pollutants. Journal of Environmental Science 40(10), 3484-3493.
- Zhao, C.-E.; Gai, P.; Song, R.; Chen, Y.; Zhang, J.; Zhu, J.-J. Nanostructured material-based biofuel cells: recent advances and future prospects. Chem. Soc. Rev. 2017, 46, 1545–1564. [Google Scholar] [CrossRef]
- Zhao, J.; Li, F.; Cao, Y.; Zhang, X.; Chen, T.; Song, H.; Wang, Z. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol. Adv. 2020, 53, 107682. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Li, X.F.; Ren, Y.P.; Wang, X.H. Effect of static magnetic field on the performances of and anode biofilms in microbial fuel cells. RSC Adv. 2016, 6, 82301–82308. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, B.; Wang, Q.; Sun, J.; Xie, G.; Ren, N.; Ren, Z.J.; Xing, D. Pulse electromagnetic fields enhance extracellular electron transfer in magnetic bioelectrochemical systems. Biotechnol. Biofuels 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Zhou, H.; Mei, X.; Liu, B.; Xie, G.; Xing, D. Magnet anode enhances extracellular electron transfer and enrichment of exoelectrogenic bacteria in bioelectrochemical systems. Biotechnol. Biofuels 2019, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wu, X.; Huang, Y.; Ni, H.; Long, Z.-E. Promoting Shewanella Bidirectional Extracellular Electron Transfer for Bioelectrocatalysis by Electropolymerized Riboflavin Interface on Carbon Electrode. Front. Microbiol. 2019, 9, 3293. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).