Introduction
The flexor digitorum superficialis (FDS) is the largest among the superficial forearm flexor muscles situated in the anterior compartment of the forearm and plays a pivotal role in finger flexion, enabling essential hand movements for daily activities [
1]. It is known for its complex structure with many anatomical variances (e.g. anomalous muscle bellies, anomalous tendon arrangement or intermediate tendons) that are a subject of ongoing research [
2,
3]. Among these variations, especially the FDS of the fifth digit has shown significant structural variability, with reports of complete absence in up to 21% of individuals as well as right and left hand asymmetry in approximately 26% of individuals [
4,
5,
6]. The clinical significance of the many different anatomic variations of the FDS includes atypical findings on physical examination and altered use patterns in the injured state [
3]. It is known that flexor tendon injuries of the hand often need surgical treatment for the best functional outcome: Partial tendon lacerations <50% are commonly managed with debridement alone, partial lacerations >50% may be treated with epitendinous repair, and complete lacerations commonly require multi-strand core repair or even tendon graft surgery [
7,
8]. Therefore a detailed understanding of FDS variability is crucial when performing procedures involving the palmar side of the forearm and the complexity of muscle anatomy should raise the watchfulness for unexpected findings at surgery [
3,
7].
The gross structure of the FDS is described as follows in anatomical textbooks: It is situated underneath the palmaris longus, flexor carpi radialis and flexor carpi ulnaris muscles and superficial to the flexor digitorum profundus and flexor pollicis longus. The FDS arises by three heads - humeral (from the medial epicondyle of the humerus), ulnar (from the medial coronoid process) and radial (from the oblique line of the radius). Its muscle belly can be separated into a superficial and a deep layer; the former dividing into two parts concluding in tendons for the third and fourth digits; the latter forming an intermediate tendon in the middle of the forearm that transitions into the distal belly for the second digit. The FDS belly for the fifth digit takes its origin from the intermediate tendon and runs in a slightly oblique course to the ulnar side of the distal forearm [
1,
2].
While the concept of the FDS/2 tendon crossing under the FDS/3 tendon was firstly described by von Luschka in 1865, this knowledge was largely forgotten until recently [
9]. In 2023, Ergün et al. provided a detailed topographical description of the bellies and tendons of the FDS based on anatomical dissection studies. Hereby, the authors demonstrated that the tendon for digit 2 runs from proximal-ulnar to distal-radial and while doing so, crosses under the third digit’s FDS tendon from the ulnar to the radial side. They coined this intersection the “chiasma antebrachii”. Moreover, the authors provided the first graphical representation of the FDS that enabled a new view on this muscle [
10].
Despite the establishment of the term "chiasma antebrachii" as an anatomical reference, it remains unclear whether this tendon intersection represents the standard anatomy or rather an uncommon variant. Therefore, the objective of the current study was to investigate its presence and detailed morphology through MRI analysis of the forearm in a clinical patient sample, hereby contributing to a better understanding of the variations of the FDS and potentially aiding clinical interventions.
Material and Methods
Study population
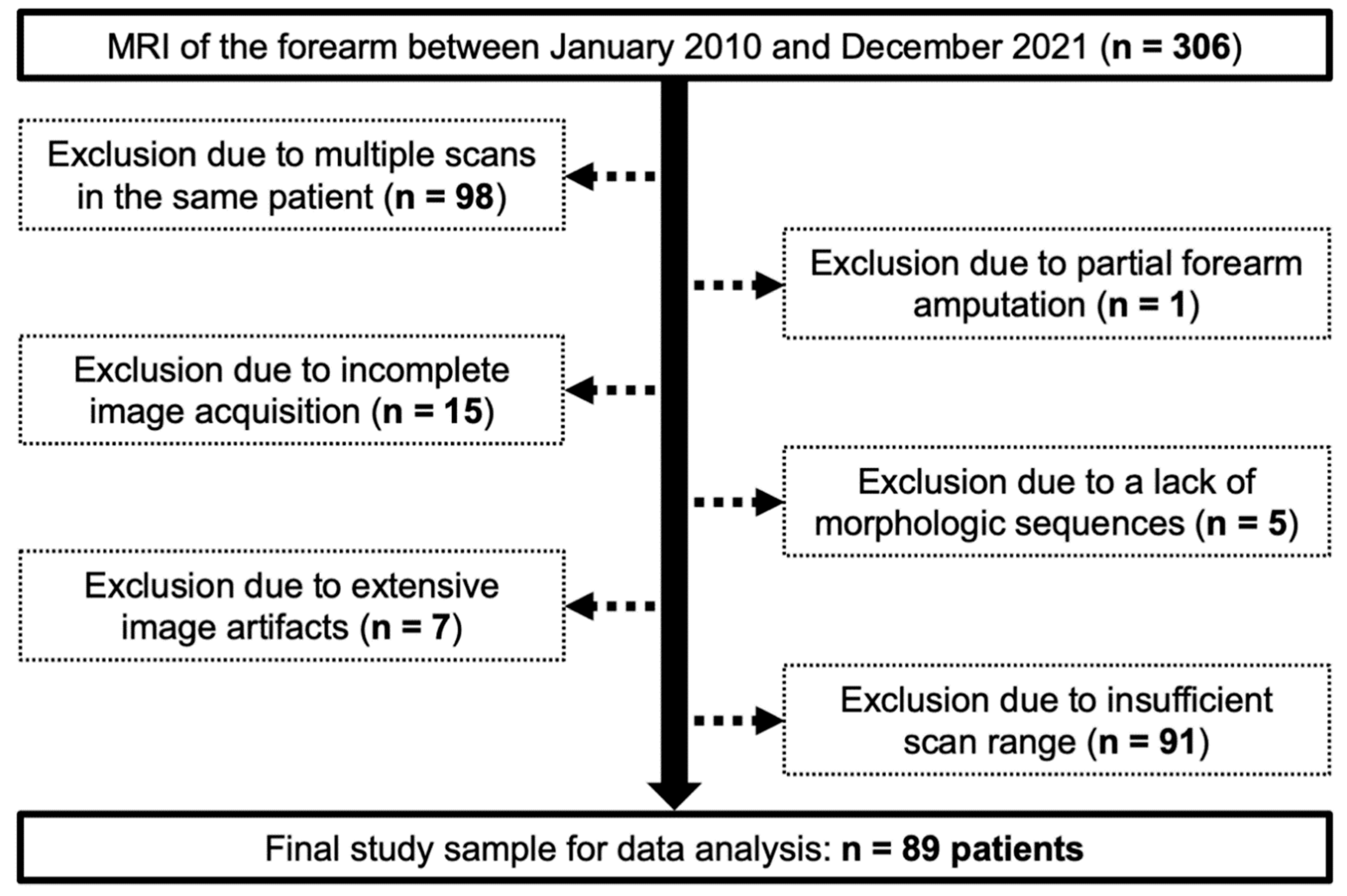
The local institutional review board approved this retrospective data analysis and waived the need for written informed consent (reference number: 20220818 01). Between January 2010 and December 2021, 306 consecutive MRI examinations of the forearm were available for radiological assessment. Of these, 217 had to be excluded from the data analysis for one of the following reasons: Multiple scans in the same patient (
n = 98), partial forearm amputation (
n = 1), incomplete image acquisition (
n = 15), lack of morphologic T1- and/or T2-weighted sequences (
n = 5), extensive image artifacts with resulting loss of diagnostic assessability (
n = 7) or insufficient scan range to evaluate the entire FDS anatomy (
n = 91). Summarizing exclusions and inclusions, a flow chart illustrates the population analyzed in this study (
Figure 1).
MRI examinations
Forearm studies were performed both on 1.5 T scanners (Magnetom Aera, Avanto or Symphony, Siemens Healthineers) in 43 scans (48.3%) and 3.0 T scanners (Magnetom Prisma or Skyra, Siemens Healthineers) in 46 scans (51.7%). All examinations were conducted in accordance with the clinically established scan protocol for the respective imaging task, including intravenous contrast application if necessary. Patients adopted a pronated position of the hand in 82 scans (92.1%), a neutral position in 5 scans (5.6%) and a supinated position in 2 scans (2.2%).
Image assessment
Two radiologists with one and six years of musculoskeletal imaging experience (C.E., J.-P.G.) collectively analyzed the above-described datasets in chronological order using a commercially available picture archiving and communication system (Merlin, Phönix-PACS) installed on a radiological workstation with a certified diagnostic monitor. The observers were given three tasks for their reads: First, to evaluate the presence of the chiasma antebrachii in dichotomous fashion (0 = no chiasma antebrachii, 1 = identifiable chiasma antebrachii). Second, if applicable, to assess the length of the chiasma. Third, to analyze the position of the FDS/2 and FDS/3 intersection by measuring the distance from proximal margin of the identified chiasma to the distal radioulnar joint as well as to the elbow joint. Both distinct landmarks were chosen to ensure reproducibility of measurements. Before commencing their reads, the radiologists reviewed five training cases not included in the study sample.
Dissected human forearms
In order to verify the image findings in anatomical dissection studies, the forearms of 11 formalin-fixated body donors from the local anatomical institute (including 6 women, age range: 55–100 years) were assessed. Before their dissection, cadaveric specimens had been fixated by intra-arterial perfusion with 4 – 4.5% formaldehyde solution, containing formaldehyde 37% (1.2 – 1.8 liters, depending on size and weight of the donors), Carlsbad salt (400 gram), chloral hydrate (400 milliliters), and Lysoformin® (400 milliliters) in 8 liters of water for several hours. Subsequently, the body donors had been stored in ethanol-(33%)-solution-filled metal containers for preservation by ethanol vapor for 1 – 2 years. The use of the human material was in full compliance with the university policy for use of body donors and recognizable body parts.
Statistics
Data analysis was supported by dedicated statistical software (SPSS version 28.0, IBM). Normal distribution of continuous items was analyzed with Kolmogorov-Smirnov tests [
11]. For parametric data with normal distribution, mean ± standard deviation is presented; otherwise, we report median and interquartile range values (IQR). Irrespective of item scale, absolute and relative frequencies are indicated for all variables.
Results
Study population
Adhering to inclusion and exclusion criteria depicted in
Figure 1, the final study group was comprised of 89 patients, including 41 women (46.1%), and had a mean age of 39.3 ± 21.3 years (range 2 – 84 years). In 47 cases (52.8 %), the left forearm was scanned. MRI examinations were performed for different clinical indications: primary or follow-up imaging of a benign or malignant tumor of the forearm (
n = 25), a history of trauma (
n = 35), diagnostic assessment of soft tissue inflammation (
n = 20), and others, such as pain without trauma history or suspected neuropathy of the median nerve (
n = 9).
MRI-based flexor digitorum superficialis assessment
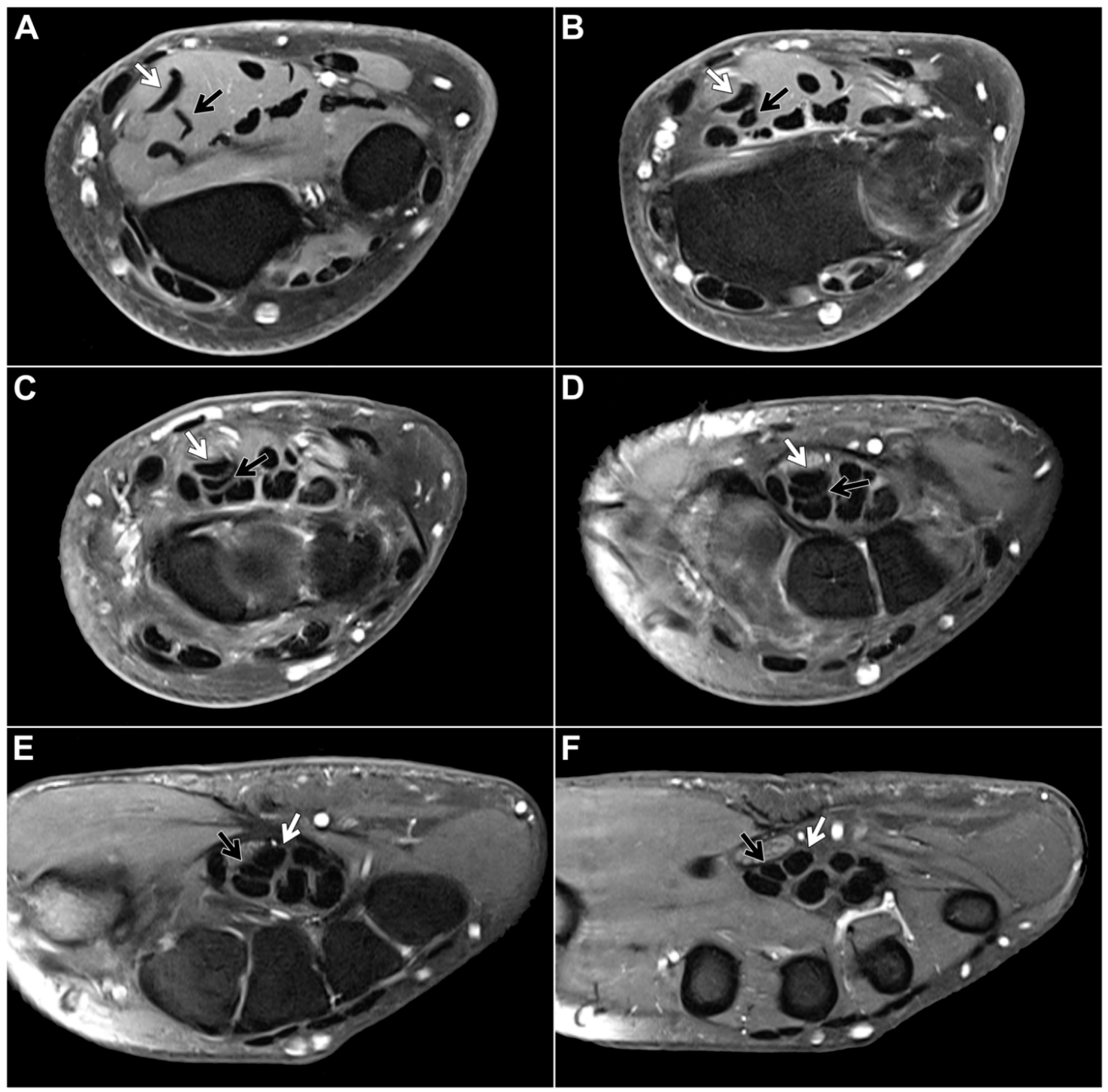
Within the study sample, the chiasma antebrachii with the above-described anatomical features was identified in 88 patients (98.9%). We defined the proximal margin of the chiasma as the first axial slice where the distal FDS/2 tendon was depicted inferiorly to the FDS/3 tendon and the distal margin as the first axial slice when the two tendons could be depicted horizontally adjacent to each other again (
Figure 2). Measuring according to these parameters, the chiasma antebrachii had a median length of 28 mm (IQR: 24 mm – 35 mm). Next, we assessed the location of the chiasma in the forearm in relation to the distal radioulnar joint and the elbow joint. We measured the respective distance from the proximal margin and documented a median distance to the distal radioulnar joint of 16 mm (IQR: 8 mm – 25 mm) and a median distance to the elbow joint of 215 mm (IQR: 187 mm – 227 mm). Measurements were performed based on axial images but were correlated in at least one other standard plane. In only one patient, the intersection was located in the middle part of the forearm. In this particular case, the intermediate tendon of the FDS crossed under the FDS3 from ulnar to the radial side. As a consequence, the distal FDS tendon segments for digits 2 and 3 could be depicted running parallel to each other in the distal third of the forearm up to their insertions at the mid phalanx of digits 2 and 3, with the FDS/2 located more radially (
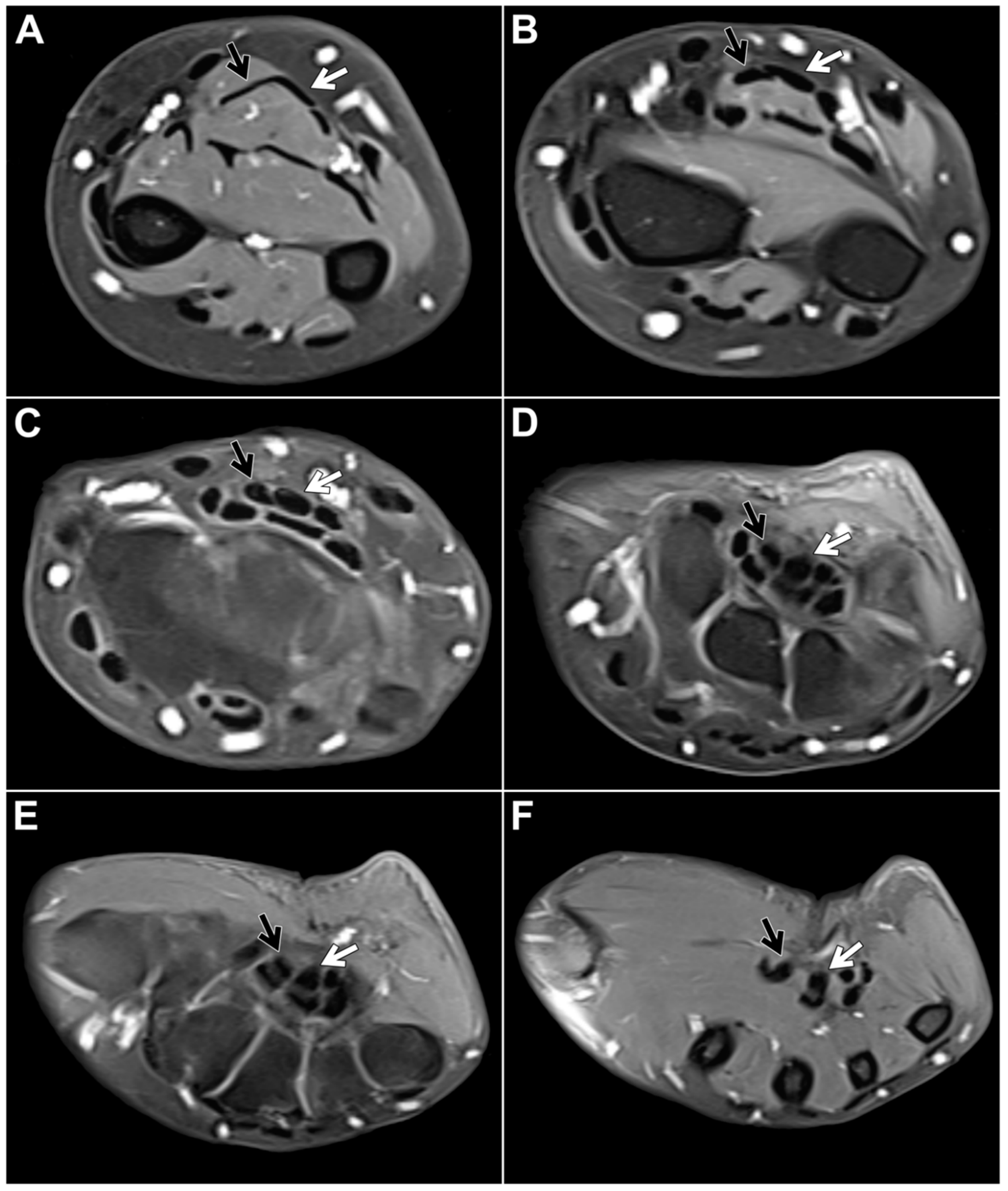
Figure 3).
Finally, the MRI sequence with the subjectively best depiction of the chiasma antebrachii was chosen by the observing radiologists. In 71 cases (79.8%), the observers deemed the T1-weighted post-contrast sequence superior, whereas a T2- or proton density-weighted sequence was preferred in 18 examinations (20.2%).
Verification of findings in cadaveric dissection studies
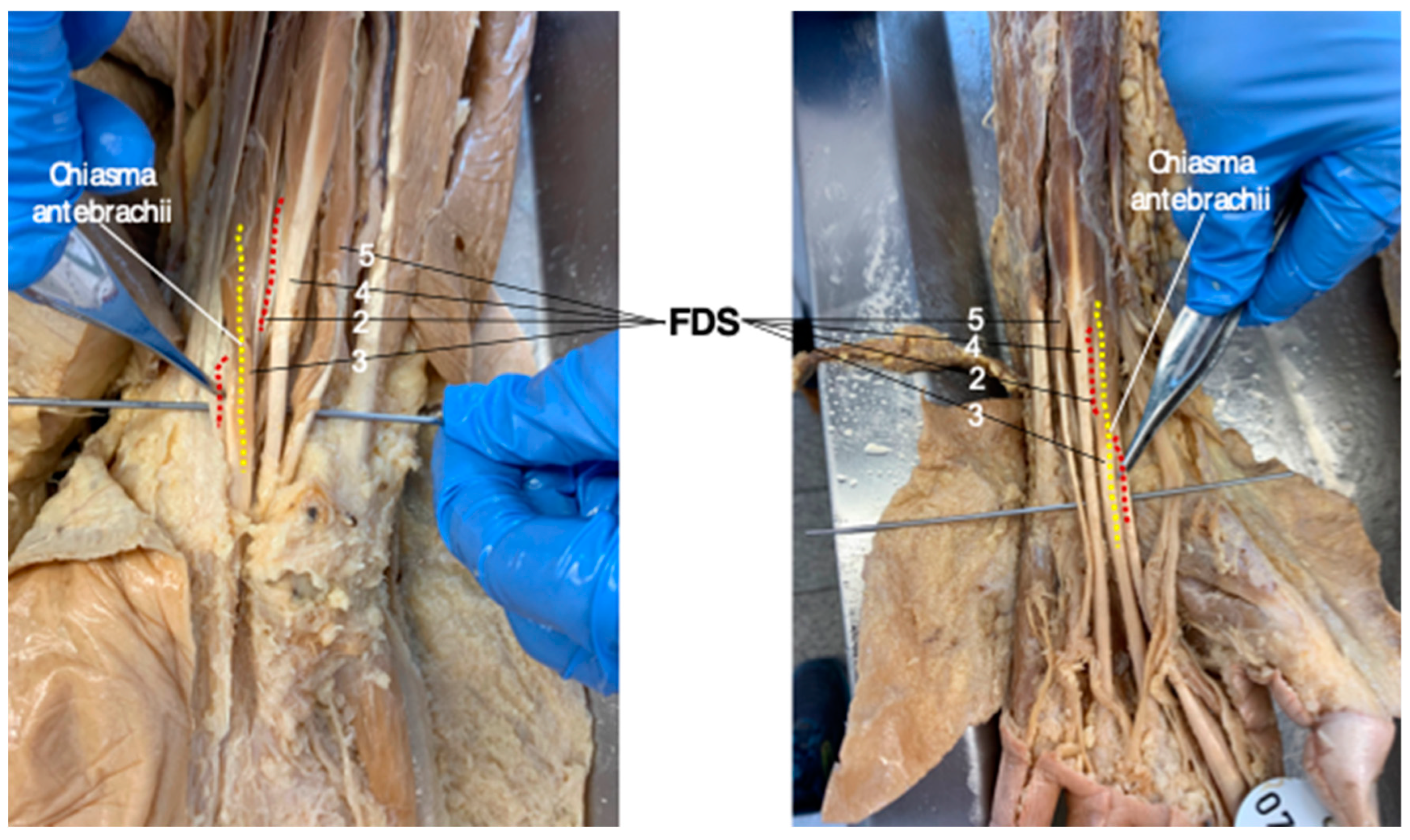
In all 11 human body donates assessed for this study, the chiasma antebrachii was present bilaterally in the distal portion of both forearms with the same anatomical configuration that was determined in the vast majority of MRI studies.
Figure 4 shows the “standard” FDS tendon anatomy, including the chiasma antebrachii located in the distal portion of the forearm, in two cadaveric specimens (
Figure 4).
Discussion
In this study, we provide a detailed MRI-based analysis of the recently introduced chiasma antebrachii, an anatomical feature defined by the distal FDS/2 tendon crossing under the FDS/3 from proximal-ulnar to distal-radial in the distal forearm. We were able to show that this intersection is typically localized in the distal portion of the forearm, where its presence could be determined in 98.9% of individuals. Only one intersection was found to be located more proximal in the middle part of the forearm. Based on our findings, we postulate that the chiasma antebrachii represents the standard anatomy of the flexor digitorum superficialis rather than an uncommon normal variant. In its standard configuration, it possesses a median length of 28 mm (IQR: 24 mm – 35 mm).
The presented analysis of detailed MR imaging supports and confirms the anatomical description of the chiasma antebrachii as introduced by Ergün et. al based on anatomical dissection studies [
10]. Our findings regarding the location of the chiasma concur with the observations in cadaveric specimens, as the distance of the tendon intersection to the elbow joint is reported with 220 ± 35 mm, while we measured a median distance of 215 mm. However, a distance to the wrist of 3-4 cm was described, as opposed to 16 mm in this study. There are several possible explanations for this incongruity: First, we strictly defined the beginning of the chiasma antebrachii (and therefore our proximal measuring point) as the first axial plane where the FDS/2 tendon was depicted inferiorly to the FDS/3 tendon, whereas Ergün et al. measured the distance “from the tendon crossing”, rendering an exact comparison difficult. Second, we chose the distal radioulnar joint, a distinct landmark, as our distal measuring point to ensure reproducibility, while the transverse carpal ligament was used in the previous anatomical study [
10]. As this ligament with its osseous attachments to the scaphoid and trapezium on the radial side and the pisiform and hamate on the ulnar side lies further distal of the distal radioulnar joint, the differing measurements seem plausible [
12]. Lastly, measurements performed on 0.6 – 3 mm thick axial slices on a diagnostic monitor might be more accurate especially in the millimeter range compared to measuring by hand in dissection studies.
We deemed the best MRI sequence for the depiction of the chiasma antebrachii to be the T1-weighted post-contrast sequence, followed by a T2- or proton density-weighted sequence. This result correlates with the latest protocol recommendations for assessment of tendon-related pathologies of the distal forearm and hand in MRI [
13,
14]. To optimize image quality, it is also recommended that patients are placed prone with the arm extended over head in the so-called “superman position”, which allows to place the wrist as close to the magnetic field’s isocenter as possible, therefore obtaining the highest signal-to-noise ratio and most homogeneous signal [
14]. Adhering to these recommendations, the superman position constitutes the diagnostic standard in our department with 82 (92.1%) of the scans analyzed in this study being performed this way. However, this position can be uncomfortable for some patients, rendering them unable to complete the examination. In these cases, other options may be considered, such as a supine position with the patient’s arm besides the body trunk, or the “prayer position” with the patient flexing the elbow while lying on their side [
14].
Exact understanding of the forearm muscle anatomy is not only important for teaching purposes in medical schools, but also for clinical interventions such as operative tendon reconstruction, minimal invasive surgery or target-specific application of therapeutics. For example, in cases of focal spasticity involving the upper limb, a precise injection of Botulinum neurotoxin into the affected muscles is considered the first-line treatment as this reduces spasticity while maintaining motor performance of the weakened spastic muscles [
15,
16]. Therefore, detailed knowledge of the functional and topographical anatomy of the FDS muscles including awareness of the location and course of nearby structures is needed to achieve a beneficial therapeutic outcome as well as prevent accidental injections into unintended areas and reduce the risk of adverse events.
Notably, the chiasma antebrachii possesses similarities to the proximal (crossing of the abductor pollicis longus and extensor pollicis brevis tendons over the extensor carpi radialis longus and brevis tendons) and distal extensor tendon intersections (crossing of the extensor pollicis longus tendon over the extensor carpi radialis longus and brevis tendons) on the dorsal side of the wrist [
13,
17]. The two listed tendon intersections are of great clinical relevance: Repetitive extension-flexion movements at these junctions commonly seen in sporting activities can result in a localized friction injury, generating tenosynovitis and characteristically leading to pain, swelling, and functional limitations; a clinical condition termed “intersection syndrome” [
18,
19]. Considering the anatomic similarities, the occurrence of a clinically relevant flexor-sided intersection syndrome in patients with intensive use of the index and middle fingers is conceivable. We therefore suggest that future studies investigate whether the FDS tendons in symptomatic patients display signs of friction-induced tenosynovitis at the level of the chiasma.
Some limitations have to be acknowledged with regard to this study. The retrospective design of the study resulted in a heterogeneous patient population in terms of sociodemographic data and clinical history. Factors such as tumor presence in the forearm, history of trauma, and soft tissue inflammation introduced variations among the participants. However, these pathologies did not have a significant impact on the specific questions addressed in this study. This is primarily because the respective conditions did not exhibit a direct topographical relationship with the FDS tendons under examination. Therefore, despite the heterogeneity within the patient population, the findings of the study remain valid and relevant to the research questions posed. Additionally, the inclusion of patients with various sociodemographic backgrounds and clinical histories contributes to the generalizability of the study findings to a broader population. As the scans were performed for different clinical indications, the scan protocols varied depending on the initial clinical question. Scans with a slice thickness of 0.6 mm might allow for a more precise evaluation and measurement than protocols with a greater slice thickness.
With this study we provide new insights into the complex anatomy of the flexor digitorum superficialis with a specific focus on the chiasma antebrachii. Our results not only support the initial description of the chiasma by Ergün et al., but also emphasize its significance as a key anatomic feature that can be observed in nearly all individuals. Furthermore, this research might serve as a foundation for future studies exploring the clinical implications of the chiasma antebrachii in conditions such as intersection syndromes.
Author Contributions
Conceptualization, Clara Elsner and Jan-Peter Grunz; Data curation, Clara Elsner, Andreas Kunz, Thorsten Bley, Nicole Wagner, Stefan Hübner and Jan-Peter Grunz; Formal analysis, Clara Elsner, Andreas Kunz, Henner Huflage and Jan-Peter Grunz; Funding acquisition, Jan-Peter Grunz; Investigation, Clara Elsner, Andreas Kunz, Henner Huflage and Karsten Luetkens; Methodology, Clara Elsner, Andreas Kunz, Nicole Wagner, Henner Huflage, Stefan Hübner, Karsten Luetkens and Jan-Peter Grunz; Project administration, Jan-Peter Grunz and Süleyman Ergün; Resources, Rainer Schmitt, Süleyman Ergün and Thorsten Bley; Supervision, Jan-Peter Grunz, Andreas Kunz and Thorsten Bley; Validation, Clara Elsner, Süleyman Ergün and Jan-Peter Grunz; Visualization, Clara Elsner and Henner Huflage; Writing – original draft, Clara Elsner; Writing – review & editing, Andreas Kunz, Nicole Wagner, Henner Huflage, Stefan Hübner, Karsten Luetkens, Thorsten Bley, Rainer Schmitt, Süleyman Ergün and Jan-Peter Grunz.
Funding
This work was partially funded by the Interdisciplinary Center of Clinical Research Würzburg [J.-P. G., grant number Z-3BC/02]. This publication was further supported by the Open Access Publication Fund of the University of Würzburg, Germany.
Institutional Review Board Statement
For this retrospective study, permission was obtained from the Institutional Review Board of the University of Würzburg, Germany (IRB number 20220818 01). All procedures were in accordance with the ethical standards of the institutional and national research committee and with the 1975 Declaration of Helsinki.
Informed Consent Statement
For this retrospective study, permission was obtained from the Institutional Review Board of the University of Würzburg, Germany.
Data Availability Statement
The datasets generated and/or analyzed during this study are not publicly available as MRI data and DICOM headers contain patient information. Data can be obtained on reasonable request from the corresponding author.
Conflicts of Interest
A.S.K., T.A.B. and J.P.G have received speaker honoraria from Siemens Healthineers within the past three years not related to the subject of this article. The Department of Diagnostic and Interventional Radiology receives ongoing research funding by Siemens Healthineers outside of the presented work. The authors of this manuscript declare no further relationships with any companies whose products of services may be related to the subject matter of the article.
References
- Gray, H. and W.H. Lewis, Anatomy of the human body. 20th ed. 1918, Philadelphia and New York: Lea & Febiger.
- Ohtani, O., Structure of the flexor digitorum superficialis. Okajimas Folia Anat Jpn, 1979. 56(5): p. 277-88.
- Tan, J.S., L. Oh, and D.S. Louis, Variations of the flexor digitorum superficialis as determined by an expanded clinical examination. J Hand Surg Am, 2009. 34(5): p. 900-6. [CrossRef]
- Gonzalez, M.H., et al., Variations of the flexor digitorum superficialis tendon of the little finger. J Hand Surg Br, 1997. 22(2): p. 277-80. [CrossRef]
- Townley, W.A., M.C. Swan, and R.L. Dunn, Congenital absence of flexor digitorum superficialis: implications for assessment of little finger lacerations. J Hand Surg Eur Vol, 2010. 35(5): p. 417-8. [CrossRef]
- Austin, G.J., B.M. Leslie, and L.K. Ruby, Variations of the flexor digitorum superficialis of the small finger. J Hand Surg Am, 1989. 14(2 Pt 1): p. 262-7. [CrossRef]
- Griffin, M., et al., An overview of the management of flexor tendon injuries. Open Orthop J, 2012. 6: p. 28-35. [CrossRef]
- Pearce, O., et al., Flexor tendon injuries: Repair & Rehabilitation. Injury, 2021. 52(8): p. 2053-2067. [CrossRef]
- von Luschka, H., Die Anatomie des Menschen in Rücksicht auf die Bedürfnisse der praktischen Heilkunde. Bd.3., 1865.
- Ergun, S., et al., Old name, new face: A systematic analysis of flexor digitorum superficialis muscle with "chiasma antebrachii". Ann Anat, 2023. 247: p. 152052. [CrossRef]
- FJ., M., The Kolmogorov-Smirnov Test for Goodness of Fit. J Am Stat Assoc, 1951(46:68–78). [CrossRef]
- Goitz, R.J., J.R. Fowler, and Z.M. Li, The transverse carpal ligament: anatomy and clinical implications. J Wrist Surg, 2014. 3(4): p. 233-4. [CrossRef]
- Schmitt, R., N. Hesse, and J.P. Grunz, Tendons and Tendon Sheaths of the Hand - An Update on MRI. Rofo, 2022. 194(12): p. 1307-1321. [CrossRef]
- Vassa, R., A. Garg, and I.M. Omar, Magnetic resonance imaging of the wrist and hand. Pol J Radiol, 2020. 85: p. e461-e488. [CrossRef]
- Simpson, D.M., et al., Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology, 2016. 86(19): p. 1818-26. [CrossRef]
- Chen, Y.T., et al., The Effects of Botulinum Toxin Injections on Spasticity and Motor Performance in Chronic Stroke with Spastic Hemiplegia. Toxins (Basel), 2020. 12(8). [CrossRef]
- Costa, C.R., W.B. Morrison, and J.A. Carrino, MRI features of intersection syndrome of the forearm. AJR Am J Roentgenol, 2003. 181(5): p. 1245-9. [CrossRef]
- Chatterjee, R. and J. Vyas, Diagnosis and management of intersection syndrome as a cause of overuse wrist pain. BMJ Case Rep, 2016. 2016. [CrossRef]
- Michols, N.J. and J. Kiel, Intersection Syndrome, in StatPearls. 2023: Treasure Island (FL).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).